537c7a9df9480b10c5174421df2a4067.ppt
- Количество слайдов: 32
 Some interesting physics in transition metal oxides: charge ordering, orbital ordering and spin-charge separation C. D. Hu Department of physics National Taiwan University 1. Introduction to double-exchange interaction (DE) 2. Introduction to charge ordering 3. Introduction to orbital ordering and orbital dynamics 4. Interplay between double-exchange interaction, charge ordering, and orbital ordering
Some interesting physics in transition metal oxides: charge ordering, orbital ordering and spin-charge separation C. D. Hu Department of physics National Taiwan University 1. Introduction to double-exchange interaction (DE) 2. Introduction to charge ordering 3. Introduction to orbital ordering and orbital dynamics 4. Interplay between double-exchange interaction, charge ordering, and orbital ordering
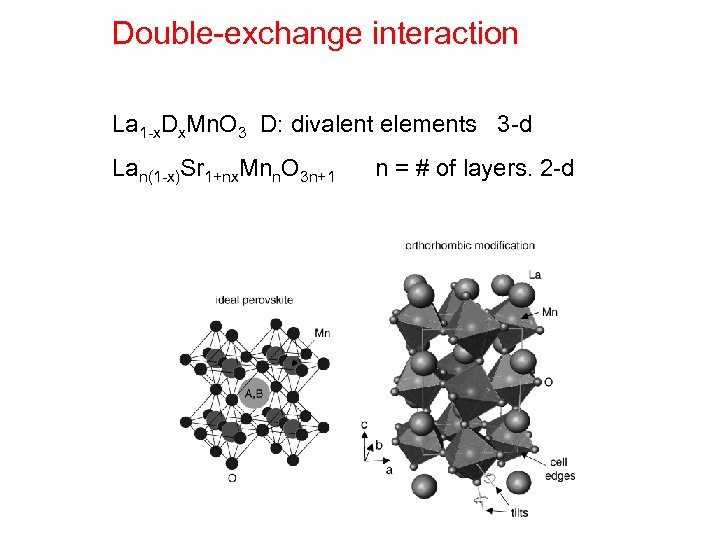 Double-exchange interaction La 1 -x. Dx. Mn. O 3 D: divalent elements 3 -d Lan(1 -x)Sr 1+nx. Mnn. O 3 n+1 n = # of layers. 2 -d
Double-exchange interaction La 1 -x. Dx. Mn. O 3 D: divalent elements 3 -d Lan(1 -x)Sr 1+nx. Mnn. O 3 n+1 n = # of layers. 2 -d
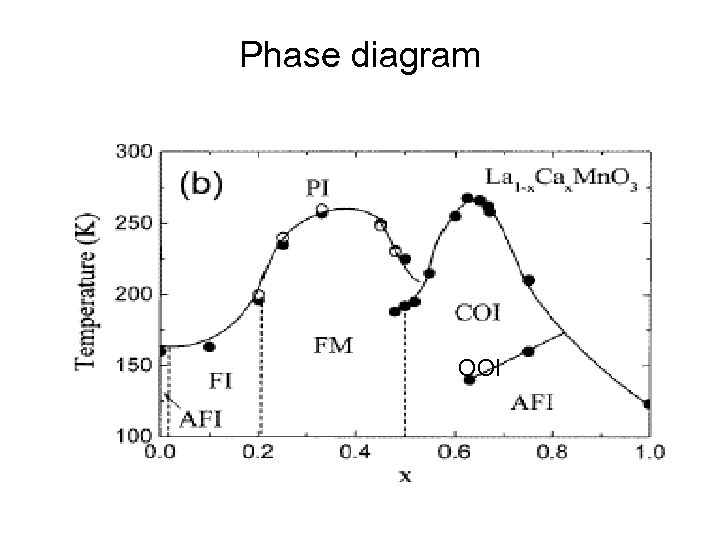 Phase diagram OOI
Phase diagram OOI
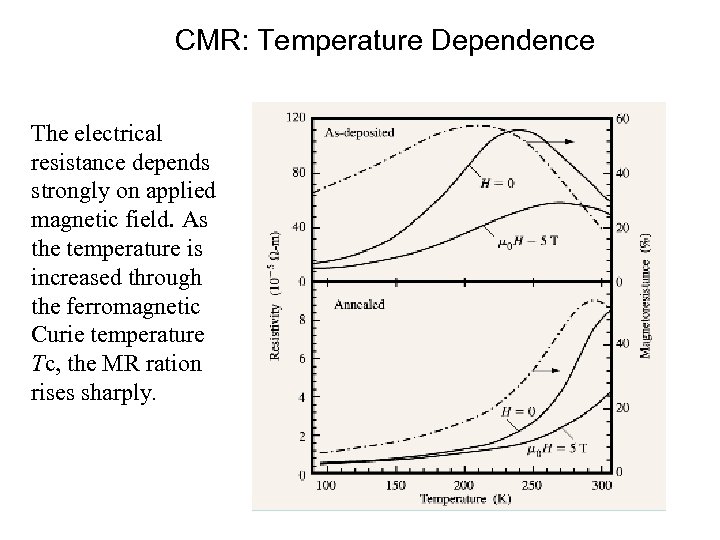 CMR: Temperature Dependence The electrical resistance depends strongly on applied magnetic field. As the temperature is increased through the ferromagnetic Curie temperature Tc, the MR ration rises sharply.
CMR: Temperature Dependence The electrical resistance depends strongly on applied magnetic field. As the temperature is increased through the ferromagnetic Curie temperature Tc, the MR ration rises sharply.
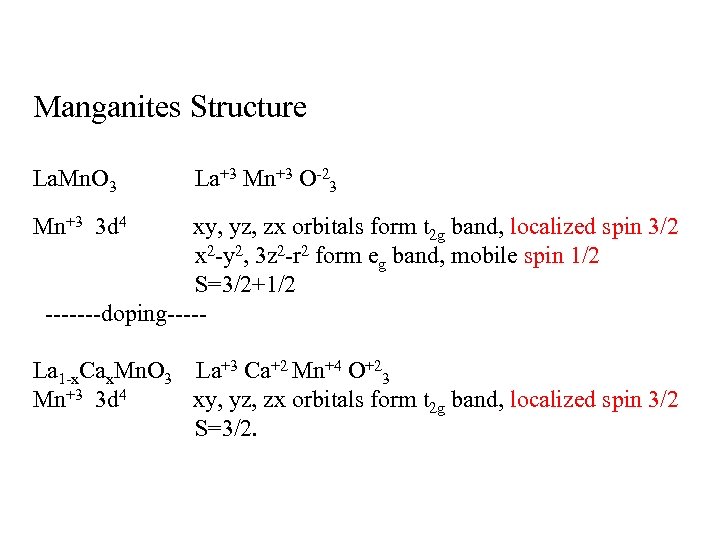 Manganites Structure La. Mn. O 3 La+3 Mn+3 O-23 Mn+3 3 d 4 xy, yz, zx orbitals form t 2 g band, localized spin 3/2 x 2 -y 2, 3 z 2 -r 2 form eg band, mobile spin 1/2 S=3/2+1/2 -------doping----- La 1 -x. Cax. Mn. O 3 La+3 Ca+2 Mn+4 O+23 Mn+3 3 d 4 xy, yz, zx orbitals form t 2 g band, localized spin 3/2 S=3/2.
Manganites Structure La. Mn. O 3 La+3 Mn+3 O-23 Mn+3 3 d 4 xy, yz, zx orbitals form t 2 g band, localized spin 3/2 x 2 -y 2, 3 z 2 -r 2 form eg band, mobile spin 1/2 S=3/2+1/2 -------doping----- La 1 -x. Cax. Mn. O 3 La+3 Ca+2 Mn+4 O+23 Mn+3 3 d 4 xy, yz, zx orbitals form t 2 g band, localized spin 3/2 S=3/2.
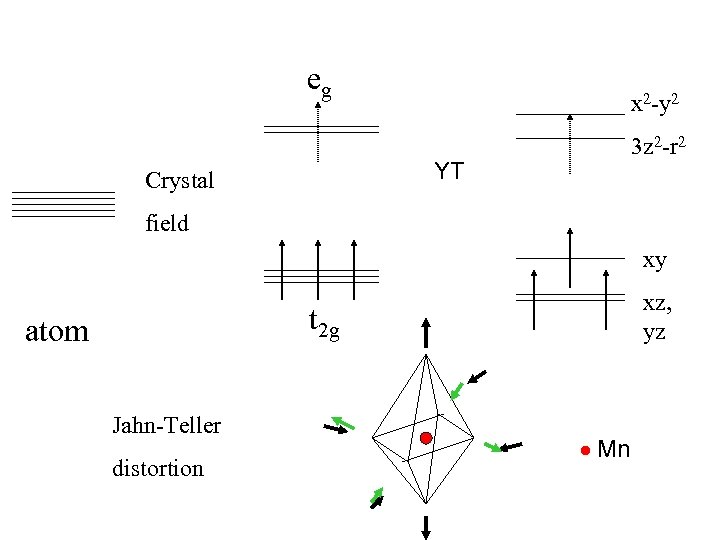 eg x 2 -y 2 3 z 2 -r 2 YT Crystal field xy xz, yz t 2 g atom Jahn-Teller distortion Mn
eg x 2 -y 2 3 z 2 -r 2 YT Crystal field xy xz, yz t 2 g atom Jahn-Teller distortion Mn
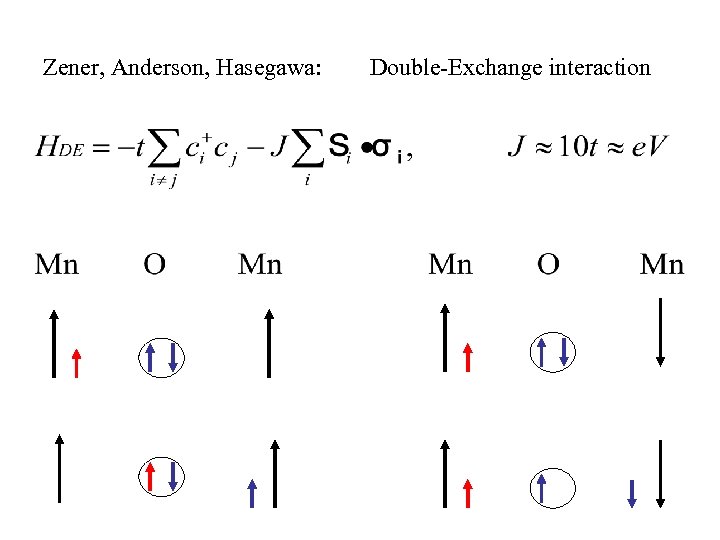 Zener, Anderson, Hasegawa: Double-Exchange interaction
Zener, Anderson, Hasegawa: Double-Exchange interaction
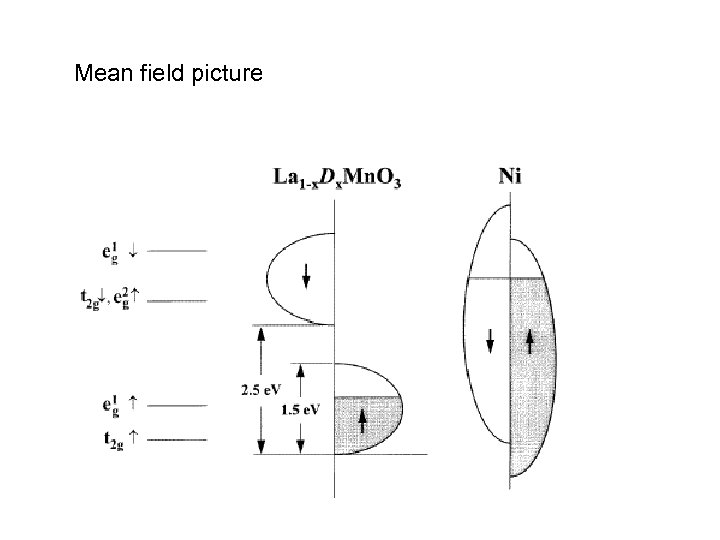 Mean field picture
Mean field picture
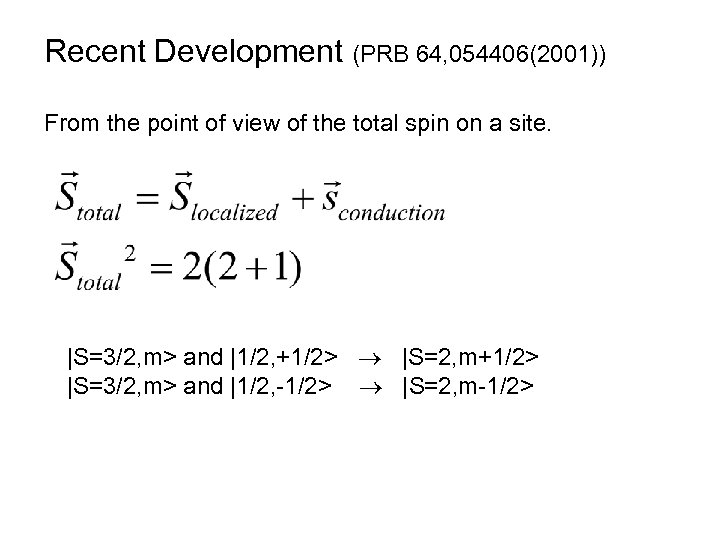 Recent Development (PRB 64, 054406(2001)) From the point of view of the total spin on a site. |S=3/2, m> and |1/2, +1/2> |S=2, m+1/2> |S=3/2, m> and |1/2, -1/2> |S=2, m-1/2>
Recent Development (PRB 64, 054406(2001)) From the point of view of the total spin on a site. |S=3/2, m> and |1/2, +1/2> |S=2, m+1/2> |S=3/2, m> and |1/2, -1/2> |S=2, m-1/2>
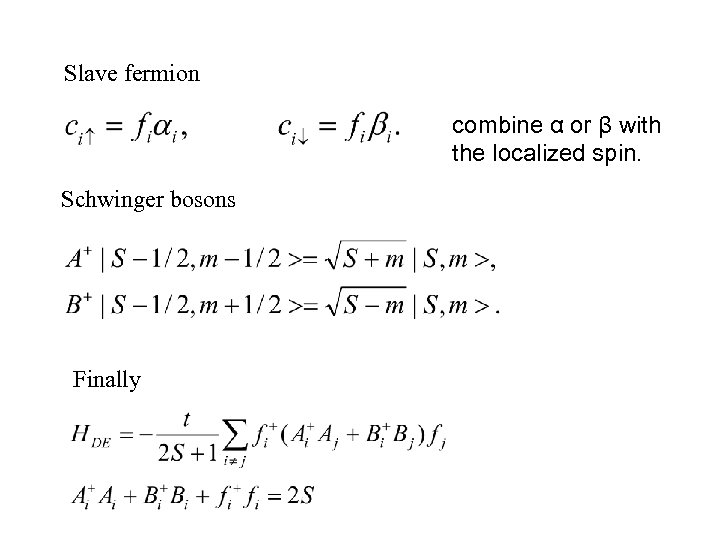 Slave fermion combine α or β with the localized spin. Schwinger bosons Finally
Slave fermion combine α or β with the localized spin. Schwinger bosons Finally
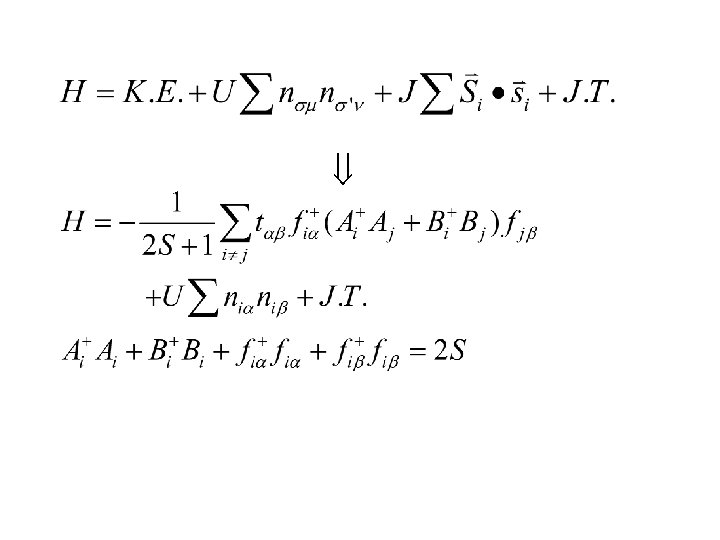
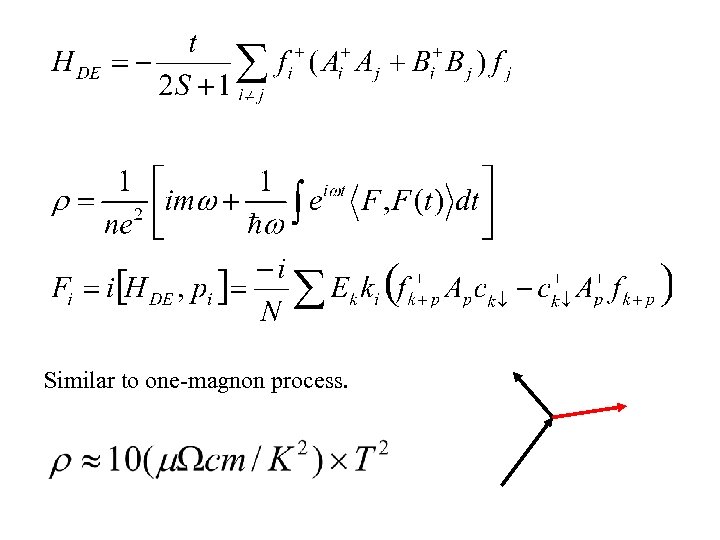 Similar to one-magnon process.
Similar to one-magnon process.
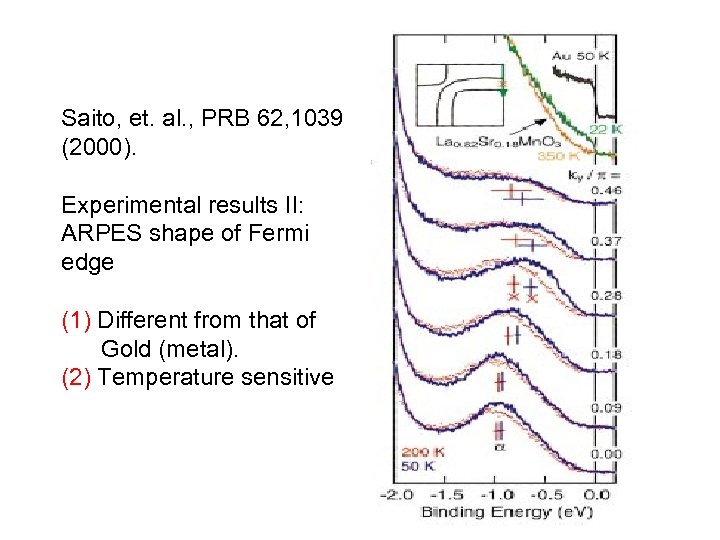 Saito, et. al. , PRB 62, 1039 (2000). Experimental results II: ARPES shape of Fermi edge (1) Different from that of Gold (metal). (2) Temperature sensitive
Saito, et. al. , PRB 62, 1039 (2000). Experimental results II: ARPES shape of Fermi edge (1) Different from that of Gold (metal). (2) Temperature sensitive
 f f f f f
f f f f f
 Charge ordering • • Many compounds cuperates manganites magnetites
Charge ordering • • Many compounds cuperates manganites magnetites
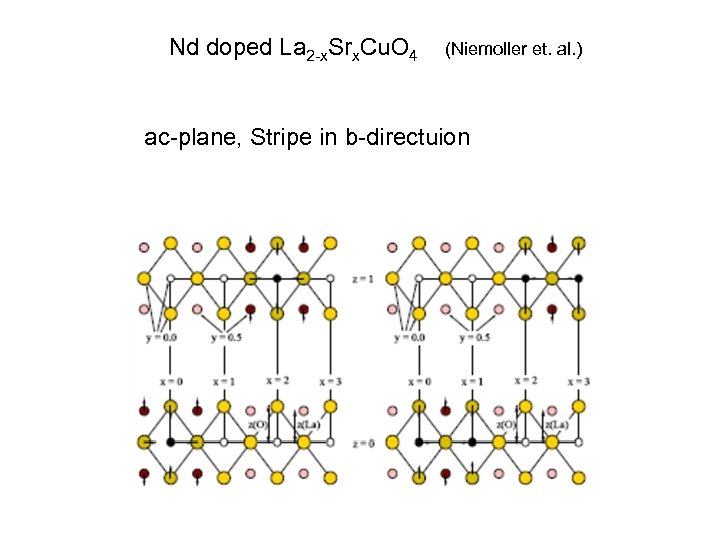 Nd doped La 2 -x. Srx. Cu. O 4 (Niemoller et. al. ) ac-plane, Stripe in b-directuion
Nd doped La 2 -x. Srx. Cu. O 4 (Niemoller et. al. ) ac-plane, Stripe in b-directuion
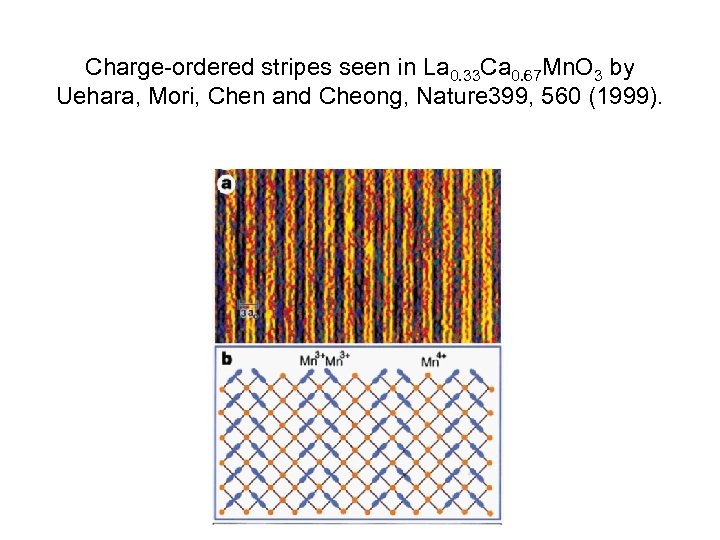 Charge-ordered stripes seen in La 0. 33 Ca 0. 67 Mn. O 3 by Uehara, Mori, Chen and Cheong, Nature 399, 560 (1999).
Charge-ordered stripes seen in La 0. 33 Ca 0. 67 Mn. O 3 by Uehara, Mori, Chen and Cheong, Nature 399, 560 (1999).
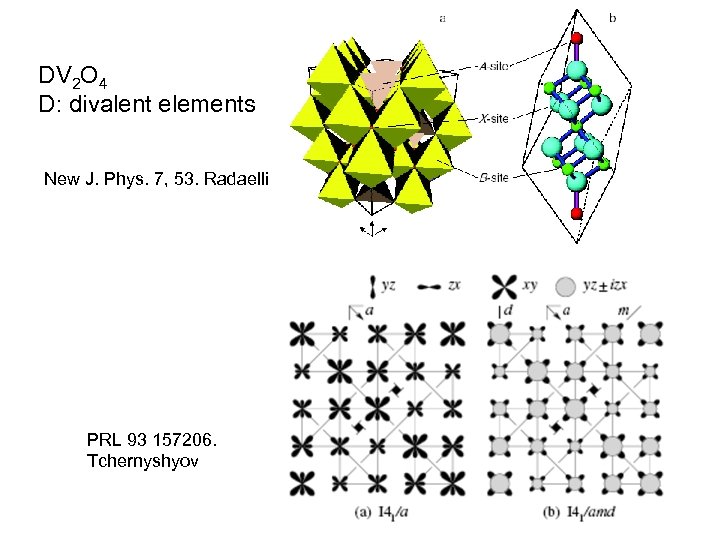 DV 2 O 4 D: divalent elements New J. Phys. 7, 53. Radaelli PRL 93 157206. Tchernyshyov
DV 2 O 4 D: divalent elements New J. Phys. 7, 53. Radaelli PRL 93 157206. Tchernyshyov
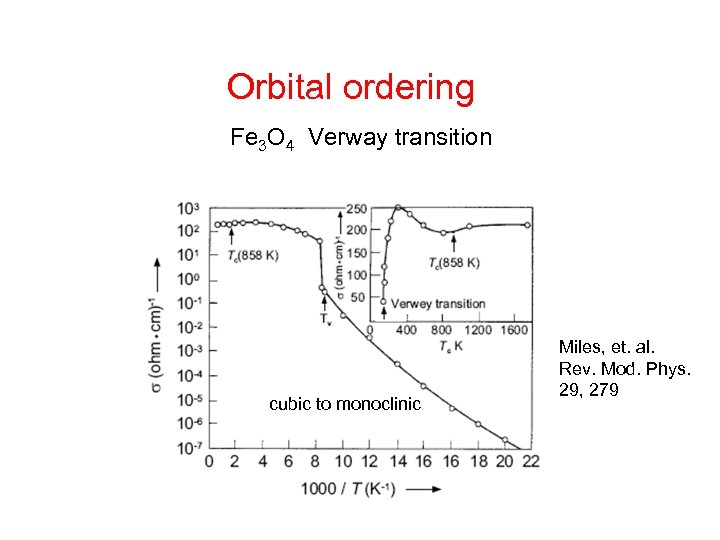 Orbital ordering Fe 3 O 4 Verway transition cubic to monoclinic Miles, et. al. Rev. Mod. Phys. 29, 279
Orbital ordering Fe 3 O 4 Verway transition cubic to monoclinic Miles, et. al. Rev. Mod. Phys. 29, 279
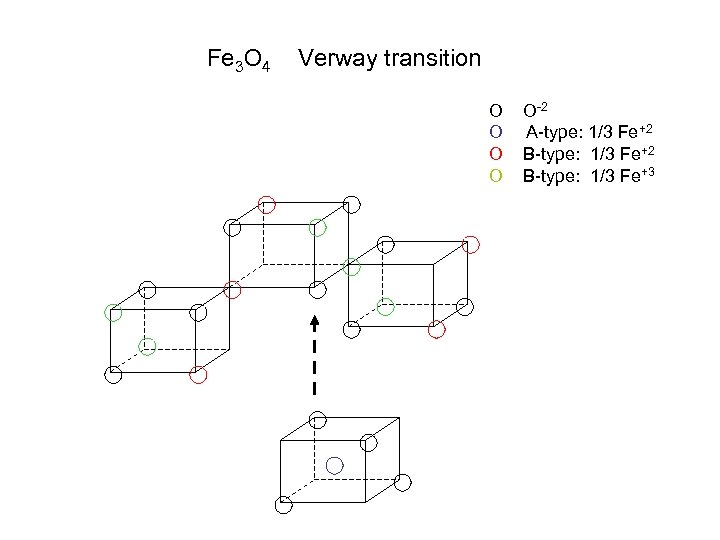 Fe 3 O 4 Verway transition O O O-2 A-type: 1/3 Fe+2 B-type: 1/3 Fe+3
Fe 3 O 4 Verway transition O O O-2 A-type: 1/3 Fe+2 B-type: 1/3 Fe+3
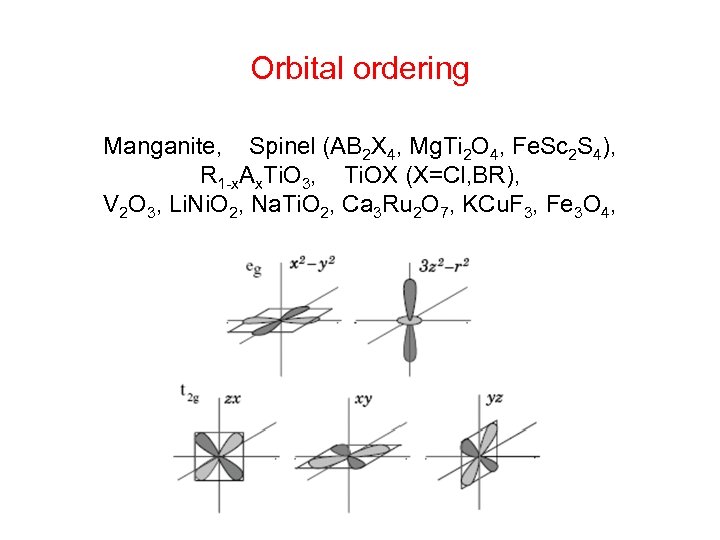 Orbital ordering Manganite, Spinel (AB 2 X 4, Mg. Ti 2 O 4, Fe. Sc 2 S 4), R 1 -x. Ax. Ti. O 3, Ti. OX (X=Cl, BR), V 2 O 3, Li. Ni. O 2, Na. Ti. O 2, Ca 3 Ru 2 O 7, KCu. F 3, Fe 3 O 4,
Orbital ordering Manganite, Spinel (AB 2 X 4, Mg. Ti 2 O 4, Fe. Sc 2 S 4), R 1 -x. Ax. Ti. O 3, Ti. OX (X=Cl, BR), V 2 O 3, Li. Ni. O 2, Na. Ti. O 2, Ca 3 Ru 2 O 7, KCu. F 3, Fe 3 O 4,
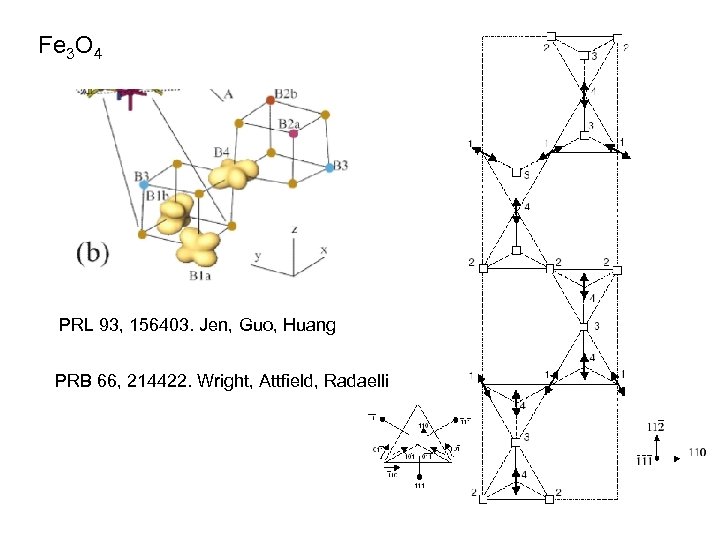 Fe 3 O 4 PRL 93, 156403. Jen, Guo, Huang PRB 66, 214422. Wright, Attfield, Radaelli
Fe 3 O 4 PRL 93, 156403. Jen, Guo, Huang PRB 66, 214422. Wright, Attfield, Radaelli
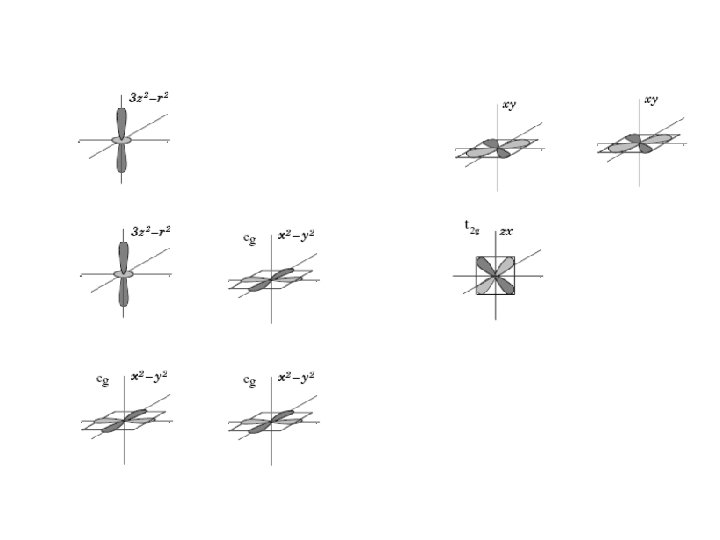
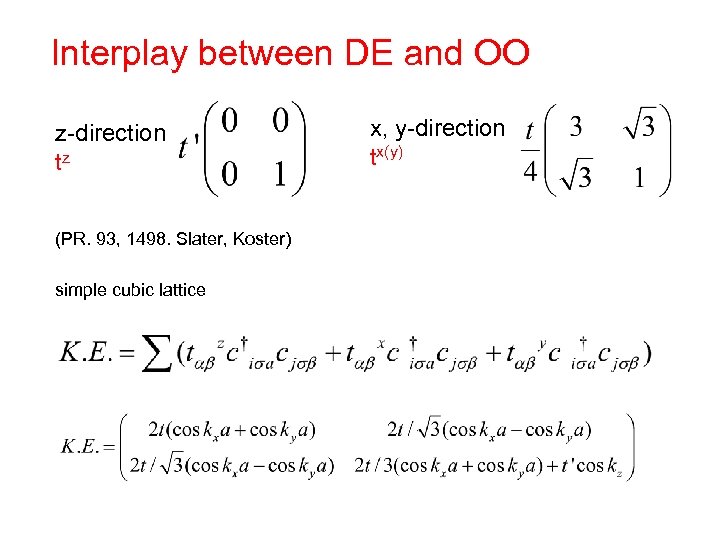 Interplay between DE and OO z-direction tz (PR. 93, 1498. Slater, Koster) simple cubic lattice x, y-direction tx(y)
Interplay between DE and OO z-direction tz (PR. 93, 1498. Slater, Koster) simple cubic lattice x, y-direction tx(y)
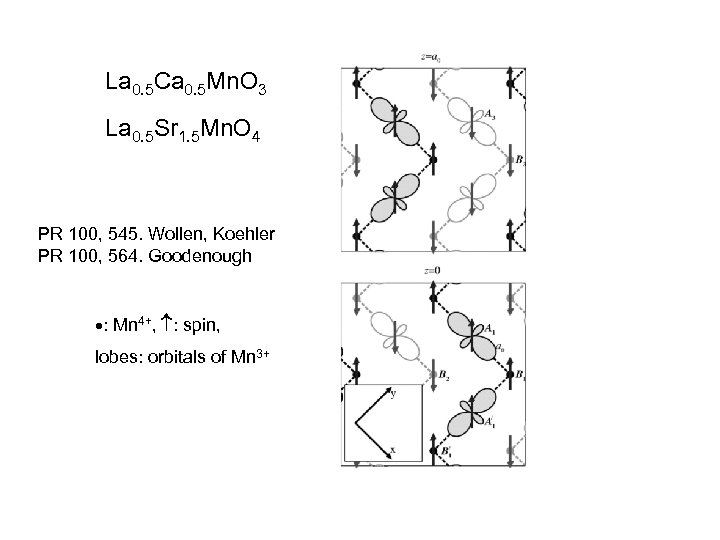 La 0. 5 Ca 0. 5 Mn. O 3 La 0. 5 Sr 1. 5 Mn. O 4 PR 100, 545. Wollen, Koehler PR 100, 564. Goodenough : Mn 4+, : spin, lobes: orbitals of Mn 3+
La 0. 5 Ca 0. 5 Mn. O 3 La 0. 5 Sr 1. 5 Mn. O 4 PR 100, 545. Wollen, Koehler PR 100, 564. Goodenough : Mn 4+, : spin, lobes: orbitals of Mn 3+
 Summary 1. A lot of interesting phenomena. 2. Orbital dynamics is simple. 3. With the new form of DE, the system seems to be managable.
Summary 1. A lot of interesting phenomena. 2. Orbital dynamics is simple. 3. With the new form of DE, the system seems to be managable.
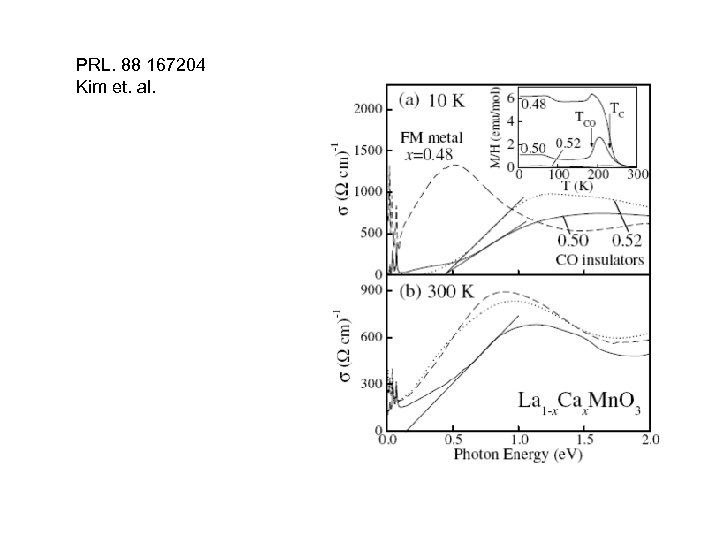 PRL. 88 167204 Kim et. al.
PRL. 88 167204 Kim et. al.
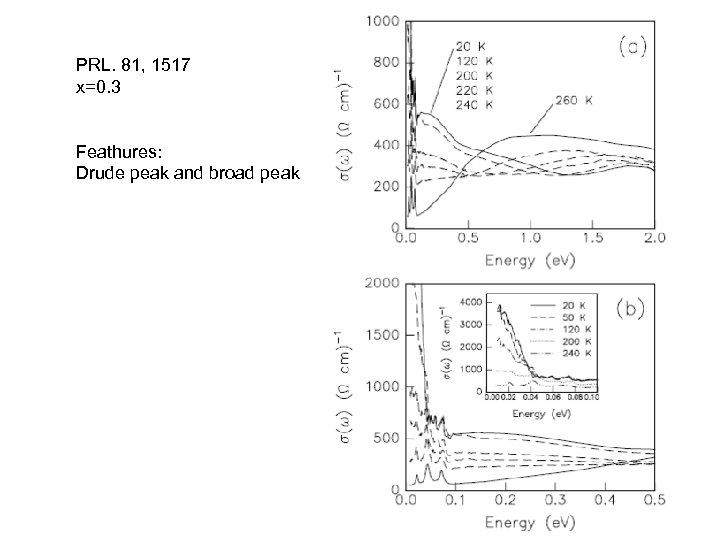 PRL. 81, 1517 x=0. 3 Feathures: Drude peak and broad peak
PRL. 81, 1517 x=0. 3 Feathures: Drude peak and broad peak
 polaron? small polaron large polaron
polaron? small polaron large polaron
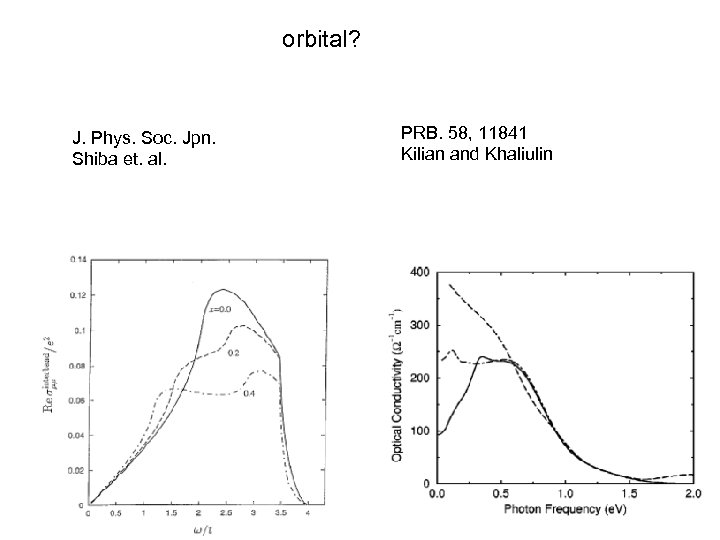 orbital? J. Phys. Soc. Jpn. Shiba et. al. PRB. 58, 11841 Kilian and Khaliulin
orbital? J. Phys. Soc. Jpn. Shiba et. al. PRB. 58, 11841 Kilian and Khaliulin
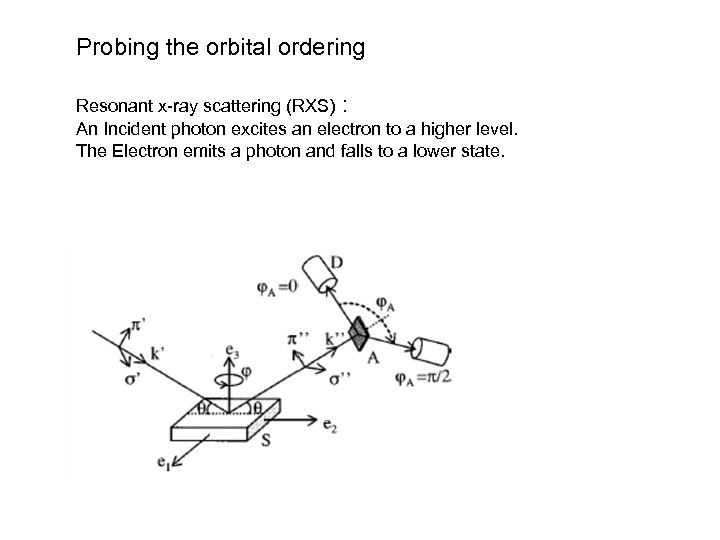 Probing the orbital ordering Resonant x-ray scattering (RXS) : An Incident photon excites an electron to a higher level. The Electron emits a photon and falls to a lower state.
Probing the orbital ordering Resonant x-ray scattering (RXS) : An Incident photon excites an electron to a higher level. The Electron emits a photon and falls to a lower state.
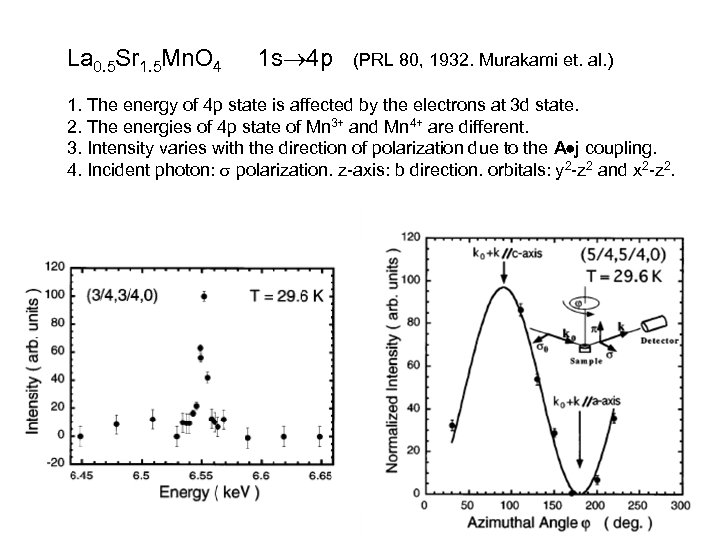 La 0. 5 Sr 1. 5 Mn. O 4 1 s 4 p (PRL 80, 1932. Murakami et. al. ) 1. The energy of 4 p state is affected by the electrons at 3 d state. 2. The energies of 4 p state of Mn 3+ and Mn 4+ are different. 3. Intensity varies with the direction of polarization due to the A j coupling. 4. Incident photon: polarization. z-axis: b direction. orbitals: y 2 -z 2 and x 2 -z 2.
La 0. 5 Sr 1. 5 Mn. O 4 1 s 4 p (PRL 80, 1932. Murakami et. al. ) 1. The energy of 4 p state is affected by the electrons at 3 d state. 2. The energies of 4 p state of Mn 3+ and Mn 4+ are different. 3. Intensity varies with the direction of polarization due to the A j coupling. 4. Incident photon: polarization. z-axis: b direction. orbitals: y 2 -z 2 and x 2 -z 2.


