385578a87b7a6c3bf0480fddc8e50763.ppt
- Количество слайдов: 42

Slide 1 © 2003 By Default! Development of an Automated Nephrotoxicity Pharmacosurveillance System Michael E. Matheny, MD MS MPH Division of General Internal Medicine Vanderbilt University, Nashville, TN Geriatrics Research, Education & Clinical Care Veteran’s Administration, Nashville, TN A Free sample background from www. powerpointbackgrounds. com

Slide 2 © 2003 By Default! Research Plan n Background & Significance n Objectives n Work Accomplished n Work Proposed n Training Activities & Objectives A Free sample background from www. powerpointbackgrounds. com

Slide 3 © 2003 By Default! Background Surveillance Rationale n Phase 3 Trials insufficient to ensure adequate safety of medications and devices – Low frequency events are not detected – Protected populations (pregnant women, children) and more ill populations not represented – Complications delayed by a number of years cannot be detected A Free sample background from www. powerpointbackgrounds. com

Slide 4 © 2003 By Default! Background Current FDA Post-Marketing Surveillance n Combination of mandatory and voluntary adverse event reporting – Mandatory reporting by manufacturers and health facilities – Voluntary Med. Watch / MAUDE reports by providers and patients 2004 Drug-Related Adverse Event Reports Total 422, 889 Manufacturer & Facility Reports 401, 396 Med. Watch 21, 493 A Free sample background from www. powerpointbackgrounds. com

Slide 5 © 2003 By Default! Background Current Post-Marketing Surveillance n ‘Phase 4’ Trials – Poor Compliance • As of March 2006 report, 797 of 1231 (65%) agreedupon trials had yet to be started – Barriers • Lack of manufacturer incentives – Expensive – Drug already on the market • Lack of regulatory enforcement A Free sample background from www. powerpointbackgrounds. com

Slide 6 © 2003 By Default! Background FDA Response n Legislation n Complementary Data Sources – Incorporate Phase 4 Trial costs into Approval Process – Increase quality of adverse event reporting – – Commissioned IOM report “The Future of Drug Safety” Public Hearing in 2006 Aggregate Existing Clinical Registry Data n Promote Use of Routine Medical Data n A Free sample background from www. powerpointbackgrounds. com

Slide 7 © 2003 By Default! Background Transition n Transition slide from surveillance to domain A Free sample background from www. powerpointbackgrounds. com
Slide 8 © 2003 By Default! Background Acute Kidney Injury Statistics n 1 -7% of all hospital admissions n 5 -20% during an ICU stay n Crude Mortality Estimates – ~15% isolated AKI – ~25% general inpatient – ~40% in ICU n National trend of increasing incidence A Free sample background from www. powerpointbackgrounds. com
Slide 9 © 2003 By Default! Background Causes of Acute Kidney Injury n Acute Medical Conditions – Rhabdomyolysis: 15 -20% – Sepsis: 40 -50% – Major Surgery: 10 -20% – Congestive Heart Failure: 20% – Myocardial Infarction – Any condition that generates a real or apparent decreased intravascular volume A Free sample background from www. powerpointbackgrounds. com
Slide 10 © 2003 By Default! Background Causes of Acute Kidney Injury n Medications – Nephrotoxic medications cause 15 -25% of AKI – Aminoglycosides: 8 -26% • Single Daily Dose: ↓ risk – Amphotericin: 50 -80% • Lipid Formulation: ↓ risk – NSAID – ACE Inhibitor – Immunosuppressive Agents n Radiocontrast Dye – Separate category, cause 10 -15% of AKI A Free sample background from www. powerpointbackgrounds. com

Slide 11 © 2003 By Default! Objectives n Validation of an Automated Data Collection System for Acute Kidney Injury Monitoring n Retrospective Evaluation of Acute Kidney Injury among Hospitalized Veterans n Pilot an Acute Kidney Injury Automated Surveillance System among Hospitalized Veterans A Free sample background from www. powerpointbackgrounds. com

Slide 12 © 2003 By Default! Prior Work A Free sample background from www. powerpointbackgrounds. com
Slide 13 © 2003 By Default! Methodology & Application Adaptation of Statistical Process Control Monitoring Methodologies Design and Implementation of a Web-Based Monitoring Application (DELTA) n n Matheny ME, Ohno-Machado L, Resnic FS. Monitoring Device Safety in Interventional Cardiology. J Am Med Inform Assoc. 2006; 13(2): 180 -7. A Free sample background from www. powerpointbackgrounds. com
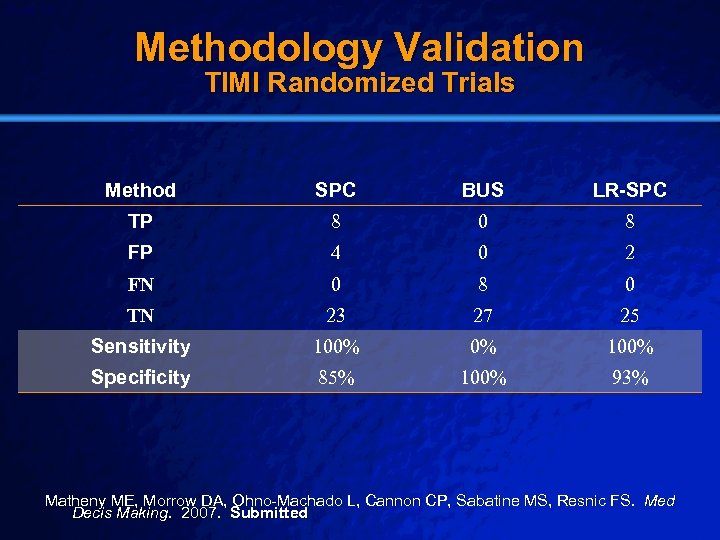
Slide 14 © 2003 By Default! Methodology Validation TIMI Randomized Trials Method SPC BUS LR-SPC TP 8 0 8 FP 4 0 2 FN 0 8 0 TN 23 27 25 Sensitivity 100% 0% 100% Specificity 85% 100% 93% Matheny ME, Morrow DA, Ohno-Machado L, Cannon CP, Sabatine MS, Resnic FS. Med Decis Making. 2007. Submitted A Free sample background from www. powerpointbackgrounds. com
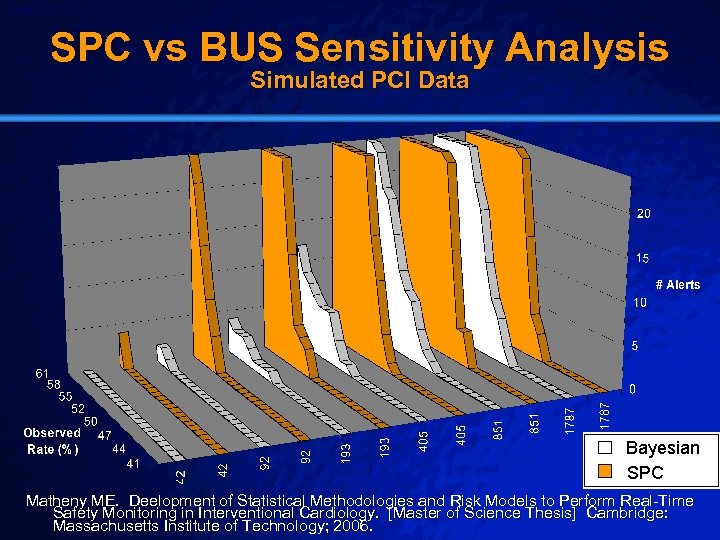
Slide 15 © 2003 By Default! SPC vs BUS Sensitivity Analysis Simulated PCI Data Matheny ME. Deelopment of Statistical Methodologies and Risk Models to Perform Real-Time Safety Monitoring in Interventional Cardiology. [Master of Science Thesis] Cambridge: Massachusetts Institute of Technology; 2006. A Free sample background from www. powerpointbackgrounds. com
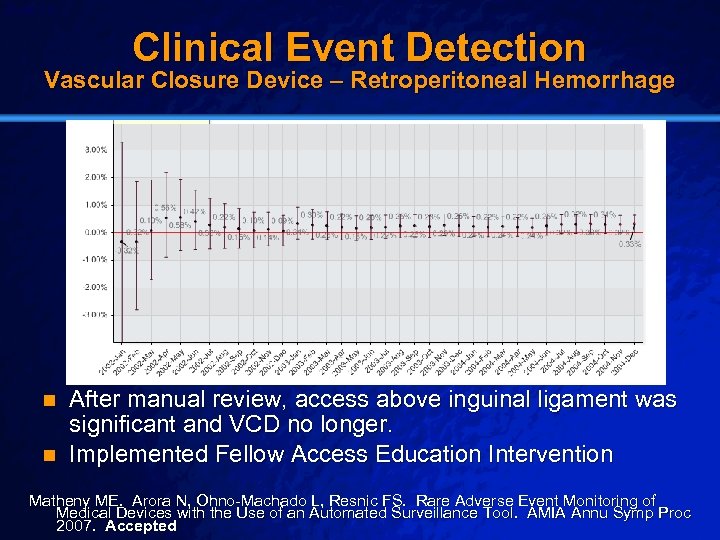
Slide 16 © 2003 By Default! Clinical Event Detection Vascular Closure Device – Retroperitoneal Hemorrhage After manual review, access above inguinal ligament was significant and VCD no longer. n Implemented Fellow Access Education Intervention n Matheny ME. Arora N, Ohno-Machado L, Resnic FS. Rare Adverse Event Monitoring of Medical Devices with the Use of an Automated Surveillance Tool. AMIA Annu Symp Proc 2007. Accepted A Free sample background from www. powerpointbackgrounds. com
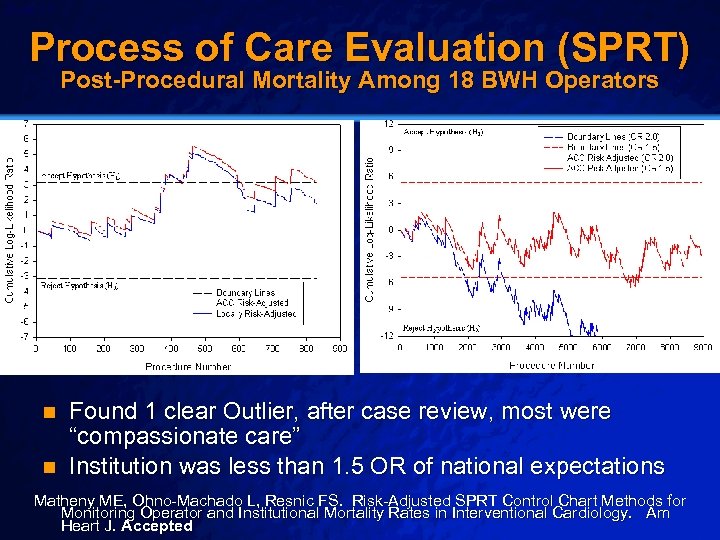
Slide 17 © 2003 By Default! Process of Care Evaluation (SPRT) Post-Procedural Mortality Among 18 BWH Operators Found 1 clear Outlier, after case review, most were “compassionate care” n Institution was less than 1. 5 OR of national expectations n Matheny ME, Ohno-Machado L, Resnic FS. Risk-Adjusted SPRT Control Chart Methods for Monitoring Operator and Institutional Mortality Rates in Interventional Cardiology. Am Heart J. Accepted A Free sample background from www. powerpointbackgrounds. com
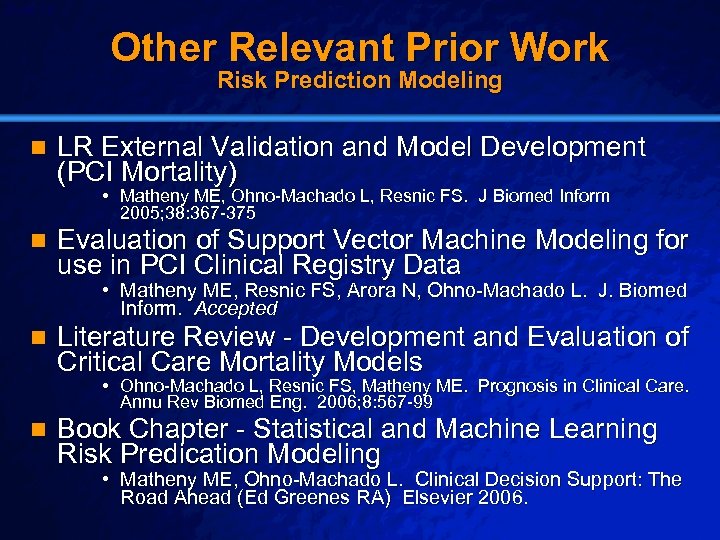
Slide 18 © 2003 By Default! Other Relevant Prior Work Risk Prediction Modeling n LR External Validation and Model Development (PCI Mortality) • Matheny ME, Ohno-Machado L, Resnic FS. J Biomed Inform 2005; 38: 367 -375 n Evaluation of Support Vector Machine Modeling for use in PCI Clinical Registry Data • Matheny ME, Resnic FS, Arora N, Ohno-Machado L. J. Biomed Inform. Accepted n Literature Review - Development and Evaluation of Critical Care Mortality Models • Ohno-Machado L, Resnic FS, Matheny ME. Prognosis in Clinical Care. Annu Rev Biomed Eng. 2006; 8: 567 -99 n Book Chapter - Statistical and Machine Learning Risk Predication Modeling • Matheny ME, Ohno-Machado L. Clinical Decision Support: The Road Ahead (Ed Greenes RA) Elsevier 2006. A Free sample background from www. powerpointbackgrounds. com

Slide 19 © 2003 By Default! Proposed Work A Free sample background from www. powerpointbackgrounds. com
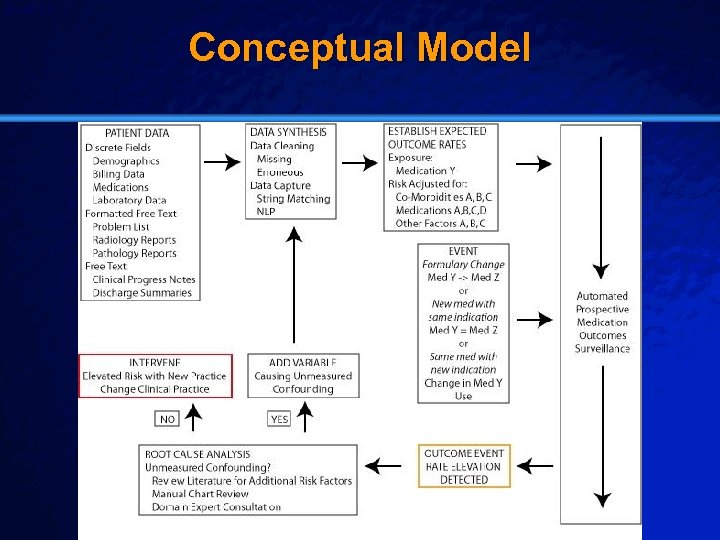
Slide 20 © 2003 By Default! Conceptual Model A Free sample background from www. powerpointbackgrounds. com

Slide 21 © 2003 By Default! Validation of an Automated Data Collection System for Acute Kidney Injury n Hypotheses: – Clinical data that does not require free text NLP, such as medication orders and laboratory tests, will achieve at least 85% specificity and sensitivity. – Clinical data that requires free text NLP, such as clinical diagnoses, will achieve at least 75% specificity and sensitivity. A Free sample background from www. powerpointbackgrounds. com

Slide 22 © 2003 By Default! Development of ADCS Data Sources n All records in 2005 -2006 for patients hospitalized in Nashville TVHS during 2006 A Free sample background from www. powerpointbackgrounds. com
Slide 23 © 2003 By Default! Data Element Identification n Literature Review of AKI Risk Factors – univariate and multivaraite risk factors n General Patient Demographics n Manual chart review from randomly selected Nashville TVHS Patients hospitalized during 2006 to identify locations and data quality for each identified data element A Free sample background from www. powerpointbackgrounds. com

Slide 24 © 2003 By Default! Data Element Selection n Complete capture of all relevant variables may be infeasible from routine clinical data. n Review of data element list with location and quality data with mentorship team n Elements will be selected based on a combination of – strength of association with AKI – data collection quality – algorithm complexity required for data extraction A Free sample background from www. powerpointbackgrounds. com
Slide 25 © 2003 By Default! Clinical Data Rule Development n Criteria must be developed for each clinical diagnosis n Example: status post nephrectomy n Example: systemic inflammatory response syndrome – Identification: Direct documentation – Possible locations: operative note, problem list, outpatient progress note, inpatient discharge summary, or CT scan of the abdomen. – Requires: free text processing of multiple sources, could use Perl word matching instead of concept-based indexing – – – Identification: Direct documentation or by clinical criteria Clinical Criteria: abnormal HR, Temp, RR or sp. O 2, or WBC. Locations of direct mention: problem list, inpatient progress note, inpatient discharge summary – Requires: use of inpatient lab and vital sign data AND/OR processed free text notes A Free sample background from www. powerpointbackgrounds. com
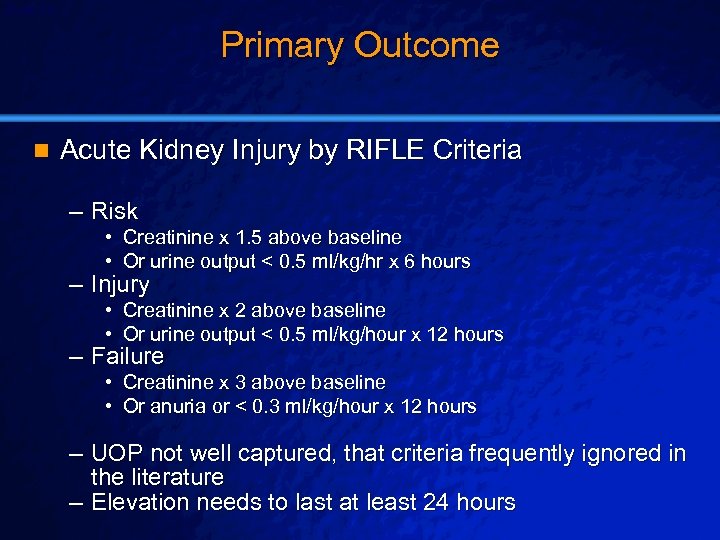
Slide 26 © 2003 By Default! Primary Outcome n Acute Kidney Injury by RIFLE Criteria – Risk • Creatinine x 1. 5 above baseline • Or urine output < 0. 5 ml/kg/hr x 6 hours – Injury • Creatinine x 2 above baseline • Or urine output < 0. 5 ml/kg/hour x 12 hours – Failure • Creatinine x 3 above baseline • Or anuria or < 0. 3 ml/kg/hour x 12 hours – UOP not well captured, that criteria frequently ignored in the literature – Elevation needs to last at least 24 hours A Free sample background from www. powerpointbackgrounds. com
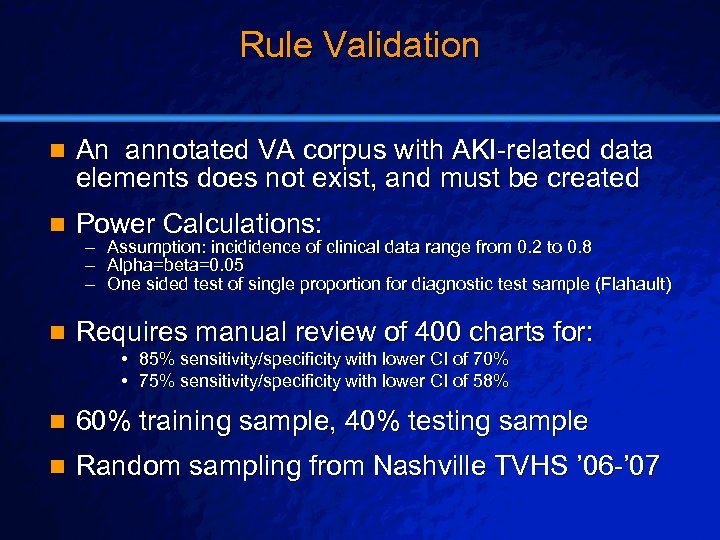
Slide 27 © 2003 By Default! Rule Validation n An annotated VA corpus with AKI-related data elements does not exist, and must be created n Power Calculations: n Requires manual review of 400 charts for: – – – Assumption: incididence of clinical data range from 0. 2 to 0. 8 Alpha=beta=0. 05 One sided test of single proportion for diagnostic test sample (Flahault) • 85% sensitivity/specificity with lower CI of 70% • 75% sensitivity/specificity with lower CI of 58% n 60% training sample, 40% testing sample n Random sampling from Nashville TVHS ’ 06 -’ 07 A Free sample background from www. powerpointbackgrounds. com
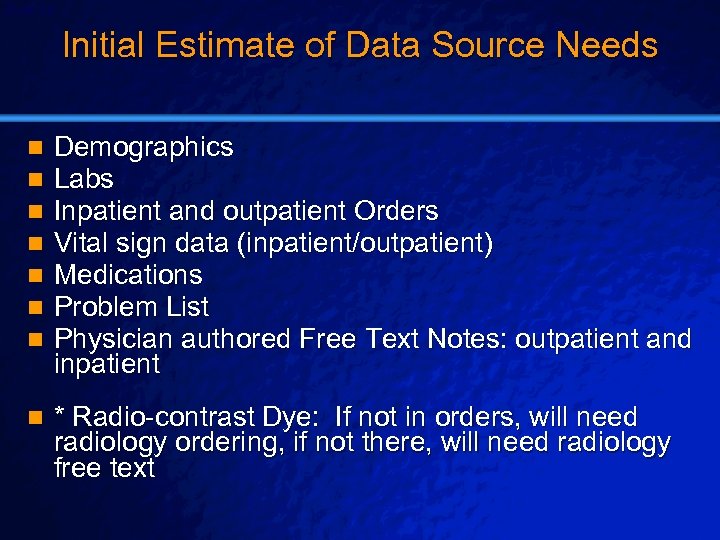
Slide 28 © 2003 By Default! Initial Estimate of Data Source Needs n n n n Demographics Labs Inpatient and outpatient Orders Vital sign data (inpatient/outpatient) Medications Problem List Physician authored Free Text Notes: outpatient and inpatient n * Radio-contrast Dye: If not in orders, will need radiology ordering, if not there, will need radiology free text A Free sample background from www. powerpointbackgrounds. com
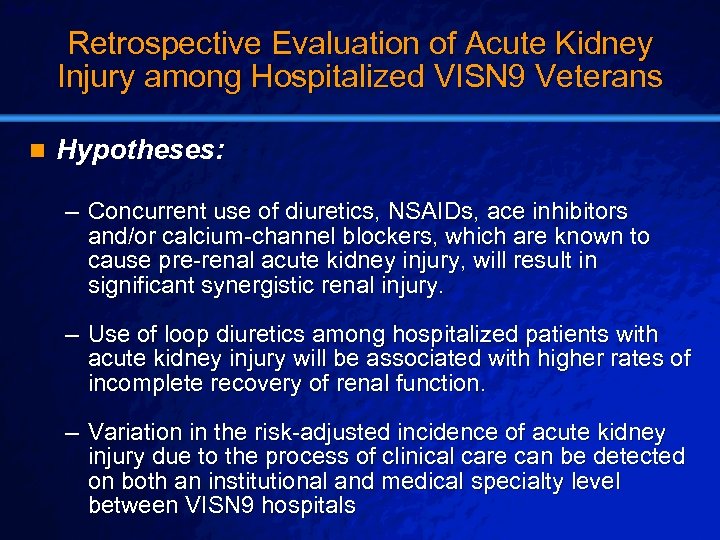
Slide 29 © 2003 By Default! Retrospective Evaluation of Acute Kidney Injury among Hospitalized VISN 9 Veterans n Hypotheses: – Concurrent use of diuretics, NSAIDs, ace inhibitors and/or calcium-channel blockers, which are known to cause pre-renal acute kidney injury, will result in significant synergistic renal injury. – Use of loop diuretics among hospitalized patients with acute kidney injury will be associated with higher rates of incomplete recovery of renal function. – Variation in the risk-adjusted incidence of acute kidney injury due to the process of clinical care can be detected on both an institutional and medical specialty level between VISN 9 hospitals A Free sample background from www. powerpointbackgrounds. com

Slide 30 © 2003 By Default! Retrospective Evaluation Data Sources n Retrospective Patient population: All VISN 9 patients hospitalized during 2006 – 2008 n Will need 2005 (maybe 2004) patient data for past medical history and baseline creatinines in order to analyze hospitalizations starting in 2006 A Free sample background from www. powerpointbackgrounds. com
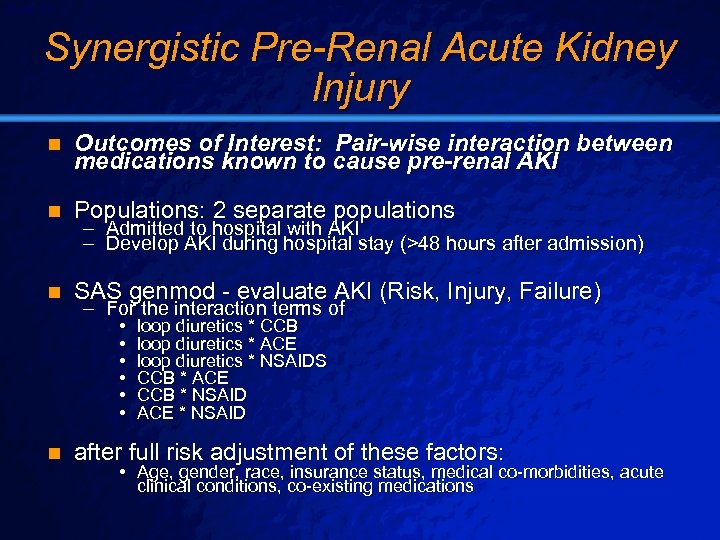
Slide 31 © 2003 By Default! Synergistic Pre-Renal Acute Kidney Injury n Outcomes of Interest: Pair-wise interaction between medications known to cause pre-renal AKI n Populations: 2 separate populations n SAS genmod - evaluate AKI (Risk, Injury, Failure) – Admitted to hospital with AKI – Develop AKI during hospital stay (>48 hours after admission) – For the interaction terms of • • • n loop diuretics * CCB loop diuretics * ACE loop diuretics * NSAIDS CCB * ACE CCB * NSAID ACE * NSAID after full risk adjustment of these factors: • Age, gender, race, insurance status, medical co-morbidities, acute clinical conditions, co-existing medications A Free sample background from www. powerpointbackgrounds. com
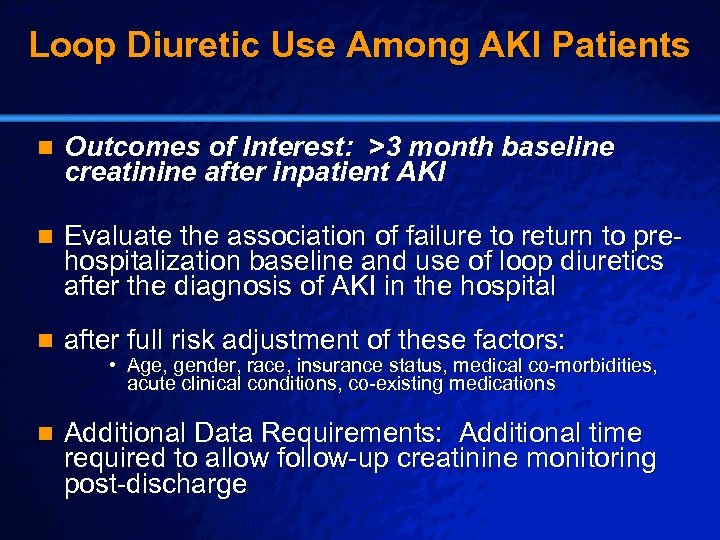
Slide 32 © 2003 By Default! Loop Diuretic Use Among AKI Patients n Outcomes of Interest: >3 month baseline creatinine after inpatient AKI n Evaluate the association of failure to return to prehospitalization baseline and use of loop diuretics after the diagnosis of AKI in the hospital n after full risk adjustment of these factors: n Additional Data Requirements: Additional time required to allow follow-up creatinine monitoring post-discharge • Age, gender, race, insurance status, medical co-morbidities, acute clinical conditions, co-existing medications A Free sample background from www. powerpointbackgrounds. com

Slide 33 © 2003 By Default! Evaluation of Variations in Clinical Care n Develop multivariable risk prediction model for development of AKI among VISN 9 patients (separate manuscript) n Evaluate risk-adjusted incidence of AKI by institution and clinical specialty to determine if outliers exist using VISN 9 (overall) data as the baseline (uses above risk prediction model). A Free sample background from www. powerpointbackgrounds. com

Slide 34 © 2003 By Default! Retrospective Evaluation of Acute Kidney Injury among Hospitalized VISN 9 Veterans n Knowledge Discovery (not hypothesis driven): – Characterize the risk for acute kidney injury with the use of the following medications: • • • NSAIDs ACE Inhibitors ARBs Radiocontrast Dye Aminoglycosides Amphotericin B – after full risk adjustment of these factors: • Age, gender, race, insurance status, medical co-morbidities, acute clinical conditions, co-existing medications A Free sample background from www. powerpointbackgrounds. com

Slide 35 © 2003 By Default! Pilot Evaluation of an Inpatient Acute Kidney Injury Surveillance System n Hypotheses – The methodology and application will detect a simulated AKI event rate elevation among retrospective routinely collected clinical data. – A prospective automated outcomes surveillance system evaluating acute kidney injury among hospitalized veterans will allow discovery of medication-related nephrotoxicity when pharmacy formularies are changed or new medications are introduced. A Free sample background from www. powerpointbackgrounds. com

Slide 36 © 2003 By Default! Surveillance System Simulated AKI event rate elevation n Use the retrospective ’ 06 -’ 08 VISN 9 data, and simulate an AKI event rate elevation in a medication not known to be nephrotoxic with a simulated outbreak algorithm frequently used in biosurveillance – Allows a sensitivity analysis to detect how significant the elevation must be (how many cases in what period of time) before the system will alert. – Good prelim data for a merit application A Free sample background from www. powerpointbackgrounds. com

Slide 37 © 2003 By Default! Prospective Pilot of an Inpatient Acute Kidney Injury Surveillance System n Having some trouble with this hypothesis. n What I really want to do is to setup the prospective system after doing the simulated detection, but there’s no way to know what, if anything, it will detect as time goes no. n How to frame that? A Free sample background from www. powerpointbackgrounds. com

Slide 38 © 2003 By Default! Training Plans Year 1 n Formal Classes n Seminar n Ongoing Funded Grants n Conferences n Journal Club – Database Development – Perl course / regular expression parsing – updates in acute kidney injury – POEM (Nashville, TN) - Attend grant meetings dealing with clinical concept tagging project – DELTA-MASSDAQ (Boston, MA) – Attend grant meetings for statistical and application development – – – AMIA NKF VA HSR&D A Free sample background from www. powerpointbackgrounds. com

Slide 39 © 2003 By Default! Training Plans Year 2 n Formal Classes n Ongoing Funded Grants n Conferences n Journal Club – Statistical Process Control – Methods for Confounding Adjustment in Prospective Cohorts – POEM (Nashville, TN) - Attend grant meetings dealing with clinical concept tagging project – DELTA-MASSDAQ (Boston, MA) – Attend grant meetings for statistical and application development – – – AMIA NKF VA HSR&D A Free sample background from www. powerpointbackgrounds. com

Slide 40 © 2003 By Default! Training Plans Year 3 n Seminar – Grant Writing n Conferences – – – AMIA NKF VA HSR&D n Journal Club A Free sample background from www. powerpointbackgrounds. com

Slide 41 © 2003 By Default! Training Plans Year 4 n Conferences – AMIA – NKF – VA HSR&D n Journal Club n Submit a HSR&D IIR Merit Grant Application A Free sample background from www. powerpointbackgrounds. com

Slide 42 © 2003 By Default! The End Michael Matheny, MD MS MPH michael. matheny@vanderbilt. edu Tennessee Valley Medical Center - Nashville Geriatric Research, Education and Clinical Care Room 4 -B 110 1310 24 th Ave. S. Nashville, TN 37212 A Free sample background from www. powerpointbackgrounds. com
385578a87b7a6c3bf0480fddc8e50763.ppt