d27727824f2f172b2cf32b86288b2c3c.ppt
- Количество слайдов: 42
 Solvent Extraction (SX) reagent selection for high temperature, high acid, high chloride and high Cu concentration leach solution at Port Pirie and its impact on electrowinning By P. Crane, M. Urbani, K. Dudley, A. Horner and M. Virnig
Solvent Extraction (SX) reagent selection for high temperature, high acid, high chloride and high Cu concentration leach solution at Port Pirie and its impact on electrowinning By P. Crane, M. Urbani, K. Dudley, A. Horner and M. Virnig
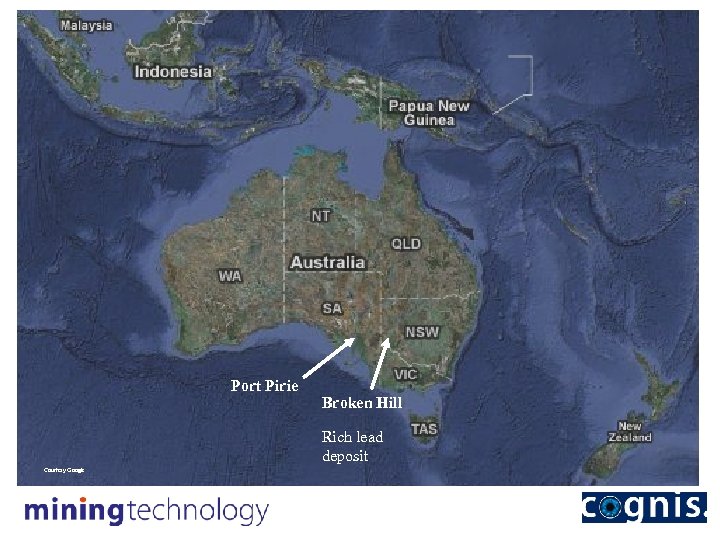 Nyrstar Port Pirie Smelter • The smelter is situated 230 km north of Adelaide in South Australia on the Spencer Gulf. Port Pirie Broken Hill Rich lead deposit Courtesy Google
Nyrstar Port Pirie Smelter • The smelter is situated 230 km north of Adelaide in South Australia on the Spencer Gulf. Port Pirie Broken Hill Rich lead deposit Courtesy Google
 The smelter was built on a natural harbour allowing ease of product export
The smelter was built on a natural harbour allowing ease of product export
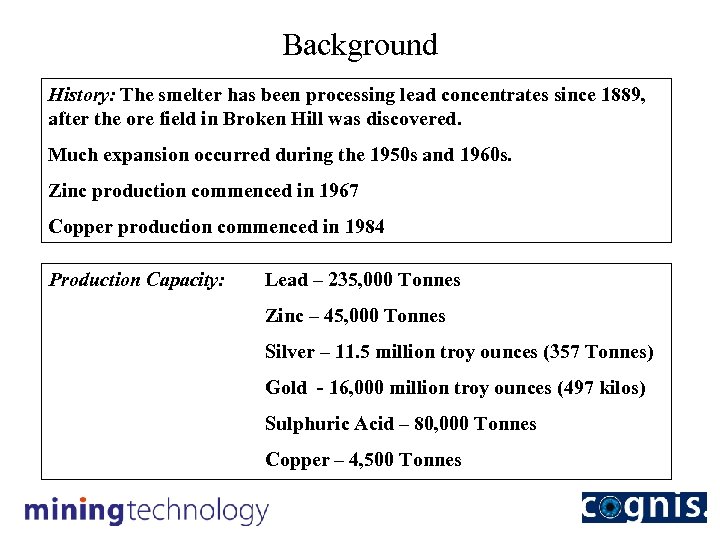 Background History: The smelter has been processing lead concentrates since 1889, after the ore field in Broken Hill was discovered. Much expansion occurred during the 1950 s and 1960 s. Zinc production commenced in 1967 Copper production commenced in 1984 Production Capacity: Lead – 235, 000 Tonnes Zinc – 45, 000 Tonnes Silver – 11. 5 million troy ounces (357 Tonnes) Gold - 16, 000 million troy ounces (497 kilos) Sulphuric Acid – 80, 000 Tonnes Copper – 4, 500 Tonnes
Background History: The smelter has been processing lead concentrates since 1889, after the ore field in Broken Hill was discovered. Much expansion occurred during the 1950 s and 1960 s. Zinc production commenced in 1967 Copper production commenced in 1984 Production Capacity: Lead – 235, 000 Tonnes Zinc – 45, 000 Tonnes Silver – 11. 5 million troy ounces (357 Tonnes) Gold - 16, 000 million troy ounces (497 kilos) Sulphuric Acid – 80, 000 Tonnes Copper – 4, 500 Tonnes
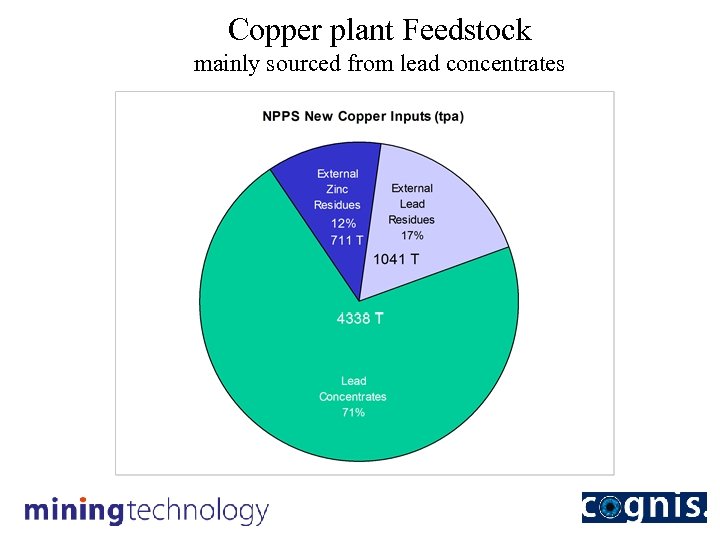 Copper plant Feedstock mainly sourced from lead concentrates
Copper plant Feedstock mainly sourced from lead concentrates
 NPPS Copper Process • Copper is recovered from the lead furnace in the form of a matte, which is treated in the copper plant • The Copper recovery process employs a unique mixed chloride-sulphate leach technology (formerly known as the BHAS process) developed by BHAS engineers. • This aggressive chloride-assisted leach is followed by conventional Cu SX / EW processes operated under fairly harsh conditions.
NPPS Copper Process • Copper is recovered from the lead furnace in the form of a matte, which is treated in the copper plant • The Copper recovery process employs a unique mixed chloride-sulphate leach technology (formerly known as the BHAS process) developed by BHAS engineers. • This aggressive chloride-assisted leach is followed by conventional Cu SX / EW processes operated under fairly harsh conditions.
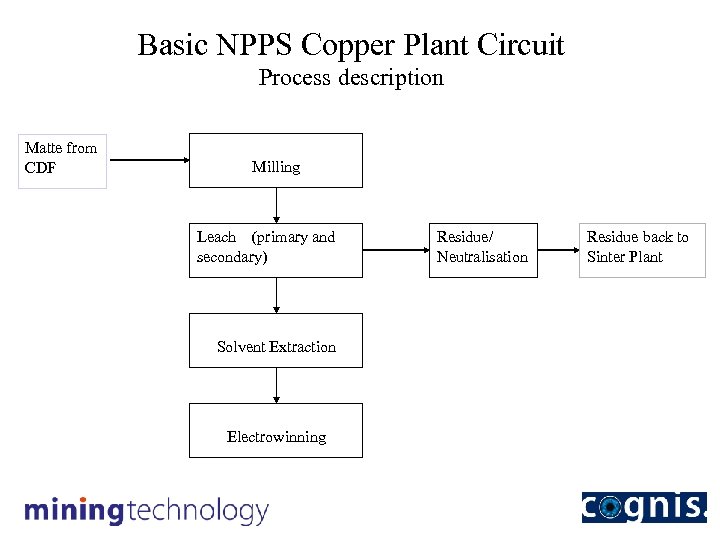 Basic NPPS Copper Plant Circuit Process description Matte from CDF Milling Leach (primary and secondary) Solvent Extraction Electrowinning Residue/ Neutralisation Residue back to Sinter Plant
Basic NPPS Copper Plant Circuit Process description Matte from CDF Milling Leach (primary and secondary) Solvent Extraction Electrowinning Residue/ Neutralisation Residue back to Sinter Plant
 • Ball Mill and Feed Hopper
• Ball Mill and Feed Hopper
 Grinding • A conventional ball mill in closed circuit with a spiral classifier, treating 12, 000 tpa matte. • Product goes to Primary Leach after thickening and storage. To leach
Grinding • A conventional ball mill in closed circuit with a spiral classifier, treating 12, 000 tpa matte. • Product goes to Primary Leach after thickening and storage. To leach
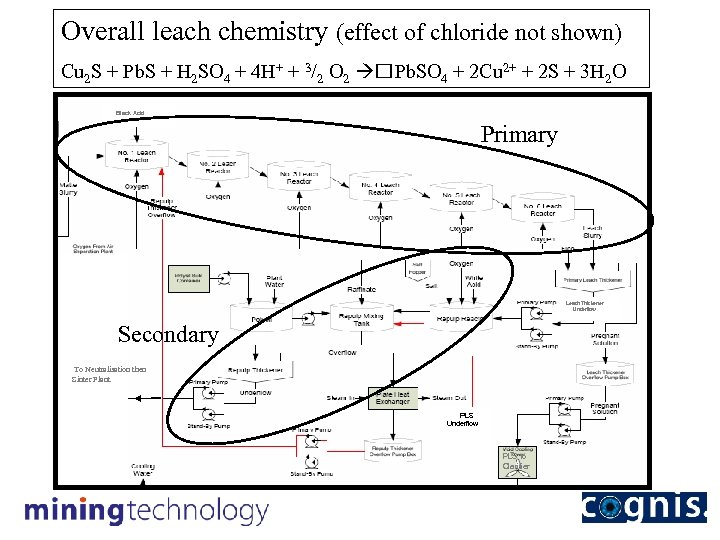 Overall leach chemistry (effect of chloride not shown) Cu 2 S + Pb. S + H 2 SO 4 + 4 H+ + 3/2 O 2 Pb. SO 4 + 2 Cu 2+ + 2 S + 3 H 2 O Black Acid Primary Leach Thickener Underflow Secondary To Neutralisation then Sinter Plant PLS Underflow PLS to Clarifier
Overall leach chemistry (effect of chloride not shown) Cu 2 S + Pb. S + H 2 SO 4 + 4 H+ + 3/2 O 2 Pb. SO 4 + 2 Cu 2+ + 2 S + 3 H 2 O Black Acid Primary Leach Thickener Underflow Secondary To Neutralisation then Sinter Plant PLS Underflow PLS to Clarifier
 • Leach Reactors
• Leach Reactors
 2 nd Stage Leach and washing • Thickener U/F from 1 st stage leach at 35 -40% solids is pumped to a one-stage repulp reactor. • Salt, white acid and Oxygen added. • 4 -5 hrs residence time. • 2 nd stage leach discharge washed with raffinate in a repulp mixer. • Polysil added in repulp mixer for silica control, and Magnafloc added to Repulp Thickener as a settling aid. • Repulp slurry is thickened; solids are sent to neutralisation and return to the sinter plant, whilst liquids go to 1 st stage leach.
2 nd Stage Leach and washing • Thickener U/F from 1 st stage leach at 35 -40% solids is pumped to a one-stage repulp reactor. • Salt, white acid and Oxygen added. • 4 -5 hrs residence time. • 2 nd stage leach discharge washed with raffinate in a repulp mixer. • Polysil added in repulp mixer for silica control, and Magnafloc added to Repulp Thickener as a settling aid. • Repulp slurry is thickened; solids are sent to neutralisation and return to the sinter plant, whilst liquids go to 1 st stage leach.
 • Salt addition to repulp reactor Na. Cl prevents precipitation of Cu. S from Cu+ and S 0.
• Salt addition to repulp reactor Na. Cl prevents precipitation of Cu. S from Cu+ and S 0.
 • Preg Clarifier • Leach Thickener
• Preg Clarifier • Leach Thickener
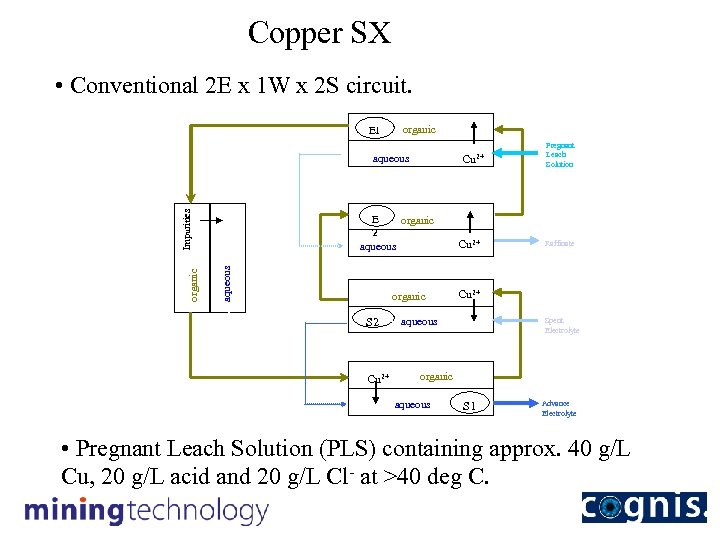 Copper SX • Conventional 2 E x 1 W x 2 S circuit. E 1 Wash Water organic aqueous Cu 2+ Pregnant Leach Solution E organic 2 aqueous organic Washing Impurities Extraction organic S 2 Cu 2+ Raffinate Cu 2+ aqueous Spent Electrolyte Wash Water Stripping Cu 2+ organic aqueous S 1 Advance Electrolyte • Pregnant Leach Solution (PLS) containing approx. 40 g/L Cu, 20 g/L acid and 20 g/L Cl- at >40 deg C.
Copper SX • Conventional 2 E x 1 W x 2 S circuit. E 1 Wash Water organic aqueous Cu 2+ Pregnant Leach Solution E organic 2 aqueous organic Washing Impurities Extraction organic S 2 Cu 2+ Raffinate Cu 2+ aqueous Spent Electrolyte Wash Water Stripping Cu 2+ organic aqueous S 1 Advance Electrolyte • Pregnant Leach Solution (PLS) containing approx. 40 g/L Cu, 20 g/L acid and 20 g/L Cl- at >40 deg C.
 • SX Mixer-settlers, S 1 and S 2
• SX Mixer-settlers, S 1 and S 2
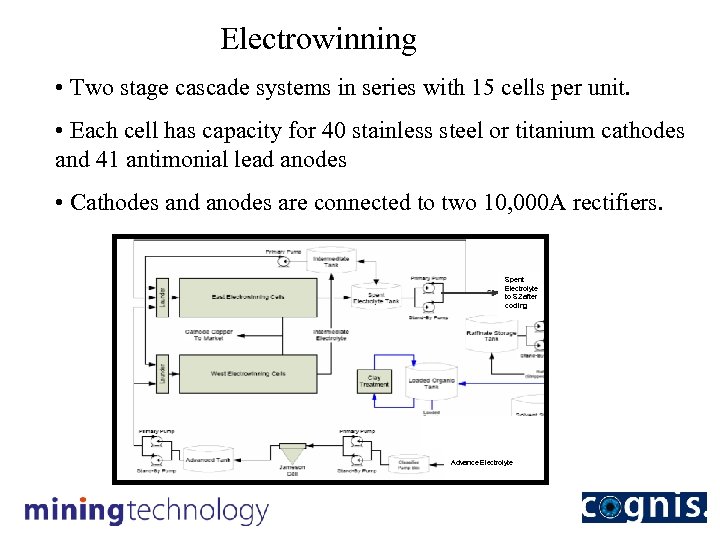 Electrowinning • Two stage cascade systems in series with 15 cells per unit. • Each cell has capacity for 40 stainless steel or titanium cathodes and 41 antimonial lead anodes • Cathodes and anodes are connected to two 10, 000 A rectifiers. Spent Electrolyte to S 2 after cooling Advance Electrolyte
Electrowinning • Two stage cascade systems in series with 15 cells per unit. • Each cell has capacity for 40 stainless steel or titanium cathodes and 41 antimonial lead anodes • Cathodes and anodes are connected to two 10, 000 A rectifiers. Spent Electrolyte to S 2 after cooling Advance Electrolyte
 • Cell house
• Cell house
 Process Difficulties The use of the highly effective chloride – sulphate leach process results in several challenges for the operation of the Cu SX-EW plant, namely it: • Produces a hot PLS, high in acid, chloride and copper content; • Requires effective silica control and solids liquid separation; • Requires a high SX extractant concentration and high O/A ratios; • Requires careful monitoring of organic health and regular, effective, organic treatment.
Process Difficulties The use of the highly effective chloride – sulphate leach process results in several challenges for the operation of the Cu SX-EW plant, namely it: • Produces a hot PLS, high in acid, chloride and copper content; • Requires effective silica control and solids liquid separation; • Requires a high SX extractant concentration and high O/A ratios; • Requires careful monitoring of organic health and regular, effective, organic treatment.
 Cognis Involvement Cognis visited Port Pirie in mid 2003 to view the plant operation and offer technical assistance. Main observations: • Operating with high concentration of Acorga® M 5640 (C 9 aldoxime with TXIB modifier) and high O/A ratio. • No phase separation under Organic Continuous conditions Forced Aqueous Continuous operation. • Evidence of silica-based crud. • Re-oximation stage due to high reagent degradation rate. • Viscous organic with dark colouration after strong acid strip. • High entrainment and resultant high Cl transfer to electrolyte (consistently >200 ppm Cl in electrolyte) titanium plates. • Poor atmospheric environment in EW (low levels Cl 2 gas).
Cognis Involvement Cognis visited Port Pirie in mid 2003 to view the plant operation and offer technical assistance. Main observations: • Operating with high concentration of Acorga® M 5640 (C 9 aldoxime with TXIB modifier) and high O/A ratio. • No phase separation under Organic Continuous conditions Forced Aqueous Continuous operation. • Evidence of silica-based crud. • Re-oximation stage due to high reagent degradation rate. • Viscous organic with dark colouration after strong acid strip. • High entrainment and resultant high Cl transfer to electrolyte (consistently >200 ppm Cl in electrolyte) titanium plates. • Poor atmospheric environment in EW (low levels Cl 2 gas).
 Cognis Involvement, cont. In August 2003, a sample of Port Pirie plant organic was analysed in Cognis’ Tucson laboratory. The main findings were: • Extremely poor OC phase break >15 minutes at 25 C • Slow stripping kinetics – 62% after 30 sec (typically 95%) • High nitro-C 9 aldoxime level (16. 2 % of total oxime) • Excessive levels of hydrolysis product (aldehyde) • High TXIB : C 9 aldoxime ratio (0. 38 compared to 0. 28 in Acorga M 5640) • Presence of 2 -cyano-4 -nonylphenol and 5 -nonylsalicylamide • The organic did not respond to clay treatment
Cognis Involvement, cont. In August 2003, a sample of Port Pirie plant organic was analysed in Cognis’ Tucson laboratory. The main findings were: • Extremely poor OC phase break >15 minutes at 25 C • Slow stripping kinetics – 62% after 30 sec (typically 95%) • High nitro-C 9 aldoxime level (16. 2 % of total oxime) • Excessive levels of hydrolysis product (aldehyde) • High TXIB : C 9 aldoxime ratio (0. 38 compared to 0. 28 in Acorga M 5640) • Presence of 2 -cyano-4 -nonylphenol and 5 -nonylsalicylamide • The organic did not respond to clay treatment
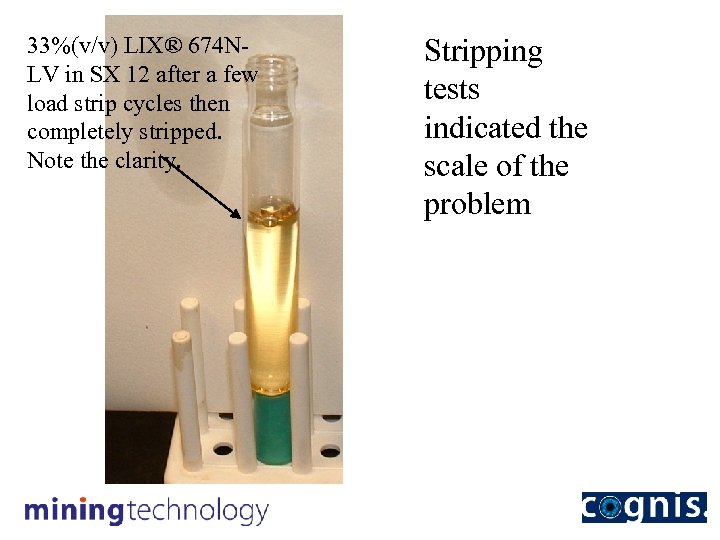 33%(v/v) LIX® 674 NLV in SX 12 after a few load strip cycles then completely stripped. Note the clarity. Stripping tests indicated the scale of the problem
33%(v/v) LIX® 674 NLV in SX 12 after a few load strip cycles then completely stripped. Note the clarity. Stripping tests indicated the scale of the problem
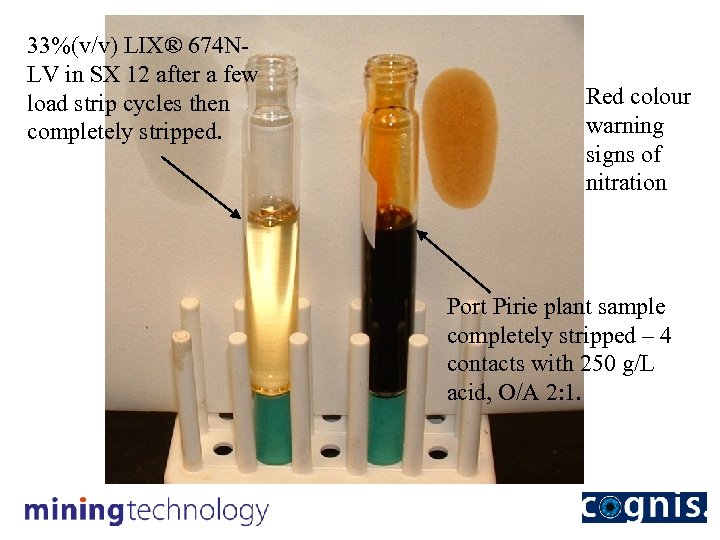 33%(v/v) LIX® 674 NLV in SX 12 after a few load strip cycles then completely stripped. Red colour warning signs of nitration Port Pirie plant sample completely stripped – 4 contacts with 250 g/L acid, O/A 2: 1.
33%(v/v) LIX® 674 NLV in SX 12 after a few load strip cycles then completely stripped. Red colour warning signs of nitration Port Pirie plant sample completely stripped – 4 contacts with 250 g/L acid, O/A 2: 1.
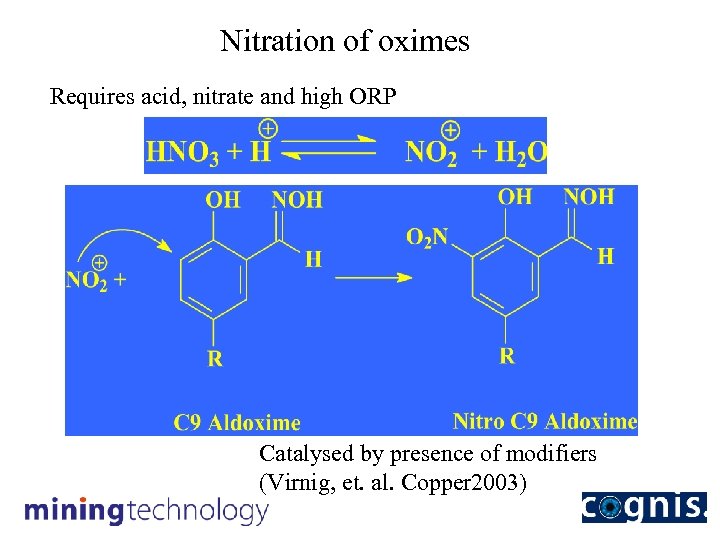 Nitration of oximes Requires acid, nitrate and high ORP Catalysed by presence of modifiers (Virnig, et. al. Copper 2003)
Nitration of oximes Requires acid, nitrate and high ORP Catalysed by presence of modifiers (Virnig, et. al. Copper 2003)
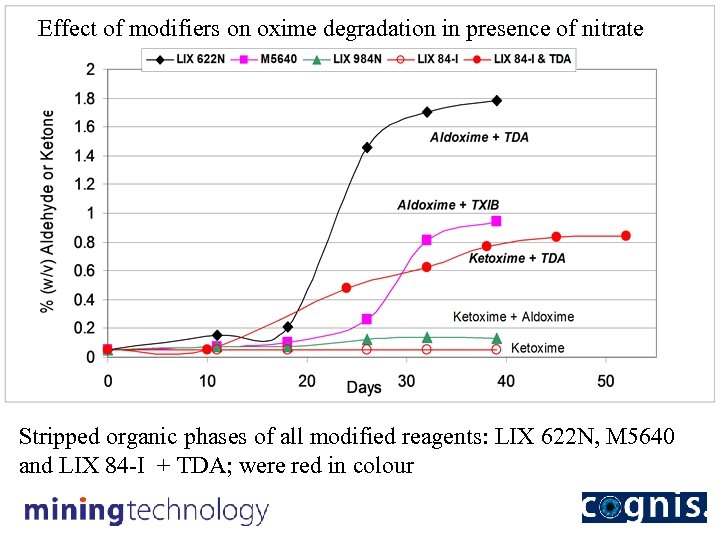 Effect of modifiers on oxime degradation in presence of nitrate Stripped organic phases of all modified reagents: LIX 622 N, M 5640 and LIX 84 -I + TDA; were red in colour
Effect of modifiers on oxime degradation in presence of nitrate Stripped organic phases of all modified reagents: LIX 622 N, M 5640 and LIX 84 -I + TDA; were red in colour
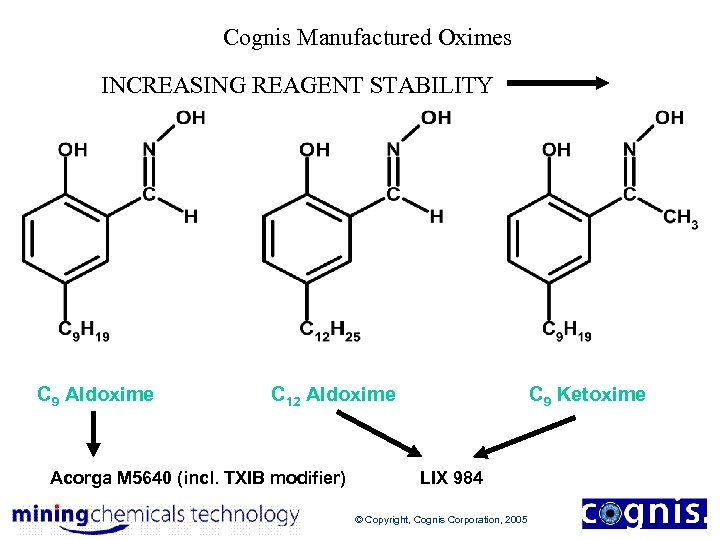 Cognis Manufactured Oximes INCREASING REAGENT STABILITY C 9 Aldoxime C 12 Aldoxime Acorga M 5640 (incl. TXIB modifier) C 9 Ketoxime LIX 984 © Copyright, Cognis Corporation, 2005
Cognis Manufactured Oximes INCREASING REAGENT STABILITY C 9 Aldoxime C 12 Aldoxime Acorga M 5640 (incl. TXIB modifier) C 9 Ketoxime LIX 984 © Copyright, Cognis Corporation, 2005
 Cognis recommendations • Convert reagent to a more stable non-modified reagent type to: – Reduce rate of nitration – Reduce rate of degradation – Reduce viscosity of organic phase • Cease re-oximation of the organic, since: – Re-oximated organics demonstrate poor physical performance – Re-oximated organics are typically difficult to clean up – Hydroxylamine may have been at least a partial source of the nitrogen oxide species Recommendations were all intended to improve the physical state and the performance of the organic phase.
Cognis recommendations • Convert reagent to a more stable non-modified reagent type to: – Reduce rate of nitration – Reduce rate of degradation – Reduce viscosity of organic phase • Cease re-oximation of the organic, since: – Re-oximated organics demonstrate poor physical performance – Re-oximated organics are typically difficult to clean up – Hydroxylamine may have been at least a partial source of the nitrogen oxide species Recommendations were all intended to improve the physical state and the performance of the organic phase.
 Port Pirie accepted the Cognis offer. LIX 984 was selected because: • 50/50 blend of LIX 84 -I (ketoxime) and LIX 860 -I (C 12 aldoxime), both of which are more stable than the C 9 aldoxime present in Acorga M 5640. • LIX 984 contains no modifier Modifiers promote nitration. • LIX 984 showed good chemical performance in initial screening tests.
Port Pirie accepted the Cognis offer. LIX 984 was selected because: • 50/50 blend of LIX 84 -I (ketoxime) and LIX 860 -I (C 12 aldoxime), both of which are more stable than the C 9 aldoxime present in Acorga M 5640. • LIX 984 contains no modifier Modifiers promote nitration. • LIX 984 showed good chemical performance in initial screening tests.
 Pre-LIX reagent addition plant profile (April 2004) • Cu Mass balance OK • High oxime concentration (43%(v/v)) • Low Cu net transfer (0. 14 g/L Cu / % oxime) • Low % Cu max load • Laboratory PDT’s – aqueous continuous - 126 seconds, organic continuous - no separation after 15 minutes • Again clay treatment had no effect, organic not filterable • Extremely high viscosity (26. 5 c. St compared to 3. 9 c. St for fresh LIX reagent at 43%(v/v)
Pre-LIX reagent addition plant profile (April 2004) • Cu Mass balance OK • High oxime concentration (43%(v/v)) • Low Cu net transfer (0. 14 g/L Cu / % oxime) • Low % Cu max load • Laboratory PDT’s – aqueous continuous - 126 seconds, organic continuous - no separation after 15 minutes • Again clay treatment had no effect, organic not filterable • Extremely high viscosity (26. 5 c. St compared to 3. 9 c. St for fresh LIX reagent at 43%(v/v)
 Acorga addition stopped in April 2004 and LIX addition commenced. Regular plant monitoring involved: • Chemical composition –C 9 and C 12 aldoxime, ketoxime, TXIB, hydrolysis and nitration products • Chemical performance – Cu recovery, % Cu ML, Cu net transfer (g/L Cu / % oxime), extraction and stripping kinetics • Physical performance – Plant PDTs, mixer continuity, temp, laboratory PDTs, Effect of clay treatment, viscosity • Monitoring the Cl- concentration in the electrolyte
Acorga addition stopped in April 2004 and LIX addition commenced. Regular plant monitoring involved: • Chemical composition –C 9 and C 12 aldoxime, ketoxime, TXIB, hydrolysis and nitration products • Chemical performance – Cu recovery, % Cu ML, Cu net transfer (g/L Cu / % oxime), extraction and stripping kinetics • Physical performance – Plant PDTs, mixer continuity, temp, laboratory PDTs, Effect of clay treatment, viscosity • Monitoring the Cl- concentration in the electrolyte
 Conversion rate tracked by decline in TXIB content
Conversion rate tracked by decline in TXIB content
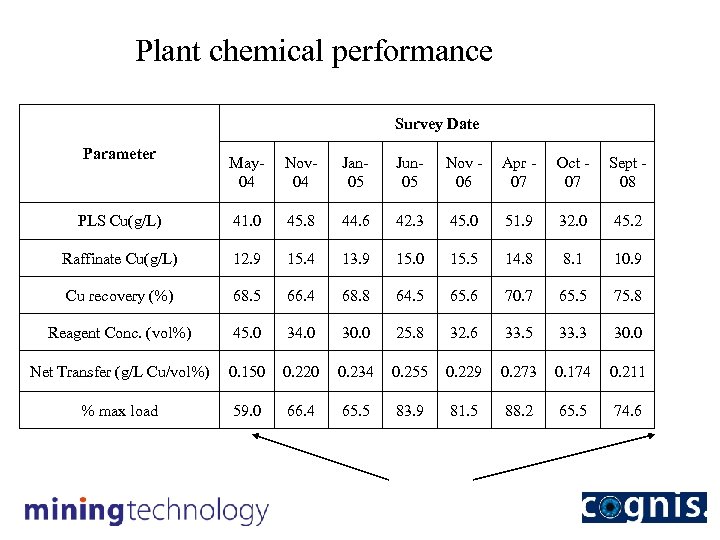 Plant chemical performance Survey Date Parameter May 04 Nov 04 Jan 05 Jun 05 Nov 06 Apr 07 Oct 07 Sept 08 PLS Cu(g/L) 41. 0 45. 8 44. 6 42. 3 45. 0 51. 9 32. 0 45. 2 Raffinate Cu(g/L) 12. 9 15. 4 13. 9 15. 0 15. 5 14. 8 8. 1 10. 9 Cu recovery (%) 68. 5 66. 4 68. 8 64. 5 65. 6 70. 7 65. 5 75. 8 Reagent Conc. (vol%) 45. 0 34. 0 30. 0 25. 8 32. 6 33. 5 33. 3 30. 0 Net Transfer (g/L Cu/vol%) 0. 150 0. 220 0. 234 0. 255 0. 229 0. 273 0. 174 0. 211 % max load 59. 0 66. 4 65. 5 83. 9 81. 5 88. 2 65. 5 74. 6
Plant chemical performance Survey Date Parameter May 04 Nov 04 Jan 05 Jun 05 Nov 06 Apr 07 Oct 07 Sept 08 PLS Cu(g/L) 41. 0 45. 8 44. 6 42. 3 45. 0 51. 9 32. 0 45. 2 Raffinate Cu(g/L) 12. 9 15. 4 13. 9 15. 0 15. 5 14. 8 8. 1 10. 9 Cu recovery (%) 68. 5 66. 4 68. 8 64. 5 65. 6 70. 7 65. 5 75. 8 Reagent Conc. (vol%) 45. 0 34. 0 30. 0 25. 8 32. 6 33. 5 33. 3 30. 0 Net Transfer (g/L Cu/vol%) 0. 150 0. 220 0. 234 0. 255 0. 229 0. 273 0. 174 0. 211 % max load 59. 0 66. 4 65. 5 83. 9 81. 5 88. 2 65. 5 74. 6
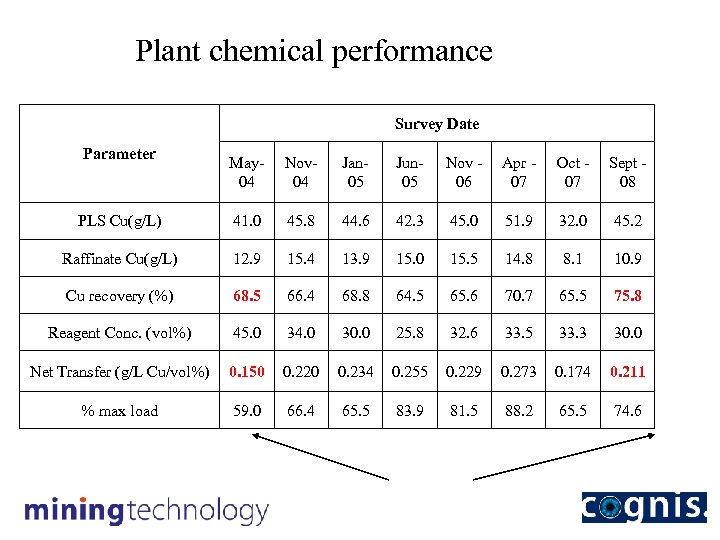 Plant chemical performance Survey Date Parameter May 04 Nov 04 Jan 05 Jun 05 Nov 06 Apr 07 Oct 07 Sept 08 PLS Cu(g/L) 41. 0 45. 8 44. 6 42. 3 45. 0 51. 9 32. 0 45. 2 Raffinate Cu(g/L) 12. 9 15. 4 13. 9 15. 0 15. 5 14. 8 8. 1 10. 9 Cu recovery (%) 68. 5 66. 4 68. 8 64. 5 65. 6 70. 7 65. 5 75. 8 Reagent Conc. (vol%) 45. 0 34. 0 30. 0 25. 8 32. 6 33. 5 33. 3 30. 0 Net Transfer (g/L Cu/vol%) 0. 150 0. 220 0. 234 0. 255 0. 229 0. 273 0. 174 0. 211 % max load 59. 0 66. 4 65. 5 83. 9 81. 5 88. 2 65. 5 74. 6
Plant chemical performance Survey Date Parameter May 04 Nov 04 Jan 05 Jun 05 Nov 06 Apr 07 Oct 07 Sept 08 PLS Cu(g/L) 41. 0 45. 8 44. 6 42. 3 45. 0 51. 9 32. 0 45. 2 Raffinate Cu(g/L) 12. 9 15. 4 13. 9 15. 0 15. 5 14. 8 8. 1 10. 9 Cu recovery (%) 68. 5 66. 4 68. 8 64. 5 65. 6 70. 7 65. 5 75. 8 Reagent Conc. (vol%) 45. 0 34. 0 30. 0 25. 8 32. 6 33. 5 33. 3 30. 0 Net Transfer (g/L Cu/vol%) 0. 150 0. 220 0. 234 0. 255 0. 229 0. 273 0. 174 0. 211 % max load 59. 0 66. 4 65. 5 83. 9 81. 5 88. 2 65. 5 74. 6
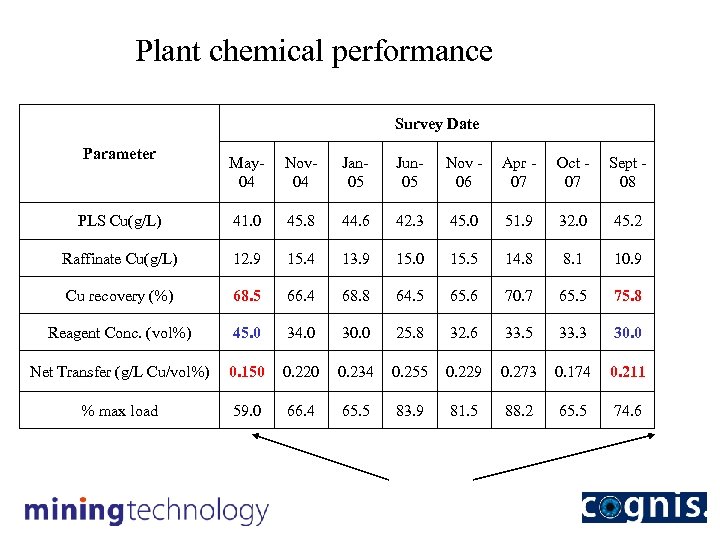 Plant chemical performance Survey Date Parameter May 04 Nov 04 Jan 05 Jun 05 Nov 06 Apr 07 Oct 07 Sept 08 PLS Cu(g/L) 41. 0 45. 8 44. 6 42. 3 45. 0 51. 9 32. 0 45. 2 Raffinate Cu(g/L) 12. 9 15. 4 13. 9 15. 0 15. 5 14. 8 8. 1 10. 9 Cu recovery (%) 68. 5 66. 4 68. 8 64. 5 65. 6 70. 7 65. 5 75. 8 Reagent Conc. (vol%) 45. 0 34. 0 30. 0 25. 8 32. 6 33. 5 33. 3 30. 0 Net Transfer (g/L Cu/vol%) 0. 150 0. 220 0. 234 0. 255 0. 229 0. 273 0. 174 0. 211 % max load 59. 0 66. 4 65. 5 83. 9 81. 5 88. 2 65. 5 74. 6
Plant chemical performance Survey Date Parameter May 04 Nov 04 Jan 05 Jun 05 Nov 06 Apr 07 Oct 07 Sept 08 PLS Cu(g/L) 41. 0 45. 8 44. 6 42. 3 45. 0 51. 9 32. 0 45. 2 Raffinate Cu(g/L) 12. 9 15. 4 13. 9 15. 0 15. 5 14. 8 8. 1 10. 9 Cu recovery (%) 68. 5 66. 4 68. 8 64. 5 65. 6 70. 7 65. 5 75. 8 Reagent Conc. (vol%) 45. 0 34. 0 30. 0 25. 8 32. 6 33. 5 33. 3 30. 0 Net Transfer (g/L Cu/vol%) 0. 150 0. 220 0. 234 0. 255 0. 229 0. 273 0. 174 0. 211 % max load 59. 0 66. 4 65. 5 83. 9 81. 5 88. 2 65. 5 74. 6
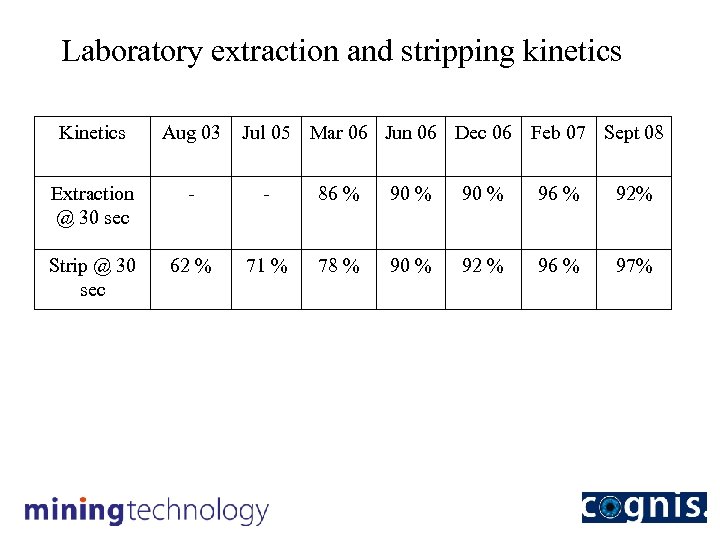 Laboratory extraction and stripping kinetics Kinetics Aug 03 Jul 05 Mar 06 Jun 06 Dec 06 Feb 07 Sept 08 Extraction @ 30 sec - - 86 % 90 % 96 % 92% Strip @ 30 sec 62 % 71 % 78 % 90 % 92 % 96 % 97%
Laboratory extraction and stripping kinetics Kinetics Aug 03 Jul 05 Mar 06 Jun 06 Dec 06 Feb 07 Sept 08 Extraction @ 30 sec - - 86 % 90 % 96 % 92% Strip @ 30 sec 62 % 71 % 78 % 90 % 92 % 96 % 97%
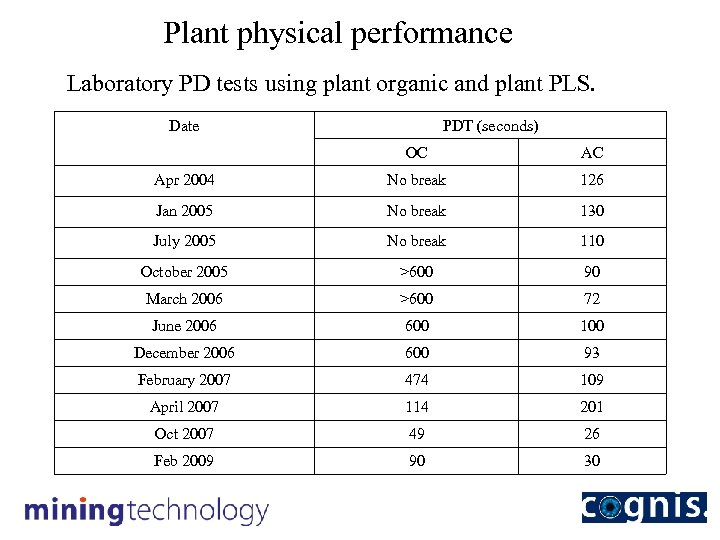 Plant physical performance Laboratory PD tests using plant organic and plant PLS. Date PDT (seconds) OC AC Apr 2004 No break 126 Jan 2005 No break 130 July 2005 No break 110 October 2005 >600 90 March 2006 >600 72 June 2006 600 100 December 2006 600 93 February 2007 474 109 April 2007 114 201 Oct 2007 49 26 Feb 2009 90 30
Plant physical performance Laboratory PD tests using plant organic and plant PLS. Date PDT (seconds) OC AC Apr 2004 No break 126 Jan 2005 No break 130 July 2005 No break 110 October 2005 >600 90 March 2006 >600 72 June 2006 600 100 December 2006 600 93 February 2007 474 109 April 2007 114 201 Oct 2007 49 26 Feb 2009 90 30
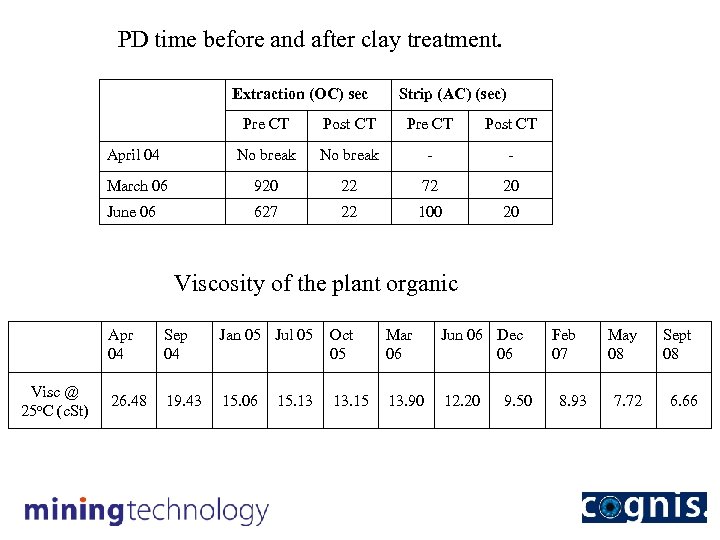 PD time before and after clay treatment. Extraction (OC) sec Strip (AC) (sec) Pre CT Post CT April 04 No break - - March 06 920 22 72 20 June 06 627 22 100 20 Viscosity of the plant organic Apr 04 Visc @ 25 o. C (c. St) Sep 04 Jan 05 Jul 05 Oct 05 Mar 06 Jun 06 Dec 06 26. 48 19. 43 15. 06 13. 15 13. 90 12. 20 15. 13 9. 50 Feb 07 8. 93 May 08 7. 72 Sept 08 6. 66
PD time before and after clay treatment. Extraction (OC) sec Strip (AC) (sec) Pre CT Post CT April 04 No break - - March 06 920 22 72 20 June 06 627 22 100 20 Viscosity of the plant organic Apr 04 Visc @ 25 o. C (c. St) Sep 04 Jan 05 Jul 05 Oct 05 Mar 06 Jun 06 Dec 06 26. 48 19. 43 15. 06 13. 15 13. 90 12. 20 15. 13 9. 50 Feb 07 8. 93 May 08 7. 72 Sept 08 6. 66
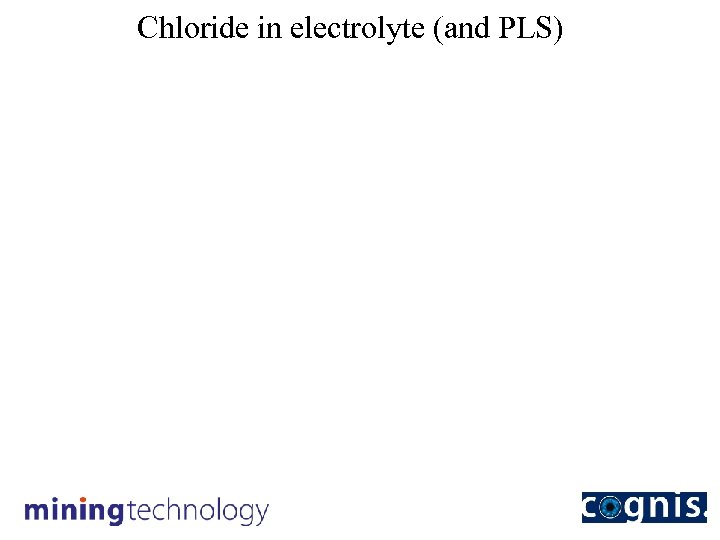 Chloride in electrolyte (and PLS)
Chloride in electrolyte (and PLS)
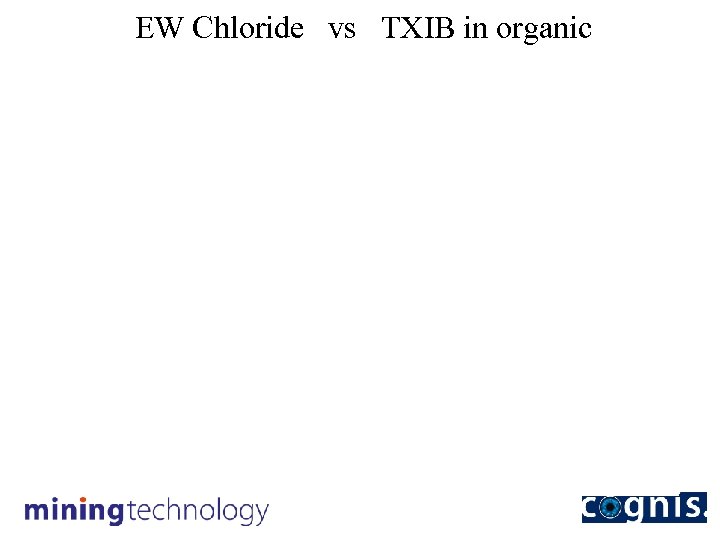 EW Chloride vs TXIB in organic
EW Chloride vs TXIB in organic
 Conclusions The conversion of Acorga modified reagent to the more stable LIX non-modified reagent resulted in: • reduction in reagent concentration to <35 vol% (now at 30%). • improvement in extraction and strip kinetics. • higher net transfer (>0. 2 gpl. Cu/vol%). • very significant improvement in phase separation behaviour. • organic phase viscosity reduced by 75%. • very significant decrease in chloride transfer to EW. • reduction in electrolyte chloride level by ~90%. • much easier plant operation.
Conclusions The conversion of Acorga modified reagent to the more stable LIX non-modified reagent resulted in: • reduction in reagent concentration to <35 vol% (now at 30%). • improvement in extraction and strip kinetics. • higher net transfer (>0. 2 gpl. Cu/vol%). • very significant improvement in phase separation behaviour. • organic phase viscosity reduced by 75%. • very significant decrease in chloride transfer to EW. • reduction in electrolyte chloride level by ~90%. • much easier plant operation.
 Acknowledgements Nyrstar Port Pirie Cognis Co-authors and investigators: Kym Dudley, Mark Urbani, Dr Michael Virnig and Anissa Horner
Acknowledgements Nyrstar Port Pirie Cognis Co-authors and investigators: Kym Dudley, Mark Urbani, Dr Michael Virnig and Anissa Horner
 Questions?
Questions?


