 Solutions and p. H Chapter 2 sec. 2. 2 pg 41 - 43
Solutions and p. H Chapter 2 sec. 2. 2 pg 41 - 43
 solutions Mixtures – a combination of substances that retain their original properties Ex. trail mix, air, salt water
solutions Mixtures – a combination of substances that retain their original properties Ex. trail mix, air, salt water
 solutions solution – mixture of one or more substances uniformly distributed in another substance. Can be solid, liquid, gas ex. Plasma (liquid) brass (solid) copper and zinc soda (liquid/gas) water and CO 2
solutions solution – mixture of one or more substances uniformly distributed in another substance. Can be solid, liquid, gas ex. Plasma (liquid) brass (solid) copper and zinc soda (liquid/gas) water and CO 2
 solutions 2 parts: 1. solute – substance being dissolved 2. solvent- substance doing dissolving Salt water: salt = solute water = solvent Aqueous solutions- water is the solvent due to hydrogen bonding Water = universal solvent because it can dissolve most things
solutions 2 parts: 1. solute – substance being dissolved 2. solvent- substance doing dissolving Salt water: salt = solute water = solvent Aqueous solutions- water is the solvent due to hydrogen bonding Water = universal solvent because it can dissolve most things
 concentration- amount of solute dissolved in a fixed amount of solution When no more solute can be dissolved…. solution is SATURATED Salt will no longer dissolve
concentration- amount of solute dissolved in a fixed amount of solution When no more solute can be dissolved…. solution is SATURATED Salt will no longer dissolve
 Acids & Bases The Homeostasis of living things depends on the degree of acidity (acid) and alkalinity (base) in certain areas
Acids & Bases The Homeostasis of living things depends on the degree of acidity (acid) and alkalinity (base) in certain areas
 Water § Acids and bases are solutions made by the dissociation of water § Water naturally breaks down into ions of opposite charges, this is called dissociation. § Water will form these ions naturally (H 3 O+ & OH-) and in EQUAL amounts. H 2 O H+ + OHThis is why water is NEUTRAL! Hydronium (H 3 O+) ions = hydroxide (OH- ) ions
Water § Acids and bases are solutions made by the dissociation of water § Water naturally breaks down into ions of opposite charges, this is called dissociation. § Water will form these ions naturally (H 3 O+ & OH-) and in EQUAL amounts. H 2 O H+ + OHThis is why water is NEUTRAL! Hydronium (H 3 O+) ions = hydroxide (OH- ) ions
 Acids & Bases Acidity= measure of the amount of Hydronium (H 3 O+) ions dissolved in a solution. Alkalinity (a/k/a basic solution)= measure of the amount of hydroxide (OH- ) ions dissolves in a solution
Acids & Bases Acidity= measure of the amount of Hydronium (H 3 O+) ions dissolved in a solution. Alkalinity (a/k/a basic solution)= measure of the amount of hydroxide (OH- ) ions dissolves in a solution
 Acids - more H+ ions released - p. H < 7 - Sour taste - Corrosive on metals - Burns skin • Ex. Orange juice, • vinegar, sulfuric acid *Homeostasis relevance- your stomach must have a certain acidity to function properly
Acids - more H+ ions released - p. H < 7 - Sour taste - Corrosive on metals - Burns skin • Ex. Orange juice, • vinegar, sulfuric acid *Homeostasis relevance- your stomach must have a certain acidity to function properly
 Bases -more OH- ions released - p. H > 7 Tastes bitter Slippery Irritate the skin - Ex. Na. OH Homeostasis relevance- your small intestine needs a certain alkalinity (basic) to function properly
Bases -more OH- ions released - p. H > 7 Tastes bitter Slippery Irritate the skin - Ex. Na. OH Homeostasis relevance- your small intestine needs a certain alkalinity (basic) to function properly
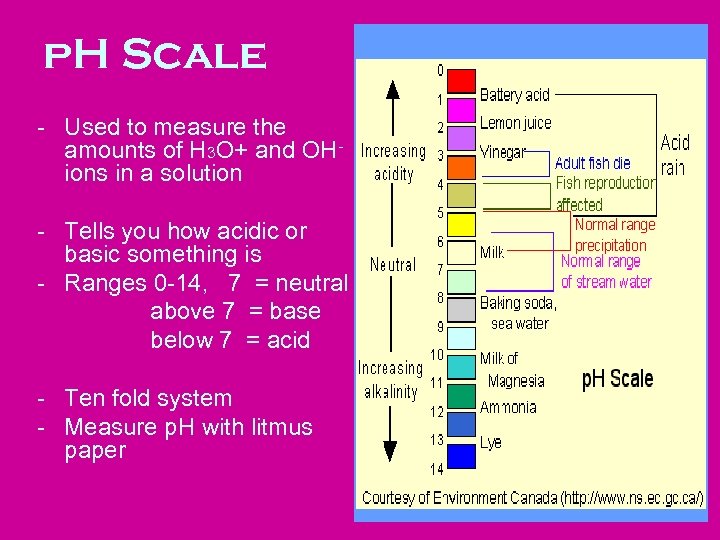 p. H Scale - Used to measure the amounts of H 3 O+ and OHions in a solution - Tells you how acidic or basic something is - Ranges 0 -14, 7 = neutral above 7 = base below 7 = acid - Ten fold system - Measure p. H with litmus paper
p. H Scale - Used to measure the amounts of H 3 O+ and OHions in a solution - Tells you how acidic or basic something is - Ranges 0 -14, 7 = neutral above 7 = base below 7 = acid - Ten fold system - Measure p. H with litmus paper
 Buffers- chemical substances that neutralize small amounts of acids and bases Ex. Acids { stomach acid & urine Ex. Bases { blood & intestinal fluid - If not at the proper p. H level, these body systems will not function properly and… you’ll need to use a Buffer ex. Alka seltzer
Buffers- chemical substances that neutralize small amounts of acids and bases Ex. Acids { stomach acid & urine Ex. Bases { blood & intestinal fluid - If not at the proper p. H level, these body systems will not function properly and… you’ll need to use a Buffer ex. Alka seltzer