7d8255fca8ddf0f34ef5452779497509.ppt
- Количество слайдов: 44
Some Basic Concepts of Energy II. Concepts relating to heat Prepared for BIO/EES 105 Energy in our World Kenneth M. Klemow, Ph. D. Wilkes University
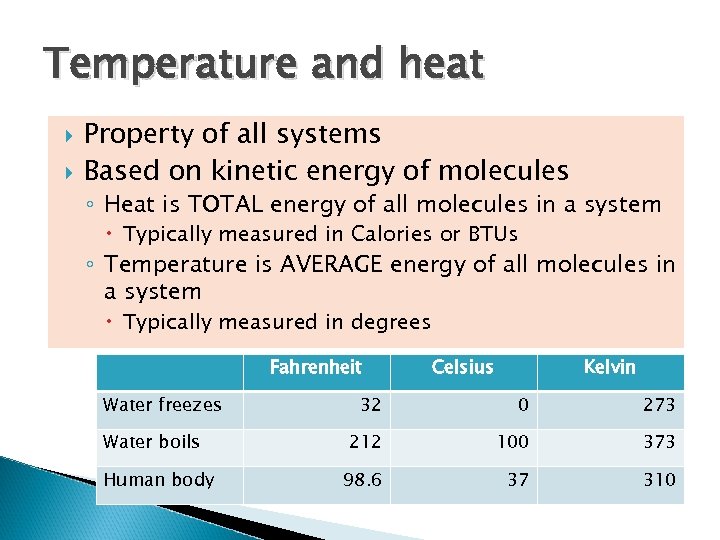
Temperature and heat Property of all systems Based on kinetic energy of molecules ◦ Heat is TOTAL energy of all molecules in a system Typically measured in Calories or BTUs ◦ Temperature is AVERAGE energy of all molecules in a system Typically measured in degrees Fahrenheit Water freezes Water boils Human body Celsius Kelvin 32 0 273 212 100 373 98. 6 37 310
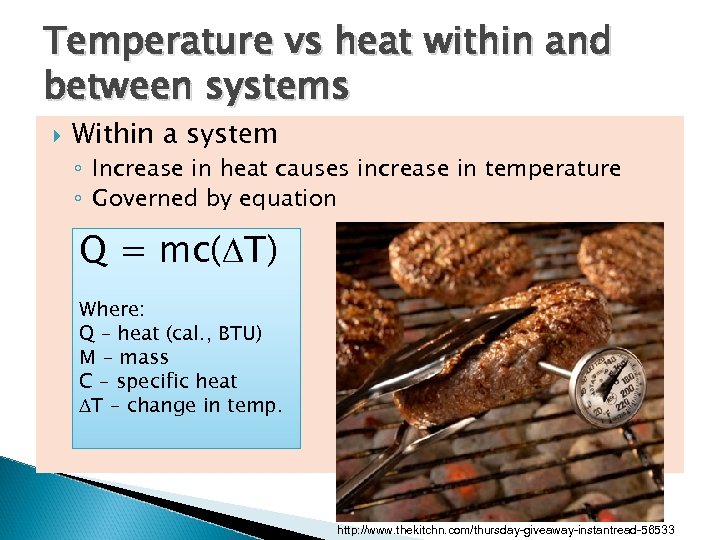
Temperature vs heat within and between systems Within a system ◦ Increase in heat causes increase in temperature ◦ Governed by equation Q = mc(DT) Where: Q – heat (cal. , BTU) M – mass C – specific heat DT – change in temp. http: //www. thekitchn. com/thursday-giveaway-instantread-56533

Temperature vs heat within and between systems Between systems ◦ Not related ◦ One system can have higher heat yet lower temperature
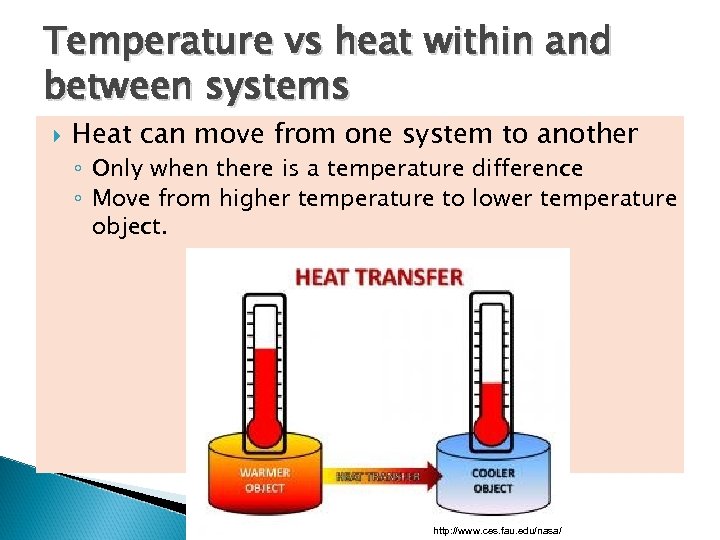
Temperature vs heat within and between systems Heat can move from one system to another ◦ Only when there is a temperature difference ◦ Move from higher temperature to lower temperature object. http: //www. ces. fau. edu/nasa/
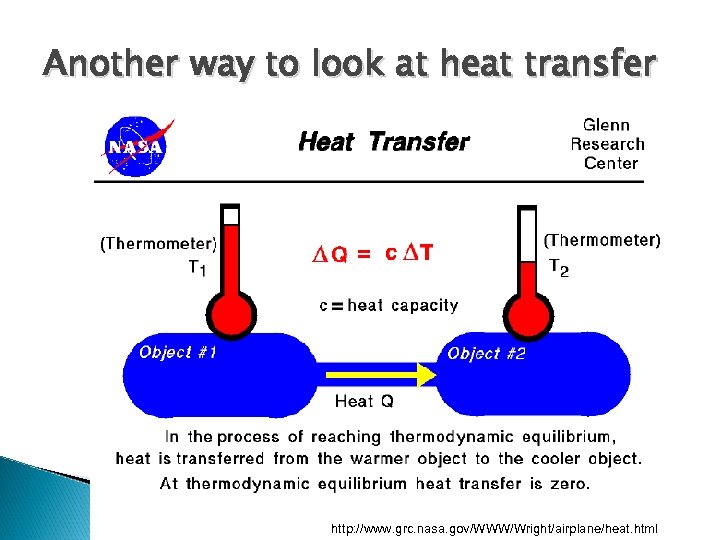
Another way to look at heat transfer http: //www. grc. nasa. gov/WWW/Wright/airplane/heat. html
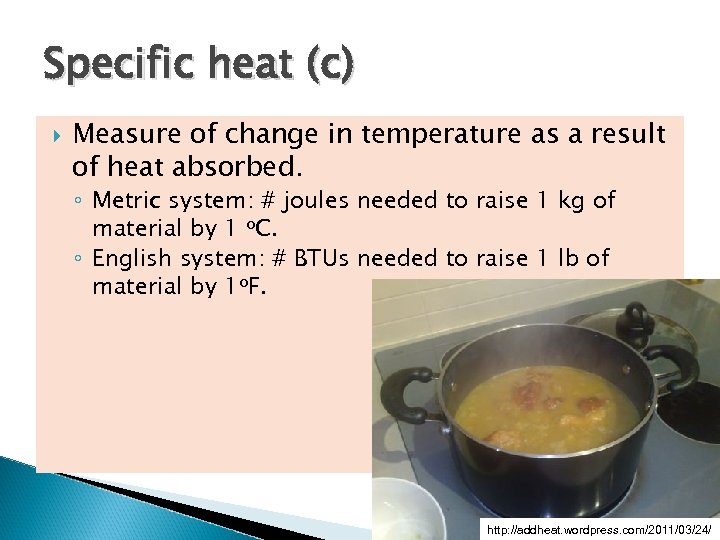
Specific heat (c) Measure of change in temperature as a result of heat absorbed. ◦ Metric system: # joules needed to raise 1 kg of material by 1 o. C. ◦ English system: # BTUs needed to raise 1 lb of material by 1 o. F. http: //addheat. wordpress. com/2011/03/24/
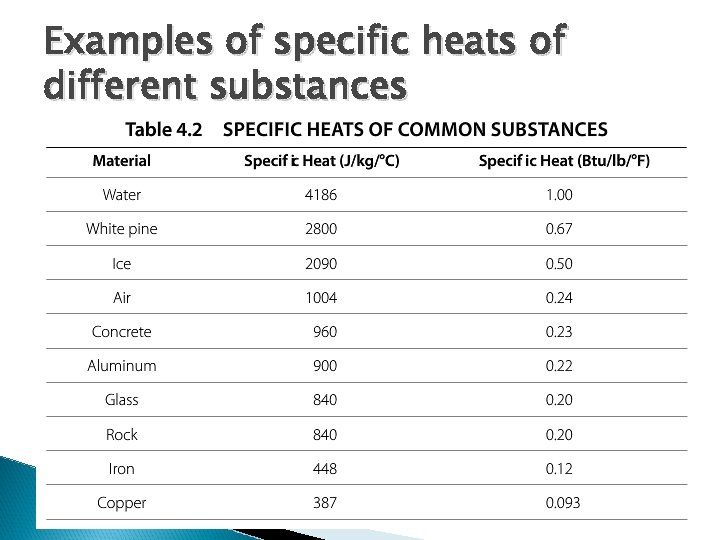
Examples of specific heats of different substances

Heat of vaporization and heat of fusion Involves phase changes Fusion solid <-> liquid For water: 80 kcal / kg http: //blogs. yis. ac. jp/19 miyoshiay/ Vaporization liquid <-> gas For water: 540 kcal / kg http: //ww. abc 6. com/story/
Heat of vaporization and heat of fusion Heat absorbed or released depending on direction Important in heat balance at earth’s surface, regulating temperatures of organisms
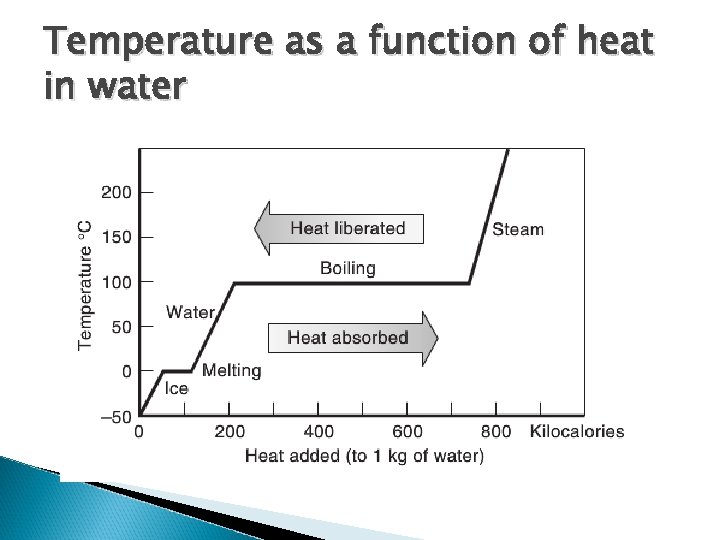
Temperature as a function of heat in water
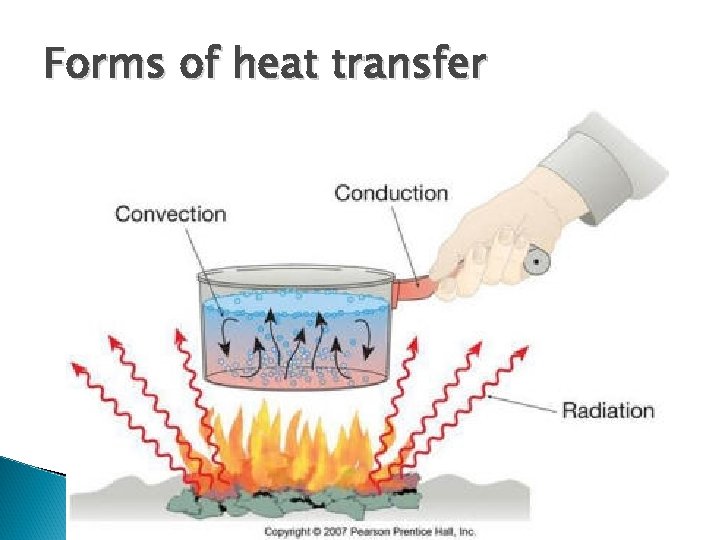
Forms of heat transfer
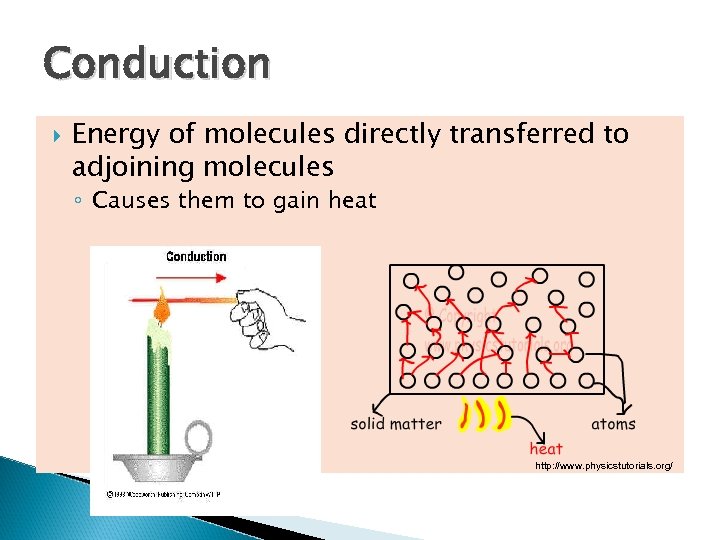
Conduction Energy of molecules directly transferred to adjoining molecules ◦ Causes them to gain heat http: //www. physicstutorials. org/
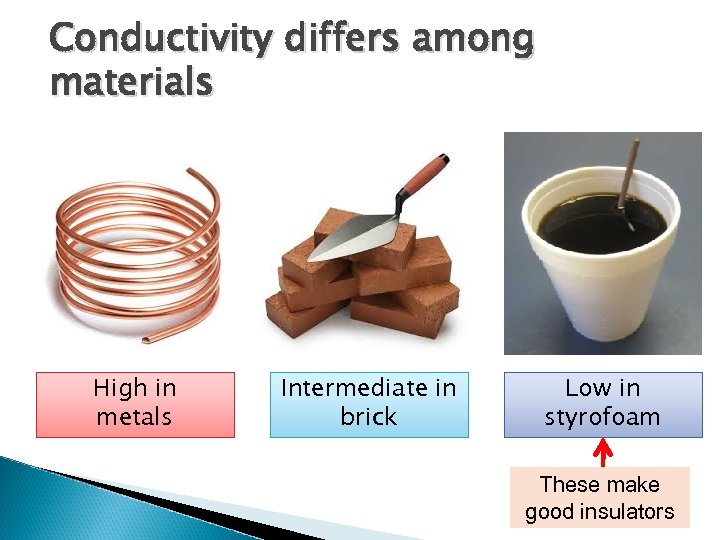
Conductivity differs among materials High in metals Intermediate in brick Low in styrofoam These make good insulators
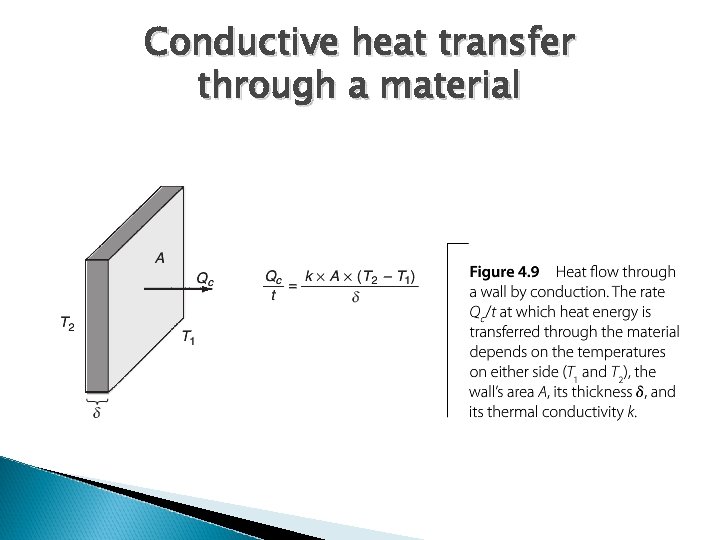
Conductive heat transfer through a material
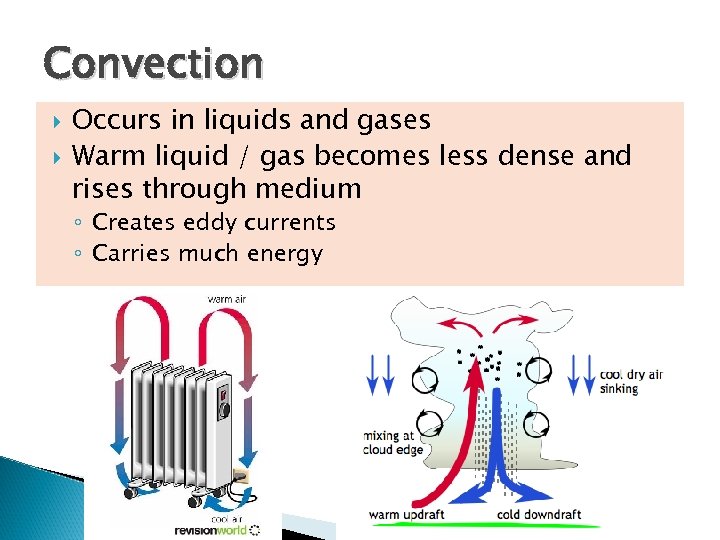
Convection Occurs in liquids and gases Warm liquid / gas becomes less dense and rises through medium ◦ Creates eddy currents ◦ Carries much energy
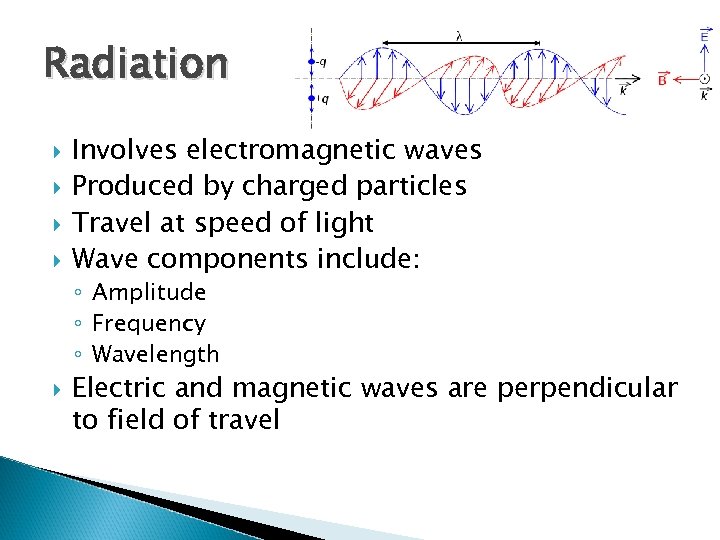
Radiation Involves electromagnetic waves Produced by charged particles Travel at speed of light Wave components include: ◦ Amplitude ◦ Frequency ◦ Wavelength Electric and magnetic waves are perpendicular to field of travel
Frequency and wavelength are inversely related Velocity (m/s) = wavelength (m) x frequency (#/second) As wavelength increases, frequency decreases
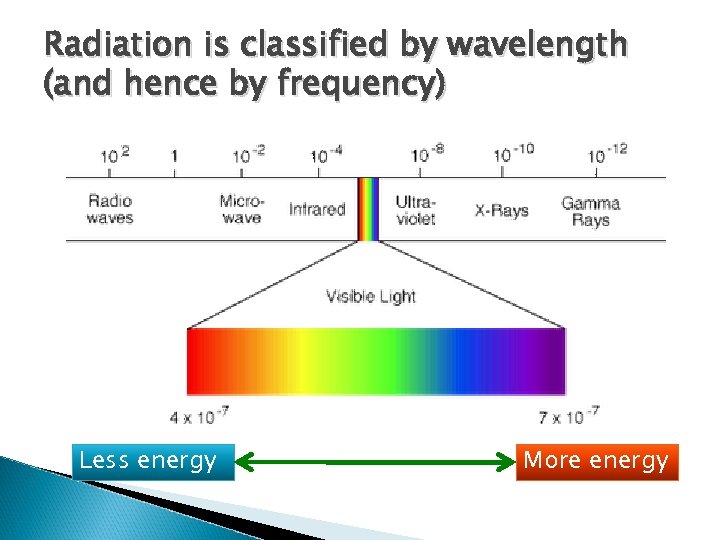
Radiation is classified by wavelength (and hence by frequency) Less energy More energy

Thought questions When radiation strikes a body, it causes that body to start radiating, itself. ◦ Will the wavelengths of that energy likely to be longer or shorter than the energy striking it? ◦ When sunlight hits the earth, will the re-radiated energy be more likely to be in the form of: Ultraviolet, Visible, Infrared energy ◦ When light strikes a chlorophyll solution, some of the energy is reradiated as visible light. What is the most likely color for that light? Blue, Green, or Red
Need to mention boudary layer effects Conduction, convection and radiation all occur in windless environment. ◦ Convection sets up eddies of moving air Adding wind can rapidly remove energy by mass transfer. Objects often covered by boundary layer of still air ◦ Conduction and convection predominate Increasing wind speed causes boundary layer to become thinner. ◦ Transfer of energy greater when wind increases
Energy consumption – thermal comfort Indoor environments often more comfortable than outdoor. ◦ Stay dry ◦ Regulate light ◦ Regulate temperature People prefer temperatures between 65 -75 o. F ◦ When T<65, we heat ◦ When T>75, we cool

Focus on Keeping Warm When cold we add heat via radiators, fireplaces, space heaters Heat generators warm the air via radiant energy If air carried away, need to warm the new air. ◦ Energy needed = 0. 018 BTU / ft 3 / o. F

Problem Imagine you come upon a small, uninhabited, single-roomed cabin in the winter ◦ Height = 10’ ◦ Width = 20’ ◦ Length = 20’ It’s 15 o. F outside, you want to heat it to 65 o. F. How many BTUs will it take?

Problem 2 If energy costs $30. 00 / million BTUs, how much will initially heating the cabin cost?

Keeping the cabin warm will be a challenge Heat losses due to conduction through the walls. Heat losses due to infiltration of cold air.

Look at conductive heat loss first Building has four walls, a ceiling, and a floor ◦ Heat will be lost through each ◦ Go back to formula Q/t = (k x A x DT)/d k = thermal conductivity of wall / floor / ceiling d = thickness For building material, we don’t consider thermal conductivity, per se. Instead we express as thermal resistance (R value), where R = d/k. ◦ Units = ft 2 -hr-o. F/Btu
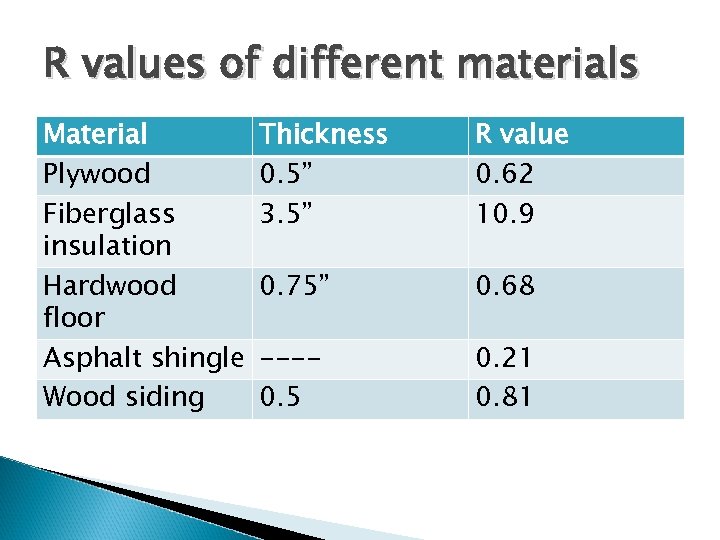
R values of different materials Material Plywood Fiberglass insulation Hardwood floor Asphalt shingle Wood siding Thickness 0. 5” 3. 5” R value 0. 62 10. 9 0. 75” 0. 68 ---0. 5 0. 21 0. 81

Formula now converts to Remember R = d/k ◦ So 1/R = k/d Remember Q/t = (k x A x DT)/d ◦ So Q/t = k/d (A x DT) ◦ And then 1/R (A x DT) ◦ And then Q = 1/R (A x DT x t) http: //www. kfiam 640. com/
Problem How much energy (in BTU) is lost through a wall measuring 20’ x 10’ in an hour. Assume: ◦ Wall covered by 0. 5” plywood ◦ It’s 65 o. F inside and 15 o. F outside How much energy is lost over the course of 24 hours?
Next problem How much energy (in BTU) is lost from the entire house by conduction in an hour? ◦ ◦ Hint 1: 2: 3: 4: Calculate loss through the four walls Calculate loss through the ceiling Calculate loss through the floor Add together Then calculate loss from the house in a 24 hour day.
Now calculate cost What is daily cost to heat house if energy = $30. 00 / million BTUs? What would be the monthly cost?
Effect of adding insulation Go back to case of wall. How much heat was lost in an hour when wall was 0. 5” plywood? Now suppose that your wall was composed of 3. 5” of fiberglass insulation. ◦ Hint 1: Find R value for 3. 5” of fiberglass ◦ Hint 2: Recalculate based on that value. ◦ Express the difference here______ If wall was 0. 5” plywood AND 3. 5” insulation, add the two R values together. ◦ Then recalculate

Now do for entire cabin. What would be hourly loss if all four walls were covered by 3. 5” insulation? What would be hourly loss if ceiling was covered by asphalt shingle above plywood? What would be hourly loss if floor covered by 0. 75” hardwood floor? Next calculate over course of a day Next calculate over course of a month

Air infiltration Premise ◦ Houses leak warm air, and allow cold air to enter ◦ That air needs to be warmed up. ◦ Formula for calculating this: Qinfil = 0. 018 x V x KDT x t

Go back to our cabin What would be energy loss in an hour, if all of the air is exchanged over the course of an hour? How much energy would be lost over the course of 24 hours? How much energy would be lost if the house leaked air at 1/10 the rate?

Total loss equal conductive and leakage added together. Basis for home energy audit!

Terminology pertaining to modern energy Renewable vs nonrenewable Traditional vs new energy Commercialized vs non-commercialized Centralized vs distributed generation On-grid vs off-grid
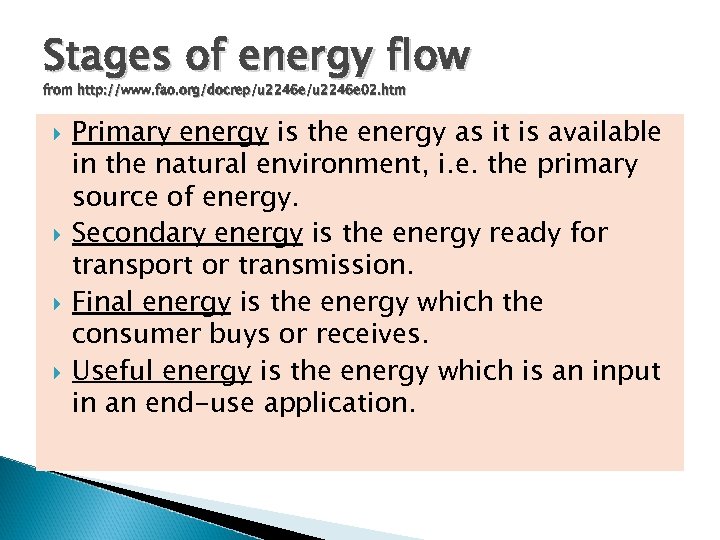
Stages of energy flow from http: //www. fao. org/docrep/u 2246 e 02. htm Primary energy is the energy as it is available in the natural environment, i. e. the primary source of energy. Secondary energy is the energy ready for transport or transmission. Final energy is the energy which the consumer buys or receives. Useful energy is the energy which is an input in an end-use application.
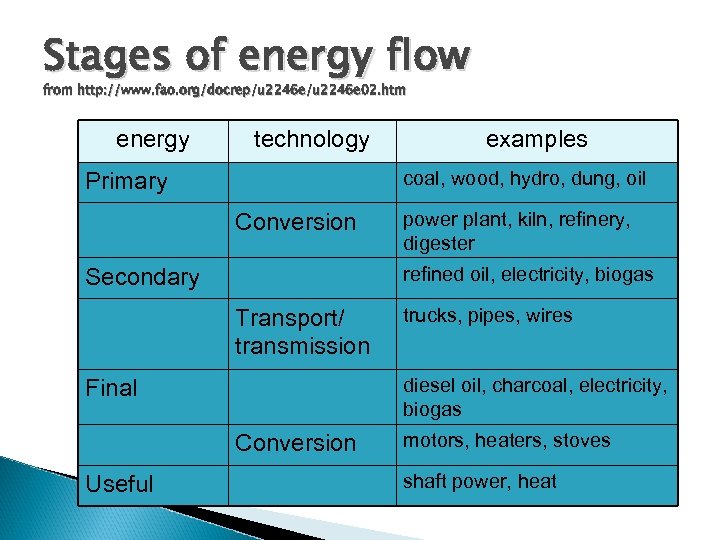
Stages of energy flow from http: //www. fao. org/docrep/u 2246 e 02. htm energy technology coal, wood, hydro, dung, oil Primary Conversion power plant, kiln, refinery, digester refined oil, electricity, biogas Secondary Transport/ transmission trucks, pipes, wires diesel oil, charcoal, electricity, biogas Final Conversion Useful examples motors, heaters, stoves shaft power, heat
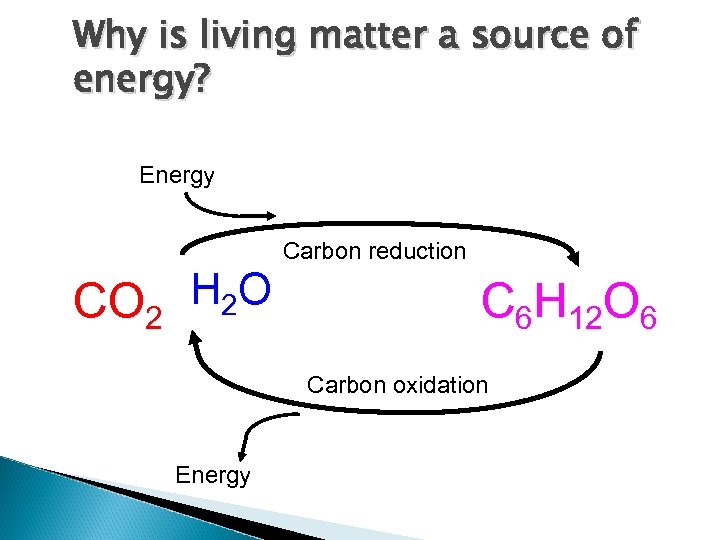
Why is living matter a source of energy? Energy Carbon reduction CO 2 H 2 O C 6 H 12 O 6 Carbon oxidation Energy
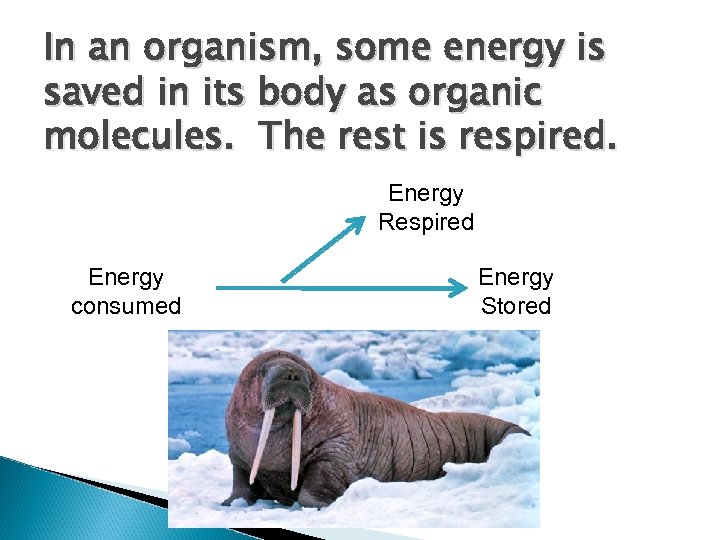
In an organism, some energy is saved in its body as organic molecules. The rest is respired. Energy Respired Energy consumed Energy Stored
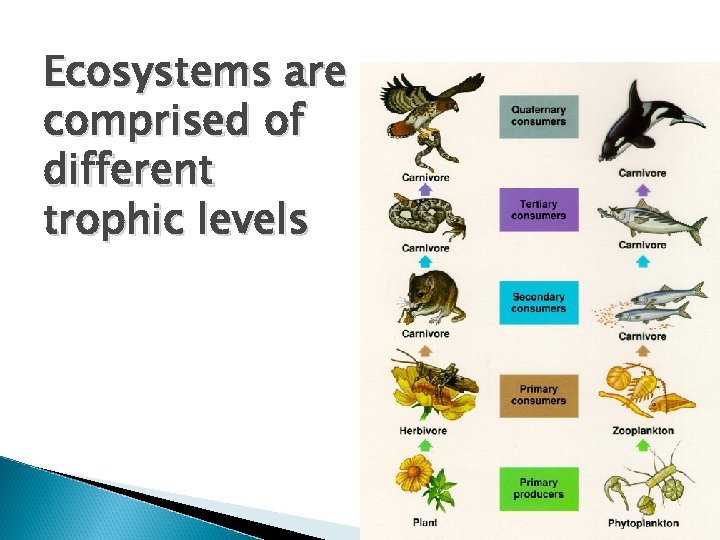
Ecosystems are comprised of different trophic levels
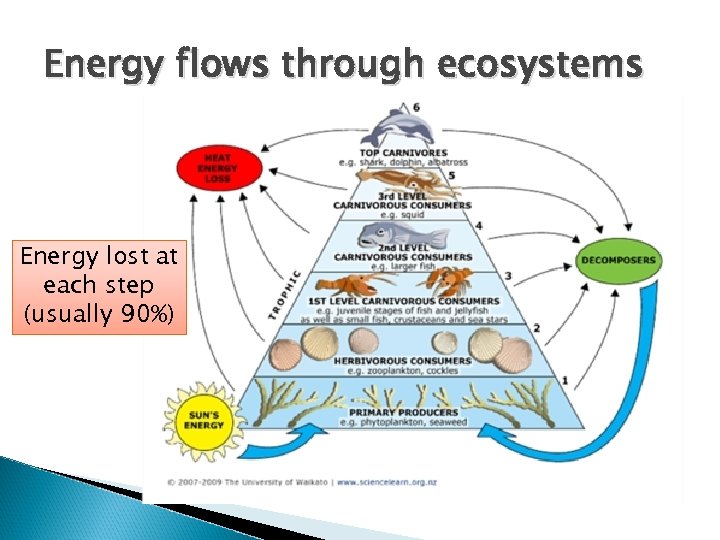
Energy flows through ecosystems Energy lost at each step (usually 90%)
7d8255fca8ddf0f34ef5452779497509.ppt