29225e390db186aae9b617d8379aa5c2.ppt
- Количество слайдов: 12
 Solution Concentration Section 15. 2
Solution Concentration Section 15. 2
 Solution Concentration is the measure of how much solute is dissolved in a specific amount of solvent. Solutions can be described qualitatively using words concentrated and dilute. -A concentrated solution would have a lot of solute compared to the amount of solvent. -A dilute solution would have very little solute compared to the amount of solvent.
Solution Concentration is the measure of how much solute is dissolved in a specific amount of solvent. Solutions can be described qualitatively using words concentrated and dilute. -A concentrated solution would have a lot of solute compared to the amount of solvent. -A dilute solution would have very little solute compared to the amount of solvent.
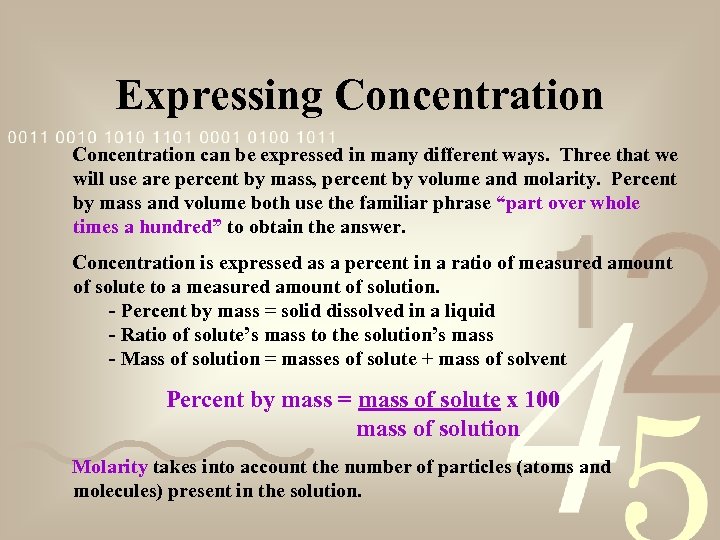 Expressing Concentration can be expressed in many different ways. Three that we will use are percent by mass, percent by volume and molarity. Percent by mass and volume both use the familiar phrase “part over whole times a hundred” to obtain the answer. Concentration is expressed as a percent in a ratio of measured amount of solute to a measured amount of solution. - Percent by mass = solid dissolved in a liquid - Ratio of solute’s mass to the solution’s mass - Mass of solution = masses of solute + mass of solvent Percent by mass = mass of solute x 100 mass of solution Molarity takes into account the number of particles (atoms and molecules) present in the solution.
Expressing Concentration can be expressed in many different ways. Three that we will use are percent by mass, percent by volume and molarity. Percent by mass and volume both use the familiar phrase “part over whole times a hundred” to obtain the answer. Concentration is expressed as a percent in a ratio of measured amount of solute to a measured amount of solution. - Percent by mass = solid dissolved in a liquid - Ratio of solute’s mass to the solution’s mass - Mass of solution = masses of solute + mass of solvent Percent by mass = mass of solute x 100 mass of solution Molarity takes into account the number of particles (atoms and molecules) present in the solution.
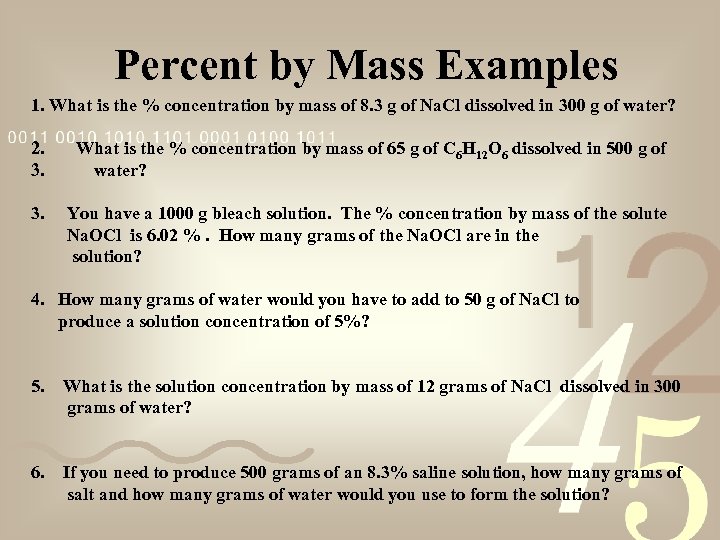 Percent by Mass Examples 1. What is the % concentration by mass of 8. 3 g of Na. Cl dissolved in 300 g of water? 2. 3. What is the % concentration by mass of 65 g of C 6 H 12 O 6 dissolved in 500 g of water? 3. You have a 1000 g bleach solution. The % concentration by mass of the solute Na. OCl is 6. 02 %. How many grams of the Na. OCl are in the solution? 4. How many grams of water would you have to add to 50 g of Na. Cl to produce a solution concentration of 5%? 5. What is the solution concentration by mass of 12 grams of Na. Cl dissolved in 300 grams of water? 6. If you need to produce 500 grams of an 8. 3% saline solution, how many grams of salt and how many grams of water would you use to form the solution?
Percent by Mass Examples 1. What is the % concentration by mass of 8. 3 g of Na. Cl dissolved in 300 g of water? 2. 3. What is the % concentration by mass of 65 g of C 6 H 12 O 6 dissolved in 500 g of water? 3. You have a 1000 g bleach solution. The % concentration by mass of the solute Na. OCl is 6. 02 %. How many grams of the Na. OCl are in the solution? 4. How many grams of water would you have to add to 50 g of Na. Cl to produce a solution concentration of 5%? 5. What is the solution concentration by mass of 12 grams of Na. Cl dissolved in 300 grams of water? 6. If you need to produce 500 grams of an 8. 3% saline solution, how many grams of salt and how many grams of water would you use to form the solution?
 Percent by Volume 1. What is the percent by volume of ethanol in a solution that contains 35 m. L of ethanol dissolved in 115 m. L of water? 2. If you have 100 m. L of a 30% aqueous solution of ethanol, what volumes of ethanol and water are in the solution? 3. What is the percent by volume of isopropyl alcohol in a solution that contains 24 m. L of isopropyl alcohol in 1. 1 L of water? 4. What is the percent by volume of 140 m. L of isopropyl alcohol dissolved in 60 m. L of water?
Percent by Volume 1. What is the percent by volume of ethanol in a solution that contains 35 m. L of ethanol dissolved in 115 m. L of water? 2. If you have 100 m. L of a 30% aqueous solution of ethanol, what volumes of ethanol and water are in the solution? 3. What is the percent by volume of isopropyl alcohol in a solution that contains 24 m. L of isopropyl alcohol in 1. 1 L of water? 4. What is the percent by volume of 140 m. L of isopropyl alcohol dissolved in 60 m. L of water?
 Diluting Solutions In the laboratory, I purchase concentrated stock solutions of chemicals because they are much more economical to buy. To use these solutions I have to dilute them to concentrations that are required by the labs we perform in class. To properly dilute these solutions I use the following equation: M 1 V 1 = M 2 V 2 Where ‘M’ = Molarity Where ‘V’ = Volume
Diluting Solutions In the laboratory, I purchase concentrated stock solutions of chemicals because they are much more economical to buy. To use these solutions I have to dilute them to concentrations that are required by the labs we perform in class. To properly dilute these solutions I use the following equation: M 1 V 1 = M 2 V 2 Where ‘M’ = Molarity Where ‘V’ = Volume
 Diluting Solutions 5. What volume of a 3 M KI solution would you use to make. 3 L of a 1. 25 M KI solution? 6. How many milliliters of a 5 M H 2 SO 4 solution would you need to prepare 100 m. L of. 25 M H 2 SO 4? 7. If you dilute 20 m. L of a 3. 5 M solution to make 100 m. L of solution, what is the molarity of the dilute solution?
Diluting Solutions 5. What volume of a 3 M KI solution would you use to make. 3 L of a 1. 25 M KI solution? 6. How many milliliters of a 5 M H 2 SO 4 solution would you need to prepare 100 m. L of. 25 M H 2 SO 4? 7. If you dilute 20 m. L of a 3. 5 M solution to make 100 m. L of solution, what is the molarity of the dilute solution?
 Molarity When chemists are working with a reaction in an aqueous solution, they must know the number of particles (atoms, molecules) that are present. Since we count the number of particles present using moles, then the number of moles of solute dissolved per liter of solution is referred to as MOLARITY (M) or molar concentration, The unit M is read as molar and a liter of solution containing one mole of solute as a 1 M solution. To calculate the molarity of a solution, we utilize the following formula: Molarity = moles of solute liters of solution
Molarity When chemists are working with a reaction in an aqueous solution, they must know the number of particles (atoms, molecules) that are present. Since we count the number of particles present using moles, then the number of moles of solute dissolved per liter of solution is referred to as MOLARITY (M) or molar concentration, The unit M is read as molar and a liter of solution containing one mole of solute as a 1 M solution. To calculate the molarity of a solution, we utilize the following formula: Molarity = moles of solute liters of solution
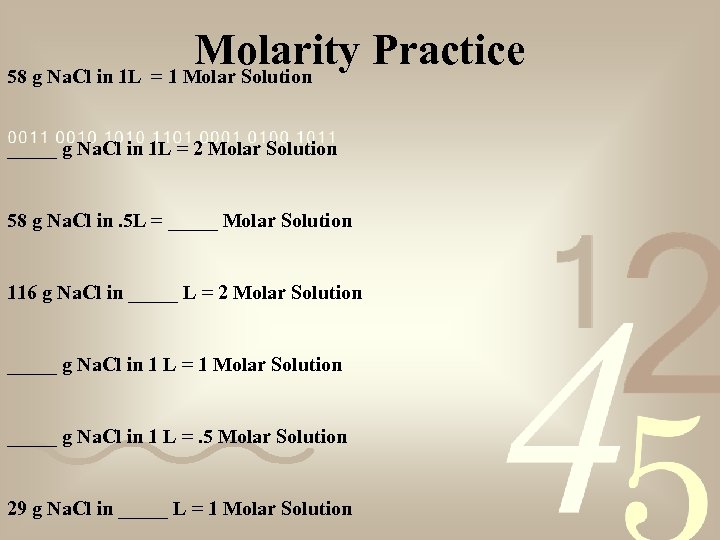 Molarity Practice 58 g Na. Cl in 1 L = 1 Molar Solution _____ g Na. Cl in 1 L = 2 Molar Solution 58 g Na. Cl in. 5 L = _____ Molar Solution 116 g Na. Cl in _____ L = 2 Molar Solution _____ g Na. Cl in 1 L = 1 Molar Solution _____ g Na. Cl in 1 L =. 5 Molar Solution 29 g Na. Cl in _____ L = 1 Molar Solution
Molarity Practice 58 g Na. Cl in 1 L = 1 Molar Solution _____ g Na. Cl in 1 L = 2 Molar Solution 58 g Na. Cl in. 5 L = _____ Molar Solution 116 g Na. Cl in _____ L = 2 Molar Solution _____ g Na. Cl in 1 L = 1 Molar Solution _____ g Na. Cl in 1 L =. 5 Molar Solution 29 g Na. Cl in _____ L = 1 Molar Solution
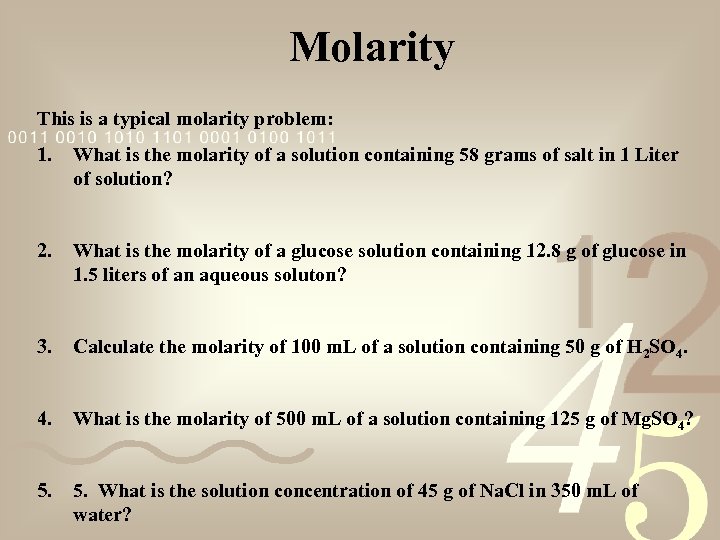 Molarity This is a typical molarity problem: 1. What is the molarity of a solution containing 58 grams of salt in 1 Liter of solution? 2. What is the molarity of a glucose solution containing 12. 8 g of glucose in 1. 5 liters of an aqueous soluton? 3. Calculate the molarity of 100 m. L of a solution containing 50 g of H 2 SO 4. What is the molarity of 500 m. L of a solution containing 125 g of Mg. SO 4? 5. What is the solution concentration of 45 g of Na. Cl in 350 m. L of water?
Molarity This is a typical molarity problem: 1. What is the molarity of a solution containing 58 grams of salt in 1 Liter of solution? 2. What is the molarity of a glucose solution containing 12. 8 g of glucose in 1. 5 liters of an aqueous soluton? 3. Calculate the molarity of 100 m. L of a solution containing 50 g of H 2 SO 4. What is the molarity of 500 m. L of a solution containing 125 g of Mg. SO 4? 5. What is the solution concentration of 45 g of Na. Cl in 350 m. L of water?
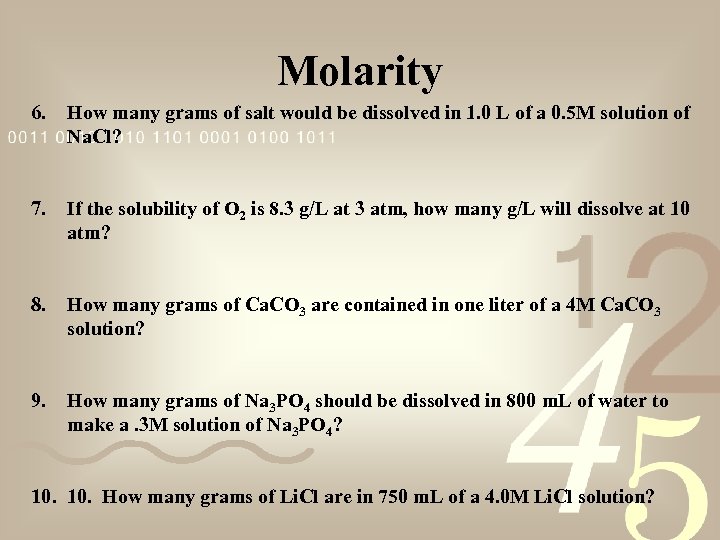 Molarity 6. How many grams of salt would be dissolved in 1. 0 L of a 0. 5 M solution of Na. Cl? 7. If the solubility of O 2 is 8. 3 g/L at 3 atm, how many g/L will dissolve at 10 atm? 8. How many grams of Ca. CO 3 are contained in one liter of a 4 M Ca. CO 3 solution? 9. How many grams of Na 3 PO 4 should be dissolved in 800 m. L of water to make a. 3 M solution of Na 3 PO 4? 10. How many grams of Li. Cl are in 750 m. L of a 4. 0 M Li. Cl solution?
Molarity 6. How many grams of salt would be dissolved in 1. 0 L of a 0. 5 M solution of Na. Cl? 7. If the solubility of O 2 is 8. 3 g/L at 3 atm, how many g/L will dissolve at 10 atm? 8. How many grams of Ca. CO 3 are contained in one liter of a 4 M Ca. CO 3 solution? 9. How many grams of Na 3 PO 4 should be dissolved in 800 m. L of water to make a. 3 M solution of Na 3 PO 4? 10. How many grams of Li. Cl are in 750 m. L of a 4. 0 M Li. Cl solution?
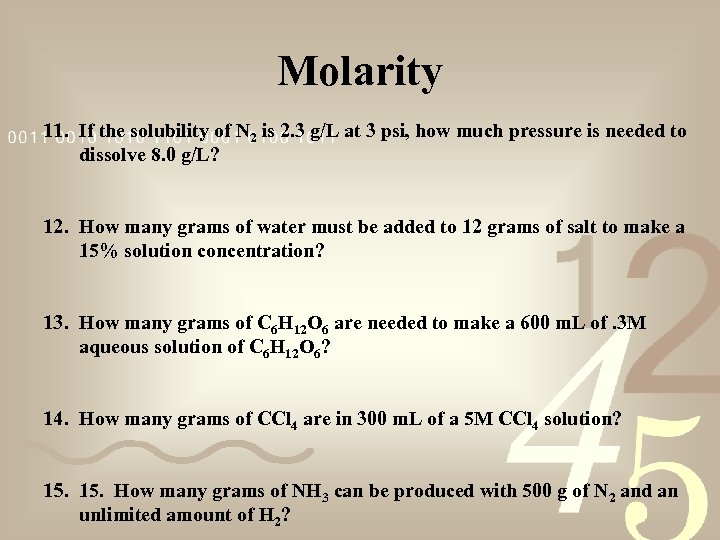 Molarity 11. If the solubility of N 2 is 2. 3 g/L at 3 psi, how much pressure is needed to dissolve 8. 0 g/L? 12. How many grams of water must be added to 12 grams of salt to make a 15% solution concentration? 13. How many grams of C 6 H 12 O 6 are needed to make a 600 m. L of. 3 M aqueous solution of C 6 H 12 O 6? 14. How many grams of CCl 4 are in 300 m. L of a 5 M CCl 4 solution? 15. How many grams of NH 3 can be produced with 500 g of N 2 and an unlimited amount of H 2?
Molarity 11. If the solubility of N 2 is 2. 3 g/L at 3 psi, how much pressure is needed to dissolve 8. 0 g/L? 12. How many grams of water must be added to 12 grams of salt to make a 15% solution concentration? 13. How many grams of C 6 H 12 O 6 are needed to make a 600 m. L of. 3 M aqueous solution of C 6 H 12 O 6? 14. How many grams of CCl 4 are in 300 m. L of a 5 M CCl 4 solution? 15. How many grams of NH 3 can be produced with 500 g of N 2 and an unlimited amount of H 2?


