a833d11b090945d7a6f8f6ce753ab014.ppt
- Количество слайдов: 51
Solutions

Solutions: Basic Definitions • Solute – substance that is being dissolved • Solvent – substance that dissolves the solute • Solution – a mixture of substances that has a uniform composition; a homogeneous mixture

Solutions: Basic Definitions • Soluble – when a substance will dissolve in another substance (salt & water) • Insoluble – when a substance will not dissolve in another substance (sand & water)

Solutions: Basic Definitions • Miscible – when two liquids are soluble in each other (alcohol & water) • Immiscible – when two liquids are not soluble in each other (oil & water) • Aqueous – dissolved in water
Solutions: Basic Definitions • unsaturated solution - If the amount of solute dissolved is less than the maximum that could be dissolved • saturated solution - solution which holds the maximum amount of solute per amount of the solution under the given conditions • supersaturated solution - solutions that contain more solute than the usual maximum amount and are unstable.

Supersaturated Solutions • They cannot permanently hold the excess solute in solution and may release it suddenly. • Supersaturated solutions, as you might imagine, have to be prepared carefully. • Generally, this is done by dissolving a solute in the solution at an elevated temperature, at which solubility is higher than at room temperature, and then slowly cooling the solution.

Solutions: Basic Definitions • Suspension – mixture containing particles that will settle out is left undisturbed (cornstarch & water) • Colloid – heterogeneous mixture that will not settle out if left alone (blood) • Emulsion – colloid in which a liquid is suspended in another liquid (mayo)

Dissolution of a Solid in a Liquid Click in this box to enter notes. Go to Slide Show View (press F 5) to play the video or animation. (To exit, press Esc. ) This media requires Power. Point® 2000 (or newer) and the Macromedia Flash Player (7 or higher). [To delete this message, click inside the box, click the border of the box, and then press delete. ] Copyright © Houghton Mifflin Company. All rights reserved.
Solutions: Basic Definitions • Electrolyte – solution that conducts an electric current • Non electrolyte – solution that does not conduct an electric current
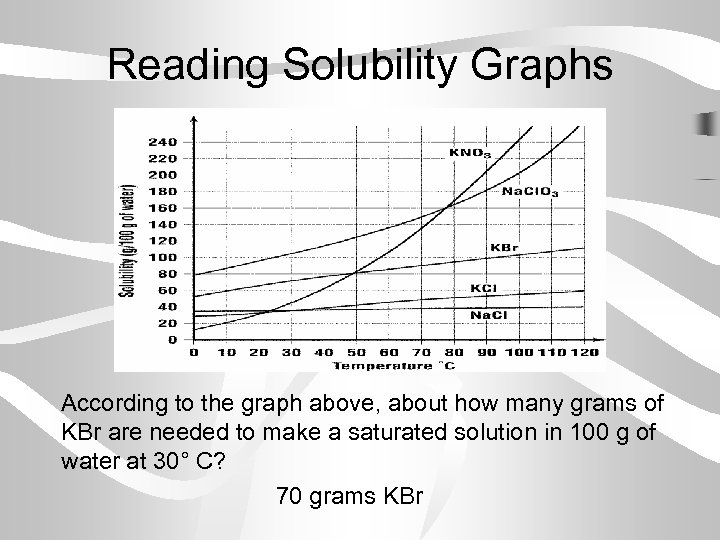
Reading Solubility Graphs According to the graph above, about how many grams of KBr are needed to make a saturated solution in 100 g of water at 30° C? 70 grams KBr
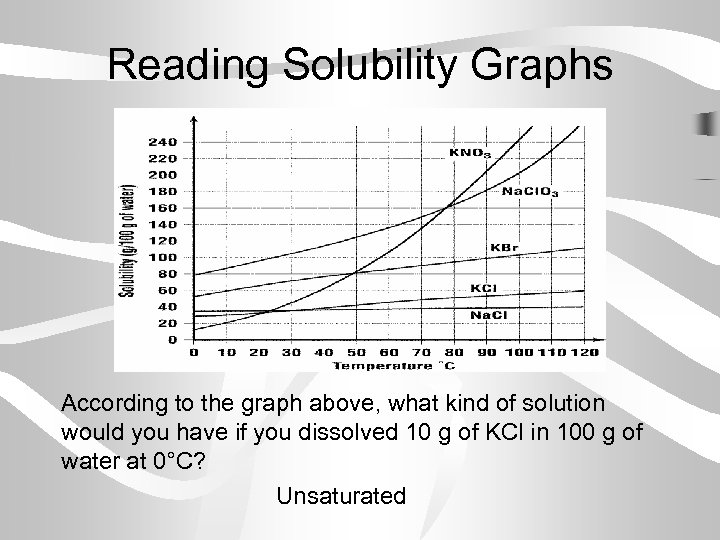
Reading Solubility Graphs According to the graph above, what kind of solution would you have if you dissolved 10 g of KCl in 100 g of water at 0°C? Unsaturated

Solubility • Solvation – process of surrounding solute particles with solvent particles to form a solution • The rule for dissolving solutions is “like dissolves like” • Polar substances will dissolve in polar solvents • Non polar substances will dissolve in non polar solvents • Non polar will NOT dissolve in polar and vice versa

Solubility Rules!!!

Increasing the Rate of Solution 1. Agitation 2. Increasing Temperature 3. Increasing Surface Area

Agitation • Increases the speed of the particles – speeds up the dissolving process in solids.
Increasing Temperature • More collisions of particles as temperature increases.

Particle Size (Increasing Surface Area) • Smaller particles dissolve faster than larger particles. – more surface area – Sugar cube vs. ½ teaspoon sugar – Teaspoon will dissolve faster

Solubility of a gas • Two main factors that affect the solubility of a gas in a liquid 1. Temperature – Normally, the higher the temperature, the faster a solute will dissolve…NOT with a gas! – In a gas, the cooler the temperature, the faster the gas will dissolve – Think of drinking a coke…what’s fizzier, a cold coke or a hot coke?

Solubility of a gas • The second factor affecting the solubility of a gas is pressure 2. Pressure – The higher the pressure, the more gas that will dissolve – Think of a coke bottle…What will happen if you leave the lid off?

Concentration • Concentration units can vary greatly. • They express a ratio that compares an amount of the solute with an amount of the solution or the solvent. • For chemistry applications, the concentration term molarity is generally the most useful. Abrv. (M)

% by Mass • Remember … • % = part x 100 whole • % by mass = mass solute x 100 mass solution

Example • What is the % by mass of a solution with 3. 6 g of Na. Cl dissolved in 100. 0 g of water? • Remember what will the Total solution be!! • % = (3. 6 / 103. 6) x 100 = 3. 5% Na. Cl

% by Volume • Remember … • % = part x 100 whole • % by volume = volume solute x 100 volume solution

Example • What is the % by volume of 75. 0 ml of ethanol dissolved in 200. 0 ml of water? • Remember what will the Total solution be!! • % = (75. 0 ml / 275. 0 ml) x 100 = 27. 3%

Molarity • Molarity is defined as the number of moles of solute per liter of solution. • Molarity = moles of solute/liter of solution • Note that the volume is the total solution volume that results, not the volume of solvent alone.
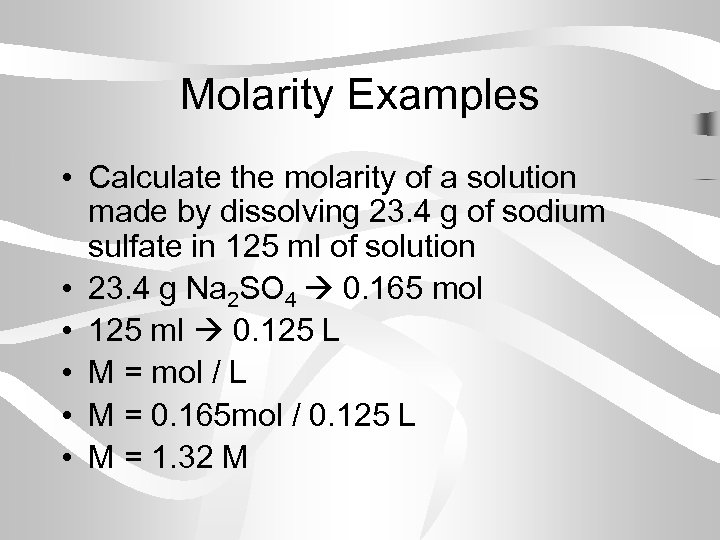
Molarity Examples • Calculate the molarity of a solution made by dissolving 23. 4 g of sodium sulfate in 125 ml of solution • 23. 4 g Na 2 SO 4 0. 165 mol • 125 ml 0. 125 L • M = mol / L • M = 0. 165 mol / 0. 125 L • M = 1. 32 M

Molarity Examples • Calculate the molarity of a solution made by dissolving 5. 00 g of C 6 H 12 O 6 in enough water to make 100. 0 ml of solution • 5 g/180 g = 0. 028 mol • 0. 028 mol/0. 100 L • 0. 280 M
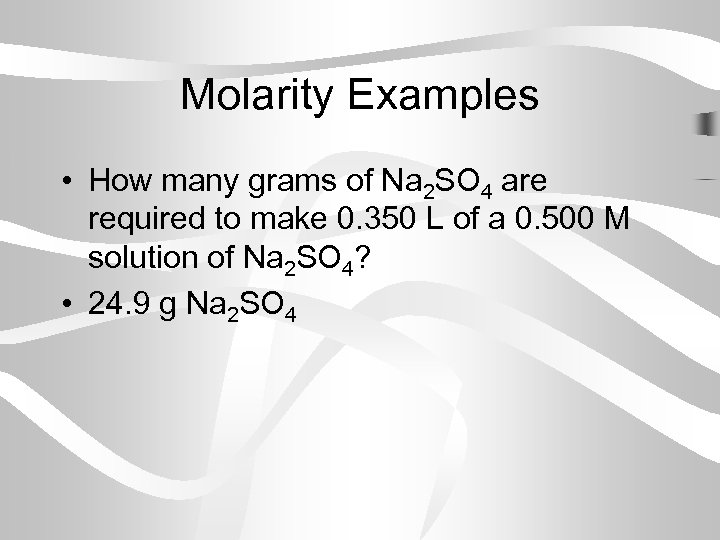
Molarity Examples • How many grams of Na 2 SO 4 are required to make 0. 350 L of a 0. 500 M solution of Na 2 SO 4? • 24. 9 g Na 2 SO 4

Dilution • When chemists purchase solutions, they generally purchase “stock solutions” which are extremely concentrated solutions • This way a chemist can dilute the strong solution to any concentration that they wish. This stops the chemist from having to buy several concentrations

Dilution Equation • • • M 1 V 1 = M 2 V 2 M 1 = initial molarity V 1 = initial volume M 2 = final molarity V 2 = final volume The units for V 1 & V 2 do not matter as long as they are the same • M 1 & M 2 MUST be in molarity

Dilution Problems • Suppose we want to make 250 ml of a 0. 10 M solution of Cu. SO 4 and we have a stock solution of 1. 0 M Cu. SO 4. How would we prepare the solution? • First do the math • M 1 V 1 = M 2 V 2 • (1 M)V 1 = (. 1 M)(250 ml) • V 1 = 25 ml • Take 25 ml of 1 M Cu. SO 4 and add it to 225 ml H 2 O

More Dilution Problems • How many ml of 3. 0 M H 2 SO 4 are required to make 450 ml of a 1. 0 M solution? • 150 m. L
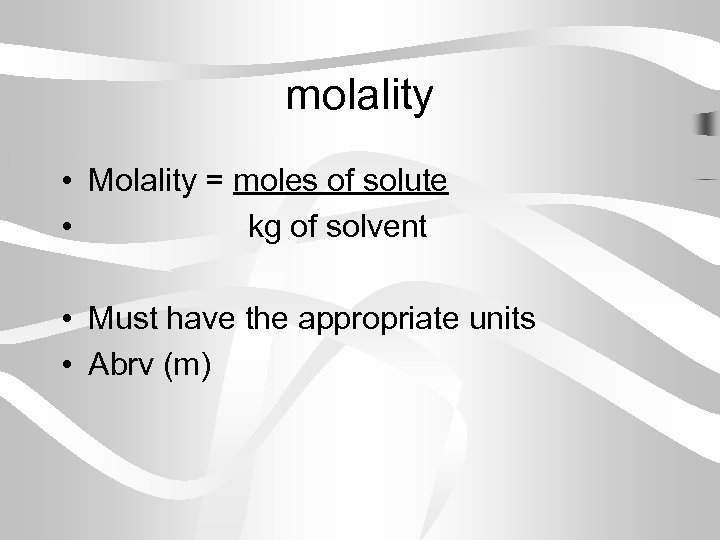
molality • Molality = moles of solute • kg of solvent • Must have the appropriate units • Abrv (m)

Colligative Properties

Colligative Properties • • • Colligative properties – physical properties of solutions that are affected only by the number of particles NOT the identity of the solute They include: 1. Vapor Pressure Lowering 2. Boiling Point Elevation 3. Freezing Point Depression 4. Osmotic Pressure In all of these we will be comparing a pure substance to a mixture

Vapor Pressure Lowering • Vapor Pressure – the pressure exerted in a closed container by liquid particles that have escaped to the surface and entered the gas phase

Vapor Pressure Lowering • The vapor pressure of a mixture is lower than a non volatile pure substance due to the fewer number of particles that are able to escape into the gas phase

Vapor Pressure Lowering: Liquid/Vapor Equilibrium Click in this box to enter notes. Go to Slide Show View (press F 5) to play the video or animation. (To exit, press Esc. ) This media requires Power. Point® 2000 (or newer) and the Macromedia Flash Player (7 or higher). [To delete this message, click inside the box, click the border of the box, and then press delete. ] Copyright © Houghton Mifflin Company. All rights reserved.

Vapor Pressure Lowering: Addition of a Solute Click in this box to enter notes. Go to Slide Show View (press F 5) to play the video or animation. (To exit, press Esc. ) This media requires Power. Point® 2000 (or newer) and the Macromedia Flash Player (7 or higher). [To delete this message, click inside the box, click the border of the box, and then press delete. ] Copyright © Houghton Mifflin Company. All rights reserved.

Boiling Point Elevation • The boiling point of a solution is the point at which enough energy has been added to overcome the intermolecular forces that hold the solute in the solution. • At this point, the molecules gain enough kinetic energy to produce a pressure that is greater than the atmospheric pressure keeping them in solution • Once this point is reached, the solution vaporizes (becomes a gas) • The boiling point of a mixture is higher that the boiling point of a pure substance

Boiling Point Elevation: Liquid/Vapor Equilibrium Click in this box to enter notes. Go to Slide Show View (press F 5) to play the video or animation. (To exit, press Esc. ) This media requires Power. Point® 2000 (or newer) and the Macromedia Flash Player (7 or higher). [To delete this message, click inside the box, click the border of the box, and then press delete. ] Copyright © Houghton Mifflin Company. All rights reserved.

Boiling Point Elevation: Addition of a Solute Click in this box to enter notes. Go to Slide Show View (press F 5) to play the video or animation. (To exit, press Esc. ) This media requires Power. Point® 2000 (or newer) and the Macromedia Flash Player (7 or higher). [To delete this message, click inside the box, click the border of the box, and then press delete. ] Copyright © Houghton Mifflin Company. All rights reserved.

Boiling Point Elevation: Solution/Vapor Equilibrium Click in this box to enter notes. Go to Slide Show View (press F 5) to play the video or animation. (To exit, press Esc. ) This media requires Power. Point® 2000 (or newer) and the Macromedia Flash Player (7 or higher). [To delete this message, click inside the box, click the border of the box, and then press delete. ] Copyright © Houghton Mifflin Company. All rights reserved.

Freezing Point Depression • The freezing point of a solution is the point where enough energy has been removed from the solution to slow the molecules down and increase intermolecular forces so the solution becomes a solid • The freezing point of a mixture is lower that the freezing point of a pure substance

Freezing Point Depression: Solid/Liquid Equilibrium Click in this box to enter notes. Go to Slide Show View (press F 5) to play the video or animation. (To exit, press Esc. ) This media requires Power. Point® 2000 (or newer) and the Macromedia Flash Player (7 or higher). [To delete this message, click inside the box, click the border of the box, and then press delete. ] Copyright © Houghton Mifflin Company. All rights reserved.

Freezing Point Depression: Addition of a Solute Click in this box to enter notes. Go to Slide Show View (press F 5) to play the video or animation. (To exit, press Esc. ) This media requires Power. Point® 2000 (or newer) and the Macromedia Flash Player (7 or higher). [To delete this message, click inside the box, click the border of the box, and then press delete. ] Copyright © Houghton Mifflin Company. All rights reserved.

Freezing Point Depression: Solid/Solution Equilibrium Click in this box to enter notes. Go to Slide Show View (press F 5) to play the video or animation. (To exit, press Esc. ) This media requires Power. Point® 2000 (or newer) and the Macromedia Flash Player (7 or higher). [To delete this message, click inside the box, click the border of the box, and then press delete. ] Copyright © Houghton Mifflin Company. All rights reserved.
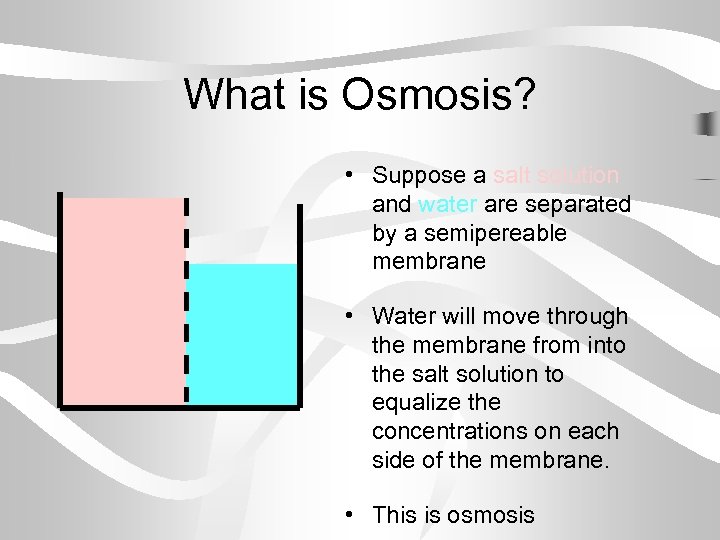
What is Osmosis? • Suppose a salt solution and water are separated by a semipereable membrane • Water will move through the membrane from into the salt solution to equalize the concentrations on each side of the membrane. • This is osmosis
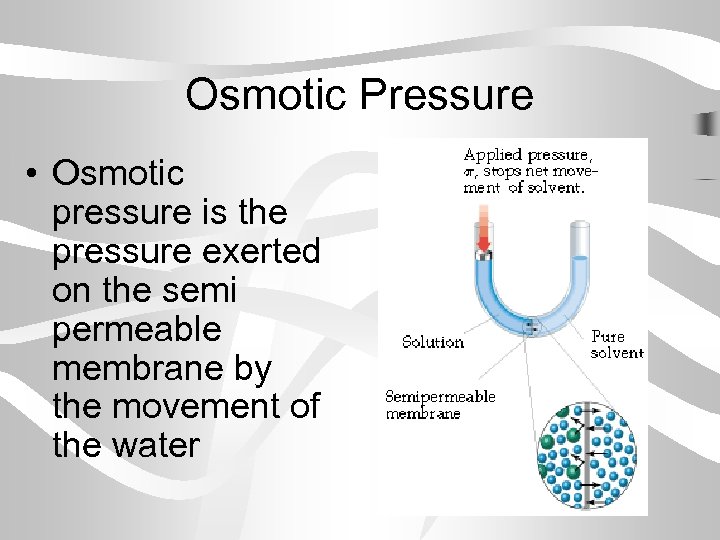
Osmotic Pressure • Osmotic pressure is the pressure exerted on the semi permeable membrane by the movement of the water

Osmosis Click in this box to enter notes. Go to Slide Show View (press F 5) to play the video or animation. (To exit, press Esc. ) This media requires Power. Point® 2000 (or newer) and the Macromedia Flash Player (7 or higher). [To delete this message, click inside the box, click the border of the box, and then press delete. ] Copyright © Houghton Mifflin Company. All rights reserved.
Micelle Formation: The Cleansing Action of Soap Click in this box to enter notes. Go to Slide Show View (press F 5) to play the video or animation. (To exit, press Esc. ) This media requires Power. Point® 2000 (or newer) and the Macromedia Flash Player (7 or higher). [To delete this message, click inside the box, click the border of the box, and then press delete. ] Copyright © Houghton Mifflin Company. All rights reserved.
a833d11b090945d7a6f8f6ce753ab014.ppt