677af020d491033f21be1bc3cfd3bfa2.ppt
- Количество слайдов: 25
 Solid state physics N. Witkowski
Solid state physics N. Witkowski
 Introduction n n Based on « Introduction to Solid State Physics » 8 th edition Charles Kittel Lecture notes from Gunnar Niklasson http: //www. teknik. uu. se/ftf/education/ftf 1/FTFI_forsta_sidan. html 40 h Lessons with N. Witkowski ¨ ¨ n 6 laboratory courses (6 x 3 h): 1 extended report + 4 limited reports ¨ ¨ ¨ n house 4, level 0, office 60111, e-mail: witkowski@insp. jussieu. fr Semiconductor physics Specific heat Superconductivity Magnetic susceptibility X-ray diffraction Band structure calculation Given between 23 rd feb-6 th march Registration : from 9 th feb on board F and Q House 4 ground level Info comes later Home work Evaluation : written examination 13 march (to be confirmed) 5 hours, 6 problems document authorized « Physics handbook for science and engineering» Carl Nordling, Jonny Osterman ¨ Calculator authorized ¨ Second chance in june ¨ ¨
Introduction n n Based on « Introduction to Solid State Physics » 8 th edition Charles Kittel Lecture notes from Gunnar Niklasson http: //www. teknik. uu. se/ftf/education/ftf 1/FTFI_forsta_sidan. html 40 h Lessons with N. Witkowski ¨ ¨ n 6 laboratory courses (6 x 3 h): 1 extended report + 4 limited reports ¨ ¨ ¨ n house 4, level 0, office 60111, e-mail: witkowski@insp. jussieu. fr Semiconductor physics Specific heat Superconductivity Magnetic susceptibility X-ray diffraction Band structure calculation Given between 23 rd feb-6 th march Registration : from 9 th feb on board F and Q House 4 ground level Info comes later Home work Evaluation : written examination 13 march (to be confirmed) 5 hours, 6 problems document authorized « Physics handbook for science and engineering» Carl Nordling, Jonny Osterman ¨ Calculator authorized ¨ Second chance in june ¨ ¨
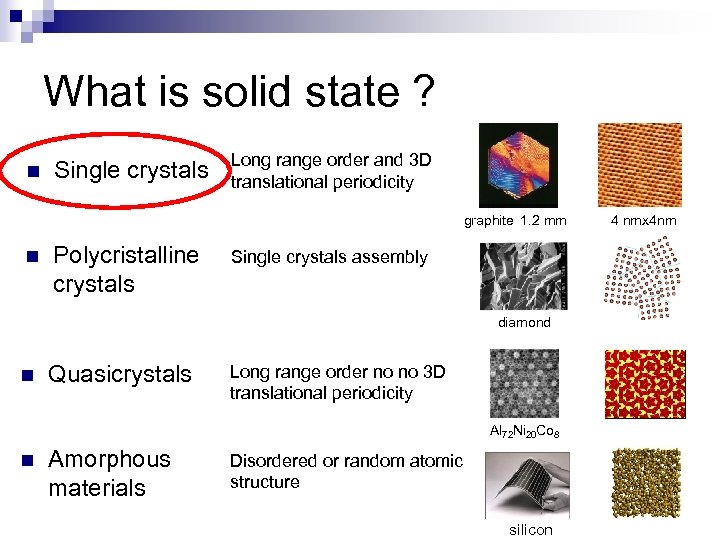 What is solid state ? n Single crystals Long range order and 3 D translational periodicity graphite 1. 2 mm n Polycristalline crystals Single crystals assembly diamond n Quasicrystals Long range order no no 3 D translational periodicity Al 72 Ni 20 Co 8 n Amorphous materials Disordered or random atomic structure silicon 4 nmx 4 nm
What is solid state ? n Single crystals Long range order and 3 D translational periodicity graphite 1. 2 mm n Polycristalline crystals Single crystals assembly diamond n Quasicrystals Long range order no no 3 D translational periodicity Al 72 Ni 20 Co 8 n Amorphous materials Disordered or random atomic structure silicon 4 nmx 4 nm
![Outline Corresponding chapter in Kittel book n n n [1] Crystal structure [2] Reciprocal Outline Corresponding chapter in Kittel book n n n [1] Crystal structure [2] Reciprocal](https://present5.com/presentation/677af020d491033f21be1bc3cfd3bfa2/image-4.jpg) Outline Corresponding chapter in Kittel book n n n [1] Crystal structure [2] Reciprocal lattice [3] Diffraction [4] Crystal binding no lecture [5] Lattice vibrations [6] Thermal properties [7] Free electron model [8] Energy band [9] Electron movement in crystals Metals and Fermi surfaces [10] Semiconductors [11] Superconductivity [12] Magnetism 1 2 2 3 4 5 6 7, 9 8 10 11
Outline Corresponding chapter in Kittel book n n n [1] Crystal structure [2] Reciprocal lattice [3] Diffraction [4] Crystal binding no lecture [5] Lattice vibrations [6] Thermal properties [7] Free electron model [8] Energy band [9] Electron movement in crystals Metals and Fermi surfaces [10] Semiconductors [11] Superconductivity [12] Magnetism 1 2 2 3 4 5 6 7, 9 8 10 11
 Chap. 1 Crystal structure
Chap. 1 Crystal structure
 Introduction n Aim : ¨ A : defining concepts and definitions ¨ B : describing the lattice types ¨ C : giving a description of crystal structures
Introduction n Aim : ¨ A : defining concepts and definitions ¨ B : describing the lattice types ¨ C : giving a description of crystal structures
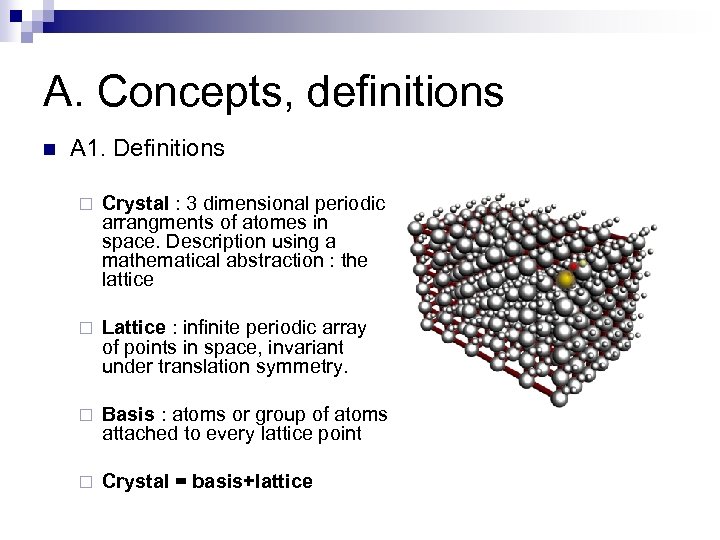 A. Concepts, definitions n A 1. Definitions ¨ Crystal : 3 dimensional periodic arrangments of atomes in space. Description using a mathematical abstraction : the lattice ¨ Lattice : infinite periodic array of points in space, invariant under translation symmetry. ¨ Basis : atoms or group of atoms attached to every lattice point ¨ Crystal = basis+lattice
A. Concepts, definitions n A 1. Definitions ¨ Crystal : 3 dimensional periodic arrangments of atomes in space. Description using a mathematical abstraction : the lattice ¨ Lattice : infinite periodic array of points in space, invariant under translation symmetry. ¨ Basis : atoms or group of atoms attached to every lattice point ¨ Crystal = basis+lattice
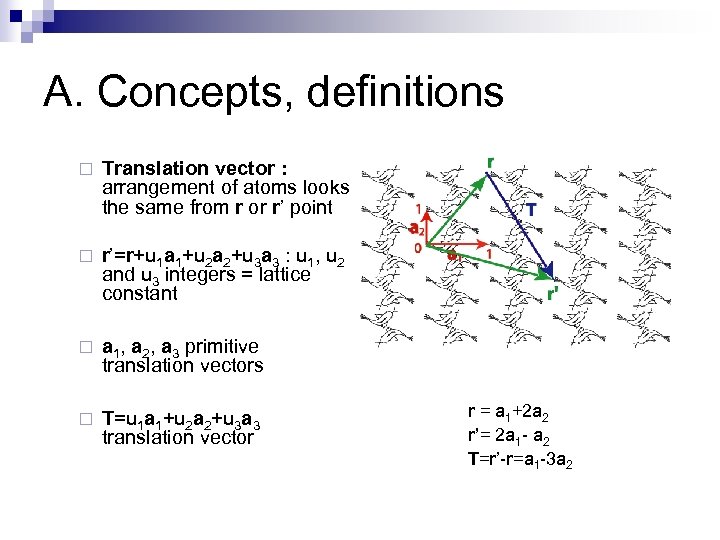 A. Concepts, definitions ¨ Translation vector : arrangement of atoms looks the same from r or r’ point ¨ r’=r+u 1 a 1+u 2 a 2+u 3 a 3 : u 1, u 2 and u 3 integers = lattice constant ¨ a 1, a 2, a 3 primitive translation vectors ¨ T=u 1 a 1+u 2 a 2+u 3 a 3 translation vector r = a 1+2 a 2 r’= 2 a 1 - a 2 T=r’-r=a 1 -3 a 2
A. Concepts, definitions ¨ Translation vector : arrangement of atoms looks the same from r or r’ point ¨ r’=r+u 1 a 1+u 2 a 2+u 3 a 3 : u 1, u 2 and u 3 integers = lattice constant ¨ a 1, a 2, a 3 primitive translation vectors ¨ T=u 1 a 1+u 2 a 2+u 3 a 3 translation vector r = a 1+2 a 2 r’= 2 a 1 - a 2 T=r’-r=a 1 -3 a 2
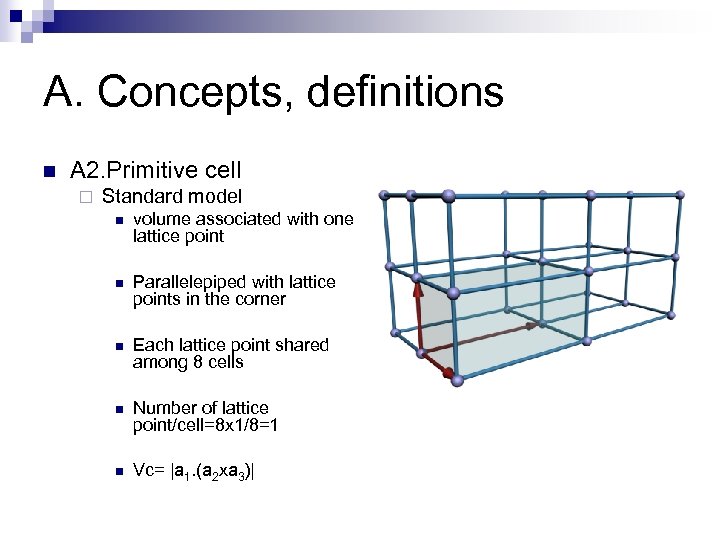 A. Concepts, definitions n A 2. Primitive cell ¨ Standard model n volume associated with one lattice point n Parallelepiped with lattice points in the corner n Each lattice point shared among 8 cells n Number of lattice point/cell=8 x 1/8=1 n Vc= |a 1. (a 2 xa 3)|
A. Concepts, definitions n A 2. Primitive cell ¨ Standard model n volume associated with one lattice point n Parallelepiped with lattice points in the corner n Each lattice point shared among 8 cells n Number of lattice point/cell=8 x 1/8=1 n Vc= |a 1. (a 2 xa 3)|
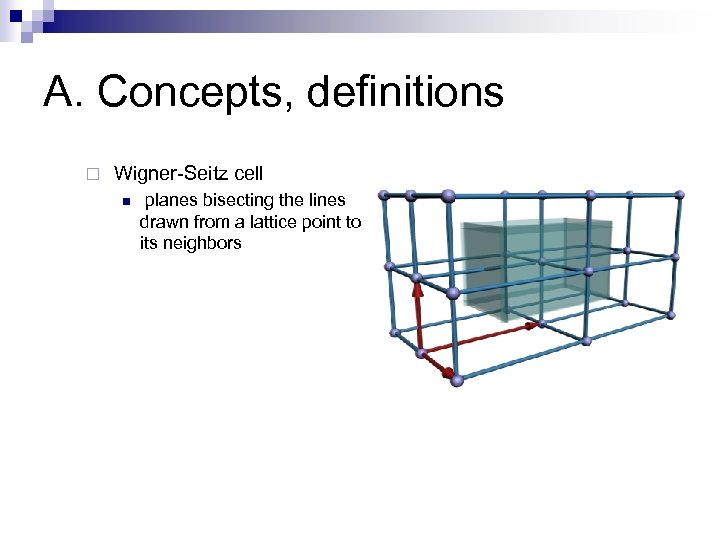 A. Concepts, definitions ¨ Wigner-Seitz cell n planes bisecting the lines drawn from a lattice point to its neighbors
A. Concepts, definitions ¨ Wigner-Seitz cell n planes bisecting the lines drawn from a lattice point to its neighbors
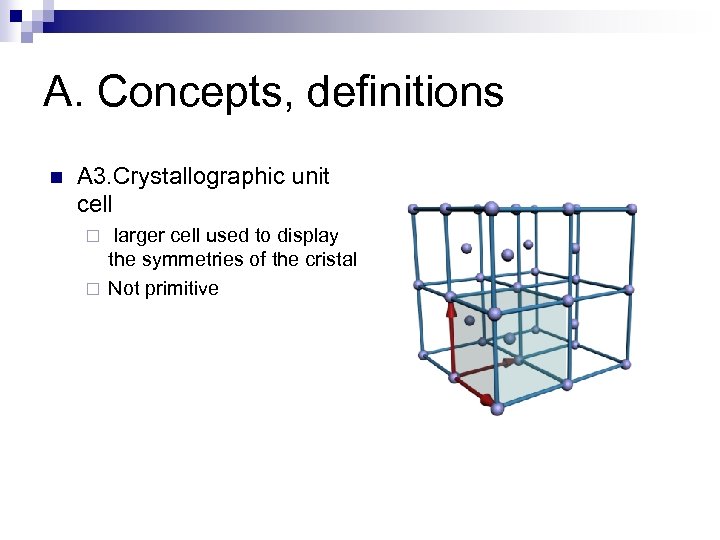 A. Concepts, definitions n A 3. Crystallographic unit cell larger cell used to display the symmetries of the cristal ¨ Not primitive ¨
A. Concepts, definitions n A 3. Crystallographic unit cell larger cell used to display the symmetries of the cristal ¨ Not primitive ¨
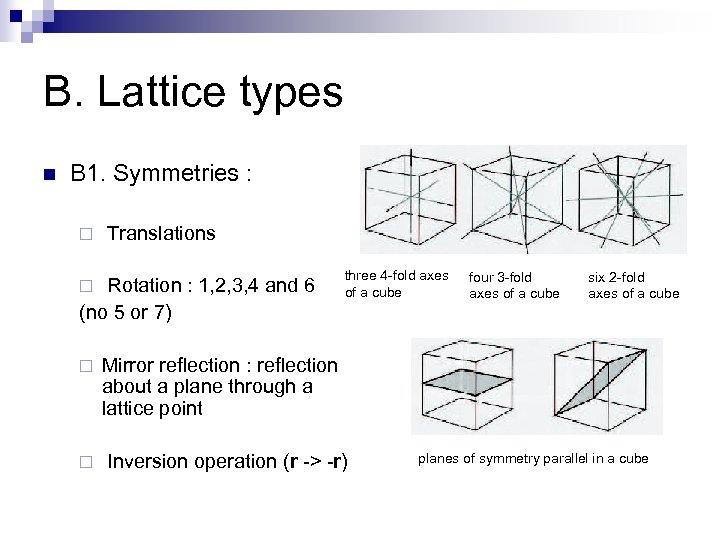 B. Lattice types n B 1. Symmetries : ¨ Translations Rotation : 1, 2, 3, 4 and 6 (no 5 or 7) ¨ three 4 -fold axes of a cube ¨ Inversion operation (r -> -r) six 2 -fold axes of a cube Mirror reflection : reflection about a plane through a lattice point ¨ four 3 -fold axes of a cube planes of symmetry parallel in a cube
B. Lattice types n B 1. Symmetries : ¨ Translations Rotation : 1, 2, 3, 4 and 6 (no 5 or 7) ¨ three 4 -fold axes of a cube ¨ Inversion operation (r -> -r) six 2 -fold axes of a cube Mirror reflection : reflection about a plane through a lattice point ¨ four 3 -fold axes of a cube planes of symmetry parallel in a cube
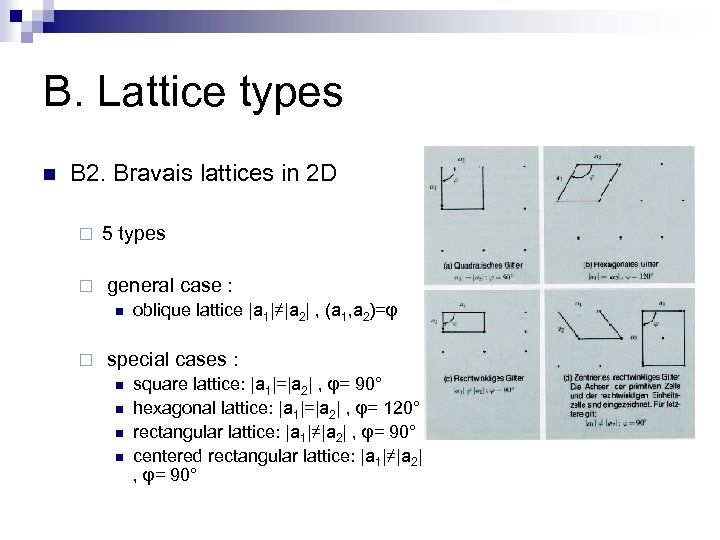 B. Lattice types n B 2. Bravais lattices in 2 D ¨ 5 types ¨ general case : n ¨ oblique lattice |a 1|≠|a 2| , (a 1, a 2)=φ special cases : n n square lattice: |a 1|=|a 2| , φ= 90° hexagonal lattice: |a 1|=|a 2| , φ= 120° rectangular lattice: |a 1|≠|a 2| , φ= 90° centered rectangular lattice: |a 1|≠|a 2| , φ= 90°
B. Lattice types n B 2. Bravais lattices in 2 D ¨ 5 types ¨ general case : n ¨ oblique lattice |a 1|≠|a 2| , (a 1, a 2)=φ special cases : n n square lattice: |a 1|=|a 2| , φ= 90° hexagonal lattice: |a 1|=|a 2| , φ= 120° rectangular lattice: |a 1|≠|a 2| , φ= 90° centered rectangular lattice: |a 1|≠|a 2| , φ= 90°
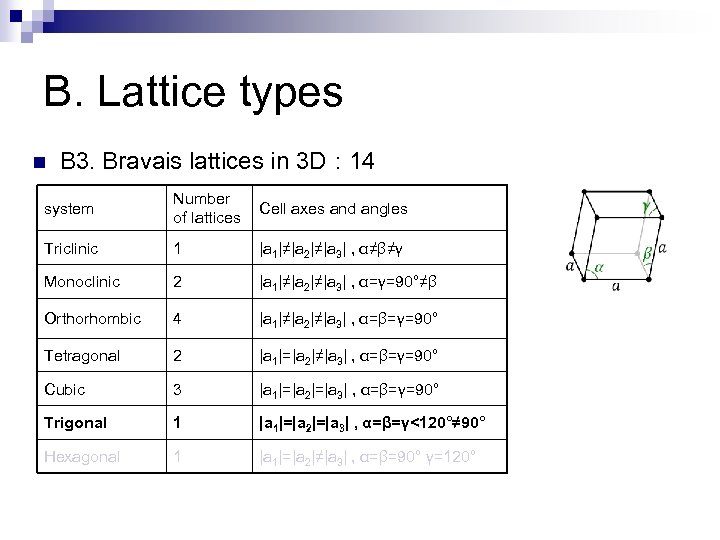
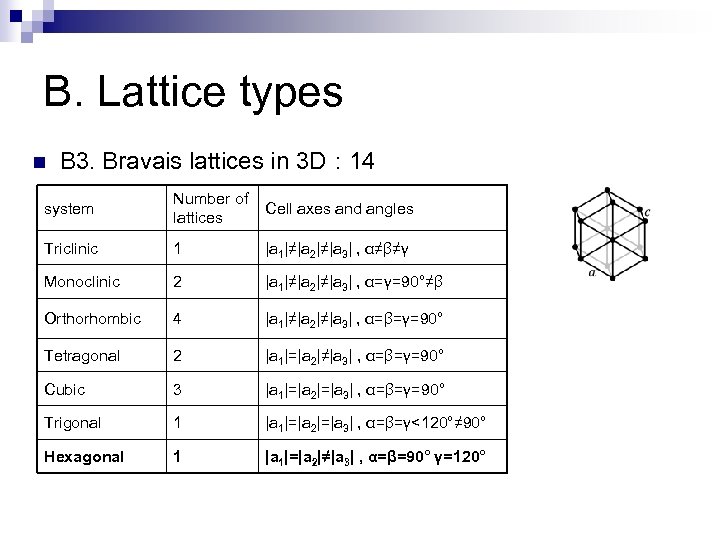
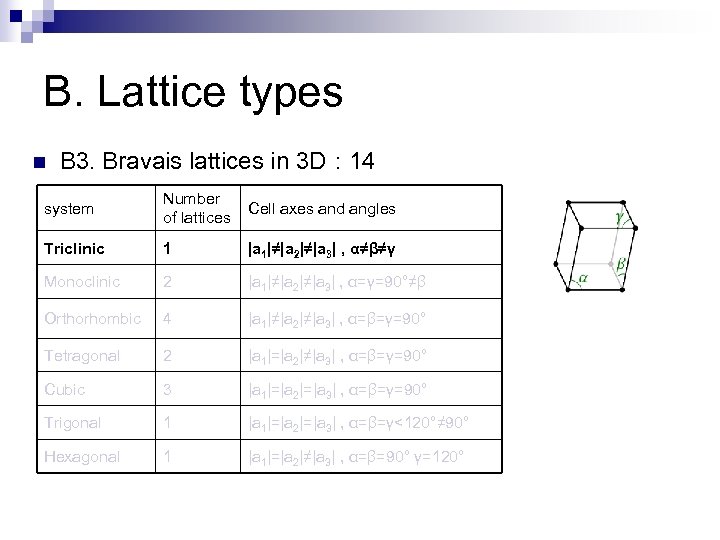 B. Lattice types n B 3. Bravais lattices in 3 D : 14 system Number of lattices Cell axes and angles Triclinic 1 |a 1|≠|a 2|≠|a 3| , α≠β≠γ Monoclinic 2 |a 1|≠|a 2|≠|a 3| , α=γ=90°≠β Orthorhombic 4 |a 1|≠|a 2|≠|a 3| , α=β=γ=90° Tetragonal 2 |a 1|=|a 2|≠|a 3| , α=β=γ=90° Cubic 3 |a 1|=|a 2|=|a 3| , α=β=γ=90° Trigonal 1 |a 1|=|a 2|=|a 3| , α=β=γ<120°≠ 90° Hexagonal 1 |a 1|=|a 2|≠|a 3| , α=β=90° γ=120°
B. Lattice types n B 3. Bravais lattices in 3 D : 14 system Number of lattices Cell axes and angles Triclinic 1 |a 1|≠|a 2|≠|a 3| , α≠β≠γ Monoclinic 2 |a 1|≠|a 2|≠|a 3| , α=γ=90°≠β Orthorhombic 4 |a 1|≠|a 2|≠|a 3| , α=β=γ=90° Tetragonal 2 |a 1|=|a 2|≠|a 3| , α=β=γ=90° Cubic 3 |a 1|=|a 2|=|a 3| , α=β=γ=90° Trigonal 1 |a 1|=|a 2|=|a 3| , α=β=γ<120°≠ 90° Hexagonal 1 |a 1|=|a 2|≠|a 3| , α=β=90° γ=120°
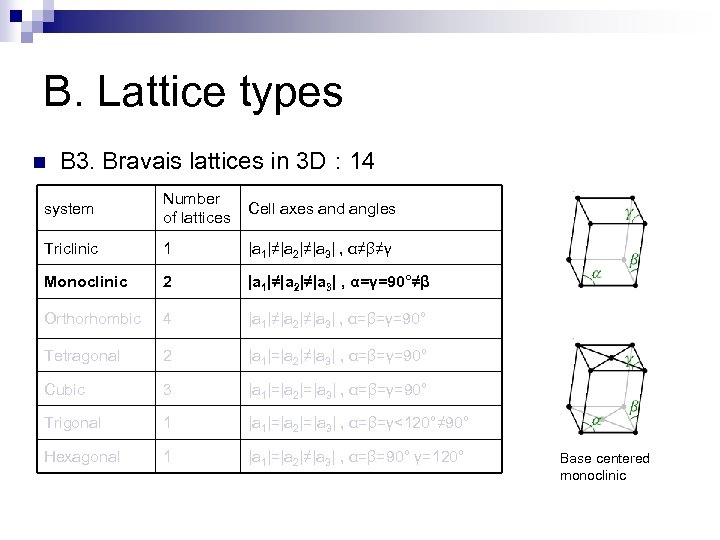 B. Lattice types n B 3. Bravais lattices in 3 D : 14 system Number of lattices Cell axes and angles Triclinic 1 |a 1|≠|a 2|≠|a 3| , α≠β≠γ Monoclinic 2 |a 1|≠|a 2|≠|a 3| , α=γ=90°≠β Orthorhombic 4 |a 1|≠|a 2|≠|a 3| , α=β=γ=90° Tetragonal 2 |a 1|=|a 2|≠|a 3| , α=β=γ=90° Cubic 3 |a 1|=|a 2|=|a 3| , α=β=γ=90° Trigonal 1 |a 1|=|a 2|=|a 3| , α=β=γ<120°≠ 90° Hexagonal 1 |a 1|=|a 2|≠|a 3| , α=β=90° γ=120° Base centered monoclinic
B. Lattice types n B 3. Bravais lattices in 3 D : 14 system Number of lattices Cell axes and angles Triclinic 1 |a 1|≠|a 2|≠|a 3| , α≠β≠γ Monoclinic 2 |a 1|≠|a 2|≠|a 3| , α=γ=90°≠β Orthorhombic 4 |a 1|≠|a 2|≠|a 3| , α=β=γ=90° Tetragonal 2 |a 1|=|a 2|≠|a 3| , α=β=γ=90° Cubic 3 |a 1|=|a 2|=|a 3| , α=β=γ=90° Trigonal 1 |a 1|=|a 2|=|a 3| , α=β=γ<120°≠ 90° Hexagonal 1 |a 1|=|a 2|≠|a 3| , α=β=90° γ=120° Base centered monoclinic
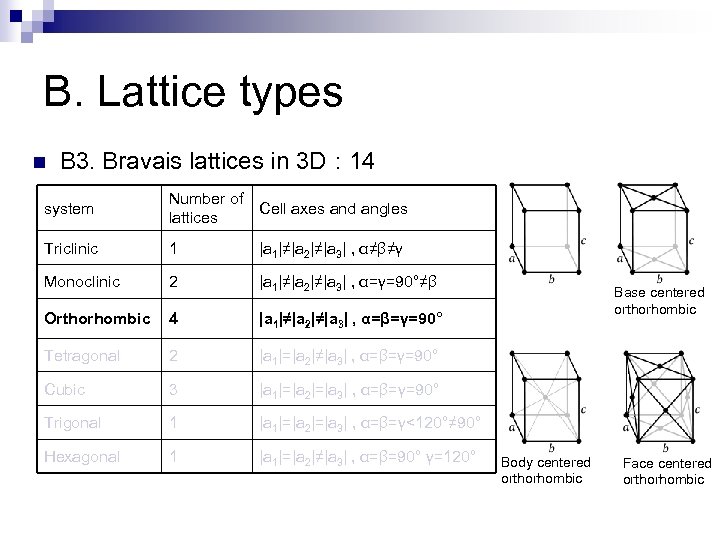 B. Lattice types n B 3. Bravais lattices in 3 D : 14 system Number of Cell axes and angles lattices Triclinic 1 |a 1|≠|a 2|≠|a 3| , α≠β≠γ Monoclinic 2 |a 1|≠|a 2|≠|a 3| , α=γ=90°≠β Orthorhombic 4 |a 1|≠|a 2|≠|a 3| , α=β=γ=90° Tetragonal 2 |a 1|=|a 2|≠|a 3| , α=β=γ=90° Cubic 3 |a 1|=|a 2|=|a 3| , α=β=γ=90° Trigonal 1 |a 1|=|a 2|=|a 3| , α=β=γ<120°≠ 90° Hexagonal 1 |a 1|=|a 2|≠|a 3| , α=β=90° γ=120° Base centered orthorhombic Body centered orthorhombic Face centered orthorhombic
B. Lattice types n B 3. Bravais lattices in 3 D : 14 system Number of Cell axes and angles lattices Triclinic 1 |a 1|≠|a 2|≠|a 3| , α≠β≠γ Monoclinic 2 |a 1|≠|a 2|≠|a 3| , α=γ=90°≠β Orthorhombic 4 |a 1|≠|a 2|≠|a 3| , α=β=γ=90° Tetragonal 2 |a 1|=|a 2|≠|a 3| , α=β=γ=90° Cubic 3 |a 1|=|a 2|=|a 3| , α=β=γ=90° Trigonal 1 |a 1|=|a 2|=|a 3| , α=β=γ<120°≠ 90° Hexagonal 1 |a 1|=|a 2|≠|a 3| , α=β=90° γ=120° Base centered orthorhombic Body centered orthorhombic Face centered orthorhombic
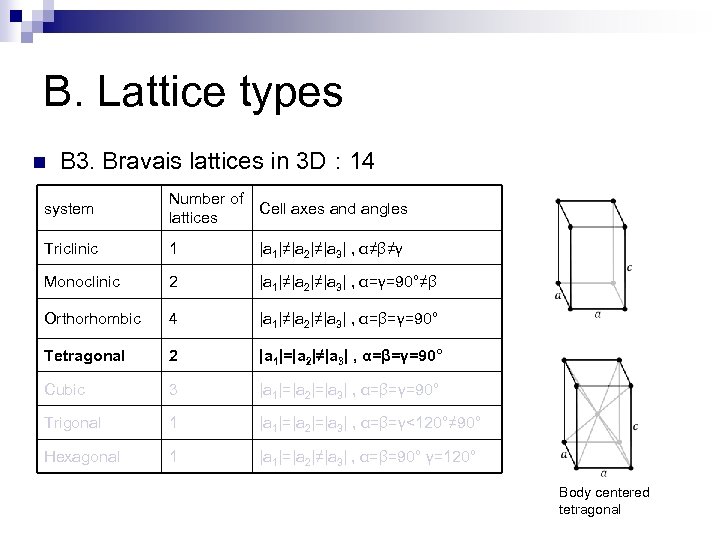 B. Lattice types n B 3. Bravais lattices in 3 D : 14 system Number of Cell axes and angles lattices Triclinic 1 |a 1|≠|a 2|≠|a 3| , α≠β≠γ Monoclinic 2 |a 1|≠|a 2|≠|a 3| , α=γ=90°≠β Orthorhombic 4 |a 1|≠|a 2|≠|a 3| , α=β=γ=90° Tetragonal 2 |a 1|=|a 2|≠|a 3| , α=β=γ=90° Cubic 3 |a 1|=|a 2|=|a 3| , α=β=γ=90° Trigonal 1 |a 1|=|a 2|=|a 3| , α=β=γ<120°≠ 90° Hexagonal 1 |a 1|=|a 2|≠|a 3| , α=β=90° γ=120° Body centered tetragonal
B. Lattice types n B 3. Bravais lattices in 3 D : 14 system Number of Cell axes and angles lattices Triclinic 1 |a 1|≠|a 2|≠|a 3| , α≠β≠γ Monoclinic 2 |a 1|≠|a 2|≠|a 3| , α=γ=90°≠β Orthorhombic 4 |a 1|≠|a 2|≠|a 3| , α=β=γ=90° Tetragonal 2 |a 1|=|a 2|≠|a 3| , α=β=γ=90° Cubic 3 |a 1|=|a 2|=|a 3| , α=β=γ=90° Trigonal 1 |a 1|=|a 2|=|a 3| , α=β=γ<120°≠ 90° Hexagonal 1 |a 1|=|a 2|≠|a 3| , α=β=90° γ=120° Body centered tetragonal
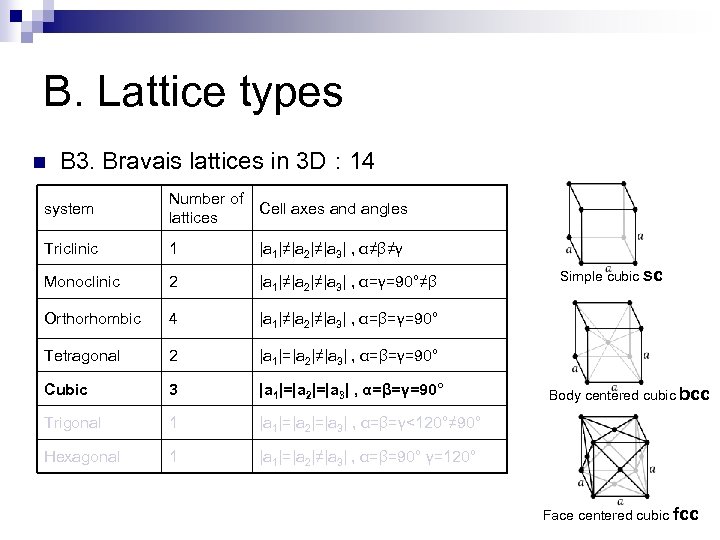 B. Lattice types n B 3. Bravais lattices in 3 D : 14 system Number of Cell axes and angles lattices Triclinic 1 |a 1|≠|a 2|≠|a 3| , α≠β≠γ Monoclinic 2 |a 1|≠|a 2|≠|a 3| , α=γ=90°≠β Orthorhombic 4 |a 1|≠|a 2|≠|a 3| , α=β=γ=90° Tetragonal 2 |a 1|=|a 2|≠|a 3| , α=β=γ=90° Cubic 3 |a 1|=|a 2|=|a 3| , α=β=γ=90° Trigonal 1 |a 1|=|a 2|=|a 3| , α=β=γ<120°≠ 90° Hexagonal 1 |a 1|=|a 2|≠|a 3| , α=β=90° γ=120° Simple cubic sc Body centered cubic bcc Face centered cubic fcc
B. Lattice types n B 3. Bravais lattices in 3 D : 14 system Number of Cell axes and angles lattices Triclinic 1 |a 1|≠|a 2|≠|a 3| , α≠β≠γ Monoclinic 2 |a 1|≠|a 2|≠|a 3| , α=γ=90°≠β Orthorhombic 4 |a 1|≠|a 2|≠|a 3| , α=β=γ=90° Tetragonal 2 |a 1|=|a 2|≠|a 3| , α=β=γ=90° Cubic 3 |a 1|=|a 2|=|a 3| , α=β=γ=90° Trigonal 1 |a 1|=|a 2|=|a 3| , α=β=γ<120°≠ 90° Hexagonal 1 |a 1|=|a 2|≠|a 3| , α=β=90° γ=120° Simple cubic sc Body centered cubic bcc Face centered cubic fcc
 B. Lattice types n B 3. Bravais lattices in 3 D : 14 system Number of lattices Cell axes and angles Triclinic 1 |a 1|≠|a 2|≠|a 3| , α≠β≠γ Monoclinic 2 |a 1|≠|a 2|≠|a 3| , α=γ=90°≠β Orthorhombic 4 |a 1|≠|a 2|≠|a 3| , α=β=γ=90° Tetragonal 2 |a 1|=|a 2|≠|a 3| , α=β=γ=90° Cubic 3 |a 1|=|a 2|=|a 3| , α=β=γ=90° Trigonal 1 |a 1|=|a 2|=|a 3| , α=β=γ<120°≠ 90° Hexagonal 1 |a 1|=|a 2|≠|a 3| , α=β=90° γ=120°
B. Lattice types n B 3. Bravais lattices in 3 D : 14 system Number of lattices Cell axes and angles Triclinic 1 |a 1|≠|a 2|≠|a 3| , α≠β≠γ Monoclinic 2 |a 1|≠|a 2|≠|a 3| , α=γ=90°≠β Orthorhombic 4 |a 1|≠|a 2|≠|a 3| , α=β=γ=90° Tetragonal 2 |a 1|=|a 2|≠|a 3| , α=β=γ=90° Cubic 3 |a 1|=|a 2|=|a 3| , α=β=γ=90° Trigonal 1 |a 1|=|a 2|=|a 3| , α=β=γ<120°≠ 90° Hexagonal 1 |a 1|=|a 2|≠|a 3| , α=β=90° γ=120°
 B. Lattice types n B 3. Bravais lattices in 3 D : 14 system Number of Cell axes and angles lattices Triclinic 1 |a 1|≠|a 2|≠|a 3| , α≠β≠γ Monoclinic 2 |a 1|≠|a 2|≠|a 3| , α=γ=90°≠β Orthorhombic 4 |a 1|≠|a 2|≠|a 3| , α=β=γ=90° Tetragonal 2 |a 1|=|a 2|≠|a 3| , α=β=γ=90° Cubic 3 |a 1|=|a 2|=|a 3| , α=β=γ=90° Trigonal 1 |a 1|=|a 2|=|a 3| , α=β=γ<120°≠ 90° Hexagonal 1 |a 1|=|a 2|≠|a 3| , α=β=90° γ=120°
B. Lattice types n B 3. Bravais lattices in 3 D : 14 system Number of Cell axes and angles lattices Triclinic 1 |a 1|≠|a 2|≠|a 3| , α≠β≠γ Monoclinic 2 |a 1|≠|a 2|≠|a 3| , α=γ=90°≠β Orthorhombic 4 |a 1|≠|a 2|≠|a 3| , α=β=γ=90° Tetragonal 2 |a 1|=|a 2|≠|a 3| , α=β=γ=90° Cubic 3 |a 1|=|a 2|=|a 3| , α=β=γ=90° Trigonal 1 |a 1|=|a 2|=|a 3| , α=β=γ<120°≠ 90° Hexagonal 1 |a 1|=|a 2|≠|a 3| , α=β=90° γ=120°
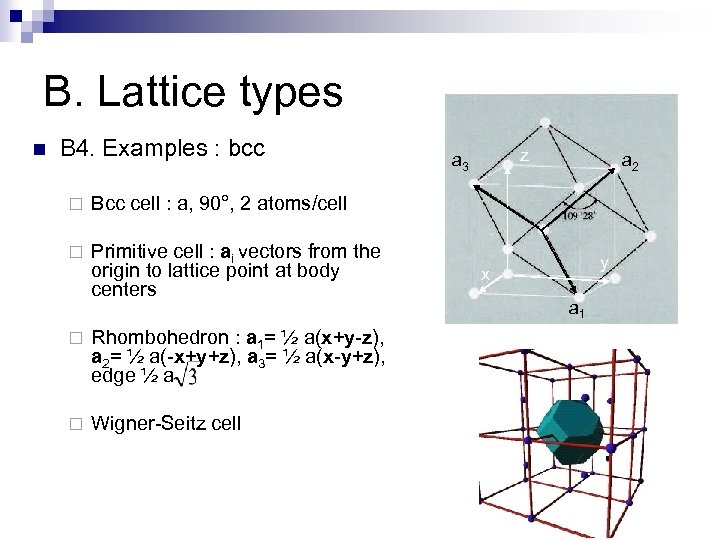 B. Lattice types n B 4. Examples : bcc ¨ Primitive cell : ai vectors from the origin to lattice point at body centers a 2 Bcc cell : a, 90°, 2 atoms/cell ¨ z a 3 ¨ Rhombohedron : a 1= ½ a(x+y-z), a 2= ½ a(-x+y+z), a 3= ½ a(x-y+z), edge ½ a ¨ Wigner-Seitz cell y x a 1
B. Lattice types n B 4. Examples : bcc ¨ Primitive cell : ai vectors from the origin to lattice point at body centers a 2 Bcc cell : a, 90°, 2 atoms/cell ¨ z a 3 ¨ Rhombohedron : a 1= ½ a(x+y-z), a 2= ½ a(-x+y+z), a 3= ½ a(x-y+z), edge ½ a ¨ Wigner-Seitz cell y x a 1
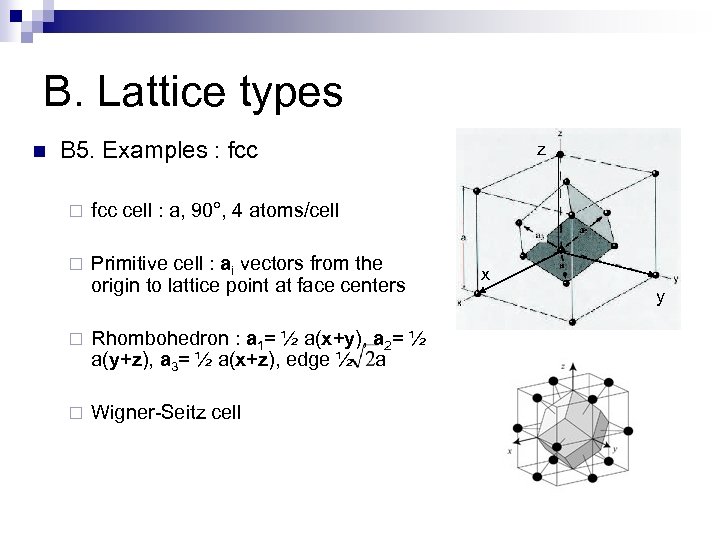 B. Lattice types n B 5. Examples : fcc ¨ fcc cell : a, 90°, 4 atoms/cell ¨ Primitive cell : ai vectors from the origin to lattice point at face centers z ¨ Rhombohedron : a 1= ½ a(x+y), a 2= ½ a(y+z), a 3= ½ a(x+z), edge ½ a ¨ Wigner-Seitz cell x y
B. Lattice types n B 5. Examples : fcc ¨ fcc cell : a, 90°, 4 atoms/cell ¨ Primitive cell : ai vectors from the origin to lattice point at face centers z ¨ Rhombohedron : a 1= ½ a(x+y), a 2= ½ a(y+z), a 3= ½ a(x+z), edge ½ a ¨ Wigner-Seitz cell x y
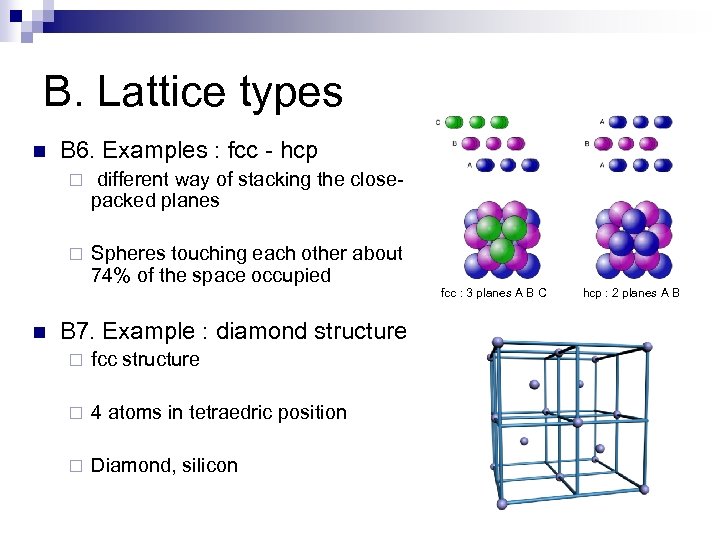 B. Lattice types n B 6. Examples : fcc - hcp ¨ ¨ n different way of stacking the closepacked planes Spheres touching each other about 74% of the space occupied B 7. Example : diamond structure ¨ fcc structure ¨ 4 atoms in tetraedric position ¨ Diamond, silicon fcc : 3 planes A B C hcp : 2 planes A B
B. Lattice types n B 6. Examples : fcc - hcp ¨ ¨ n different way of stacking the closepacked planes Spheres touching each other about 74% of the space occupied B 7. Example : diamond structure ¨ fcc structure ¨ 4 atoms in tetraedric position ¨ Diamond, silicon fcc : 3 planes A B C hcp : 2 planes A B
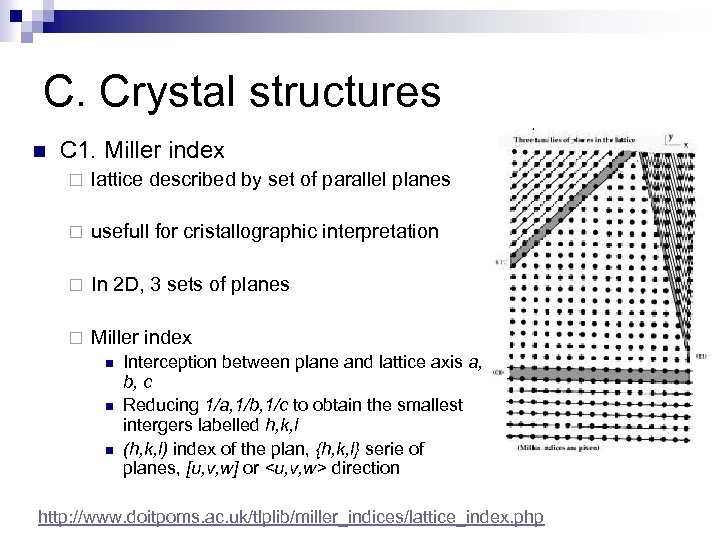 C. Crystal structures n C 1. Miller index ¨ lattice described by set of parallel planes ¨ usefull for cristallographic interpretation ¨ In 2 D, 3 sets of planes ¨ Miller index n n n Interception between plane and lattice axis a, b, c Reducing 1/a, 1/b, 1/c to obtain the smallest intergers labelled h, k, l (h, k, l) index of the plan, {h, k, l} serie of planes, [u, v, w] or
C. Crystal structures n C 1. Miller index ¨ lattice described by set of parallel planes ¨ usefull for cristallographic interpretation ¨ In 2 D, 3 sets of planes ¨ Miller index n n n Interception between plane and lattice axis a, b, c Reducing 1/a, 1/b, 1/c to obtain the smallest intergers labelled h, k, l (h, k, l) index of the plan, {h, k, l} serie of planes, [u, v, w] or
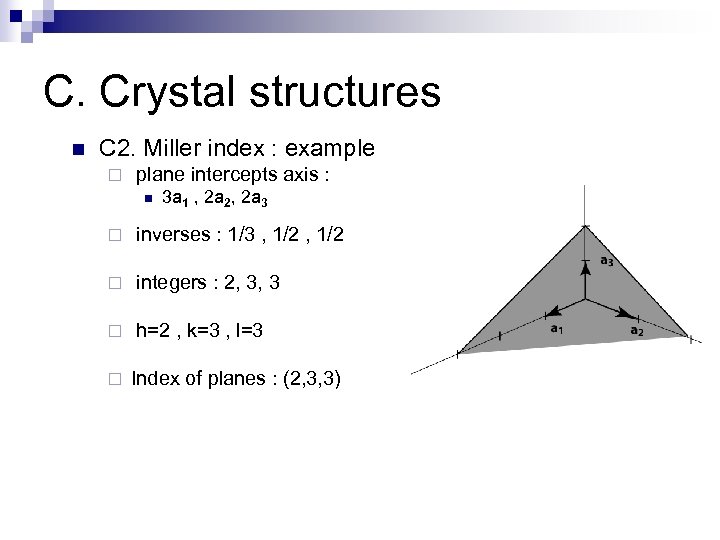 C. Crystal structures n C 2. Miller index : example ¨ plane intercepts axis : n 3 a 1 , 2 a 2, 2 a 3 ¨ inverses : 1/3 , 1/2 ¨ integers : 2, 3, 3 ¨ h=2 , k=3 , l=3 ¨ Index of planes : (2, 3, 3)
C. Crystal structures n C 2. Miller index : example ¨ plane intercepts axis : n 3 a 1 , 2 a 2, 2 a 3 ¨ inverses : 1/3 , 1/2 ¨ integers : 2, 3, 3 ¨ h=2 , k=3 , l=3 ¨ Index of planes : (2, 3, 3)


