8e0cc99a2d25e8d8797ff4d6b87bdcba.ppt
- Количество слайдов: 19
 Soft. PAP® A Novel Collection Device for Cervical Cytology
Soft. PAP® A Novel Collection Device for Cervical Cytology
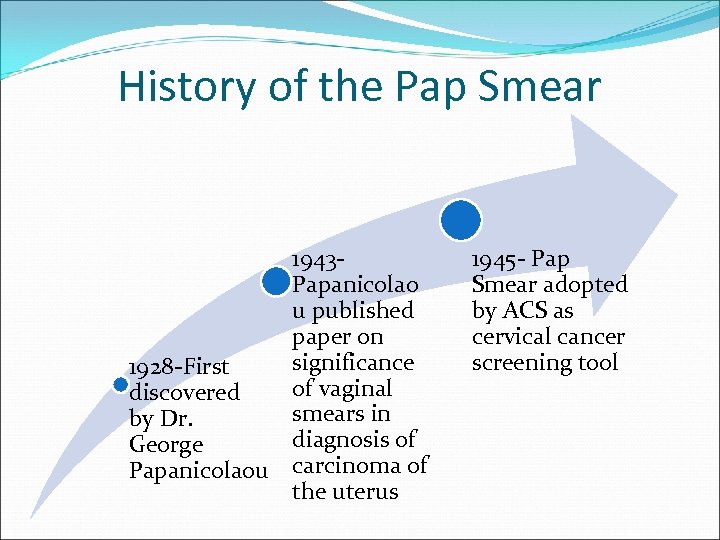 History of the Pap Smear 1943 Papanicolao u published paper on significance 1928 -First of vaginal discovered smears in by Dr. diagnosis of George Papanicolaou carcinoma of the uterus 1945 - Pap Smear adopted by ACS as cervical cancer screening tool
History of the Pap Smear 1943 Papanicolao u published paper on significance 1928 -First of vaginal discovered smears in by Dr. diagnosis of George Papanicolaou carcinoma of the uterus 1945 - Pap Smear adopted by ACS as cervical cancer screening tool
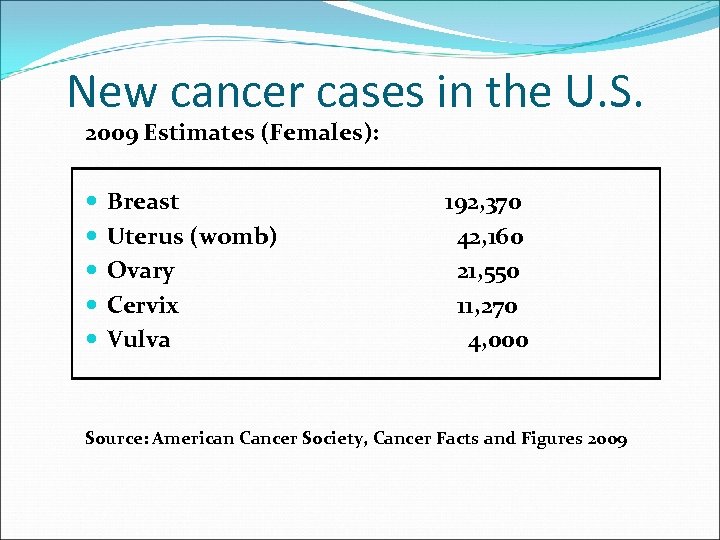 New cancer cases in the U. S. 2009 Estimates (Females): Breast Uterus (womb) Ovary Cervix Vulva 192, 370 42, 160 21, 550 11, 270 4, 000 Source: American Cancer Society, Cancer Facts and Figures 2009
New cancer cases in the U. S. 2009 Estimates (Females): Breast Uterus (womb) Ovary Cervix Vulva 192, 370 42, 160 21, 550 11, 270 4, 000 Source: American Cancer Society, Cancer Facts and Figures 2009
 How common is cervical cancer? 500, 000 women worldwide die of cervical cancer annually 60 -65 million women in the U. S. have a Pap test each year 3 -5 million women in the U. S. have an abnormal result 11, 270 new cervical cancers diagnosed in the U. S. in 2009 4, 100 deaths from cervical cancer in the U. S. per year Most cervical cancer can be prevented
How common is cervical cancer? 500, 000 women worldwide die of cervical cancer annually 60 -65 million women in the U. S. have a Pap test each year 3 -5 million women in the U. S. have an abnormal result 11, 270 new cervical cancers diagnosed in the U. S. in 2009 4, 100 deaths from cervical cancer in the U. S. per year Most cervical cancer can be prevented
 Pap Smears Conventional Liquid based monolayer (e. g. . Thin Prep) Spatula, Brush or Broom Collection HPV testing Automated Analysis
Pap Smears Conventional Liquid based monolayer (e. g. . Thin Prep) Spatula, Brush or Broom Collection HPV testing Automated Analysis
 Classification Bethesda System Based on the probability of invasive cancer Originally developed in 1998 System revised in 2001 Is the international gold standard for classifying Pap specimens
Classification Bethesda System Based on the probability of invasive cancer Originally developed in 1998 System revised in 2001 Is the international gold standard for classifying Pap specimens
 Bethesda Classification (2001) Squamous Cell Atypical squamous cells (ASC) Undetermined Significance (ASC-US) Not exclude High Grade (ASC-H) Low Grade Squamous Intraepithelial lesion (LSIL) High Grade Squamous Intraepithelial lesion (HSIL) Squamous Cell Carcinoma Glandular Cell Atypical Glandular cells (AG) Undetermined Significance (AG-US) Favors Neoplasm Adenocarcinoma In Situ (AIS) Adenocarcinoma
Bethesda Classification (2001) Squamous Cell Atypical squamous cells (ASC) Undetermined Significance (ASC-US) Not exclude High Grade (ASC-H) Low Grade Squamous Intraepithelial lesion (LSIL) High Grade Squamous Intraepithelial lesion (HSIL) Squamous Cell Carcinoma Glandular Cell Atypical Glandular cells (AG) Undetermined Significance (AG-US) Favors Neoplasm Adenocarcinoma In Situ (AIS) Adenocarcinoma
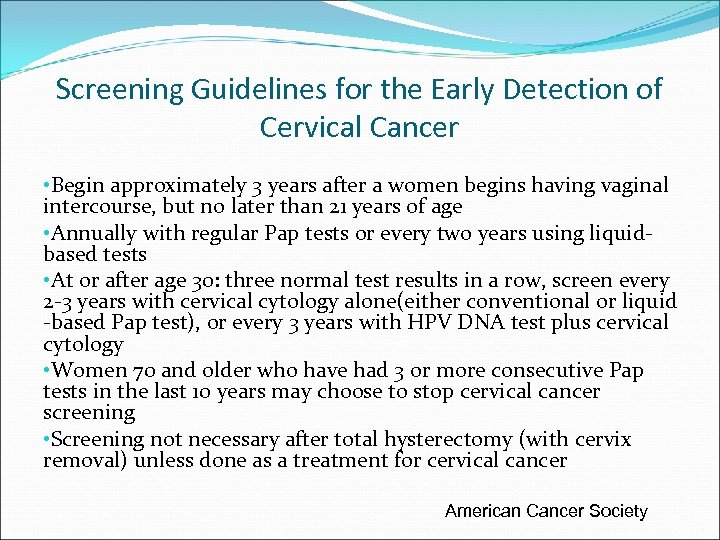 Screening Guidelines for the Early Detection of Cervical Cancer • Begin approximately 3 years after a women begins having vaginal intercourse, but no later than 21 years of age • Annually with regular Pap tests or every two years using liquidbased tests • At or after age 30: three normal test results in a row, screen every 2 -3 years with cervical cytology alone(either conventional or liquid -based Pap test), or every 3 years with HPV DNA test plus cervical cytology • Women 70 and older who have had 3 or more consecutive Pap tests in the last 10 years may choose to stop cervical cancer screening • Screening not necessary after total hysterectomy (with cervix removal) unless done as a treatment for cervical cancer American Cancer Society
Screening Guidelines for the Early Detection of Cervical Cancer • Begin approximately 3 years after a women begins having vaginal intercourse, but no later than 21 years of age • Annually with regular Pap tests or every two years using liquidbased tests • At or after age 30: three normal test results in a row, screen every 2 -3 years with cervical cytology alone(either conventional or liquid -based Pap test), or every 3 years with HPV DNA test plus cervical cytology • Women 70 and older who have had 3 or more consecutive Pap tests in the last 10 years may choose to stop cervical cancer screening • Screening not necessary after total hysterectomy (with cervix removal) unless done as a treatment for cervical cancer American Cancer Society
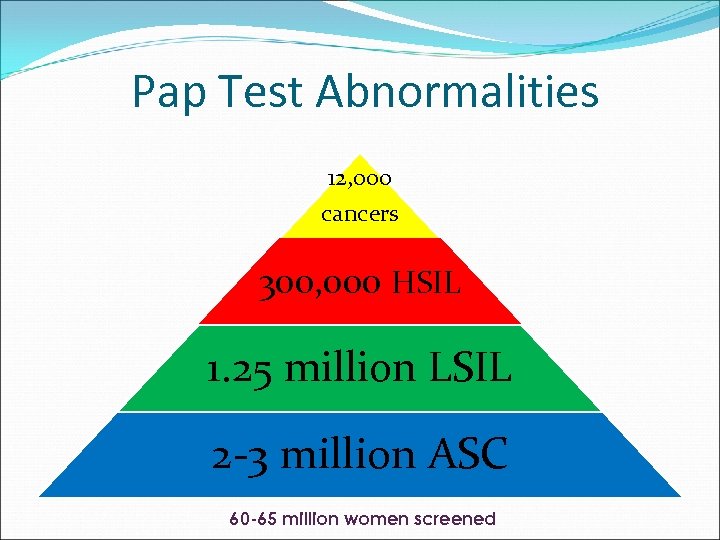 Pap Test Abnormalities 12, 000 cancers 300, 000 HSIL 1. 25 million LSIL 2 -3 million ASC 60 -65 million women screened
Pap Test Abnormalities 12, 000 cancers 300, 000 HSIL 1. 25 million LSIL 2 -3 million ASC 60 -65 million women screened
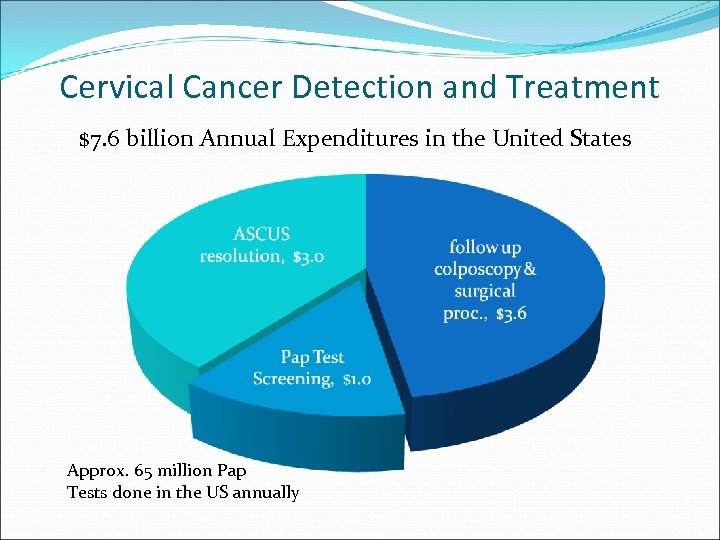 Cervical Cancer Detection and Treatment $7. 6 billion Annual Expenditures in the United States Approx. 65 million Pap Tests done in the US annually
Cervical Cancer Detection and Treatment $7. 6 billion Annual Expenditures in the United States Approx. 65 million Pap Tests done in the US annually
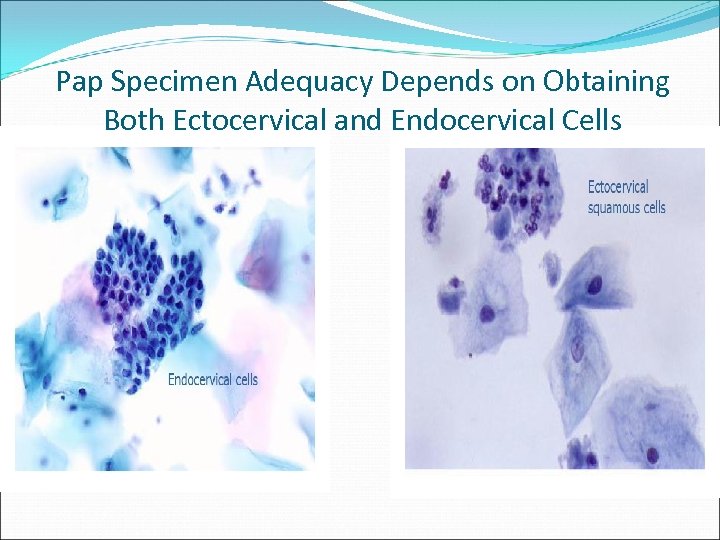 Pap Specimen Adequacy Depends on Obtaining Both Ectocervical and Endocervical Cells
Pap Specimen Adequacy Depends on Obtaining Both Ectocervical and Endocervical Cells
 Sources of Errors with Conventional Pap Sampling errors Cells not collected on sampling device Collected cells not transferred to slide Cells poorly preserved Screening errors Cytologist misses abnormal cells Incorrect classification of cells *These errors all contribute to false negative results, with reported estimates ranging from 6 -50%
Sources of Errors with Conventional Pap Sampling errors Cells not collected on sampling device Collected cells not transferred to slide Cells poorly preserved Screening errors Cytologist misses abnormal cells Incorrect classification of cells *These errors all contribute to false negative results, with reported estimates ranging from 6 -50%
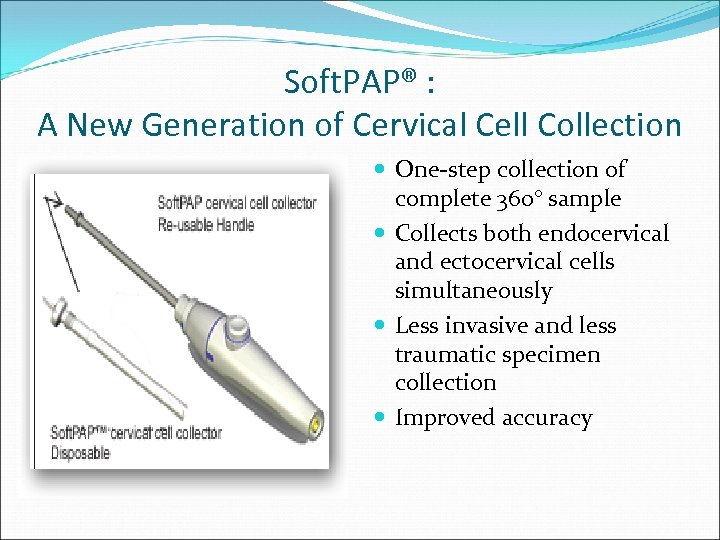 Soft. PAP® : A New Generation of Cervical Cell Collection One-step collection of complete 360° sample Collects both endocervical and ectocervical cells simultaneously Less invasive and less traumatic specimen collection Improved accuracy
Soft. PAP® : A New Generation of Cervical Cell Collection One-step collection of complete 360° sample Collects both endocervical and ectocervical cells simultaneously Less invasive and less traumatic specimen collection Improved accuracy
 Soft. PAP® : Advantages over Conventional Methods Statistically significant improvements vs. standard Spatula/Cytobrush Improved sensitivity Ø Reduces false negatives by 26% Improved specificity Ø Reduction of false positives of 33% Ease of use for the provider Greater patient comfort
Soft. PAP® : Advantages over Conventional Methods Statistically significant improvements vs. standard Spatula/Cytobrush Improved sensitivity Ø Reduces false negatives by 26% Improved specificity Ø Reduction of false positives of 33% Ease of use for the provider Greater patient comfort
 Comparison of the Adequacy and Efficacy of Soft. PAP® to Standard Specimen Collection for Cervical Cytology Trial Objective To demonstrate equivalency of cervical cytology sampling using Soft. PAP to conventional spatula/brush for cervical CA screening (Pap, HPV testing) Patient Population Methods 703 colposcopy clinic patients Randomized collection of cervical cytology specimens (compared for Pap, HPV testing) • specimen adequacy • sensitivity • specificity Study Endpoints
Comparison of the Adequacy and Efficacy of Soft. PAP® to Standard Specimen Collection for Cervical Cytology Trial Objective To demonstrate equivalency of cervical cytology sampling using Soft. PAP to conventional spatula/brush for cervical CA screening (Pap, HPV testing) Patient Population Methods 703 colposcopy clinic patients Randomized collection of cervical cytology specimens (compared for Pap, HPV testing) • specimen adequacy • sensitivity • specificity Study Endpoints
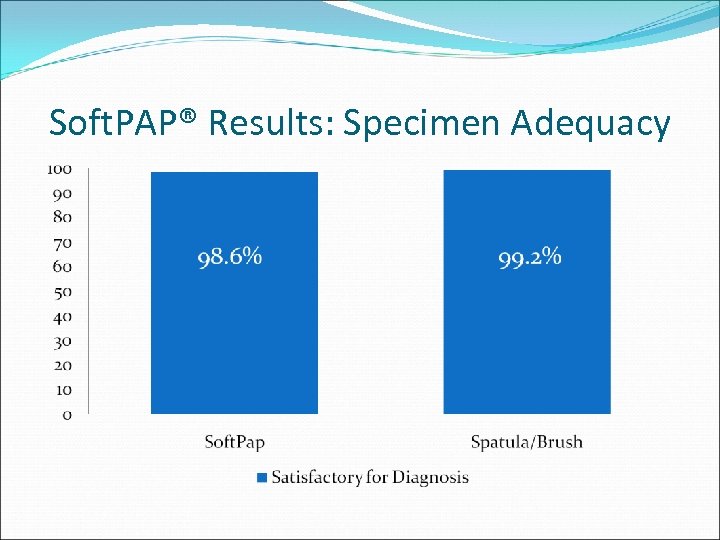 Soft. PAP® Results: Specimen Adequacy
Soft. PAP® Results: Specimen Adequacy
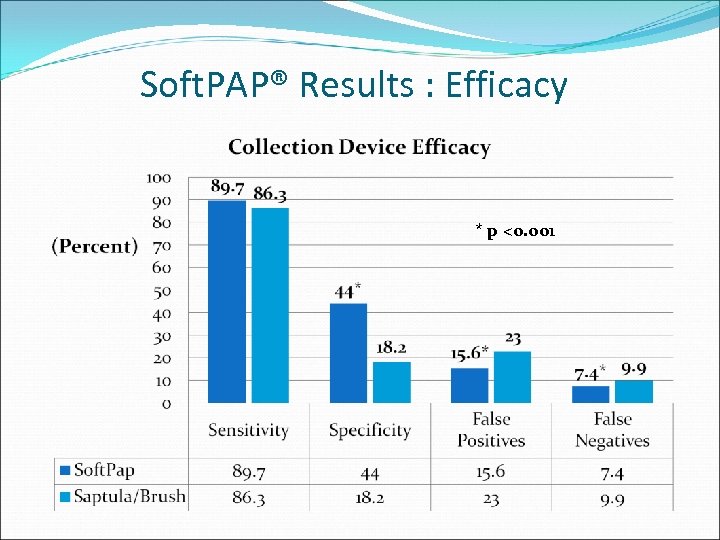 Soft. PAP® Results : Efficacy * p <0. 001
Soft. PAP® Results : Efficacy * p <0. 001
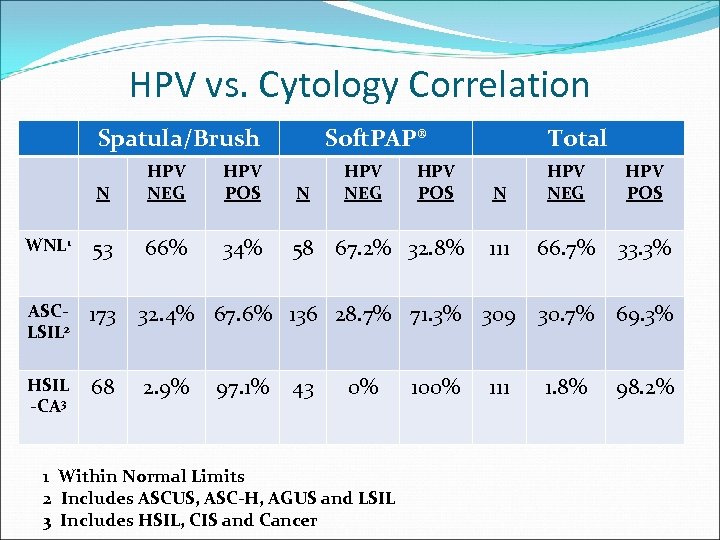 HPV vs. Cytology Correlation Spatula/Brush Soft. PAP® N HPV NEG HPV POS N WNL 1 53 66% 34% 58 67. 2% 32. 8% ASCLSIL 2 173 32. 4% 67. 6% 136 28. 7% 71. 3% 309 30. 7% 69. 3% HSIL -CA 3 68 2. 9% 97. 1% 43 HPV NEG 0% 1 Within Normal Limits 2 Includes ASCUS, ASC-H, AGUS and LSIL 3 Includes HSIL, CIS and Cancer HPV POS Total 100% N HPV NEG HPV POS 111 66. 7% 33. 3% 111 1. 8% 98. 2%
HPV vs. Cytology Correlation Spatula/Brush Soft. PAP® N HPV NEG HPV POS N WNL 1 53 66% 34% 58 67. 2% 32. 8% ASCLSIL 2 173 32. 4% 67. 6% 136 28. 7% 71. 3% 309 30. 7% 69. 3% HSIL -CA 3 68 2. 9% 97. 1% 43 HPV NEG 0% 1 Within Normal Limits 2 Includes ASCUS, ASC-H, AGUS and LSIL 3 Includes HSIL, CIS and Cancer HPV POS Total 100% N HPV NEG HPV POS 111 66. 7% 33. 3% 111 1. 8% 98. 2%
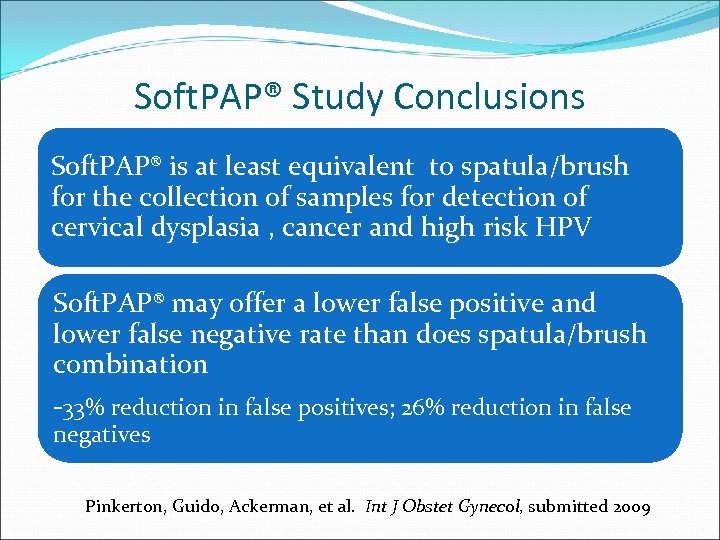 Soft. PAP® Study Conclusions Soft. PAP® is at least equivalent to spatula/brush for the collection of samples for detection of cervical dysplasia , cancer and high risk HPV Soft. PAP® may offer a lower false positive and lower false negative rate than does spatula/brush combination -33% reduction in false positives; 26% reduction in false negatives Pinkerton, Guido, Ackerman, et al. Int J Obstet Gynecol, submitted 2009
Soft. PAP® Study Conclusions Soft. PAP® is at least equivalent to spatula/brush for the collection of samples for detection of cervical dysplasia , cancer and high risk HPV Soft. PAP® may offer a lower false positive and lower false negative rate than does spatula/brush combination -33% reduction in false positives; 26% reduction in false negatives Pinkerton, Guido, Ackerman, et al. Int J Obstet Gynecol, submitted 2009


