3845d9b9c0622890f93728335f1d0f10.ppt
- Количество слайдов: 16
 So. GAT and HIV NAT Standards 2 nd International Standard for HIV-1 RNA Harvey Holmes*, Clare Davis* and Alan Heath** Divisions of Retrovirology* and Informatics** National Institute for Biological Standards and Control (NIBSC) UK XVIII So. GAT Washington 24 May 2005
So. GAT and HIV NAT Standards 2 nd International Standard for HIV-1 RNA Harvey Holmes*, Clare Davis* and Alan Heath** Divisions of Retrovirology* and Informatics** National Institute for Biological Standards and Control (NIBSC) UK XVIII So. GAT Washington 24 May 2005
 • So. GAT and NAT Standards available from NIBSC So. GAT and NIBSC have collaborated in establishing: International Standards: – 1997: 1 st International Standard for HCV RNA (96/790) – 1999: 1 st International Standard for HIV-1 RNA (97/656) – 1999: 1 st international Standard for HBV DNA (97/746) – 2000: 1 st International Standard for B 19 DNA (99/800) – 2002: 1 st International Standard for HAV RNA (00/560) – 2003: 1 st International Reference Panel for HIV-1 Genotypes (01/466) – genotypes A-H – 2003: 2 nd International Standard for HCV RNA (96/798) Working Reagents: • HIV-1 RNA PWS-1, PWS-2, PWS-3 • HCV RNA • HBV DNA • HAV RNA • B 19 DNA • Multiplex (HCV, HBV, HIV, HAV, B 19) • HCV Genotype Panel (genotypes 1 -6) XVIII So. GAT Washington 24 May 2005
• So. GAT and NAT Standards available from NIBSC So. GAT and NIBSC have collaborated in establishing: International Standards: – 1997: 1 st International Standard for HCV RNA (96/790) – 1999: 1 st International Standard for HIV-1 RNA (97/656) – 1999: 1 st international Standard for HBV DNA (97/746) – 2000: 1 st International Standard for B 19 DNA (99/800) – 2002: 1 st International Standard for HAV RNA (00/560) – 2003: 1 st International Reference Panel for HIV-1 Genotypes (01/466) – genotypes A-H – 2003: 2 nd International Standard for HCV RNA (96/798) Working Reagents: • HIV-1 RNA PWS-1, PWS-2, PWS-3 • HCV RNA • HBV DNA • HAV RNA • B 19 DNA • Multiplex (HCV, HBV, HIV, HAV, B 19) • HCV Genotype Panel (genotypes 1 -6) XVIII So. GAT Washington 24 May 2005
 Retrovirology Standards and Working Reagents XVIII So. GAT Washington 24 May 2005
Retrovirology Standards and Working Reagents XVIII So. GAT Washington 24 May 2005
 Replacement of 1 st International Standard for HIV-1 RNA (NIBSC Code 97/656) • Sample YY established as 1 st International Standard (code 97/656) in 1999 • Assigned a concentration of 100, 000 IU per vial/ml • Standard made from HIV-1 PCR-positive, antibody-negative, plasmapheresis donation diluted in defibrinated plasma • 2300 vials freeze-dried and stored at -200 C • So. GAT 2003 - agreement to replace standard due to: – low Stocks (~ 450) – presence of HBV DNA – may affect suitability for multiplex assays • Interim report on collaborative study presented by Clare Davis at So. GAT 2004 XVIII So. GAT Washington 24 May 2005
Replacement of 1 st International Standard for HIV-1 RNA (NIBSC Code 97/656) • Sample YY established as 1 st International Standard (code 97/656) in 1999 • Assigned a concentration of 100, 000 IU per vial/ml • Standard made from HIV-1 PCR-positive, antibody-negative, plasmapheresis donation diluted in defibrinated plasma • 2300 vials freeze-dried and stored at -200 C • So. GAT 2003 - agreement to replace standard due to: – low Stocks (~ 450) – presence of HBV DNA – may affect suitability for multiplex assays • Interim report on collaborative study presented by Clare Davis at So. GAT 2004 XVIII So. GAT Washington 24 May 2005
 Candidate 2 nd International Standard • So. GAT recommended that sample XX from first collaborative study be re-evaluated as candidate 2 nd International Standard (IS) • Characteristics: – HIV-1 subtype B low passage field isolate (CCR 5/NSI) from Edinburgh (P Simmonds) – Propagated on human PBMCs - stock stored under LN – Virus diluted in human cryosupernatant to suitable concentration – 2200 vials of material freeze-dried – Fill CV = 0. 2% XVIII So. GAT Washington 24 May 2005
Candidate 2 nd International Standard • So. GAT recommended that sample XX from first collaborative study be re-evaluated as candidate 2 nd International Standard (IS) • Characteristics: – HIV-1 subtype B low passage field isolate (CCR 5/NSI) from Edinburgh (P Simmonds) – Propagated on human PBMCs - stock stored under LN – Virus diluted in human cryosupernatant to suitable concentration – 2200 vials of material freeze-dried – Fill CV = 0. 2% XVIII So. GAT Washington 24 May 2005
 International Collaborative Study to Establish 2 nd International Standard for HIV-1 RNA • Aims of study: – Calibrate XX against 1 st International Standard (1 st IS) – Confirm stability of XX – Determine values in current assays • 10 laboratories participated • Candidate XX and the 1 st International Standard recoded (samples 1 and 2 respectively) • Participants asked to test samples in 3 independent assays. • Initially at 10 -fold dilutions • Subsequently at half log dilutions around the end point • 8 data sets received, 5 from quantitative assays and 3 from qualitative assays • NIBSC collated analysed data XVIII So. GAT Washington 24 May 2005
International Collaborative Study to Establish 2 nd International Standard for HIV-1 RNA • Aims of study: – Calibrate XX against 1 st International Standard (1 st IS) – Confirm stability of XX – Determine values in current assays • 10 laboratories participated • Candidate XX and the 1 st International Standard recoded (samples 1 and 2 respectively) • Participants asked to test samples in 3 independent assays. • Initially at 10 -fold dilutions • Subsequently at half log dilutions around the end point • 8 data sets received, 5 from quantitative assays and 3 from qualitative assays • NIBSC collated analysed data XVIII So. GAT Washington 24 May 2005
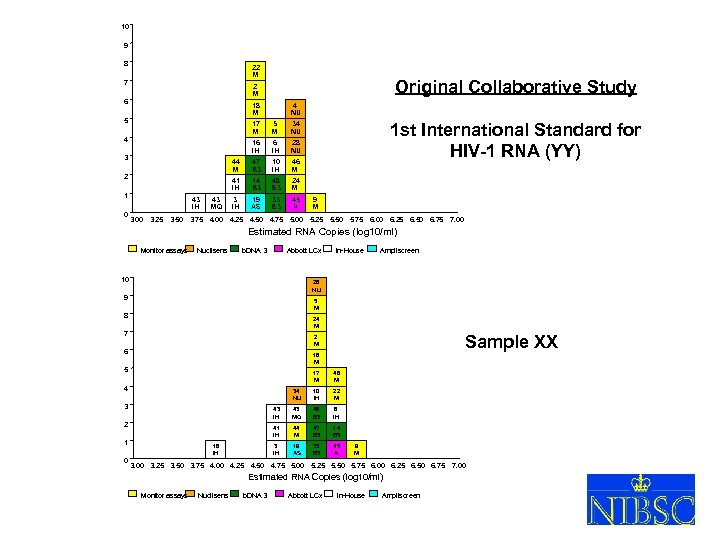 10 9 8 22 M 7 18 M 5 4 NU 17 M 5 M 16 IH 28 NU 44 M 47 B 3 10 IH 46 M 41 IH 3 2 14 B 3 48 B 3 1 43 IH 43 MQ 24 M 3 IH 19 AS 33 B 3 45 A 1 st International Standard for HIV-1 RNA (YY) 34 NU 4 0 Original Collaborative Study 2 M 6 9 M 3. 00 3. 25 3. 50 3. 75 4. 00 4. 25 4. 50 4. 75 5. 00 5. 25 5. 50 5. 75 6. 00 6. 25 6. 50 6. 75 7. 00 Estimated RNA Copies (log 10/ml) Monitor assays Nuclisens b. DNA 3 Abbott LCx 10 Ampliscreen 28 NU 9 5 M 8 24 M 7 Sample XX 2 M 6 18 M 5 17 M 46 M 34 NU 10 IH 22 M 43 IH 43 MQ 48 B 3 6 IH 41 IH 44 M 47 B 3 14 B 3 3 IH 19 AS 33 B 3 45 A 4 3 2 1 0 In-House 16 IH 9 M 3. 00 3. 25 3. 50 3. 75 4. 00 4. 25 4. 50 4. 75 5. 00 5. 25 5. 50 5. 75 6. 00 6. 25 6. 50 6. 75 7. 00 Estimated RNA Copies (log 10/ml) Monitor assays Nuclisens b. DNA 3 Abbott LCx In-House Ampliscreen XVIII So. GAT Washington 24 May 2005
10 9 8 22 M 7 18 M 5 4 NU 17 M 5 M 16 IH 28 NU 44 M 47 B 3 10 IH 46 M 41 IH 3 2 14 B 3 48 B 3 1 43 IH 43 MQ 24 M 3 IH 19 AS 33 B 3 45 A 1 st International Standard for HIV-1 RNA (YY) 34 NU 4 0 Original Collaborative Study 2 M 6 9 M 3. 00 3. 25 3. 50 3. 75 4. 00 4. 25 4. 50 4. 75 5. 00 5. 25 5. 50 5. 75 6. 00 6. 25 6. 50 6. 75 7. 00 Estimated RNA Copies (log 10/ml) Monitor assays Nuclisens b. DNA 3 Abbott LCx 10 Ampliscreen 28 NU 9 5 M 8 24 M 7 Sample XX 2 M 6 18 M 5 17 M 46 M 34 NU 10 IH 22 M 43 IH 43 MQ 48 B 3 6 IH 41 IH 44 M 47 B 3 14 B 3 3 IH 19 AS 33 B 3 45 A 4 3 2 1 0 In-House 16 IH 9 M 3. 00 3. 25 3. 50 3. 75 4. 00 4. 25 4. 50 4. 75 5. 00 5. 25 5. 50 5. 75 6. 00 6. 25 6. 50 6. 75 7. 00 Estimated RNA Copies (log 10/ml) Monitor assays Nuclisens b. DNA 3 Abbott LCx In-House Ampliscreen XVIII So. GAT Washington 24 May 2005
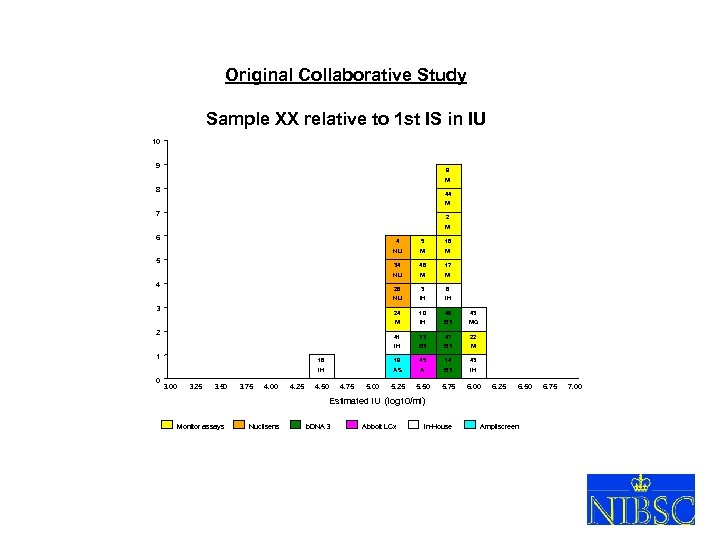 Original Collaborative Study Sample XX relative to 1 st IS in IU 10 9 9 M 8 44 M 7 2 M 6 4 M M 3 6 NU IH IH 24 10 48 43 M 2 17 28 3 M 46 NU 4 M 34 5 5 NU 18 IH B 3 MQ 22 41 3. 00 3. 25 3. 50 3. 75 4. 00 4. 25 B 3 M 19 45 14 43 IH 0 47 B 3 16 1 33 IH AS A B 3 IH 5. 25 5. 50 5. 75 6. 00 4. 50 4. 75 5. 00 6. 25 6. 50 Estimated IU (log 10/ml) Monitor assays Nuclisens b. DNA 3 Abbott LCx In-House XVIII So. GAT Washington 24 May 2005 Ampliscreen 6. 75 7. 00
Original Collaborative Study Sample XX relative to 1 st IS in IU 10 9 9 M 8 44 M 7 2 M 6 4 M M 3 6 NU IH IH 24 10 48 43 M 2 17 28 3 M 46 NU 4 M 34 5 5 NU 18 IH B 3 MQ 22 41 3. 00 3. 25 3. 50 3. 75 4. 00 4. 25 B 3 M 19 45 14 43 IH 0 47 B 3 16 1 33 IH AS A B 3 IH 5. 25 5. 50 5. 75 6. 00 4. 50 4. 75 5. 00 6. 25 6. 50 Estimated IU (log 10/ml) Monitor assays Nuclisens b. DNA 3 Abbott LCx In-House XVIII So. GAT Washington 24 May 2005 Ampliscreen 6. 75 7. 00
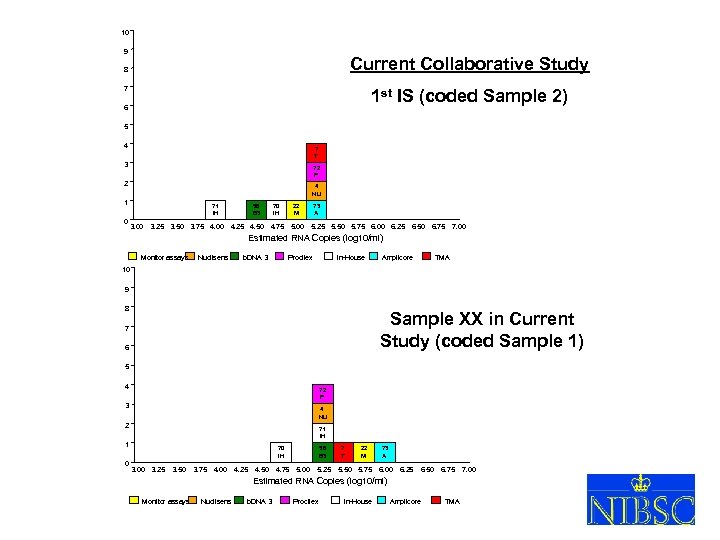 10 9 Current Collaborative Study 8 7 1 st IS (coded Sample 2) 6 5 4 7 T 3 72 P 2 4 NU 1 0 71 IH 58 B 3 70 IH 22 M 73 A 3. 00 3. 25 3. 50 3. 75 4. 00 4. 25 4. 50 4. 75 5. 00 5. 25 5. 50 5. 75 6. 00 6. 25 6. 50 6. 75 7. 00 Estimated RNA Copies (log 10/ml) Monitor assays Nuclisens b. DNA 3 Procilex In-House Amplicore TMA 10 9 8 Sample XX in Current Study (coded Sample 1) 7 6 5 4 72 P 3 4 NU 2 71 IH 1 0 70 IH 3. 00 3. 25 3. 50 3. 75 4. 00 4. 25 4. 50 4. 75 58 B 3 5. 00 5. 25 7 T 22 M 73 A 5. 50 5. 75 6. 00 6. 25 6. 50 6. 75 7. 00 Estimated RNA Copies (log 10/ml) Monitor assays Nuclisens b. DNA 3 Procilex In-House Amplicore TMA XVIII So. GAT Washington 24 May 2005
10 9 Current Collaborative Study 8 7 1 st IS (coded Sample 2) 6 5 4 7 T 3 72 P 2 4 NU 1 0 71 IH 58 B 3 70 IH 22 M 73 A 3. 00 3. 25 3. 50 3. 75 4. 00 4. 25 4. 50 4. 75 5. 00 5. 25 5. 50 5. 75 6. 00 6. 25 6. 50 6. 75 7. 00 Estimated RNA Copies (log 10/ml) Monitor assays Nuclisens b. DNA 3 Procilex In-House Amplicore TMA 10 9 8 Sample XX in Current Study (coded Sample 1) 7 6 5 4 72 P 3 4 NU 2 71 IH 1 0 70 IH 3. 00 3. 25 3. 50 3. 75 4. 00 4. 25 4. 50 4. 75 58 B 3 5. 00 5. 25 7 T 22 M 73 A 5. 50 5. 75 6. 00 6. 25 6. 50 6. 75 7. 00 Estimated RNA Copies (log 10/ml) Monitor assays Nuclisens b. DNA 3 Procilex In-House Amplicore TMA XVIII So. GAT Washington 24 May 2005
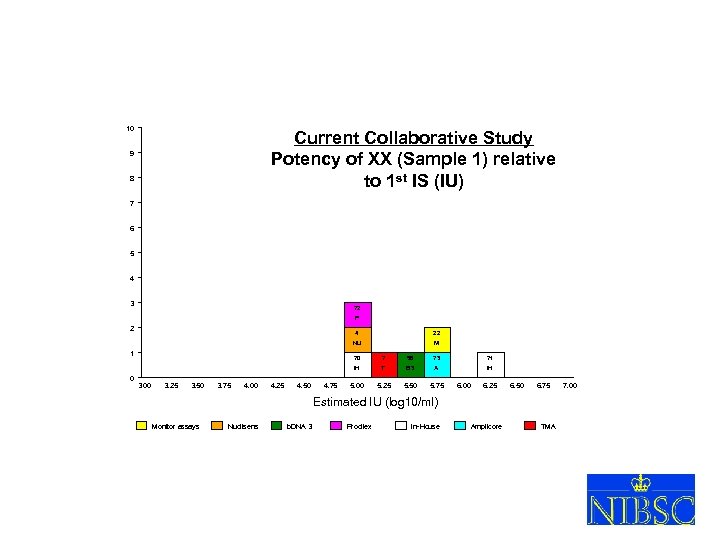 10 Current Collaborative Study Potency of XX (Sample 1) relative to 1 st IS (IU) 9 8 7 6 5 4 3 72 P 2 4 22 NU 1 M 3. 00 3. 25 3. 50 3. 75 4. 00 4. 25 4. 50 4. 75 7 58 73 71 IH 0 70 T B 3 A IH 5. 00 5. 25 5. 50 5. 75 6. 00 6. 25 6. 50 6. 75 Estimated IU (log 10/ml) Monitor assays Nuclisens b. DNA 3 Procilex In-House Amplicore XVIII So. GAT Washington 24 May 2005 TMA 7. 00
10 Current Collaborative Study Potency of XX (Sample 1) relative to 1 st IS (IU) 9 8 7 6 5 4 3 72 P 2 4 22 NU 1 M 3. 00 3. 25 3. 50 3. 75 4. 00 4. 25 4. 50 4. 75 7 58 73 71 IH 0 70 T B 3 A IH 5. 00 5. 25 5. 50 5. 75 6. 00 6. 25 6. 50 6. 75 Estimated IU (log 10/ml) Monitor assays Nuclisens b. DNA 3 Procilex In-House Amplicore XVIII So. GAT Washington 24 May 2005 TMA 7. 00
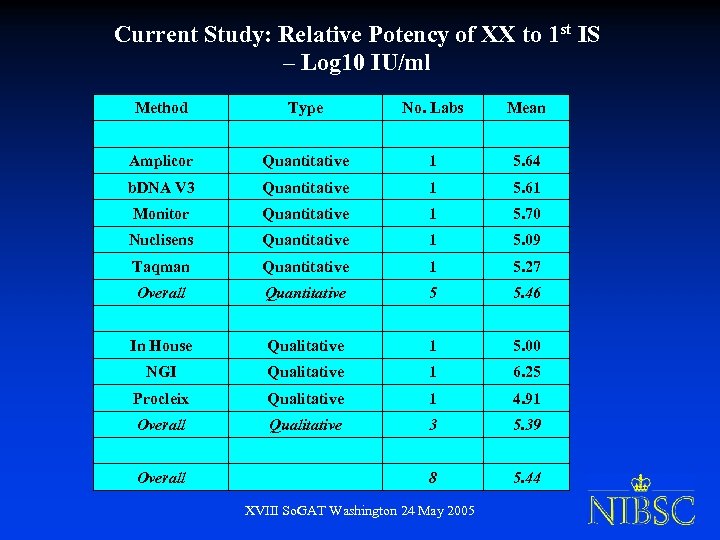 Current Study: Relative Potency of XX to 1 st IS – Log 10 IU/ml Method Type No. Labs Mean Amplicor Quantitative 1 5. 64 b. DNA V 3 Quantitative 1 5. 61 Monitor Quantitative 1 5. 70 Nuclisens Quantitative 1 5. 09 Taqman Quantitative 1 5. 27 Overall Quantitative 5 5. 46 In House Qualitative 1 5. 00 NGI Qualitative 1 6. 25 Procleix Qualitative 1 4. 91 Overall Qualitative 3 5. 39 8 5. 44 Overall XVIII So. GAT Washington 24 May 2005
Current Study: Relative Potency of XX to 1 st IS – Log 10 IU/ml Method Type No. Labs Mean Amplicor Quantitative 1 5. 64 b. DNA V 3 Quantitative 1 5. 61 Monitor Quantitative 1 5. 70 Nuclisens Quantitative 1 5. 09 Taqman Quantitative 1 5. 27 Overall Quantitative 5 5. 46 In House Qualitative 1 5. 00 NGI Qualitative 1 6. 25 Procleix Qualitative 1 4. 91 Overall Qualitative 3 5. 39 8 5. 44 Overall XVIII So. GAT Washington 24 May 2005
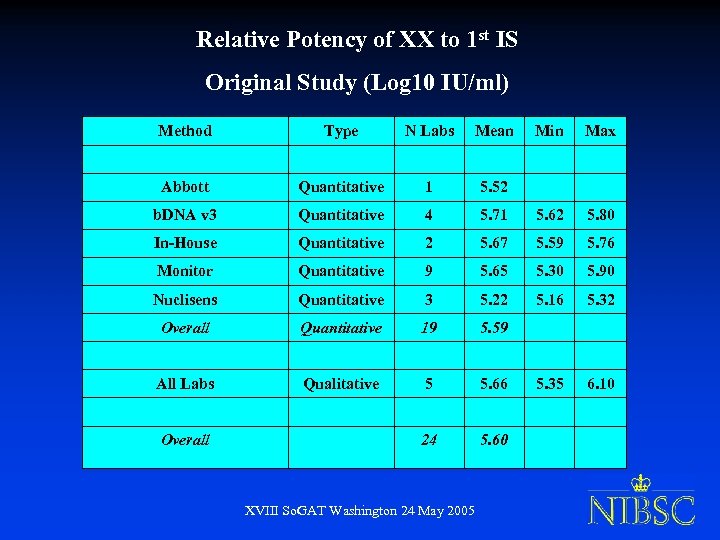 Relative Potency of XX to 1 st IS Original Study (Log 10 IU/ml) Method Type N Labs Mean Min Max Abbott Quantitative 1 5. 52 b. DNA v 3 Quantitative 4 5. 71 5. 62 5. 80 In-House Quantitative 2 5. 67 5. 59 5. 76 Monitor Quantitative 9 5. 65 5. 30 5. 90 Nuclisens Quantitative 3 5. 22 5. 16 5. 32 Overall Quantitative 19 5. 59 All Labs Qualitative 5 5. 66 5. 35 6. 10 24 5. 60 Overall XVIII So. GAT Washington 24 May 2005
Relative Potency of XX to 1 st IS Original Study (Log 10 IU/ml) Method Type N Labs Mean Min Max Abbott Quantitative 1 5. 52 b. DNA v 3 Quantitative 4 5. 71 5. 62 5. 80 In-House Quantitative 2 5. 67 5. 59 5. 76 Monitor Quantitative 9 5. 65 5. 30 5. 90 Nuclisens Quantitative 3 5. 22 5. 16 5. 32 Overall Quantitative 19 5. 59 All Labs Qualitative 5 5. 66 5. 35 6. 10 24 5. 60 Overall XVIII So. GAT Washington 24 May 2005
 2 nd International Standard for HIV-1 RNA • No evidence of any drift in values between the two studies - no stability issues • Four options for assigning value to XX: – 1: Use results from original study (5. 60 log 10 IU/ml) – 2: Use results from current study (5. 44 log 10 IU/ml) – 3: Pool data from both studies (5. 56 log 10 IU/ml) – 4: Determine overall mean for each method and obtain compromise between methods – pragmatic rather than statistically based • Option 3 favoured by NIBSC statistician XVIII So. GAT Washington 24 May 2005
2 nd International Standard for HIV-1 RNA • No evidence of any drift in values between the two studies - no stability issues • Four options for assigning value to XX: – 1: Use results from original study (5. 60 log 10 IU/ml) – 2: Use results from current study (5. 44 log 10 IU/ml) – 3: Pool data from both studies (5. 56 log 10 IU/ml) – 4: Determine overall mean for each method and obtain compromise between methods – pragmatic rather than statistically based • Option 3 favoured by NIBSC statistician XVIII So. GAT Washington 24 May 2005
 International Collaborative Study to Establish 2 nd International Standard for HIV-1 RNA Participants • • • T Cuypers Sanquin – CLB, The Netherlands M Chudy Paul Ehrlich Institute, Germany J Docker Gen Probe, USA K Fransen Institute of Tropical Medicine, Belgium I Hewlett CBER, FDA, USA F Intelmann Baxter, Austria D Jardine National Reference Laboratory, Australia J Turczyn Bayer Testing lab, USA Richard Smith NGI, USA C Davis/H Holmes NIBSC, UK XVIII So. GAT Washington 24 May 2005
International Collaborative Study to Establish 2 nd International Standard for HIV-1 RNA Participants • • • T Cuypers Sanquin – CLB, The Netherlands M Chudy Paul Ehrlich Institute, Germany J Docker Gen Probe, USA K Fransen Institute of Tropical Medicine, Belgium I Hewlett CBER, FDA, USA F Intelmann Baxter, Austria D Jardine National Reference Laboratory, Australia J Turczyn Bayer Testing lab, USA Richard Smith NGI, USA C Davis/H Holmes NIBSC, UK XVIII So. GAT Washington 24 May 2005
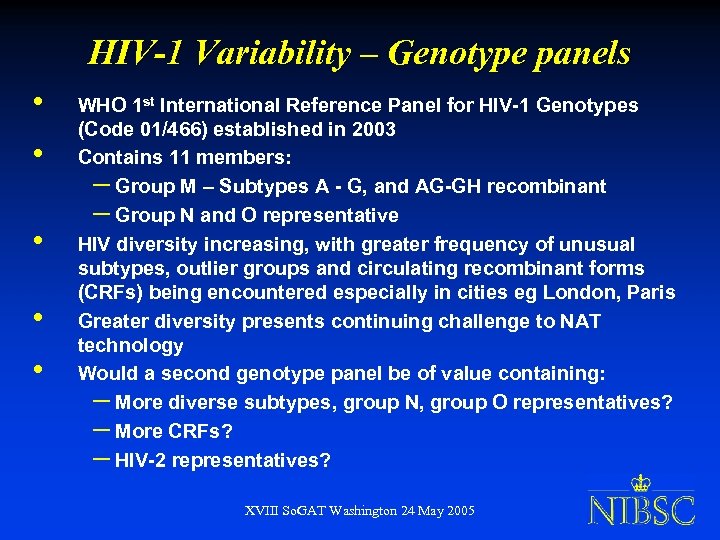 HIV-1 Variability – Genotype panels • • • WHO 1 st International Reference Panel for HIV-1 Genotypes (Code 01/466) established in 2003 Contains 11 members: – Group M – Subtypes A - G, and AG-GH recombinant – Group N and O representative HIV diversity increasing, with greater frequency of unusual subtypes, outlier groups and circulating recombinant forms (CRFs) being encountered especially in cities eg London, Paris Greater diversity presents continuing challenge to NAT technology Would a second genotype panel be of value containing: – More diverse subtypes, group N, group O representatives? – More CRFs? – HIV-2 representatives? XVIII So. GAT Washington 24 May 2005
HIV-1 Variability – Genotype panels • • • WHO 1 st International Reference Panel for HIV-1 Genotypes (Code 01/466) established in 2003 Contains 11 members: – Group M – Subtypes A - G, and AG-GH recombinant – Group N and O representative HIV diversity increasing, with greater frequency of unusual subtypes, outlier groups and circulating recombinant forms (CRFs) being encountered especially in cities eg London, Paris Greater diversity presents continuing challenge to NAT technology Would a second genotype panel be of value containing: – More diverse subtypes, group N, group O representatives? – More CRFs? – HIV-2 representatives? XVIII So. GAT Washington 24 May 2005
 Conclusions • Report on candidate 2 nd International Standard for HIV-1 RNA to WHO ECBS • Recommendation on unitage – option 3? ? • Need for 2 nd HIV Genotype Panel containing more diverse field isolates? ? XVIII So. GAT Washington 24 May 2005
Conclusions • Report on candidate 2 nd International Standard for HIV-1 RNA to WHO ECBS • Recommendation on unitage – option 3? ? • Need for 2 nd HIV Genotype Panel containing more diverse field isolates? ? XVIII So. GAT Washington 24 May 2005


