17b53c639b2d685c70ccd4cfbd193435.ppt
- Количество слайдов: 29
Solubility & Equilibrium Ksp
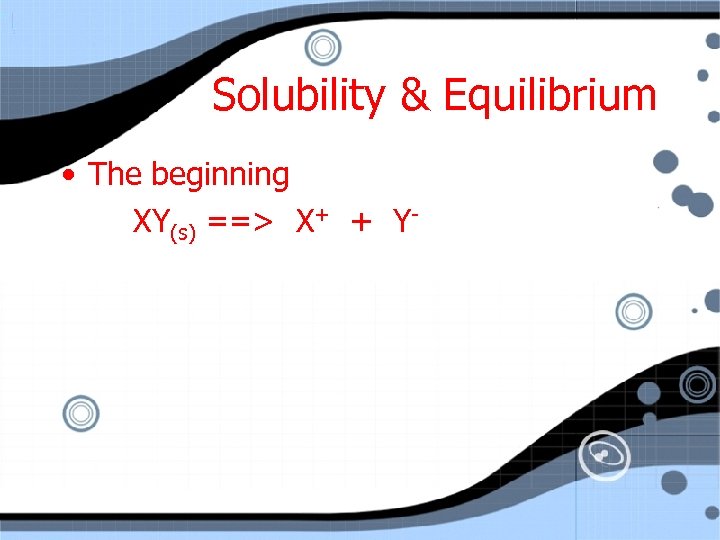
Solubility & Equilibrium • The beginning XY(s) ==> X+ + Y-

Solubility & Equilibrium • The beginning XY(s) ==> X+ + YAs time continues the [ions] begins to increase.
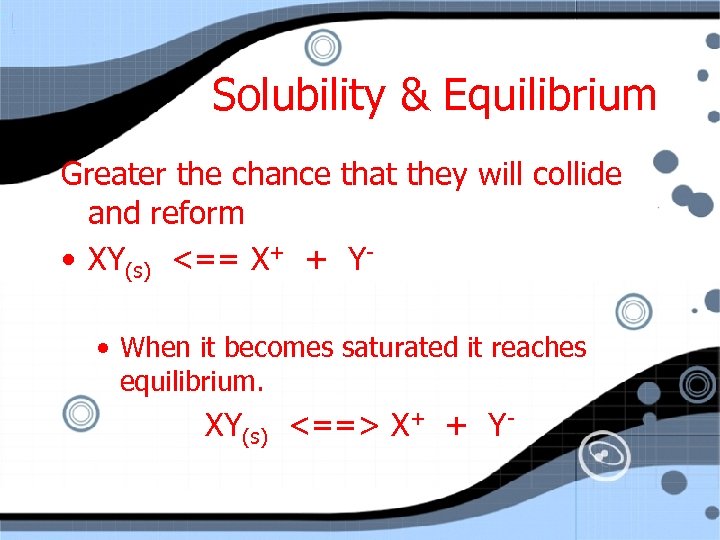
Solubility & Equilibrium Greater the chance that they will collide and reform • XY(s) <== X+ + Y • When it becomes saturated it reaches equilibrium. XY(s) <==> X+ + Y-
![Solubility & Equilibrium Constant for Solubility-Ksp = [X+]x [Y-]y Solubility = molar solubility = Solubility & Equilibrium Constant for Solubility-Ksp = [X+]x [Y-]y Solubility = molar solubility =](https://present5.com/presentation/17b53c639b2d685c70ccd4cfbd193435/image-5.jpg)
Solubility & Equilibrium Constant for Solubility-Ksp = [X+]x [Y-]y Solubility = molar solubility = mol/L • Be careful ~ sometimes given g/L or have to solve for g/L
![Solubility & Equilibrium Constant for Solubility-Ksp = [X+]x [Y-]y 1) Just ions, no solids Solubility & Equilibrium Constant for Solubility-Ksp = [X+]x [Y-]y 1) Just ions, no solids](https://present5.com/presentation/17b53c639b2d685c70ccd4cfbd193435/image-6.jpg)
Solubility & Equilibrium Constant for Solubility-Ksp = [X+]x [Y-]y 1) Just ions, no solids
![Solubility & Equilibrium Constant for Solubility-Ksp = [X+]x [Y-]y 2) It doesn’t matter if Solubility & Equilibrium Constant for Solubility-Ksp = [X+]x [Y-]y 2) It doesn’t matter if](https://present5.com/presentation/17b53c639b2d685c70ccd4cfbd193435/image-7.jpg)
Solubility & Equilibrium Constant for Solubility-Ksp = [X+]x [Y-]y 2) It doesn’t matter if you have stuff on the bottom.
![Solubility & Equilibrium Constant for Solubility-Ksp = [X+]x [Y-]y 3) Not solubility - that’s Solubility & Equilibrium Constant for Solubility-Ksp = [X+]x [Y-]y 3) Not solubility - that’s](https://present5.com/presentation/17b53c639b2d685c70ccd4cfbd193435/image-8.jpg)
Solubility & Equilibrium Constant for Solubility-Ksp = [X+]x [Y-]y 3) Not solubility - that’s an equilibrium position(Q) (will more dissolve or not? )
![Solubility & Equilibrium Constant for Solubility-Ksp = [X+]x [Y-]y 4) Equilibrium so remember ICE Solubility & Equilibrium Constant for Solubility-Ksp = [X+]x [Y-]y 4) Equilibrium so remember ICE](https://present5.com/presentation/17b53c639b2d685c70ccd4cfbd193435/image-9.jpg)
Solubility & Equilibrium Constant for Solubility-Ksp = [X+]x [Y-]y 4) Equilibrium so remember ICE
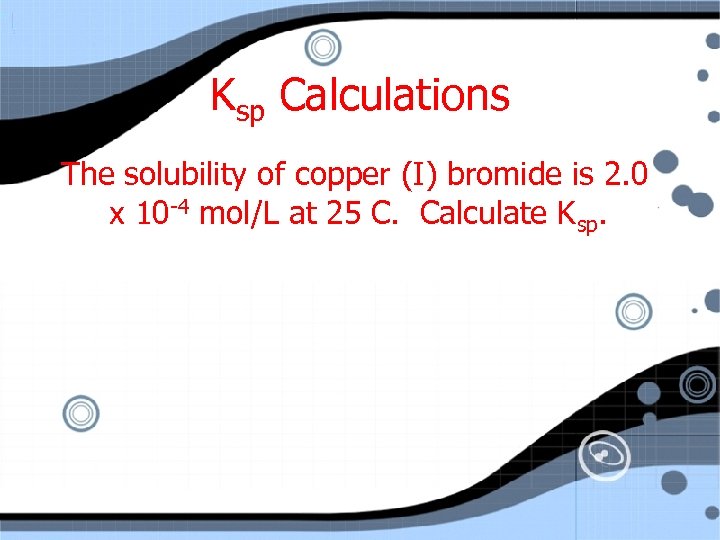
Ksp Calculations The solubility of copper (I) bromide is 2. 0 x 10 -4 mol/L at 25 C. Calculate Ksp.
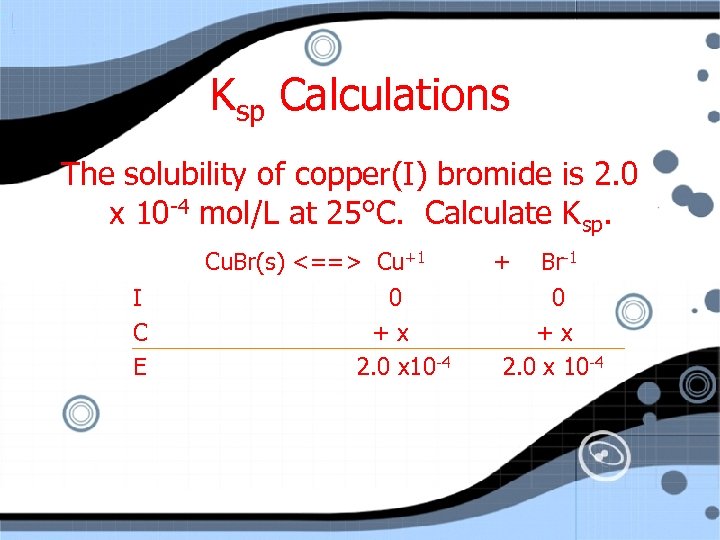
Ksp Calculations The solubility of copper(I) bromide is 2. 0 x 10 -4 mol/L at 25°C. Calculate Ksp. Cu. Br(s) <==> Cu+1 I C E 0 +x 2. 0 x 10 -4 + Br-1 0 +x 2. 0 x 10 -4
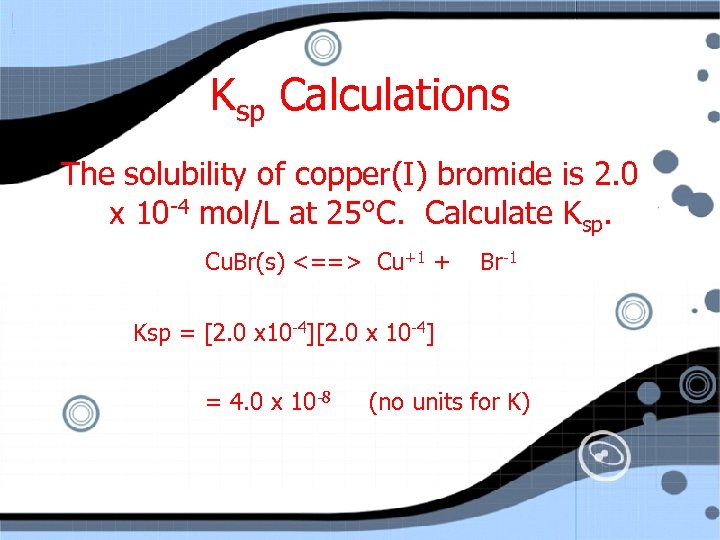
Ksp Calculations The solubility of copper(I) bromide is 2. 0 x 10 -4 mol/L at 25°C. Calculate Ksp. Cu. Br(s) <==> Cu+1 + Br-1 Ksp = [2. 0 x 10 -4] = 4. 0 x 10 -8 (no units for K)
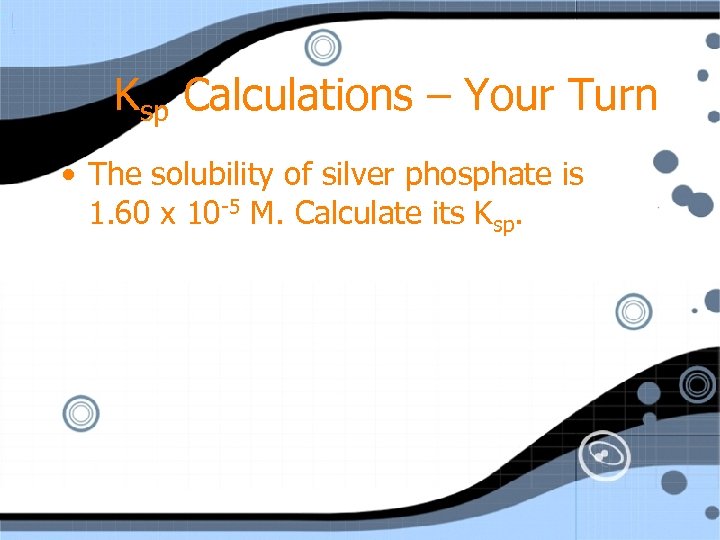
Ksp Calculations – Your Turn • The solubility of silver phosphate is 1. 60 x 10 -5 M. Calculate its Ksp.
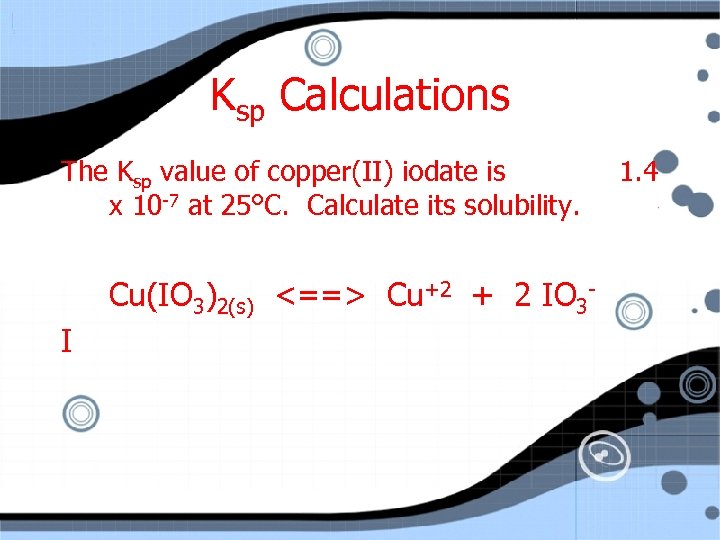
Ksp Calculations The Ksp value of copper(II) iodate is x 10 -7 at 25°C. Calculate its solubility. Cu(IO 3)2(s) <==> Cu+2 + 2 IO 3 I 1. 4
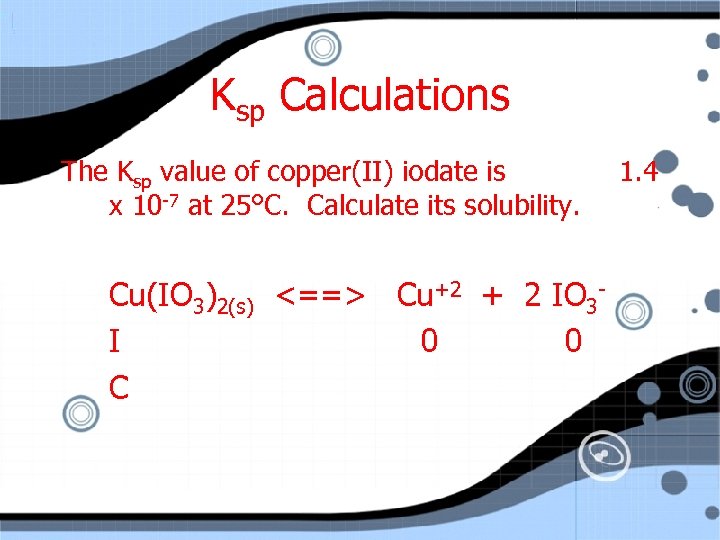
Ksp Calculations The Ksp value of copper(II) iodate is x 10 -7 at 25°C. Calculate its solubility. Cu(IO 3)2(s) <==> Cu+2 + 2 IO 3 I 0 0 C 1. 4
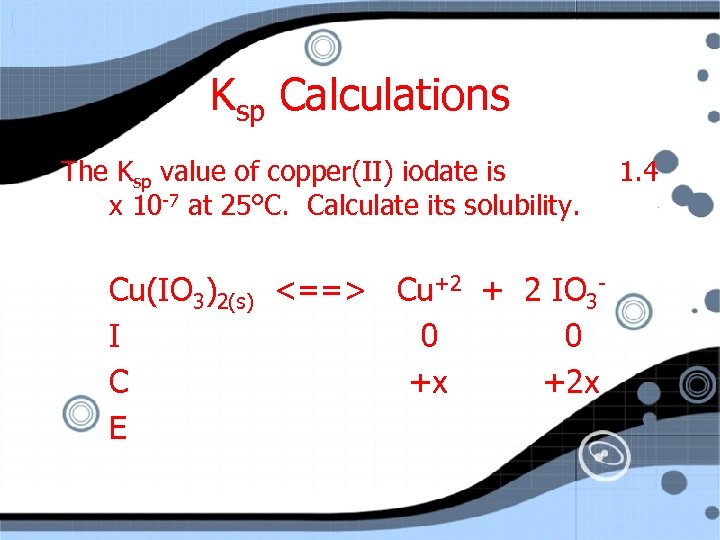
Ksp Calculations The Ksp value of copper(II) iodate is x 10 -7 at 25°C. Calculate its solubility. Cu(IO 3)2(s) <==> Cu+2 + 2 IO 3 I 0 0 C +x +2 x E 1. 4
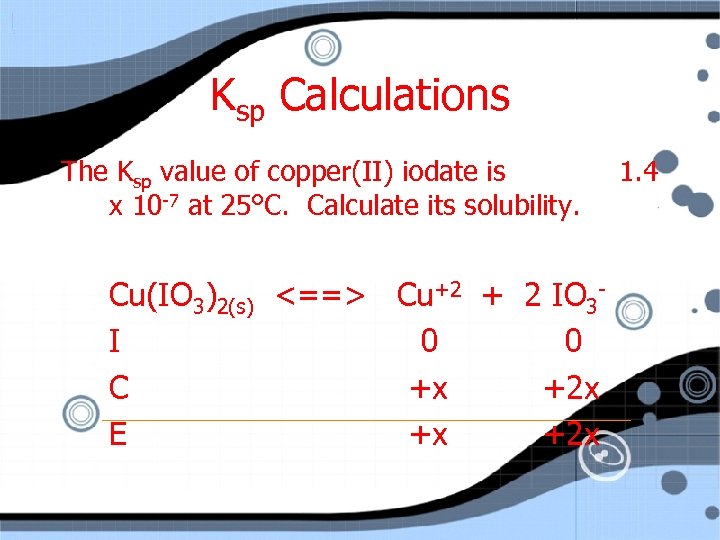
Ksp Calculations The Ksp value of copper(II) iodate is x 10 -7 at 25°C. Calculate its solubility. Cu(IO 3)2(s) <==> Cu+2 + 2 IO 3 I 0 0 C +x +2 x E +x +2 x 1. 4
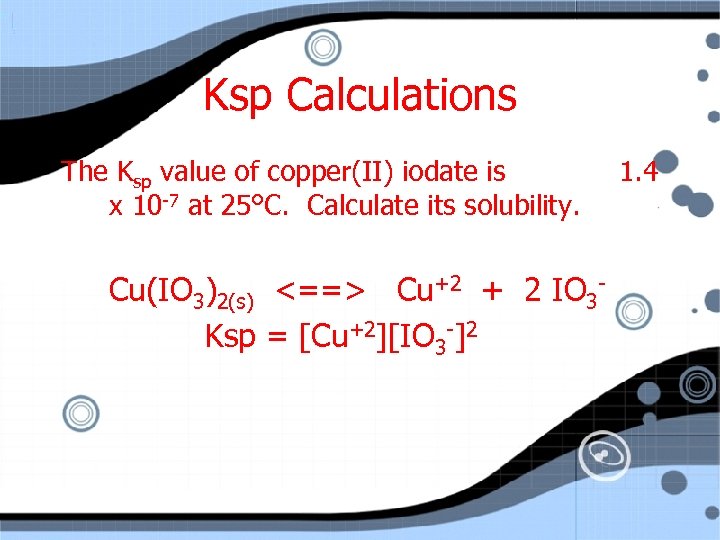
Ksp Calculations The Ksp value of copper(II) iodate is x 10 -7 at 25°C. Calculate its solubility. Cu(IO 3)2(s) <==> Cu+2 + 2 IO 3 Ksp = [Cu+2][IO 3 -]2 1. 4
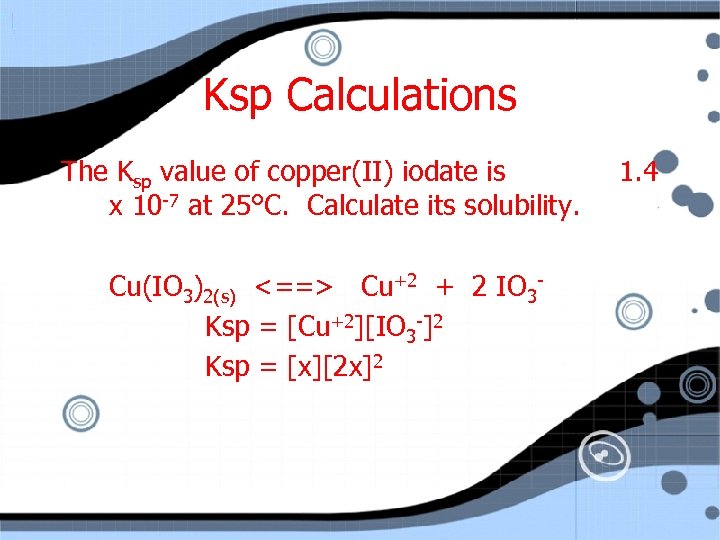
Ksp Calculations The Ksp value of copper(II) iodate is x 10 -7 at 25°C. Calculate its solubility. Cu(IO 3)2(s) <==> Cu+2 + 2 IO 3 Ksp = [Cu+2][IO 3 -]2 Ksp = [x][2 x]2 1. 4
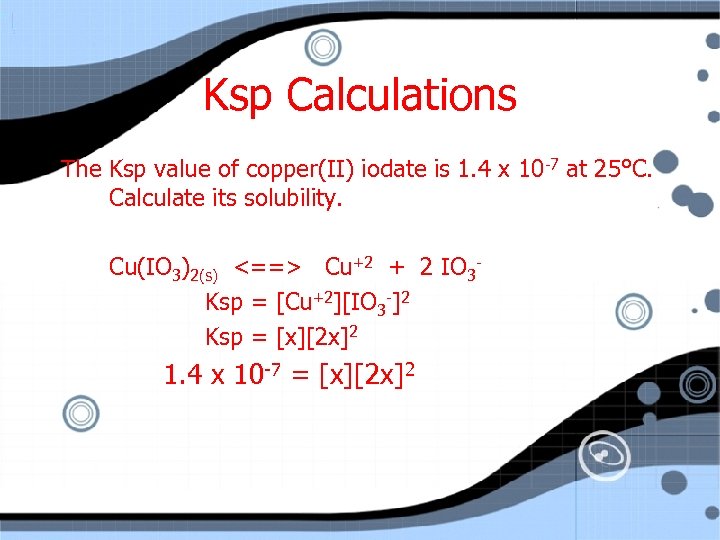
Ksp Calculations The Ksp value of copper(II) iodate is 1. 4 x 10 -7 at 25°C. Calculate its solubility. Cu(IO 3)2(s) <==> Cu+2 + 2 IO 3 Ksp = [Cu+2][IO 3 -]2 Ksp = [x][2 x]2 1. 4 x 10 -7 = [x][2 x]2
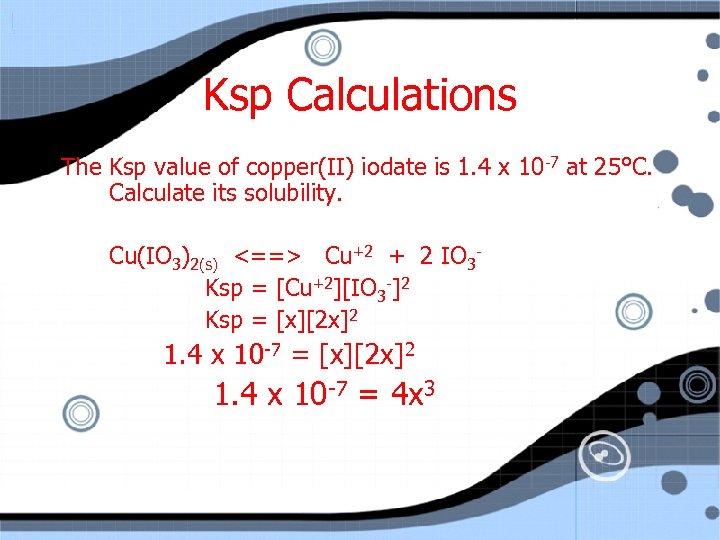
Ksp Calculations The Ksp value of copper(II) iodate is 1. 4 x 10 -7 at 25°C. Calculate its solubility. Cu(IO 3)2(s) <==> Cu+2 + 2 IO 3 Ksp = [Cu+2][IO 3 -]2 Ksp = [x][2 x]2 1. 4 x 10 -7 = 4 x 3
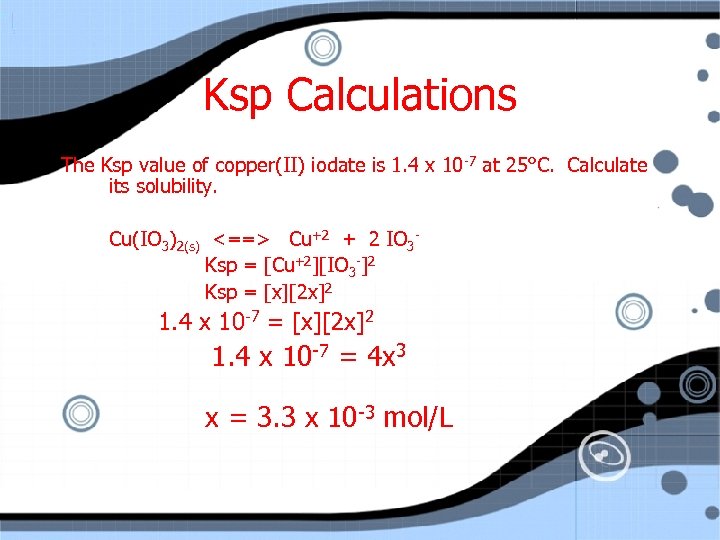
Ksp Calculations The Ksp value of copper(II) iodate is 1. 4 x 10 -7 at 25°C. Calculate its solubility. Cu(IO 3)2(s) <==> Cu+2 + 2 IO 3 Ksp = [Cu+2][IO 3 -]2 Ksp = [x][2 x]2 1. 4 x 10 -7 = 4 x 3 x = 3. 3 x 10 -3 mol/L
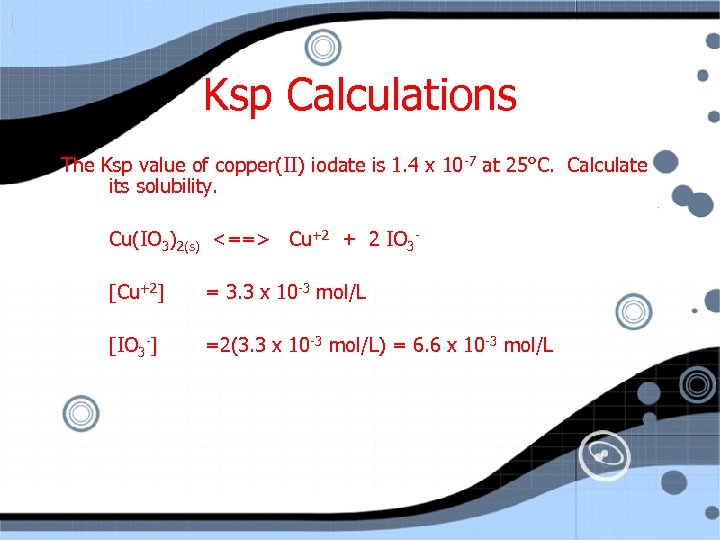
Ksp Calculations The Ksp value of copper(II) iodate is 1. 4 x 10 -7 at 25°C. Calculate its solubility. Cu(IO 3)2(s) <==> Cu+2 + 2 IO 3[Cu+2] = 3. 3 x 10 -3 mol/L [IO 3 -] =2(3. 3 x 10 -3 mol/L) = 6. 6 x 10 -3 mol/L
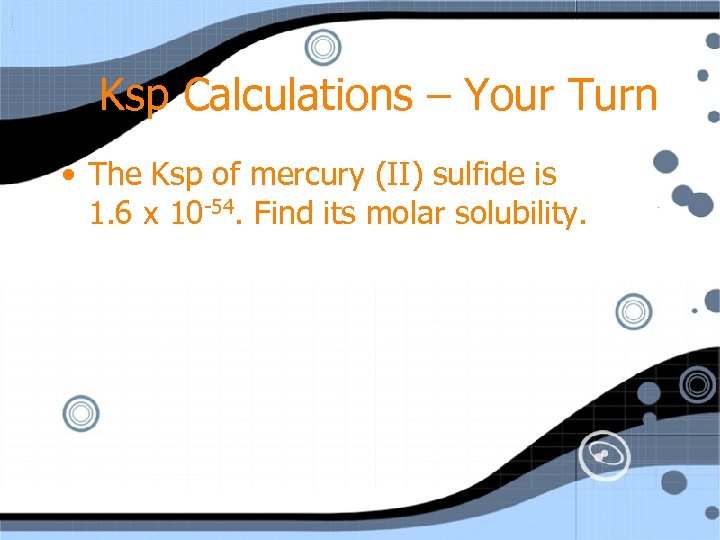
Ksp Calculations – Your Turn • The Ksp of mercury (II) sulfide is 1. 6 x 10 -54. Find its molar solubility.

Ksp Calculations What does it all mean? According to Ksp, EVERYTHING is at least very, very slightly soluble. Looking at the Ksp can help you figure out the solubility of compounds compared to each other – with a couple key rules about doing it!
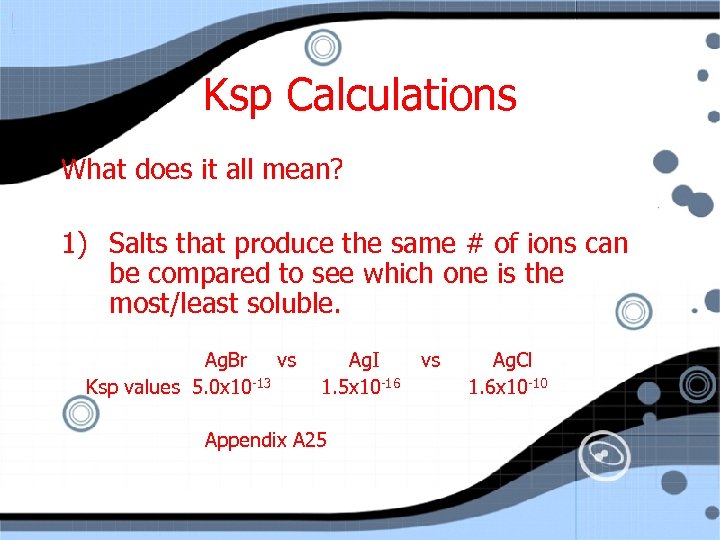
Ksp Calculations What does it all mean? 1) Salts that produce the same # of ions can be compared to see which one is the most/least soluble. Ag. Br vs Ksp values 5. 0 x 10 -13 Ag. I 1. 5 x 10 -16 Appendix A 25 vs Ag. Cl 1. 6 x 10 -10

Ksp Calculations What does it all mean? 2) If the salts break into different # of ions, you can’t just look. Must calculate.
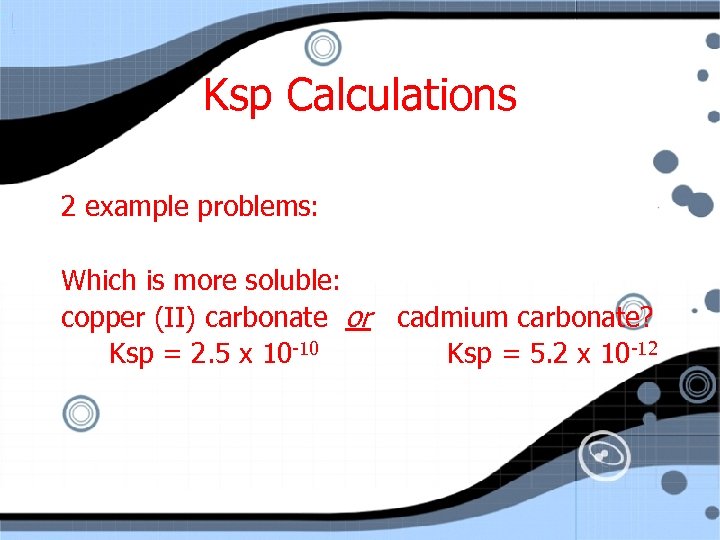
Ksp Calculations 2 example problems: Which is more soluble: copper (II) carbonate or cadmium carbonate? Ksp = 2. 5 x 10 -10 Ksp = 5. 2 x 10 -12
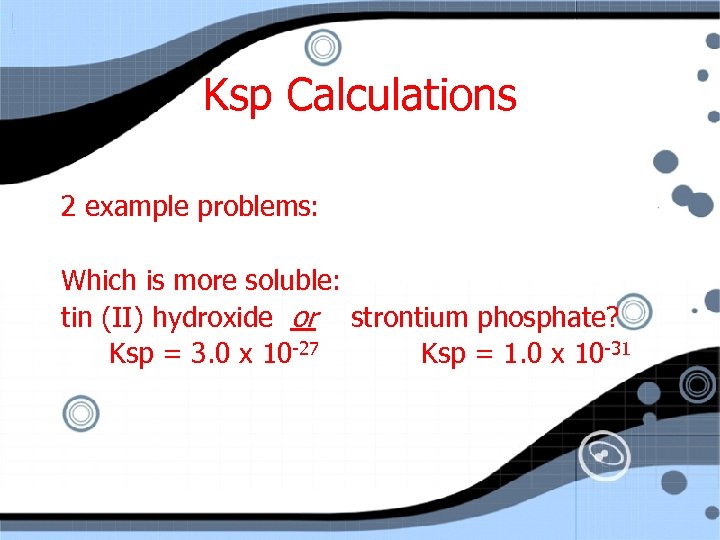
Ksp Calculations 2 example problems: Which is more soluble: tin (II) hydroxide or strontium phosphate? Ksp = 3. 0 x 10 -27 Ksp = 1. 0 x 10 -31
17b53c639b2d685c70ccd4cfbd193435.ppt