8-Silicates.ppt
- Количество слайдов: 147
 Silicate Minerals Modified from curse GLY 4310, Spring 2012 1
Silicate Minerals Modified from curse GLY 4310, Spring 2012 1
 Silicates u u Silicates are composed of silicon-oxygen tetrahedrons, an arrangement which contains four oxygen atoms surrounding a silicon atom (Si. O 4 -4). Silicates are often divided into two major groups: ferromagnesian silicates and non -ferromagnesian silicates u Ferromagnesian silicates contain iron or magnesium ions joined to the silicate structure. They are darker and have a heavier specific gravity than nonferromagnesian silicate minerals. u Ferromagnesians include minerals such as olivine, pyroxene, hornblende, and biotite u Non-ferromagnesians include muscovite, feldspar, and quartz Silicates comprise the majority of minerals in the Earth’s crust and upper mantle. Over 25% of all minerals are included in this group, with over 40% of those accounting for the most common and abundant minerals. Feldspar, Quartz, Biotite, and Amphibole are the most common silicates Quartz Copyright©Stonetrust, Inc. 2 Table of Contents
Silicates u u Silicates are composed of silicon-oxygen tetrahedrons, an arrangement which contains four oxygen atoms surrounding a silicon atom (Si. O 4 -4). Silicates are often divided into two major groups: ferromagnesian silicates and non -ferromagnesian silicates u Ferromagnesian silicates contain iron or magnesium ions joined to the silicate structure. They are darker and have a heavier specific gravity than nonferromagnesian silicate minerals. u Ferromagnesians include minerals such as olivine, pyroxene, hornblende, and biotite u Non-ferromagnesians include muscovite, feldspar, and quartz Silicates comprise the majority of minerals in the Earth’s crust and upper mantle. Over 25% of all minerals are included in this group, with over 40% of those accounting for the most common and abundant minerals. Feldspar, Quartz, Biotite, and Amphibole are the most common silicates Quartz Copyright©Stonetrust, Inc. 2 Table of Contents
 Crustal Chemistry • The earth’s crust is composed of three common elements, on an atom percent basis § Oxygen, 62. 5% § Silicon, 21. 2% § Aluminum, 6. 47% • Silicates are the most common minerals on the planet • They are called “rock-forming” minerals for this reason 3
Crustal Chemistry • The earth’s crust is composed of three common elements, on an atom percent basis § Oxygen, 62. 5% § Silicon, 21. 2% § Aluminum, 6. 47% • Silicates are the most common minerals on the planet • They are called “rock-forming” minerals for this reason 3
 Other Common Cations • Metal cations also contribute to minerals • On an atom % basis: § § § Sodium, 2. 64 Calcium, 1. 94 Iron, 1. 92 Magnesium, 1. 84 Potassium, 1. 42 4
Other Common Cations • Metal cations also contribute to minerals • On an atom % basis: § § § Sodium, 2. 64 Calcium, 1. 94 Iron, 1. 92 Magnesium, 1. 84 Potassium, 1. 42 4
 Types of Silicate Minerals in the Earth’s Crust • Silicates make up 92% of the crust § § § § Plagioclase, 39% Alkali feldspar, 12% Quartz, 12% Pyroxene, 11% Amphiboles, 5% Micas, 5% Clays, 5% Other silicates, 3% 5
Types of Silicate Minerals in the Earth’s Crust • Silicates make up 92% of the crust § § § § Plagioclase, 39% Alkali feldspar, 12% Quartz, 12% Pyroxene, 11% Amphiboles, 5% Micas, 5% Clays, 5% Other silicates, 3% 5
 Whole Earth • When the mantle and core are included, the compositional picture changes • Olivine is the main constituent of the upper mantle, and may be the most common mineral on earth • The lower mantle is composed of other silicates • The core is believed to be an Fe-Ni mix 6
Whole Earth • When the mantle and core are included, the compositional picture changes • Olivine is the main constituent of the upper mantle, and may be the most common mineral on earth • The lower mantle is composed of other silicates • The core is believed to be an Fe-Ni mix 6
 Silicate Nomenclature Silicate Subclass Alternative Name Neso – (or Ortho) Silicates Sorosilicates Island Cyclosilicates Ring Inosilicates Chain Phyllosilicates Layer Couplet Tectosilicates (or Tekto Framework -) 7
Silicate Nomenclature Silicate Subclass Alternative Name Neso – (or Ortho) Silicates Sorosilicates Island Cyclosilicates Ring Inosilicates Chain Phyllosilicates Layer Couplet Tectosilicates (or Tekto Framework -) 7
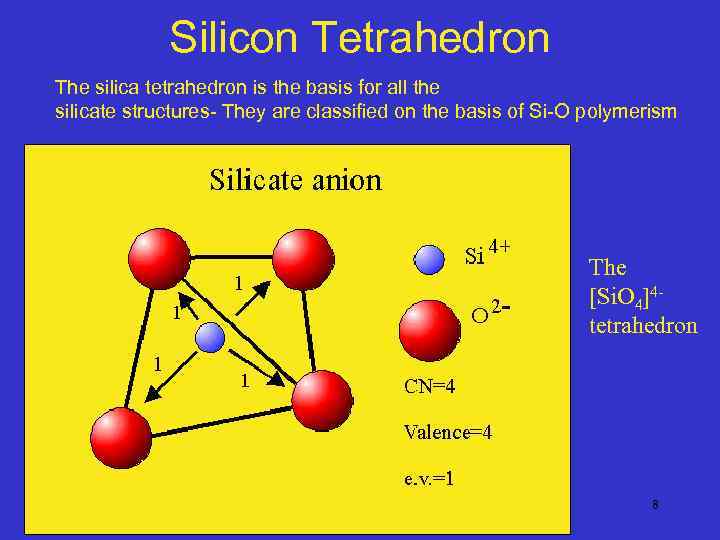 Silicon Tetrahedron The silica tetrahedron is the basis for all the silicate structures- They are classified on the basis of Si-O polymerism The [Si. O 4]4 tetrahedron 8
Silicon Tetrahedron The silica tetrahedron is the basis for all the silicate structures- They are classified on the basis of Si-O polymerism The [Si. O 4]4 tetrahedron 8
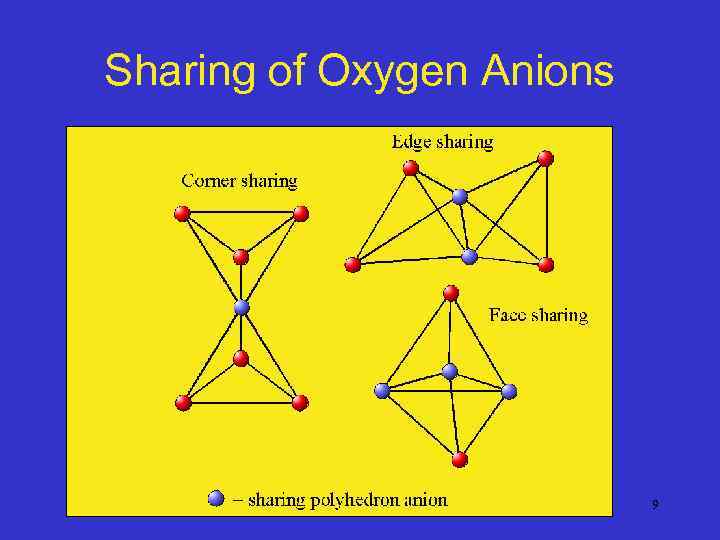 Sharing of Oxygen Anions 9
Sharing of Oxygen Anions 9
![Mineral Structures Silicates are classified on the basis of Si-O polymerism [Si. O 4]4 Mineral Structures Silicates are classified on the basis of Si-O polymerism [Si. O 4]4](https://present5.com/presentation/68703897_135084041/image-10.jpg) Mineral Structures Silicates are classified on the basis of Si-O polymerism [Si. O 4]4 - Independent tetrahedra Examples: olivine [Si 2 O 7]6 - Nesosilicates garnet Double tetrahedra Sorosilicates Examples: lawsonite n[Si. O 3]2 - n = 3, 4, 6 Cyclosilicates Examples: benitoite Ba. Ti[Si 3 O 9] axinite Ca 3 Al 2 BO 3[Si 4 O 12]OH beryl Be 3 Al 2[Si 6 O 18]
Mineral Structures Silicates are classified on the basis of Si-O polymerism [Si. O 4]4 - Independent tetrahedra Examples: olivine [Si 2 O 7]6 - Nesosilicates garnet Double tetrahedra Sorosilicates Examples: lawsonite n[Si. O 3]2 - n = 3, 4, 6 Cyclosilicates Examples: benitoite Ba. Ti[Si 3 O 9] axinite Ca 3 Al 2 BO 3[Si 4 O 12]OH beryl Be 3 Al 2[Si 6 O 18]
![Mineral Structures Silicates are classified on the basis of Si-O polymerism [Si. O 3]2 Mineral Structures Silicates are classified on the basis of Si-O polymerism [Si. O 3]2](https://present5.com/presentation/68703897_135084041/image-11.jpg) Mineral Structures Silicates are classified on the basis of Si-O polymerism [Si. O 3]2 - single chains tetrahedra pryoxenes pyroxenoids Inosilicates [Si 4 O 11]4 - Double amphiboles
Mineral Structures Silicates are classified on the basis of Si-O polymerism [Si. O 3]2 - single chains tetrahedra pryoxenes pyroxenoids Inosilicates [Si 4 O 11]4 - Double amphiboles
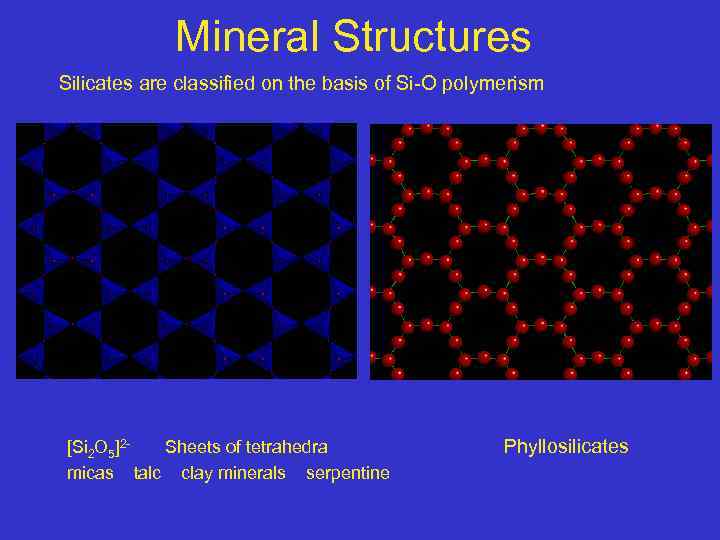 Mineral Structures Silicates are classified on the basis of Si-O polymerism [Si 2 O 5]2 Sheets of tetrahedra micas talc clay minerals serpentine Phyllosilicates
Mineral Structures Silicates are classified on the basis of Si-O polymerism [Si 2 O 5]2 Sheets of tetrahedra micas talc clay minerals serpentine Phyllosilicates
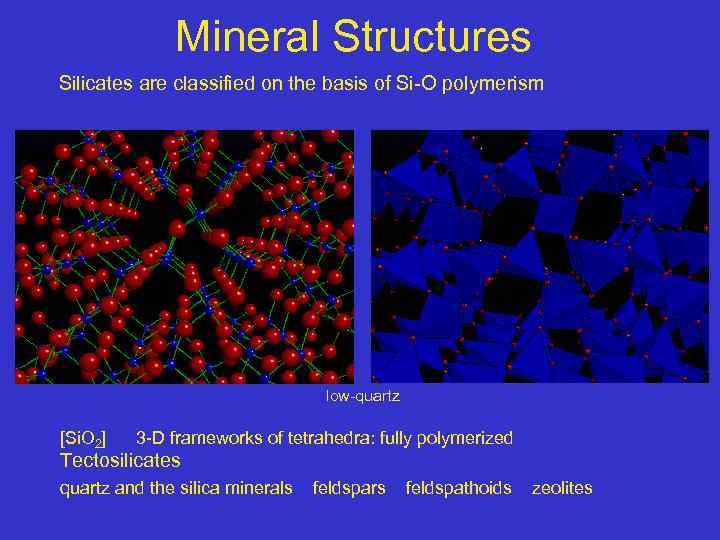 Mineral Structures Silicates are classified on the basis of Si-O polymerism low-quartz [Si. O 2] 3 -D frameworks of tetrahedra: fully polymerized Tectosilicates quartz and the silica minerals feldspars feldspathoids zeolites
Mineral Structures Silicates are classified on the basis of Si-O polymerism low-quartz [Si. O 2] 3 -D frameworks of tetrahedra: fully polymerized Tectosilicates quartz and the silica minerals feldspars feldspathoids zeolites
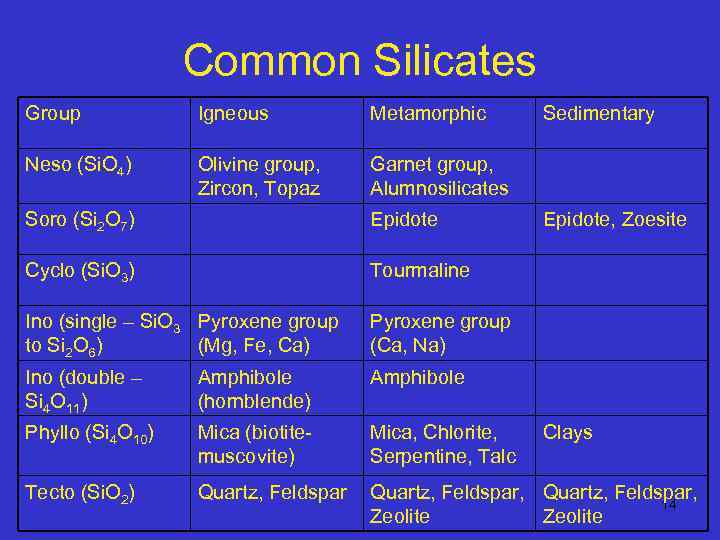 Common Silicates Group Igneous Metamorphic Neso (Si. O 4) Olivine group, Zircon, Topaz Sedimentary Garnet group, Alumnosilicates Soro (Si 2 O 7) Epidote, Zoesite Cyclo (Si. O 3) Tourmaline Ino (single – Si. O 3 Pyroxene group to Si 2 O 6) (Mg, Fe, Ca) Pyroxene group (Ca, Na) Ino (double – Si 4 O 11) Amphibole (hornblende) Amphibole Phyllo (Si 4 O 10) Mica (biotitemuscovite) Mica, Chlorite, Serpentine, Talc Tecto (Si. O 2) Quartz, Feldspar, 14 Zeolite Clays
Common Silicates Group Igneous Metamorphic Neso (Si. O 4) Olivine group, Zircon, Topaz Sedimentary Garnet group, Alumnosilicates Soro (Si 2 O 7) Epidote, Zoesite Cyclo (Si. O 3) Tourmaline Ino (single – Si. O 3 Pyroxene group to Si 2 O 6) (Mg, Fe, Ca) Pyroxene group (Ca, Na) Ino (double – Si 4 O 11) Amphibole (hornblende) Amphibole Phyllo (Si 4 O 10) Mica (biotitemuscovite) Mica, Chlorite, Serpentine, Talc Tecto (Si. O 2) Quartz, Feldspar, 14 Zeolite Clays
 Structural Formulas and Silicates • The key to understanding silicate mineral groups, solid solution, and miscibility • Symbology – W = large cations, C. N. >6 (with oxygen) • Ca, Na, K – X = medium-sized, bivalent cations, C. N. = 6 (with oxygen) • Mg, Fe+2 , & Ca (sort of) – Y = medium-sized, trivalent cation, C. N. = 6 (with oxygen) • Typically Al and sometimes Fe+3 – Z = small cations, C. N. = 4 (with oxygen) • Mainly Si+4, but also Al+3 15
Structural Formulas and Silicates • The key to understanding silicate mineral groups, solid solution, and miscibility • Symbology – W = large cations, C. N. >6 (with oxygen) • Ca, Na, K – X = medium-sized, bivalent cations, C. N. = 6 (with oxygen) • Mg, Fe+2 , & Ca (sort of) – Y = medium-sized, trivalent cation, C. N. = 6 (with oxygen) • Typically Al and sometimes Fe+3 – Z = small cations, C. N. = 4 (with oxygen) • Mainly Si+4, but also Al+3 15
![Structural Formulas • Olivine Group – Nesosilicates [Si. O 4] – X 2 Si. Structural Formulas • Olivine Group – Nesosilicates [Si. O 4] – X 2 Si.](https://present5.com/presentation/68703897_135084041/image-16.jpg) Structural Formulas • Olivine Group – Nesosilicates [Si. O 4] – X 2 Si. O 4 – X = Mg, Fe • Pyroxene Group – Single chain inosilicates [Si. O 3 or Si 2 O 6] – X 2 Si. O 3 to (W, X, Y)2 Z 2 O 6 • Feldspar Group – Tecto (framework) silicates [Si. O 2] – WZ 4 O 8 • Garnet Group – – Nesosilicates [Si. O 4] X 3 Y 2 (Si. O 4)3 X = Ca, Mn, Fe, Mg Y = Fe+3, Cr • Amphibole Group – Double chain inosilicates [Si 8 O 22] – W 0 -1 X 2 Y 5(Z 8 O 22)(OH, F)2 • Mica Group – Phylo (sheet) silicates [Si 4 O 10] – W(X, Y)2 -3(Z 4 O 10)(OH, F)2 16
Structural Formulas • Olivine Group – Nesosilicates [Si. O 4] – X 2 Si. O 4 – X = Mg, Fe • Pyroxene Group – Single chain inosilicates [Si. O 3 or Si 2 O 6] – X 2 Si. O 3 to (W, X, Y)2 Z 2 O 6 • Feldspar Group – Tecto (framework) silicates [Si. O 2] – WZ 4 O 8 • Garnet Group – – Nesosilicates [Si. O 4] X 3 Y 2 (Si. O 4)3 X = Ca, Mn, Fe, Mg Y = Fe+3, Cr • Amphibole Group – Double chain inosilicates [Si 8 O 22] – W 0 -1 X 2 Y 5(Z 8 O 22)(OH, F)2 • Mica Group – Phylo (sheet) silicates [Si 4 O 10] – W(X, Y)2 -3(Z 4 O 10)(OH, F)2 16
 Nesosilicates • Characterized by independent Si 04 tetrahedra, which are not linked together directly • They are bonded together by ionic bonds to interstitial cations • The structures of the nesosilicates are therefore, very dependent on the size and charge of the interstitial cations • Because the tetrahedral do not share oxygen, the Si: 0 ratio is 1: 4. 17
Nesosilicates • Characterized by independent Si 04 tetrahedra, which are not linked together directly • They are bonded together by ionic bonds to interstitial cations • The structures of the nesosilicates are therefore, very dependent on the size and charge of the interstitial cations • Because the tetrahedral do not share oxygen, the Si: 0 ratio is 1: 4. 17
 Interstitial Cations • Since the Si. O 4 tetrahedron has a charge of 4, two divalent cations, a trivalent and a monovalent, or a quadravalent cation are required to maintain electrical neutrality • Several structure types are possible – in the silicate structures the letter A = non-silicon cations with lower valency then Si 4+ , B = Si or Al or other higher valency cations, O = oxygen 18
Interstitial Cations • Since the Si. O 4 tetrahedron has a charge of 4, two divalent cations, a trivalent and a monovalent, or a quadravalent cation are required to maintain electrical neutrality • Several structure types are possible – in the silicate structures the letter A = non-silicon cations with lower valency then Si 4+ , B = Si or Al or other higher valency cations, O = oxygen 18
 A 2 Si. O 4 • This group includes the olivine series • Structure is based on an nearly HCP arrangement of the O 2 - ions • A ions are in octahedral voids • B ion in a tetrahedral void • ½ of the octahedral voids are occupied, 1/8 of the tetrahedral voids are occupied 19
A 2 Si. O 4 • This group includes the olivine series • Structure is based on an nearly HCP arrangement of the O 2 - ions • A ions are in octahedral voids • B ion in a tetrahedral void • ½ of the octahedral voids are occupied, 1/8 of the tetrahedral voids are occupied 19
 Olivine Series • Olivine itself is the compound (Fe, Mg)2 Si 04 with a complete solid solution series § As with other solid solution series the two end members are the most important § Fayalite – Fe 2 Si 04 Fa § Forsterite – Mg 2 Si 04 Fo 20
Olivine Series • Olivine itself is the compound (Fe, Mg)2 Si 04 with a complete solid solution series § As with other solid solution series the two end members are the most important § Fayalite – Fe 2 Si 04 Fa § Forsterite – Mg 2 Si 04 Fo 20
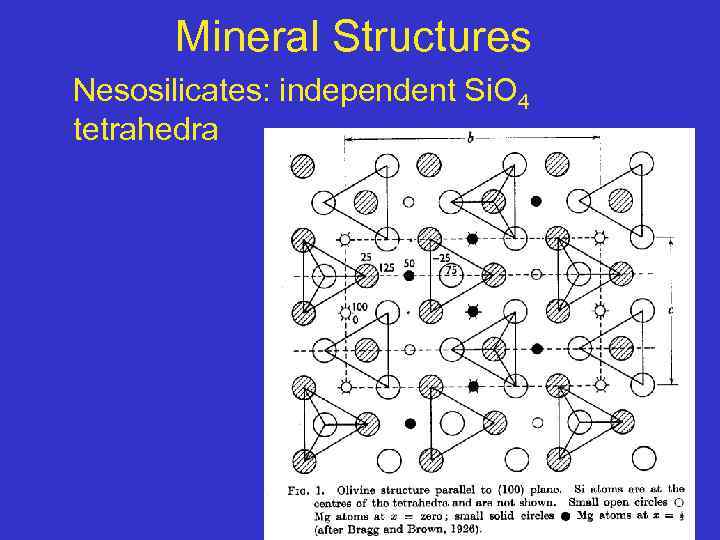 Mineral Structures Nesosilicates: independent Si. O 4 tetrahedra
Mineral Structures Nesosilicates: independent Si. O 4 tetrahedra
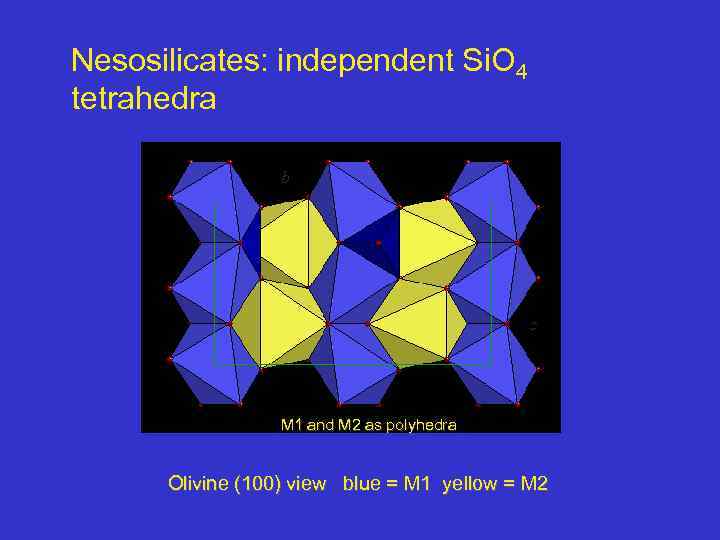 Nesosilicates: independent Si. O 4 tetrahedra b c M 1 and M 2 as polyhedra Olivine (100) view blue = M 1 yellow = M 2
Nesosilicates: independent Si. O 4 tetrahedra b c M 1 and M 2 as polyhedra Olivine (100) view blue = M 1 yellow = M 2
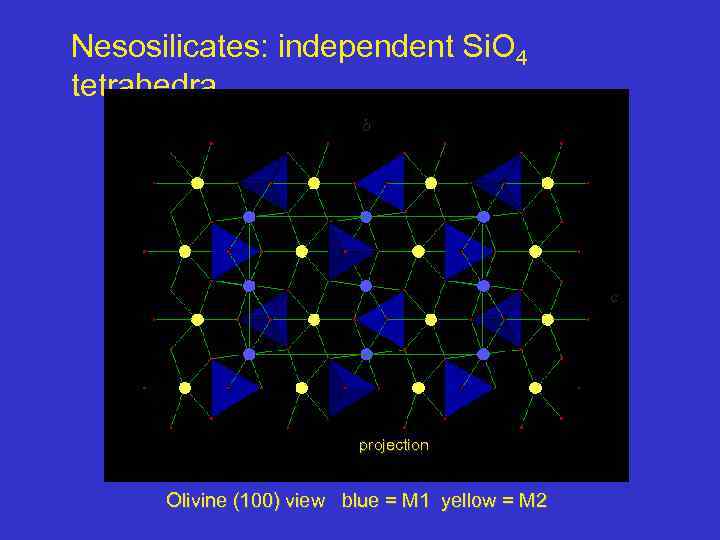 Nesosilicates: independent Si. O 4 tetrahedra b c projection Olivine (100) view blue = M 1 yellow = M 2
Nesosilicates: independent Si. O 4 tetrahedra b c projection Olivine (100) view blue = M 1 yellow = M 2
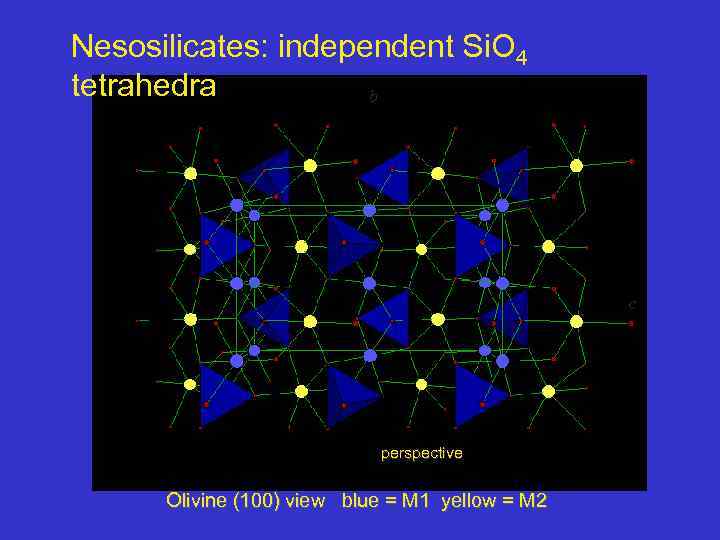 Nesosilicates: independent Si. O 4 tetrahedra b c perspective Olivine (100) view blue = M 1 yellow = M 2
Nesosilicates: independent Si. O 4 tetrahedra b c perspective Olivine (100) view blue = M 1 yellow = M 2
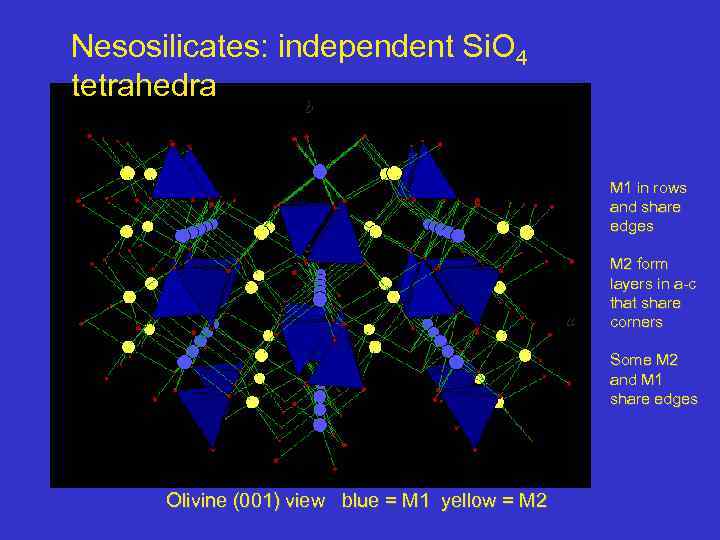 Nesosilicates: independent Si. O 4 tetrahedra b M 1 in rows and share edges a M 2 form layers in a-c that share corners Some M 2 and M 1 share edges Olivine (001) view blue = M 1 yellow = M 2
Nesosilicates: independent Si. O 4 tetrahedra b M 1 in rows and share edges a M 2 form layers in a-c that share corners Some M 2 and M 1 share edges Olivine (001) view blue = M 1 yellow = M 2
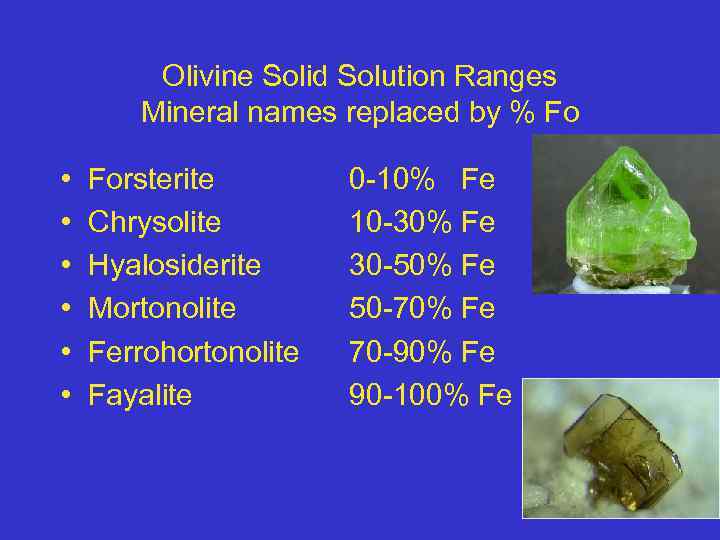 Olivine Solid Solution Ranges Mineral names replaced by % Fo • • • Forsterite Chrysolite Hyalosiderite Mortonolite Ferrohortonolite Fayalite 0 -10% Fe 10 -30% Fe 30 -50% Fe 50 -70% Fe 70 -90% Fe 90 -100% Fe 26
Olivine Solid Solution Ranges Mineral names replaced by % Fo • • • Forsterite Chrysolite Hyalosiderite Mortonolite Ferrohortonolite Fayalite 0 -10% Fe 10 -30% Fe 30 -50% Fe 50 -70% Fe 70 -90% Fe 90 -100% Fe 26
 Solid Solution Nomenclature • As with some other important series an abbreviation is used for the end members – compositions can be expressed using abbreviated symbols • Example Fe 0. 6 Mg 1. 4 Si 04 = Fa 30 Fo 70 27
Solid Solution Nomenclature • As with some other important series an abbreviation is used for the end members – compositions can be expressed using abbreviated symbols • Example Fe 0. 6 Mg 1. 4 Si 04 = Fa 30 Fo 70 27
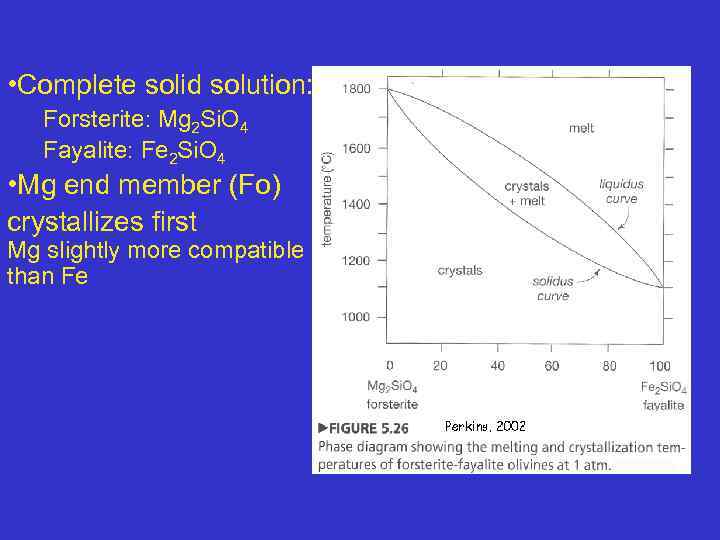 • Complete solid solution: Forsterite: Mg 2 Si. O 4 Fayalite: Fe 2 Si. O 4 • Mg end member (Fo) crystallizes first Mg slightly more compatible than Fe
• Complete solid solution: Forsterite: Mg 2 Si. O 4 Fayalite: Fe 2 Si. O 4 • Mg end member (Fo) crystallizes first Mg slightly more compatible than Fe
 Other Olivine Group Minerals (Fe, Mg) ---> (Ca, Mn) • • Ca. Mg. Si 04 Mn 2 Si 04 Ca. Mn. Si 04 Ca. Fe. Si 04 Monticellite Tephroite Glaucochroite Kirschsteinite 29
Other Olivine Group Minerals (Fe, Mg) ---> (Ca, Mn) • • Ca. Mg. Si 04 Mn 2 Si 04 Ca. Mn. Si 04 Ca. Fe. Si 04 Monticellite Tephroite Glaucochroite Kirschsteinite 29
 Nesosilicates: independent Si. O 4 tetrahedra Olivine Occurrences: – – – Principally in mafic and ultramafic igneous and meta-igneous rocks Fayalite in meta-ironstones and in some alkalic granitoids Forsterite in some siliceous dolomitic marbles Monticellite Ca. Mg. Si. O 4 Ca M 2 (larger ion, larger site) High grade metamorphic siliceous carbonates
Nesosilicates: independent Si. O 4 tetrahedra Olivine Occurrences: – – – Principally in mafic and ultramafic igneous and meta-igneous rocks Fayalite in meta-ironstones and in some alkalic granitoids Forsterite in some siliceous dolomitic marbles Monticellite Ca. Mg. Si. O 4 Ca M 2 (larger ion, larger site) High grade metamorphic siliceous carbonates
 ASi. O 4 • The most common mineral of this group is the mineral zircon, Zr. Si 04 • In zircon, the A ions are in distorted cubic coordination with 4 oxygens at one distance, 4 further away • Zircon always contains some Hf and sometimes Th or U (may be metamict) • Thorite, Th. Si 04, is isostructural but is often metamict because of radioactive decay 31
ASi. O 4 • The most common mineral of this group is the mineral zircon, Zr. Si 04 • In zircon, the A ions are in distorted cubic coordination with 4 oxygens at one distance, 4 further away • Zircon always contains some Hf and sometimes Th or U (may be metamict) • Thorite, Th. Si 04, is isostructural but is often metamict because of radioactive decay 31
 ASi. O 4 : zircon, an Accessory minerals in rocks • Commonly contains uranium and thorium • (and daughter product: lead) as minor • atomic substitution components • Highly useful for geochronology: • radiometric dating using the unstable • isotopes U & Th -> Pb • Also common as an accessory mineral • in metamorphic and sedimentary • (highly resistant) rocks
ASi. O 4 : zircon, an Accessory minerals in rocks • Commonly contains uranium and thorium • (and daughter product: lead) as minor • atomic substitution components • Highly useful for geochronology: • radiometric dating using the unstable • isotopes U & Th -> Pb • Also common as an accessory mineral • in metamorphic and sedimentary • (highly resistant) rocks
 Garnets, A 3 B 2(Si. O 4)3 • Larger A site is occupied by divalent cations (2+) which are relatively large, with a coordination number of VIII (octahedra) § Typical cations are Ca 2+, Mg 2+, Fe 2+, Mn 2+, and some trivalent lanthanides • The smaller B site is occupied by trivalent cations which are smaller, with a CN of VI (polyhedra) § Typical cations Al 3+, Cr 3+, Fe 3+, and Ti 4+ 33
Garnets, A 3 B 2(Si. O 4)3 • Larger A site is occupied by divalent cations (2+) which are relatively large, with a coordination number of VIII (octahedra) § Typical cations are Ca 2+, Mg 2+, Fe 2+, Mn 2+, and some trivalent lanthanides • The smaller B site is occupied by trivalent cations which are smaller, with a CN of VI (polyhedra) § Typical cations Al 3+, Cr 3+, Fe 3+, and Ti 4+ 33
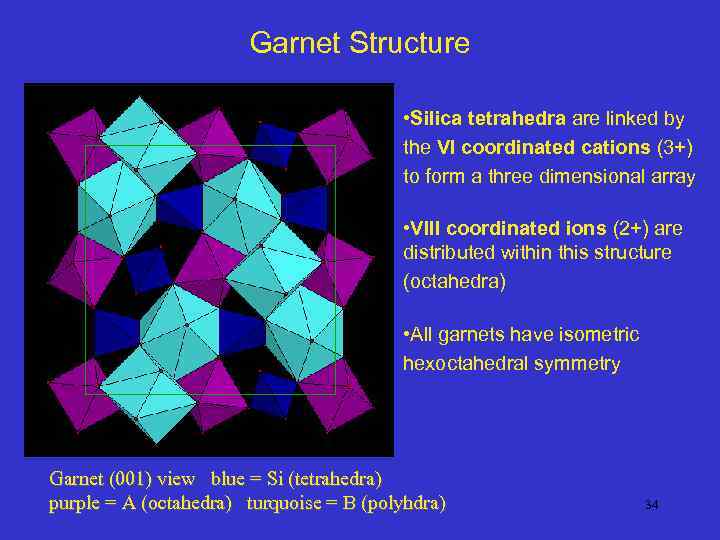 Garnet Structure • Silica tetrahedra are linked by the VI coordinated cations (3+) to form a three dimensional array • VIII coordinated ions (2+) are distributed within this structure (octahedra) • All garnets have isometric hexoctahedral symmetry Garnet (001) view blue = Si (tetrahedra) purple = A (octahedra) turquoise = B (polyhdra) 34
Garnet Structure • Silica tetrahedra are linked by the VI coordinated cations (3+) to form a three dimensional array • VIII coordinated ions (2+) are distributed within this structure (octahedra) • All garnets have isometric hexoctahedral symmetry Garnet (001) view blue = Si (tetrahedra) purple = A (octahedra) turquoise = B (polyhdra) 34
 Calcium and Noncalcium Garnets • Ca 2+ is larger than Mg 2+, Fe 2+ and Mn 2+ • Garnets can be split into two groups, the Ca ( Ugrandites) and non-Ca garnets • A similar division may be made for the B ions into Al (pyralspites) , Fe 3+ and Cr 3+ garnets. 35
Calcium and Noncalcium Garnets • Ca 2+ is larger than Mg 2+, Fe 2+ and Mn 2+ • Garnets can be split into two groups, the Ca ( Ugrandites) and non-Ca garnets • A similar division may be made for the B ions into Al (pyralspites) , Fe 3+ and Cr 3+ garnets. 35
![Ugrandites (A=Ca) and pyralspite (B=Al) Garnets Garnet: A 2+3 B 3+2 [Si. O 4]3 Ugrandites (A=Ca) and pyralspite (B=Al) Garnets Garnet: A 2+3 B 3+2 [Si. O 4]3](https://present5.com/presentation/68703897_135084041/image-36.jpg) Ugrandites (A=Ca) and pyralspite (B=Al) Garnets Garnet: A 2+3 B 3+2 [Si. O 4]3 “Pyralspites” - B = Al Pyrope: Mg 3 Al 2 [Si. O 4]3 Almandine: Fe 3 Al 2 [Si. O 4]3 Spessartine: Mn 3 Al 2 [Si. O 4]3 “Ugrandites” - A = Ca Uvarovite: Ca 3 Cr 2 [Si. O 4]3 Grossularite: Ca 3 Al 2 [Si. O 4]3 Andradite: Ca 3 Fe 2 [Si. O 4]3 a 2 a 1 a 3 Occurrence: Mostly metamorphic Some high-Al igneous Also in some mantle Garnet (001) view blue = Si purple = A turquoise = B peridotites 36
Ugrandites (A=Ca) and pyralspite (B=Al) Garnets Garnet: A 2+3 B 3+2 [Si. O 4]3 “Pyralspites” - B = Al Pyrope: Mg 3 Al 2 [Si. O 4]3 Almandine: Fe 3 Al 2 [Si. O 4]3 Spessartine: Mn 3 Al 2 [Si. O 4]3 “Ugrandites” - A = Ca Uvarovite: Ca 3 Cr 2 [Si. O 4]3 Grossularite: Ca 3 Al 2 [Si. O 4]3 Andradite: Ca 3 Fe 2 [Si. O 4]3 a 2 a 1 a 3 Occurrence: Mostly metamorphic Some high-Al igneous Also in some mantle Garnet (001) view blue = Si purple = A turquoise = B peridotites 36
 Ca Garnets Name Formula Color Uvarovite Ca 3 Cr 2(Si 04)3 Emerald green Grossularite, also called cinnamon stone, essonite Andradite Ca 3 Al 2(Si 04)3 White green, yellow, cinnamon brown, pale red Ca 3 Fe 2(Si 04)3 Yellow, green, brown, black 37
Ca Garnets Name Formula Color Uvarovite Ca 3 Cr 2(Si 04)3 Emerald green Grossularite, also called cinnamon stone, essonite Andradite Ca 3 Al 2(Si 04)3 White green, yellow, cinnamon brown, pale red Ca 3 Fe 2(Si 04)3 Yellow, green, brown, black 37
 Non-Ca Garnets Name Formula Color Pyrope Mg 3 Al 2(Si 04)3 Deep red to black Almandine Fe 3 Al 2(Si 04)3 Deep red to brown Spessartite Mn 3 Al 2(Si 04)3 Brownish to red 38
Non-Ca Garnets Name Formula Color Pyrope Mg 3 Al 2(Si 04)3 Deep red to black Almandine Fe 3 Al 2(Si 04)3 Deep red to brown Spessartite Mn 3 Al 2(Si 04)3 Brownish to red 38
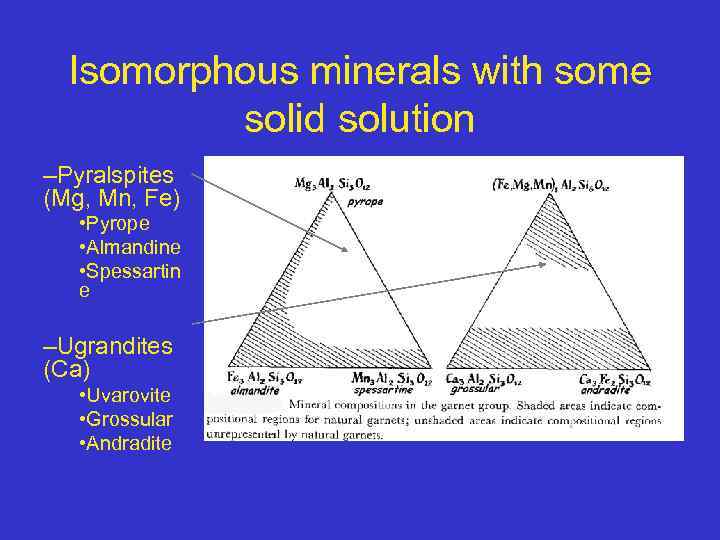 Isomorphous minerals with some solid solution –Pyralspites (Mg, Mn, Fe) • Pyrope • Almandine • Spessartin e –Ugrandites (Ca) • Uvarovite • Grossular • Andradite
Isomorphous minerals with some solid solution –Pyralspites (Mg, Mn, Fe) • Pyrope • Almandine • Spessartin e –Ugrandites (Ca) • Uvarovite • Grossular • Andradite
 Ca Garnet Photos Uvarovite Garnet (above) Ca 3 Cr 2(Si 04)3 Grossular garnet (above) Ca 3 Al 2(Si 04)3 Grossular, variety hessonite (left) 40
Ca Garnet Photos Uvarovite Garnet (above) Ca 3 Cr 2(Si 04)3 Grossular garnet (above) Ca 3 Al 2(Si 04)3 Grossular, variety hessonite (left) 40
 Garnet Photos Andradite garnet (above) Ca 3 Fe 2(Si 04)3 Almandine garnet (left and right) Fe 3 Al 2(Si 04)3 41
Garnet Photos Andradite garnet (above) Ca 3 Fe 2(Si 04)3 Almandine garnet (left and right) Fe 3 Al 2(Si 04)3 41
 Aluminosilicates • Aluminosilicates have aluminum in addition to silicon in the structure • They may belong to any silicate subclass 42
Aluminosilicates • Aluminosilicates have aluminum in addition to silicon in the structure • They may belong to any silicate subclass 42
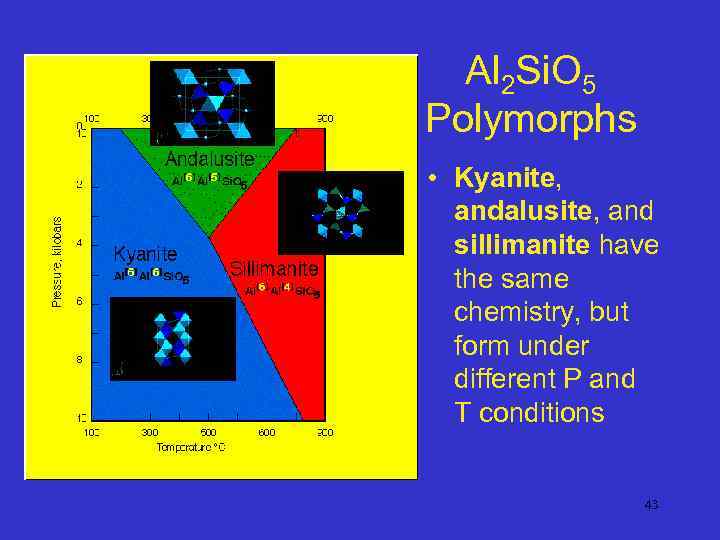 Al 2 Si. O 5 Polymorphs • Kyanite, andalusite, and sillimanite have the same chemistry, but form under different P and T conditions 43
Al 2 Si. O 5 Polymorphs • Kyanite, andalusite, and sillimanite have the same chemistry, but form under different P and T conditions 43
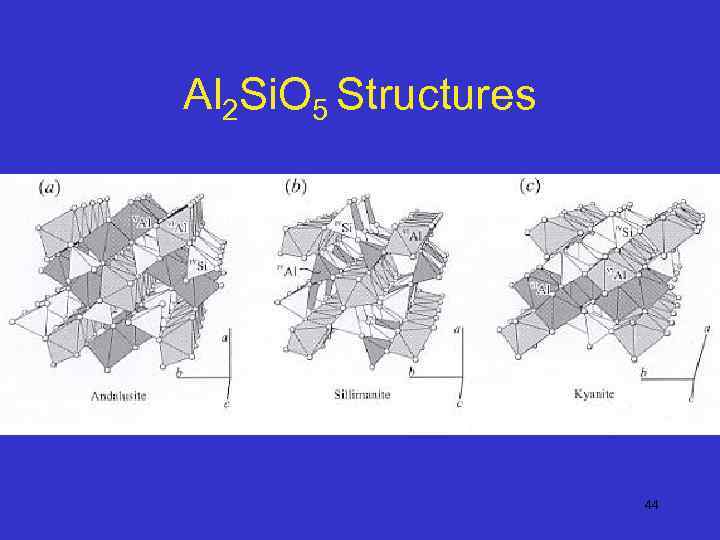 Al 2 Si. O 5 Structures 44
Al 2 Si. O 5 Structures 44
 Topaz • • Al 2 (Si 04)(F, OH)2 H=8 {001} perfect Used as a gemstone (hard mineral) • In pegmatite and/or hydrothermal deposits (accessory phase) 45
Topaz • • Al 2 (Si 04)(F, OH)2 H=8 {001} perfect Used as a gemstone (hard mineral) • In pegmatite and/or hydrothermal deposits (accessory phase) 45
 Staurolite • Fe 2 Al 906(Si 04)4(O, OH)2 • Crystals are prismatic • Often twinned (penetration twins), with two varieties of cruciform twins 46
Staurolite • Fe 2 Al 906(Si 04)4(O, OH)2 • Crystals are prismatic • Often twinned (penetration twins), with two varieties of cruciform twins 46
 Titanite • Ca. Ti. O(Si 04) • Formerly known as sphene • An example of a titanosilicate • N = 1. 91 – • luster resinous to adamantine 47
Titanite • Ca. Ti. O(Si 04) • Formerly known as sphene • An example of a titanosilicate • N = 1. 91 – • luster resinous to adamantine 47
 Willimite Willemite with Franklinite and Quartz New Jersey • Zn 2 Si. O 4 • Associated with other Zn ores • Mn may replace Zn • Often fluorescence 48
Willimite Willemite with Franklinite and Quartz New Jersey • Zn 2 Si. O 4 • Associated with other Zn ores • Mn may replace Zn • Often fluorescence 48
 Sorosilicates • Characterized by two Si 04 tetrahedra joined through a single oxygen to give an Si: O ratio of 2: 7 49
Sorosilicates • Characterized by two Si 04 tetrahedra joined through a single oxygen to give an Si: O ratio of 2: 7 49
 Epidote Group • Contains both Si 04 and Si 207 groups • General formula is X 2 VIIIY 3 VI(Si 207)(Si 04)O(OH) • X = Ca 2+, Na+ • Y = Al 3+, Fe 3+, Mn 3+, Cr 3+ • Epidote: Ca 2(Al, Fe)Al 2(Si 2 O 7)(Si. O 4)O(OH) • Clinozoisite: Ca 2 Al 3(Si 2 O 7)(Si. O 4)O(OH) Common in regional metamorphism 50
Epidote Group • Contains both Si 04 and Si 207 groups • General formula is X 2 VIIIY 3 VI(Si 207)(Si 04)O(OH) • X = Ca 2+, Na+ • Y = Al 3+, Fe 3+, Mn 3+, Cr 3+ • Epidote: Ca 2(Al, Fe)Al 2(Si 2 O 7)(Si. O 4)O(OH) • Clinozoisite: Ca 2 Al 3(Si 2 O 7)(Si. O 4)O(OH) Common in regional metamorphism 50
 Vesuvianite • Formerly called Idocrase • Ca 10(Mg, Fe)2 Al 4(Si 04)5(Si 207)2(OH)7 • Tetragonal H = 6 ½ • Brown or green 51
Vesuvianite • Formerly called Idocrase • Ca 10(Mg, Fe)2 Al 4(Si 04)5(Si 207)2(OH)7 • Tetragonal H = 6 ½ • Brown or green 51
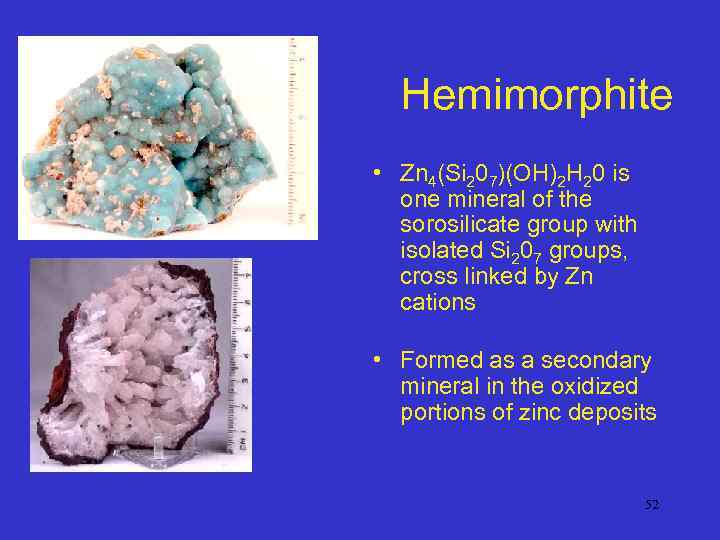 Hemimorphite • Zn 4(Si 207)(OH)2 H 20 is one mineral of the sorosilicate group with isolated Si 207 groups, cross linked by Zn cations • Formed as a secondary mineral in the oxidized portions of zinc deposits 52
Hemimorphite • Zn 4(Si 207)(OH)2 H 20 is one mineral of the sorosilicate group with isolated Si 207 groups, cross linked by Zn cations • Formed as a secondary mineral in the oxidized portions of zinc deposits 52
 Lawsonite • Ca. Al 2 Si 2 O 7(OH)2 H 2 O • Found only in metamorphic blue (glaucophane)-schist or similar low temperature, moderate to high pressure environments. 53
Lawsonite • Ca. Al 2 Si 2 O 7(OH)2 H 2 O • Found only in metamorphic blue (glaucophane)-schist or similar low temperature, moderate to high pressure environments. 53
 Cyclosilicates • When three or more Si tetrahedral groups are linked, a cyclical structure is possible • The Si: O ratio is 1: 3 • Rings containing 3, 4, or 6 Si are possible, but only the rings with 6 Si are at all common 54
Cyclosilicates • When three or more Si tetrahedral groups are linked, a cyclical structure is possible • The Si: O ratio is 1: 3 • Rings containing 3, 4, or 6 Si are possible, but only the rings with 6 Si are at all common 54
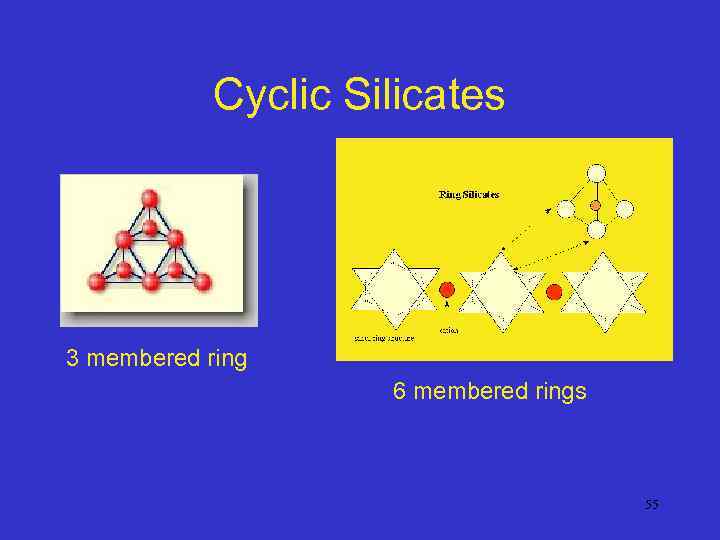 Cyclic Silicates 3 membered ring 6 membered rings 55
Cyclic Silicates 3 membered ring 6 membered rings 55
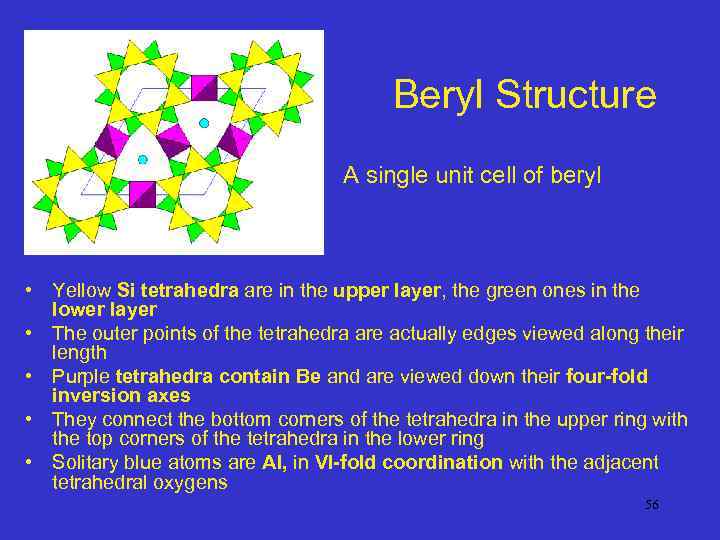 Beryl Structure A single unit cell of beryl • Yellow Si tetrahedra are in the upper layer, the green ones in the lower layer • The outer points of the tetrahedra are actually edges viewed along their length • Purple tetrahedra contain Be and are viewed down their four-fold inversion axes • They connect the bottom corners of the tetrahedra in the upper ring with the top corners of the tetrahedra in the lower ring • Solitary blue atoms are Al, in VI-fold coordination with the adjacent tetrahedral oxygens 56
Beryl Structure A single unit cell of beryl • Yellow Si tetrahedra are in the upper layer, the green ones in the lower layer • The outer points of the tetrahedra are actually edges viewed along their length • Purple tetrahedra contain Be and are viewed down their four-fold inversion axes • They connect the bottom corners of the tetrahedra in the upper ring with the top corners of the tetrahedra in the lower ring • Solitary blue atoms are Al, in VI-fold coordination with the adjacent tetrahedral oxygens 56
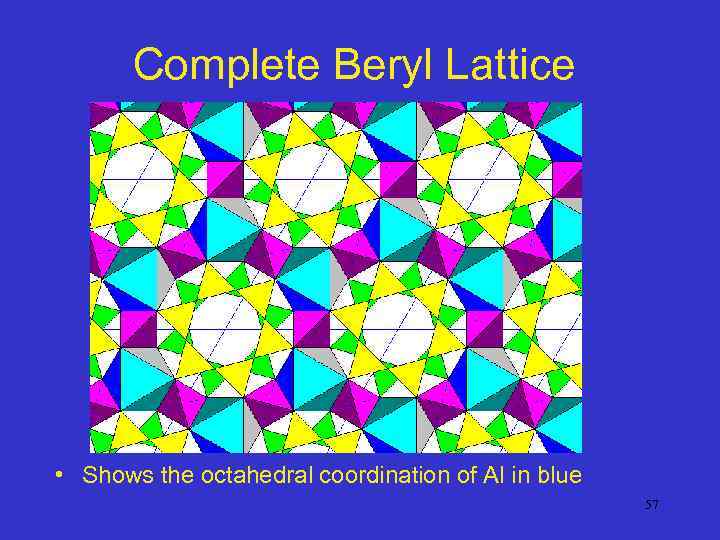 Complete Beryl Lattice • Shows the octahedral coordination of Al in blue 57
Complete Beryl Lattice • Shows the octahedral coordination of Al in blue 57
 Gem Beryl • Upper left, emerald Upper right, aquamarine • Lower left, morganite Lower right, golden beryl 58
Gem Beryl • Upper left, emerald Upper right, aquamarine • Lower left, morganite Lower right, golden beryl 58
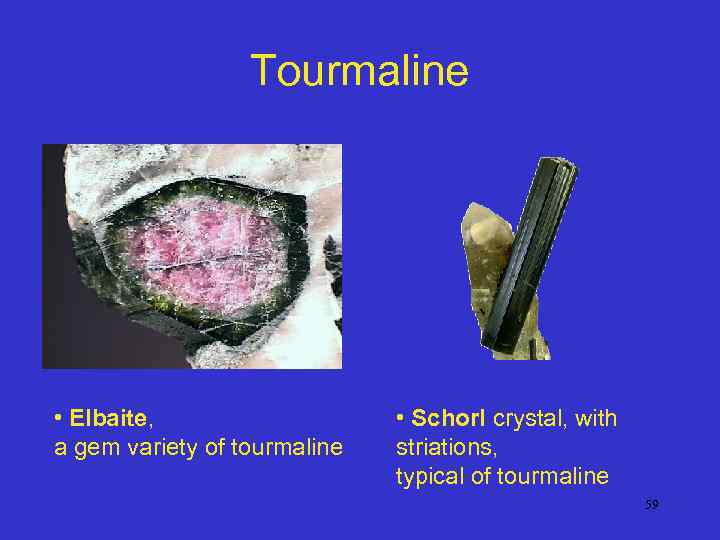 Tourmaline • Elbaite, a gem variety of tourmaline • Schorl crystal, with striations, typical of tourmaline 59
Tourmaline • Elbaite, a gem variety of tourmaline • Schorl crystal, with striations, typical of tourmaline 59
 Chrysocolla • Amorphous but similar to dioptase, a six-membered cyclosilicate • May contain Si 4 O 10 units, which would make it a phyllosilicate 60
Chrysocolla • Amorphous but similar to dioptase, a six-membered cyclosilicate • May contain Si 4 O 10 units, which would make it a phyllosilicate 60
 Inosilicates • Inosilicates include two very important groups of silicates, the pyroxenes and the amphiboles • Both have chain structures • Si 04 tetrahedra link together to form either a single chain or a double chain composed of two linked, parallel single chains 61
Inosilicates • Inosilicates include two very important groups of silicates, the pyroxenes and the amphiboles • Both have chain structures • Si 04 tetrahedra link together to form either a single chain or a double chain composed of two linked, parallel single chains 61
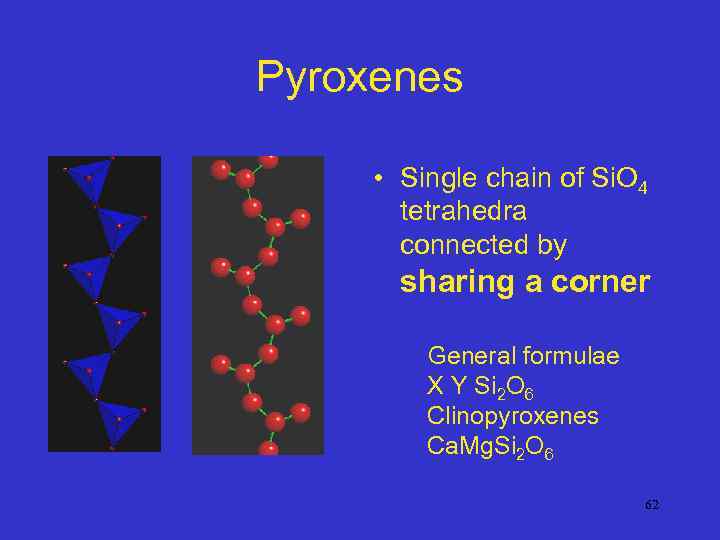 Pyroxenes • Single chain of Si. O 4 tetrahedra connected by sharing a corner General formulae X Y Si 2 O 6 Clinopyroxenes Ca. Mg. Si 2 O 6 62
Pyroxenes • Single chain of Si. O 4 tetrahedra connected by sharing a corner General formulae X Y Si 2 O 6 Clinopyroxenes Ca. Mg. Si 2 O 6 62
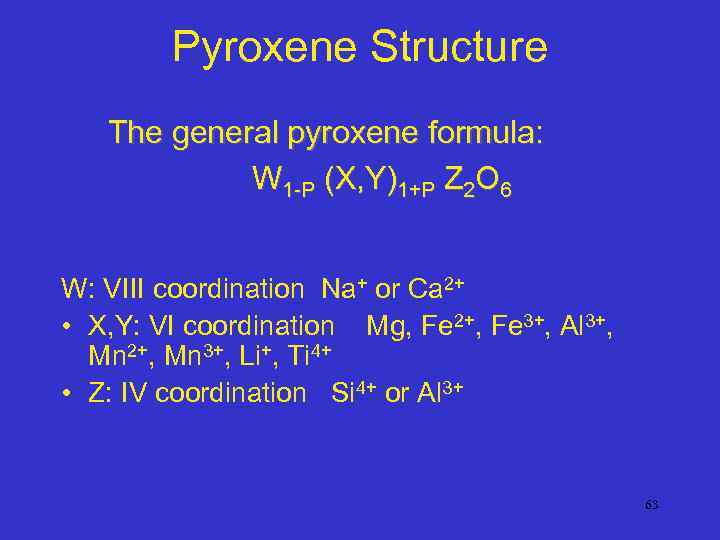 Pyroxene Structure The general pyroxene formula: W 1 -P (X, Y)1+P Z 2 O 6 W: VIII coordination Na+ or Ca 2+ • X, Y: VI coordination Mg, Fe 2+, Fe 3+, Al 3+, Mn 2+, Mn 3+, Li+, Ti 4+ • Z: IV coordination Si 4+ or Al 3+ 63
Pyroxene Structure The general pyroxene formula: W 1 -P (X, Y)1+P Z 2 O 6 W: VIII coordination Na+ or Ca 2+ • X, Y: VI coordination Mg, Fe 2+, Fe 3+, Al 3+, Mn 2+, Mn 3+, Li+, Ti 4+ • Z: IV coordination Si 4+ or Al 3+ 63
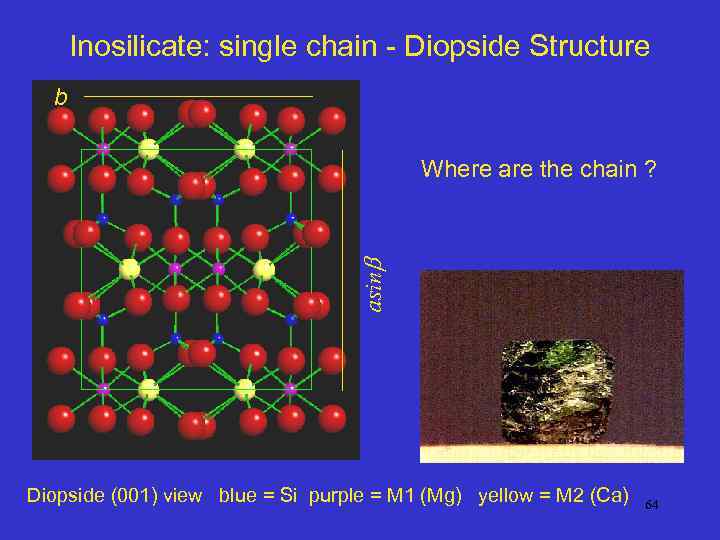 Inosilicate: single chain - Diopside Structure b asin Where are the chain ? Diopside (001) view blue = Si purple = M 1 (Mg) yellow = M 2 (Ca) 64
Inosilicate: single chain - Diopside Structure b asin Where are the chain ? Diopside (001) view blue = Si purple = M 1 (Mg) yellow = M 2 (Ca) 64
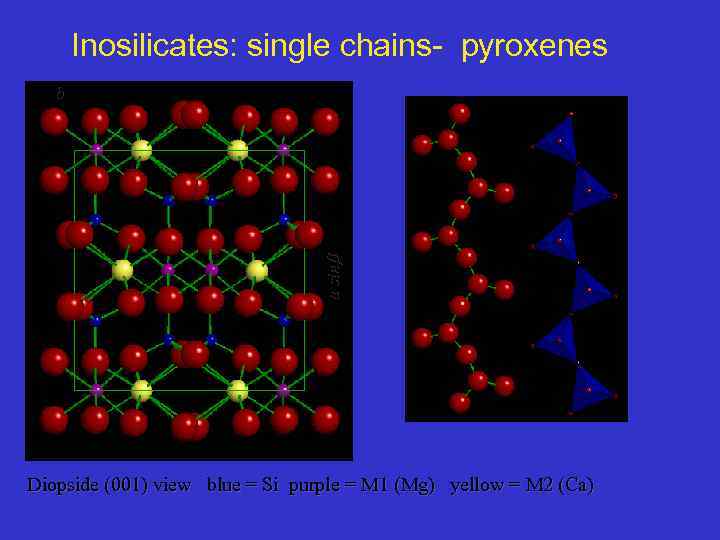 Inosilicates: single chains- pyroxenes a sin b Diopside (001) view blue = Si purple = M 1 (Mg) yellow = M 2 (Ca)
Inosilicates: single chains- pyroxenes a sin b Diopside (001) view blue = Si purple = M 1 (Mg) yellow = M 2 (Ca)
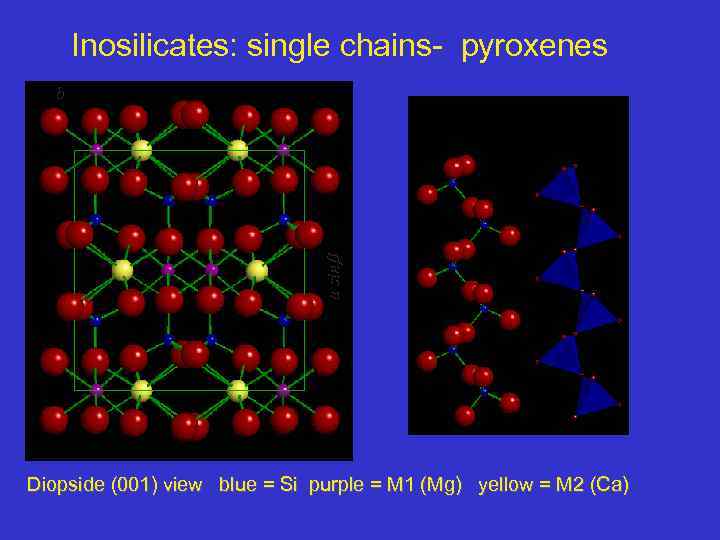 Inosilicates: single chains- pyroxenes a sin b Diopside (001) view blue = Si purple = M 1 (Mg) yellow = M 2 (Ca)
Inosilicates: single chains- pyroxenes a sin b Diopside (001) view blue = Si purple = M 1 (Mg) yellow = M 2 (Ca)
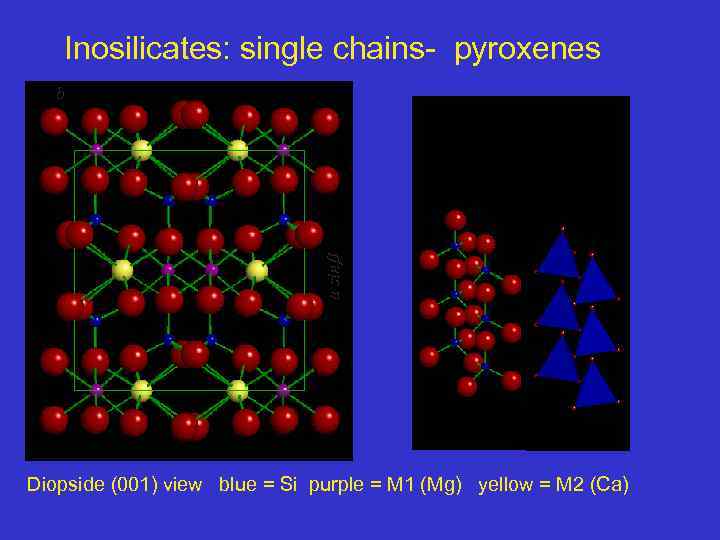 Inosilicates: single chains- pyroxenes a sin b Diopside (001) view blue = Si purple = M 1 (Mg) yellow = M 2 (Ca)
Inosilicates: single chains- pyroxenes a sin b Diopside (001) view blue = Si purple = M 1 (Mg) yellow = M 2 (Ca)
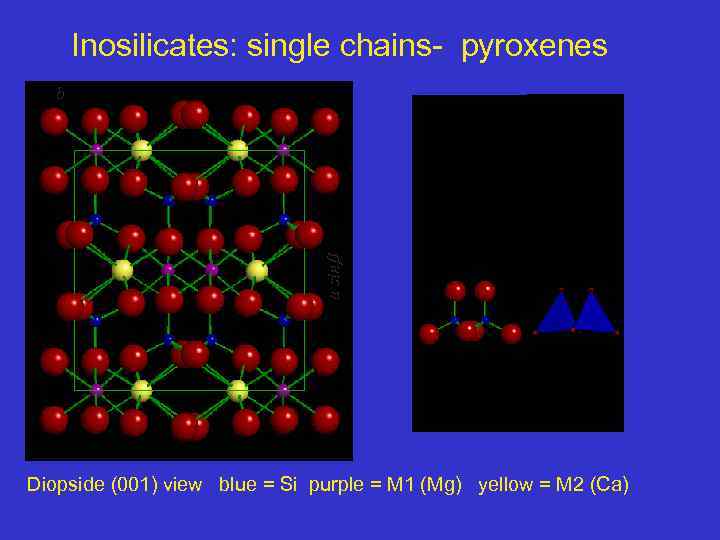 Inosilicates: single chains- pyroxenes a sin b Diopside (001) view blue = Si purple = M 1 (Mg) yellow = M 2 (Ca)
Inosilicates: single chains- pyroxenes a sin b Diopside (001) view blue = Si purple = M 1 (Mg) yellow = M 2 (Ca)
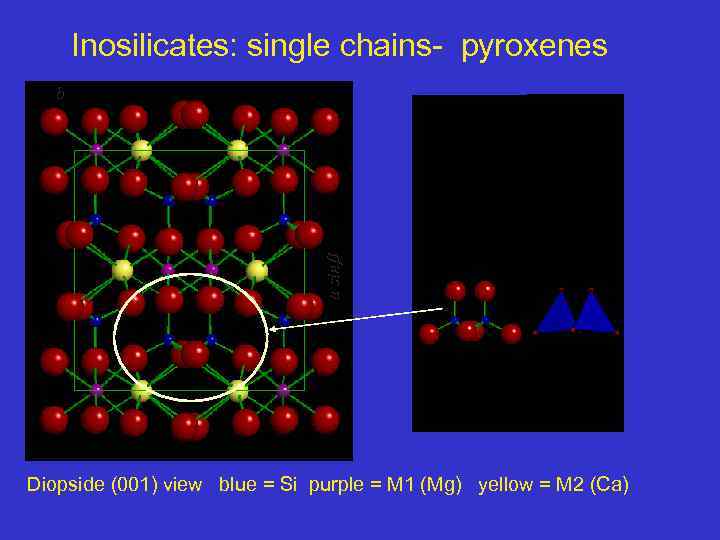 Inosilicates: single chains- pyroxenes a sin b Diopside (001) view blue = Si purple = M 1 (Mg) yellow = M 2 (Ca)
Inosilicates: single chains- pyroxenes a sin b Diopside (001) view blue = Si purple = M 1 (Mg) yellow = M 2 (Ca)
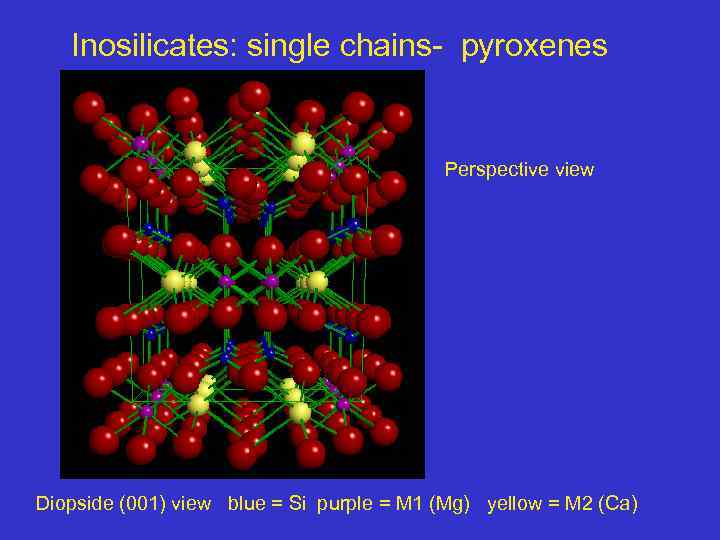 Inosilicates: single chains- pyroxenes Perspective view Diopside (001) view blue = Si purple = M 1 (Mg) yellow = M 2 (Ca)
Inosilicates: single chains- pyroxenes Perspective view Diopside (001) view blue = Si purple = M 1 (Mg) yellow = M 2 (Ca)
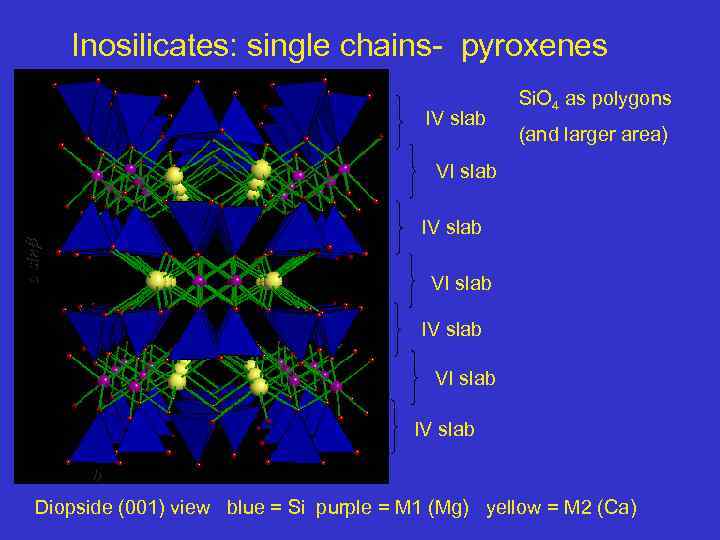 Inosilicates: single chains- pyroxenes IV slab Si. O 4 as polygons (and larger area) VI slab a sin IV slab VI slab IV slab b Diopside (001) view blue = Si purple = M 1 (Mg) yellow = M 2 (Ca)
Inosilicates: single chains- pyroxenes IV slab Si. O 4 as polygons (and larger area) VI slab a sin IV slab VI slab IV slab b Diopside (001) view blue = Si purple = M 1 (Mg) yellow = M 2 (Ca)
 Inosilicates: single chains- pyroxenes M 1 octahedron
Inosilicates: single chains- pyroxenes M 1 octahedron
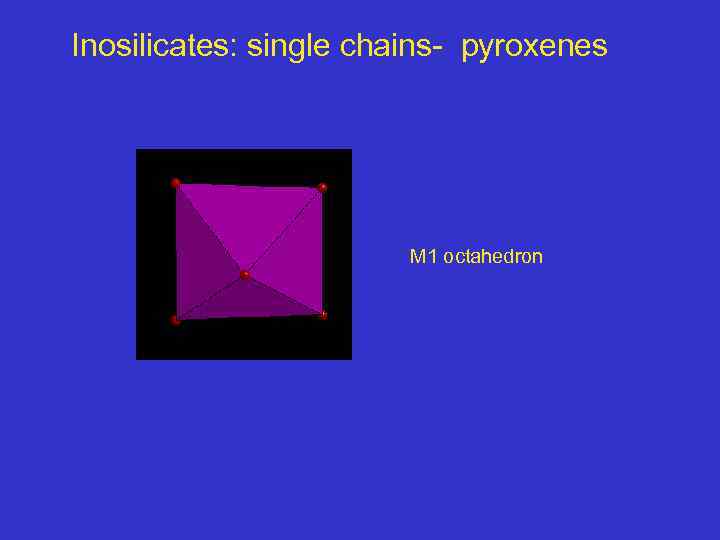 Inosilicates: single chains- pyroxenes M 1 octahedron
Inosilicates: single chains- pyroxenes M 1 octahedron
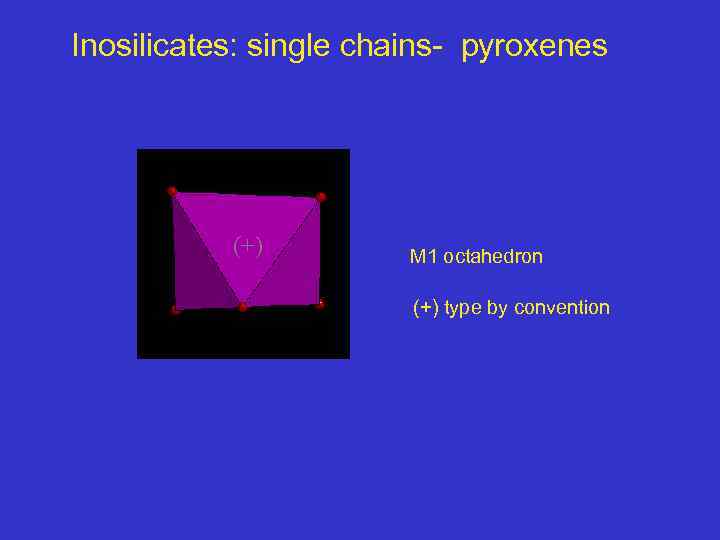 Inosilicates: single chains- pyroxenes (+) M 1 octahedron (+) type by convention
Inosilicates: single chains- pyroxenes (+) M 1 octahedron (+) type by convention
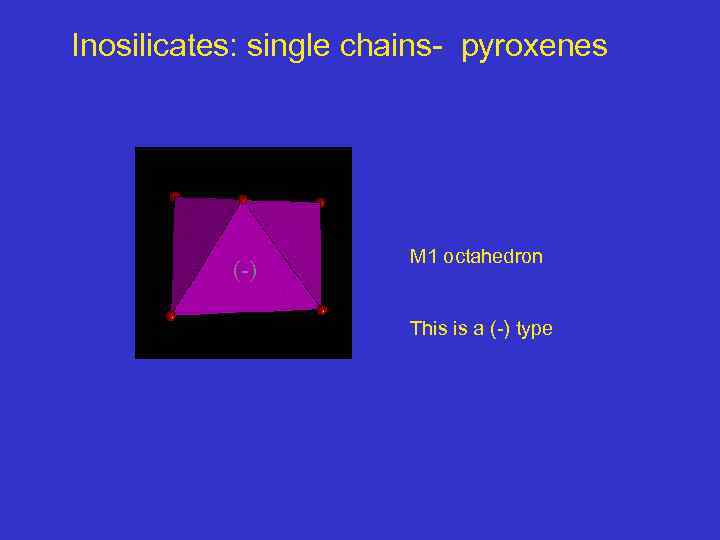 Inosilicates: single chains- pyroxenes (-) M 1 octahedron This is a (-) type
Inosilicates: single chains- pyroxenes (-) M 1 octahedron This is a (-) type
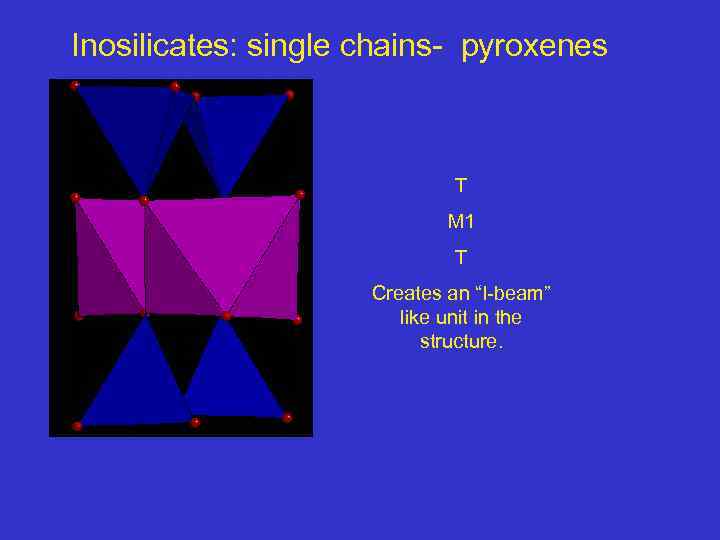 Inosilicates: single chains- pyroxenes T M 1 T Creates an “I-beam” like unit in the structure.
Inosilicates: single chains- pyroxenes T M 1 T Creates an “I-beam” like unit in the structure.
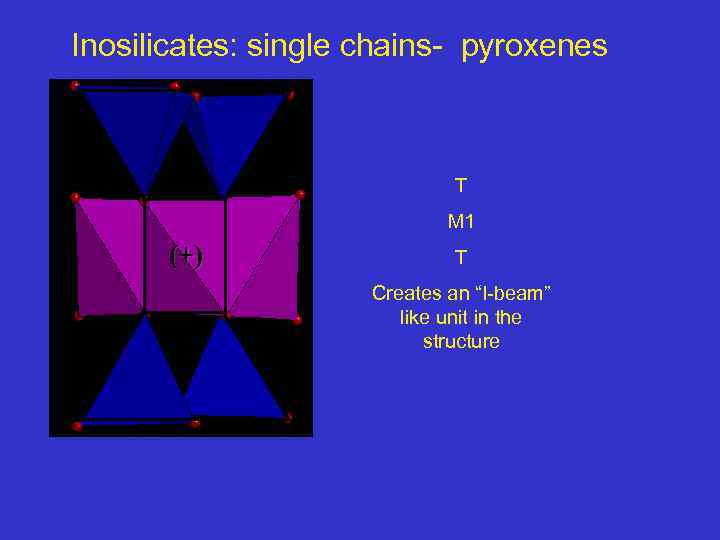 Inosilicates: single chains- pyroxenes T M 1 (+) T Creates an “I-beam” like unit in the structure
Inosilicates: single chains- pyroxenes T M 1 (+) T Creates an “I-beam” like unit in the structure
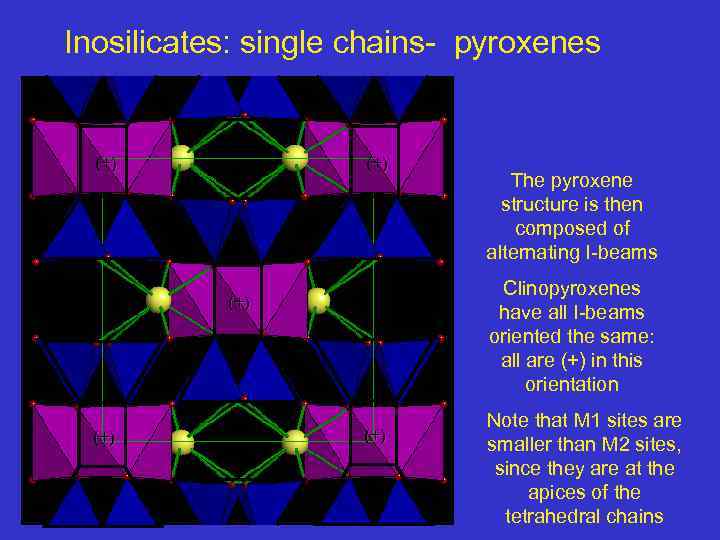 Inosilicates: single chains- pyroxenes (+) Clinopyroxenes have all I-beams oriented the same: all are (+) in this orientation (+) The pyroxene structure is then composed of alternating I-beams (+) Note that M 1 sites are smaller than M 2 sites, since they are at the apices of the tetrahedral chains
Inosilicates: single chains- pyroxenes (+) Clinopyroxenes have all I-beams oriented the same: all are (+) in this orientation (+) The pyroxene structure is then composed of alternating I-beams (+) Note that M 1 sites are smaller than M 2 sites, since they are at the apices of the tetrahedral chains
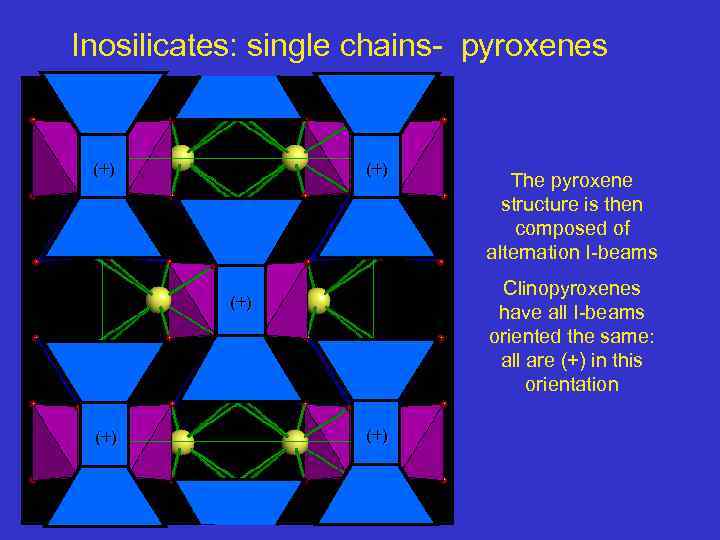 Inosilicates: single chains- pyroxenes (+) Clinopyroxenes have all I-beams oriented the same: all are (+) in this orientation (+) The pyroxene structure is then composed of alternation I-beams (+)
Inosilicates: single chains- pyroxenes (+) Clinopyroxenes have all I-beams oriented the same: all are (+) in this orientation (+) The pyroxene structure is then composed of alternation I-beams (+)
 Inosilicates: single chains- pyroxenes Tetrehedra and M 1 octahedra share tetrahedral apical oxygen atoms
Inosilicates: single chains- pyroxenes Tetrehedra and M 1 octahedra share tetrahedral apical oxygen atoms
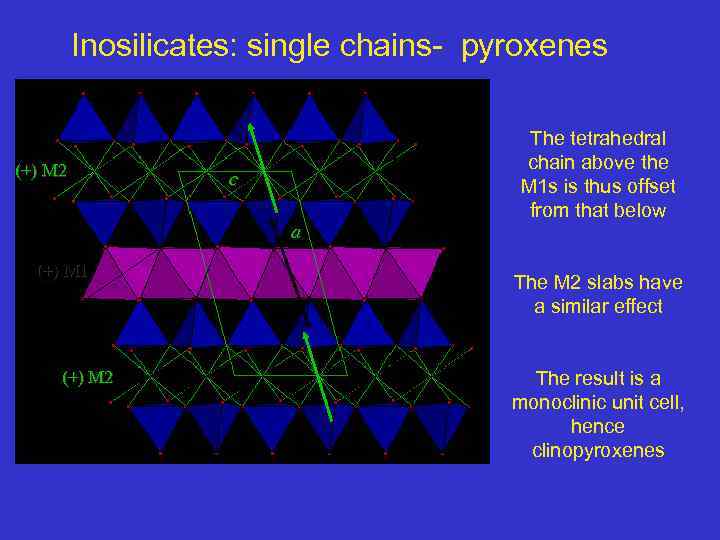 Inosilicates: single chains- pyroxenes (+) M 2 c a (+) M 1 (+) M 2 The tetrahedral chain above the M 1 s is thus offset from that below The M 2 slabs have a similar effect The result is a monoclinic unit cell, hence clinopyroxenes
Inosilicates: single chains- pyroxenes (+) M 2 c a (+) M 1 (+) M 2 The tetrahedral chain above the M 1 s is thus offset from that below The M 2 slabs have a similar effect The result is a monoclinic unit cell, hence clinopyroxenes
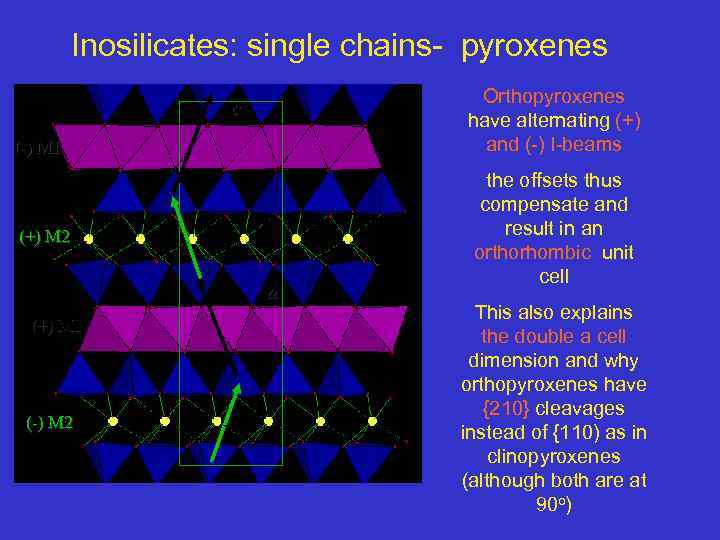 Inosilicates: single chains- pyroxenes Orthopyroxenes have alternating (+) and (-) I-beams c (-) M 1 (+) M 2 a (+) M 1 (-) M 2 the offsets thus compensate and result in an orthorhombic unit cell This also explains the double a cell dimension and why orthopyroxenes have {210} cleavages instead of {110) as in clinopyroxenes (although both are at 90 o)
Inosilicates: single chains- pyroxenes Orthopyroxenes have alternating (+) and (-) I-beams c (-) M 1 (+) M 2 a (+) M 1 (-) M 2 the offsets thus compensate and result in an orthorhombic unit cell This also explains the double a cell dimension and why orthopyroxenes have {210} cleavages instead of {110) as in clinopyroxenes (although both are at 90 o)
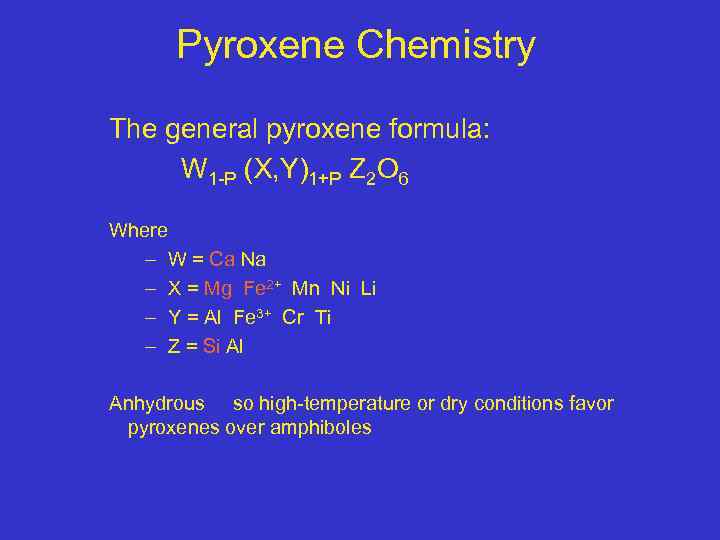 Pyroxene Chemistry The general pyroxene formula: W 1 -P (X, Y)1+P Z 2 O 6 Where – W = Ca Na – X = Mg Fe 2+ Mn Ni Li – Y = Al Fe 3+ Cr Ti – Z = Si Al Anhydrous so high-temperature or dry conditions favor pyroxenes over amphiboles
Pyroxene Chemistry The general pyroxene formula: W 1 -P (X, Y)1+P Z 2 O 6 Where – W = Ca Na – X = Mg Fe 2+ Mn Ni Li – Y = Al Fe 3+ Cr Ti – Z = Si Al Anhydrous so high-temperature or dry conditions favor pyroxenes over amphiboles
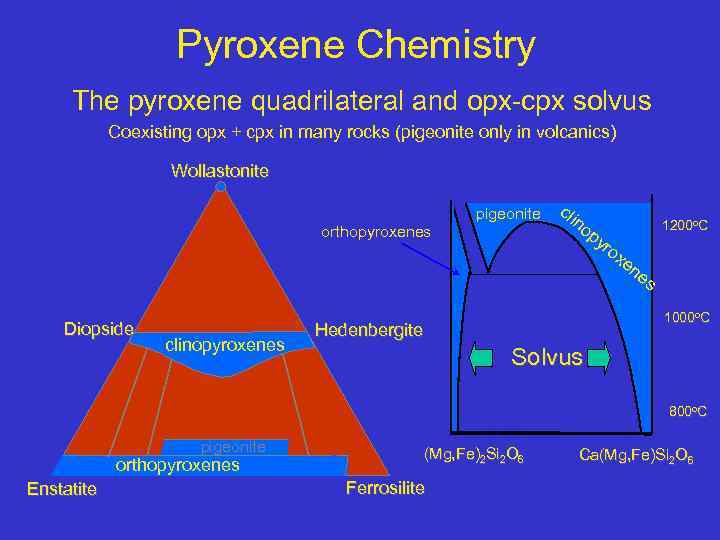 Pyroxene Chemistry The pyroxene quadrilateral and opx-cpx solvus Coexisting opx + cpx in many rocks (pigeonite only in volcanics) Wollastonite pigeonite orthopyroxenes Diopside clinopyroxenes cli no py 1200 o. C ro xe ne s 1000 o. C Hedenbergite Solvus 800 o. C pigeonite orthopyroxenes Enstatite (Mg, Fe)2 Si 2 O 6 Ferrosilite Ca(Mg, Fe)Si 2 O 6
Pyroxene Chemistry The pyroxene quadrilateral and opx-cpx solvus Coexisting opx + cpx in many rocks (pigeonite only in volcanics) Wollastonite pigeonite orthopyroxenes Diopside clinopyroxenes cli no py 1200 o. C ro xe ne s 1000 o. C Hedenbergite Solvus 800 o. C pigeonite orthopyroxenes Enstatite (Mg, Fe)2 Si 2 O 6 Ferrosilite Ca(Mg, Fe)Si 2 O 6
 Orthopyroxenes • Enstatite • Hypersthene • Orthoferrosilite Mg. Si. O 3 (Mg, Fe)Si. O 3 Fe Si. O 3 85
Orthopyroxenes • Enstatite • Hypersthene • Orthoferrosilite Mg. Si. O 3 (Mg, Fe)Si. O 3 Fe Si. O 3 85
 Enstatite • Brownish orthopyroxene (opx) • Lower photo is of Bronzite, an opx containing some Fe, and displaying an iridescence known as Schiller luster 86
Enstatite • Brownish orthopyroxene (opx) • Lower photo is of Bronzite, an opx containing some Fe, and displaying an iridescence known as Schiller luster 86
 Clinopyroxenes X Diopside Hedenbergite Augite Pigeonite Aegirine Jadeite Spodumene Y Ca Mg Ca Fe 2+ Ca (Mg, Fe 2+) (Al, Fe 3+, Ti) (Mg, Fe 2+, Ca) (Mg, Fe 2+) (Al, Fe 3+) Na Fe 3+ Na Al Li Al Si 2 O 6 Si 2 O 6 87
Clinopyroxenes X Diopside Hedenbergite Augite Pigeonite Aegirine Jadeite Spodumene Y Ca Mg Ca Fe 2+ Ca (Mg, Fe 2+) (Al, Fe 3+, Ti) (Mg, Fe 2+, Ca) (Mg, Fe 2+) (Al, Fe 3+) Na Fe 3+ Na Al Li Al Si 2 O 6 Si 2 O 6 87
 Augite • Augite is distinguished by 2 D cleavage at 90° • Al occurs at tetrahedral sites, so trivalent cations are present at normally divalent sites 88
Augite • Augite is distinguished by 2 D cleavage at 90° • Al occurs at tetrahedral sites, so trivalent cations are present at normally divalent sites 88
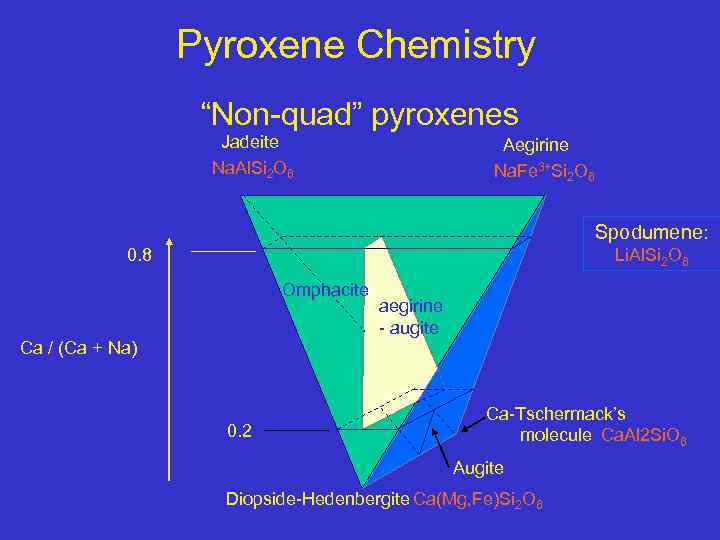 Pyroxene Chemistry “Non-quad” pyroxenes Jadeite Na. Al. Si 2 O 6 Aegirine Na. Fe 3+Si 2 O 6 Spodumene: 0. 8 Li. Al. Si 2 O 6 Omphacite aegirine - augite Ca / (Ca + Na) 0. 2 Ca-Tschermack’s molecule Ca. Al 2 Si. O 6 Augite Diopside-Hedenbergite Ca(Mg, Fe)Si 2 O 6
Pyroxene Chemistry “Non-quad” pyroxenes Jadeite Na. Al. Si 2 O 6 Aegirine Na. Fe 3+Si 2 O 6 Spodumene: 0. 8 Li. Al. Si 2 O 6 Omphacite aegirine - augite Ca / (Ca + Na) 0. 2 Ca-Tschermack’s molecule Ca. Al 2 Si. O 6 Augite Diopside-Hedenbergite Ca(Mg, Fe)Si 2 O 6
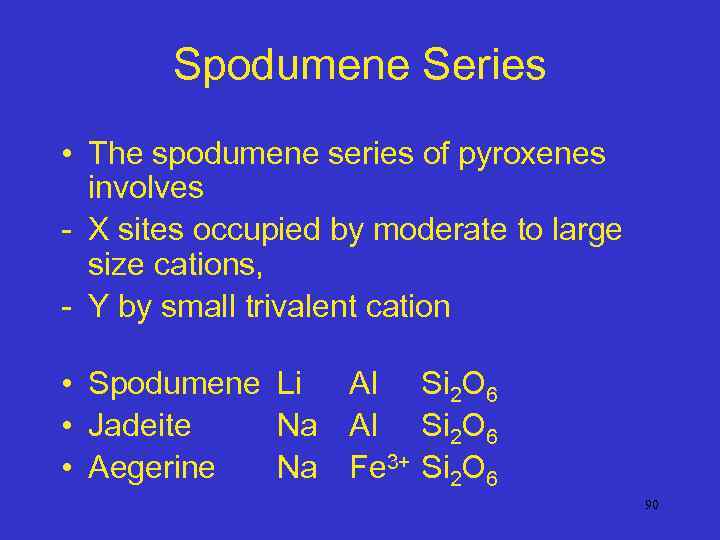 Spodumene Series • The spodumene series of pyroxenes involves - X sites occupied by moderate to large size cations, - Y by small trivalent cation • Spodumene Li Al Si 2 O 6 • Jadeite Na Al Si 2 O 6 • Aegerine Na Fe 3+ Si 2 O 6 90
Spodumene Series • The spodumene series of pyroxenes involves - X sites occupied by moderate to large size cations, - Y by small trivalent cation • Spodumene Li Al Si 2 O 6 • Jadeite Na Al Si 2 O 6 • Aegerine Na Fe 3+ Si 2 O 6 90
 Pyroxenoid Structure • Large cations occupy both X and Y, producing the triclinic structure of the pyroxenoids • Chains made of Si 2 O 7 and Si. O 4 groups linked together are present, and the chains are parallel to b • Si: O = 1: 3 91
Pyroxenoid Structure • Large cations occupy both X and Y, producing the triclinic structure of the pyroxenoids • Chains made of Si 2 O 7 and Si. O 4 groups linked together are present, and the chains are parallel to b • Si: O = 1: 3 91
 Pyroxene vs. Pyroxenoid • “Ideal” pyroxene chains with 5. 2 Å repeat (2 tetrahedra) become distorted as other cations occupy 7. 1 A 12. 5 A • Note presence of Si 2 O 7 couplets 5. 2 A Pyroxene 2 -tet repeat Wollastonite (Ca M 1) 3 -tet repeat Rhodonite Mn. Si. O 3 5 -tet repeat 92
Pyroxene vs. Pyroxenoid • “Ideal” pyroxene chains with 5. 2 Å repeat (2 tetrahedra) become distorted as other cations occupy 7. 1 A 12. 5 A • Note presence of Si 2 O 7 couplets 5. 2 A Pyroxene 2 -tet repeat Wollastonite (Ca M 1) 3 -tet repeat Rhodonite Mn. Si. O 3 5 -tet repeat 92
 Pyroxenoids • Top, pectolite • Middle, wollastonite • Bottom, rhodonite 93
Pyroxenoids • Top, pectolite • Middle, wollastonite • Bottom, rhodonite 93
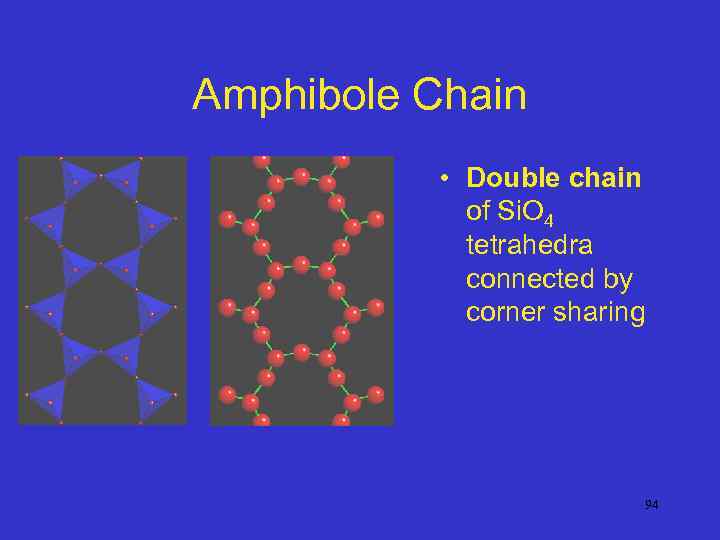 Amphibole Chain • Double chain of Si. O 4 tetrahedra connected by corner sharing 94
Amphibole Chain • Double chain of Si. O 4 tetrahedra connected by corner sharing 94
 Amphibole Structure • Amphiboles have a double chain structure formed by sharing three corners • All have the basic Si 4 O 11 double chains, with larger X ions are in VIII coordination, while smaller Y cations are in VI coordination • Si: O = 1: 2. 75 95
Amphibole Structure • Amphiboles have a double chain structure formed by sharing three corners • All have the basic Si 4 O 11 double chains, with larger X ions are in VIII coordination, while smaller Y cations are in VI coordination • Si: O = 1: 2. 75 95
 Amphibole Formula • The general formula is: W 0 -1 X 0 -7 Y 7 -14 Z 16 O 44(OH)4 • W: Na+, K+, • X = Ca 2+, Na+, Mn 2+, Fe 2+, Mg 2+, Li+ • Y: Mg 2+, Fe 3+, Al 3+, Mn 2+, Mn 3+, Ti 4+ • Z: Si 4+, Al 3+ 96
Amphibole Formula • The general formula is: W 0 -1 X 0 -7 Y 7 -14 Z 16 O 44(OH)4 • W: Na+, K+, • X = Ca 2+, Na+, Mn 2+, Fe 2+, Mg 2+, Li+ • Y: Mg 2+, Fe 3+, Al 3+, Mn 2+, Mn 3+, Ti 4+ • Z: Si 4+, Al 3+ 96
 Amphiboles : W 0 -1 X 0 -7 Y 7 -14 Z 16 O 44(OH)4 • Closely related to pyroxenes –Same cations; amphiboles have water –Complete and partial solid solution –Coupled substitution –Orthorhombic and monoclinic
Amphiboles : W 0 -1 X 0 -7 Y 7 -14 Z 16 O 44(OH)4 • Closely related to pyroxenes –Same cations; amphiboles have water –Complete and partial solid solution –Coupled substitution –Orthorhombic and monoclinic
![Amphibole Double Chain Tremolite • Ca 2 Mg 5 [Si 8 O 22] (OH)2 Amphibole Double Chain Tremolite • Ca 2 Mg 5 [Si 8 O 22] (OH)2](https://present5.com/presentation/68703897_135084041/image-98.jpg) Amphibole Double Chain Tremolite • Ca 2 Mg 5 [Si 8 O 22] (OH)2 • (001) view • Blue = Si • Purple = M 1 • Rose = M 2 • Gray = M 3 (all Mg) • Yellow = M 4 (Ca) 98
Amphibole Double Chain Tremolite • Ca 2 Mg 5 [Si 8 O 22] (OH)2 • (001) view • Blue = Si • Purple = M 1 • Rose = M 2 • Gray = M 3 (all Mg) • Yellow = M 4 (Ca) 98
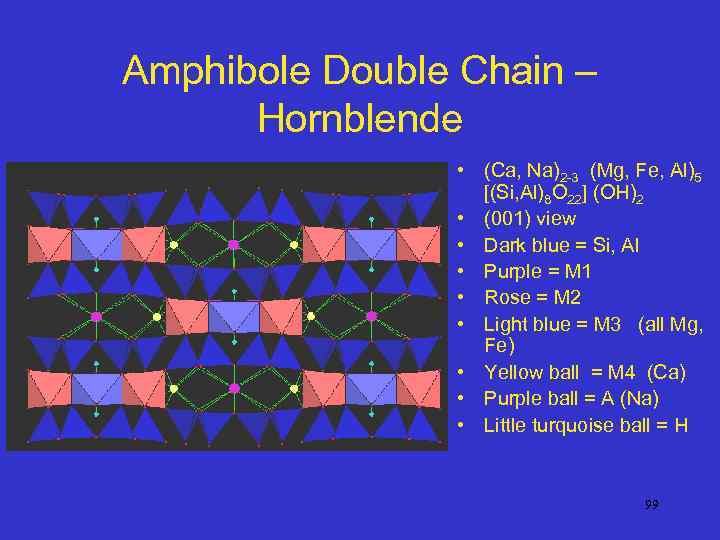 Amphibole Double Chain – Hornblende • (Ca, Na)2 -3 (Mg, Fe, Al)5 [(Si, Al)8 O 22] (OH)2 • (001) view • Dark blue = Si, Al • Purple = M 1 • Rose = M 2 • Light blue = M 3 (all Mg, Fe) • Yellow ball = M 4 (Ca) • Purple ball = A (Na) • Little turquoise ball = H 99
Amphibole Double Chain – Hornblende • (Ca, Na)2 -3 (Mg, Fe, Al)5 [(Si, Al)8 O 22] (OH)2 • (001) view • Dark blue = Si, Al • Purple = M 1 • Rose = M 2 • Light blue = M 3 (all Mg, Fe) • Yellow ball = M 4 (Ca) • Purple ball = A (Na) • Little turquoise ball = H 99
 Amphibole Site Size Hornblende (001) view Dark blue = Si, Al, Purple = M 1, Rose = M 2, Light blue = M 3 (all Mg, Fe) Yellow ball = M 4 (Ca) Purple ball = A (Na) Little turquoise ball = H • M 1 -M 3 are small sites • M 4 is larger (Ca) • A-site is really big • Variety of sites great chemical range 100
Amphibole Site Size Hornblende (001) view Dark blue = Si, Al, Purple = M 1, Rose = M 2, Light blue = M 3 (all Mg, Fe) Yellow ball = M 4 (Ca) Purple ball = A (Na) Little turquoise ball = H • M 1 -M 3 are small sites • M 4 is larger (Ca) • A-site is really big • Variety of sites great chemical range 100
 Pyroxene Cleavage • Aegirine – a sodic pyroxene 101
Pyroxene Cleavage • Aegirine – a sodic pyroxene 101
 Amphibole Cleavage • Hornblende 102
Amphibole Cleavage • Hornblende 102
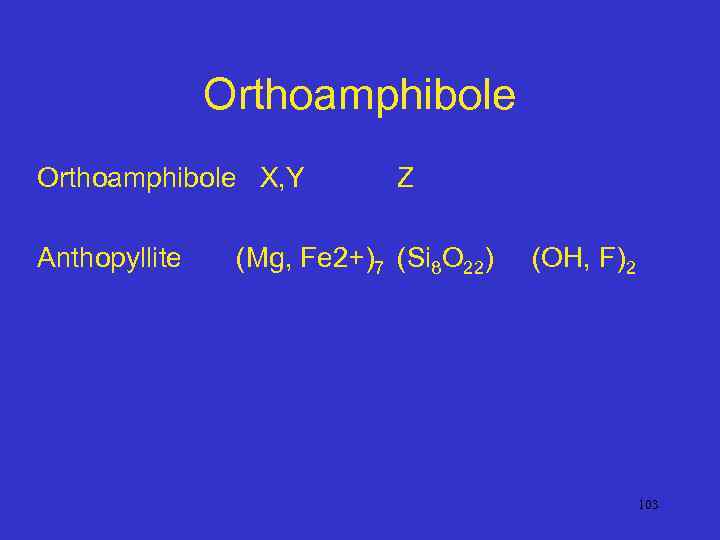 Orthoamphibole X, Y Anthopyllite Z (Mg, Fe 2+)7 (Si 8 O 22) (OH, F)2 103
Orthoamphibole X, Y Anthopyllite Z (Mg, Fe 2+)7 (Si 8 O 22) (OH, F)2 103
 Clinoamphiboles 104
Clinoamphiboles 104
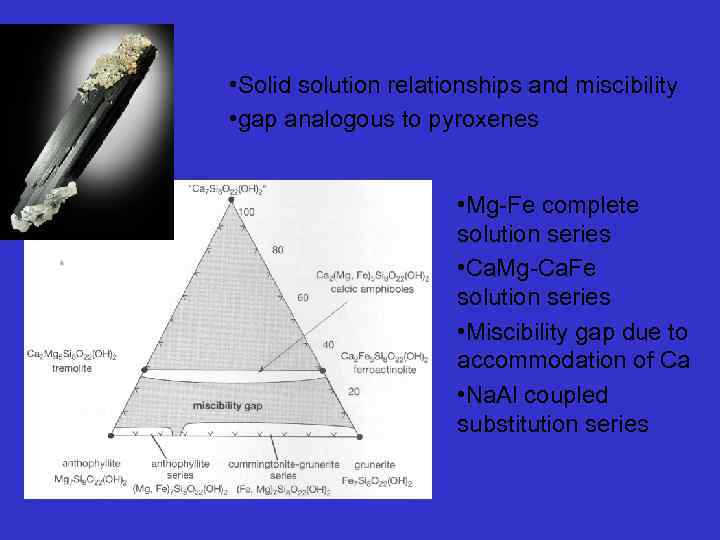 • Solid solution relationships and miscibility • gap analogous to pyroxenes • Mg-Fe complete solution series • Ca. Mg-Ca. Fe solution series • Miscibility gap due to accommodation of Ca • Na. Al coupled substitution series
• Solid solution relationships and miscibility • gap analogous to pyroxenes • Mg-Fe complete solution series • Ca. Mg-Ca. Fe solution series • Miscibility gap due to accommodation of Ca • Na. Al coupled substitution series
 Hornblende • Hornblende: the most common (and a complicated) amphibole • “Any black amphibole” • Typical in intermediate igneous rocks • Also common high temperature metamorphic rocks (K, Na)0 -1(Ca, Na, Fe, Mg)2 (Mg, Fe, Al)5(Si, Al)8 O 22(OH)2
Hornblende • Hornblende: the most common (and a complicated) amphibole • “Any black amphibole” • Typical in intermediate igneous rocks • Also common high temperature metamorphic rocks (K, Na)0 -1(Ca, Na, Fe, Mg)2 (Mg, Fe, Al)5(Si, Al)8 O 22(OH)2
 Phyllosilicates • Phyllon is the Greek word for leaf – phyllosilicates are thus "leaf-like", platy or flaky minerals which have a layered structure • The basic silicate sheet structure is composed of a hexagonal grouping of tetrahedra 107
Phyllosilicates • Phyllon is the Greek word for leaf – phyllosilicates are thus "leaf-like", platy or flaky minerals which have a layered structure • The basic silicate sheet structure is composed of a hexagonal grouping of tetrahedra 107
 Micas • Micas are the chief minerals of schist's and are also commonly found in igneous rocks • They form at lower temperatures than the inosilicates (pyroxenes and amphiboles) and are frequently formed as replacement minerals after hydrothermal alteration • Ratio of Si: O is 2: 5 108
Micas • Micas are the chief minerals of schist's and are also commonly found in igneous rocks • They form at lower temperatures than the inosilicates (pyroxenes and amphiboles) and are frequently formed as replacement minerals after hydrothermal alteration • Ratio of Si: O is 2: 5 108
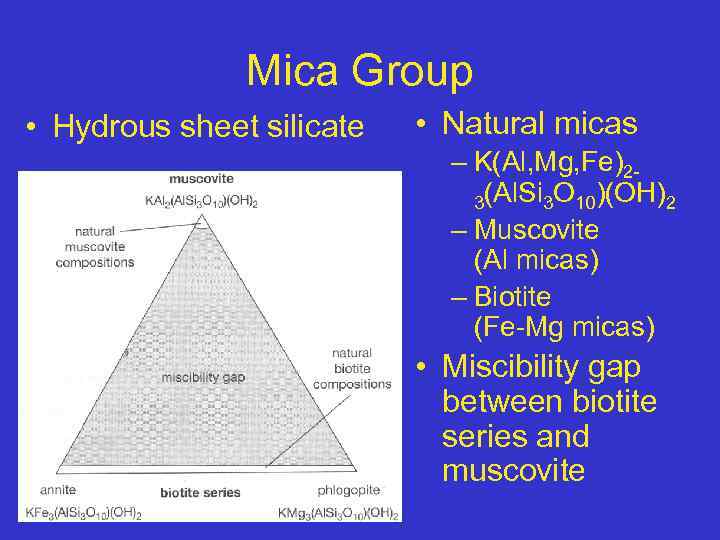 Mica Group • Hydrous sheet silicate • Natural micas – K(Al, Mg, Fe)23(Al. Si 3 O 10)(OH)2 – Muscovite (Al micas) – Biotite (Fe-Mg micas) • Miscibility gap between biotite series and muscovite
Mica Group • Hydrous sheet silicate • Natural micas – K(Al, Mg, Fe)23(Al. Si 3 O 10)(OH)2 – Muscovite (Al micas) – Biotite (Fe-Mg micas) • Miscibility gap between biotite series and muscovite
 Biotite • Essential minerals in – Igneous rocks • Muscovite: Felsic igneous rocks, Granites • Biotite: Felsic to intermediate rocks – Metamorphic rocks • Schists pseudo-hexagonal crystalline aggregate of muscovite
Biotite • Essential minerals in – Igneous rocks • Muscovite: Felsic igneous rocks, Granites • Biotite: Felsic to intermediate rocks – Metamorphic rocks • Schists pseudo-hexagonal crystalline aggregate of muscovite
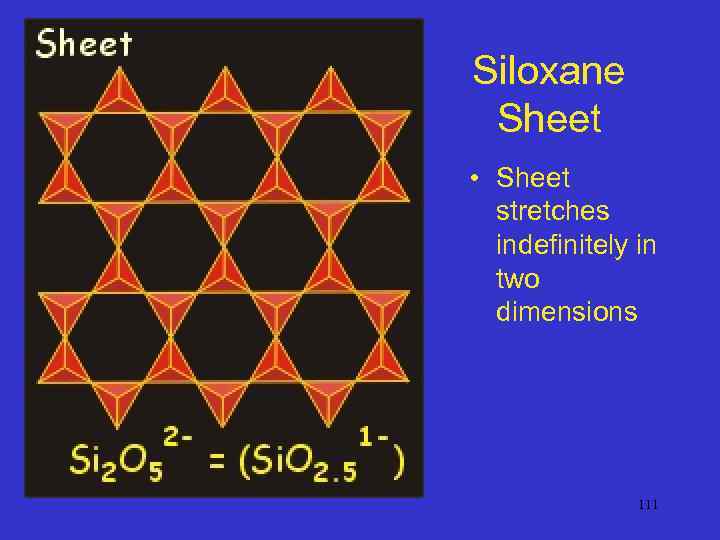 Siloxane Sheet • Sheet stretches indefinitely in two dimensions 111
Siloxane Sheet • Sheet stretches indefinitely in two dimensions 111
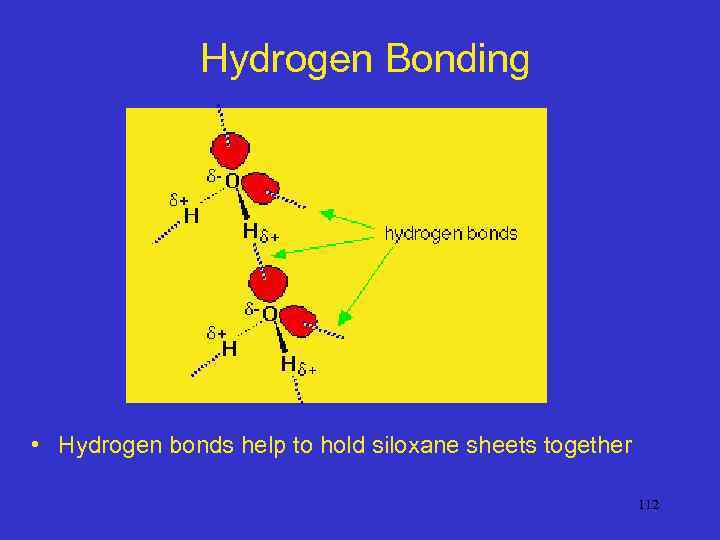 Hydrogen Bonding • Hydrogen bonds help to hold siloxane sheets together 112
Hydrogen Bonding • Hydrogen bonds help to hold siloxane sheets together 112
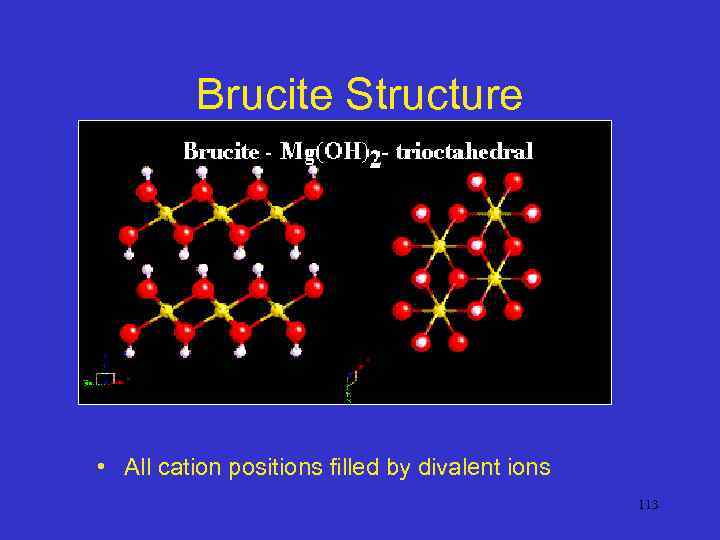 Brucite Structure • All cation positions filled by divalent ions 113
Brucite Structure • All cation positions filled by divalent ions 113
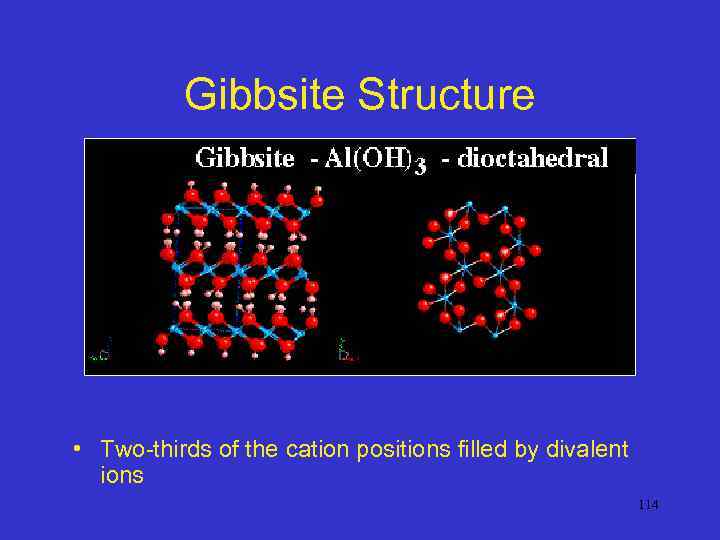 Gibbsite Structure • Two-thirds of the cation positions filled by divalent ions 114
Gibbsite Structure • Two-thirds of the cation positions filled by divalent ions 114
 Diphormic Phyllosilicates • One t-layer, one o-layer • 0. 7 nm repeat distance • Kaolinite - dioctahedral Al 4[Si 4 O 10](OH)8 • Serpentine – trioctahedral Mg 6[Si 4 O 10](OH)8 115
Diphormic Phyllosilicates • One t-layer, one o-layer • 0. 7 nm repeat distance • Kaolinite - dioctahedral Al 4[Si 4 O 10](OH)8 • Serpentine – trioctahedral Mg 6[Si 4 O 10](OH)8 115
 Serpentines • Fibrous diphormic • phyllosilicate Massive serpentine More often antigorite –Mg 3 Si 2 O 5(OH)4 –Low-grade alteration of olivine, pyroxene, and amphibole 116
Serpentines • Fibrous diphormic • phyllosilicate Massive serpentine More often antigorite –Mg 3 Si 2 O 5(OH)4 –Low-grade alteration of olivine, pyroxene, and amphibole 116
 Triphormic Phyllosilicates • In this phyllosilicate the ratio of tetrahedral : octahedral layers is 2: 1 • Basal spacing is generally around 0. 9 nm • The structure is a t-o-t sandwoch, with apical oxygens pointing inward • Pyrophyllite – dioctahedral Al 2{Si 4 O 10}(OH)2 • Talc - trioctahedral Mg 3{Si 4 O 10}(OH)2 117
Triphormic Phyllosilicates • In this phyllosilicate the ratio of tetrahedral : octahedral layers is 2: 1 • Basal spacing is generally around 0. 9 nm • The structure is a t-o-t sandwoch, with apical oxygens pointing inward • Pyrophyllite – dioctahedral Al 2{Si 4 O 10}(OH)2 • Talc - trioctahedral Mg 3{Si 4 O 10}(OH)2 117
 • Talc –Mg 3 Si 4 O 10(OH)2 –Low-grade –metamorphic rocks
• Talc –Mg 3 Si 4 O 10(OH)2 –Low-grade –metamorphic rocks
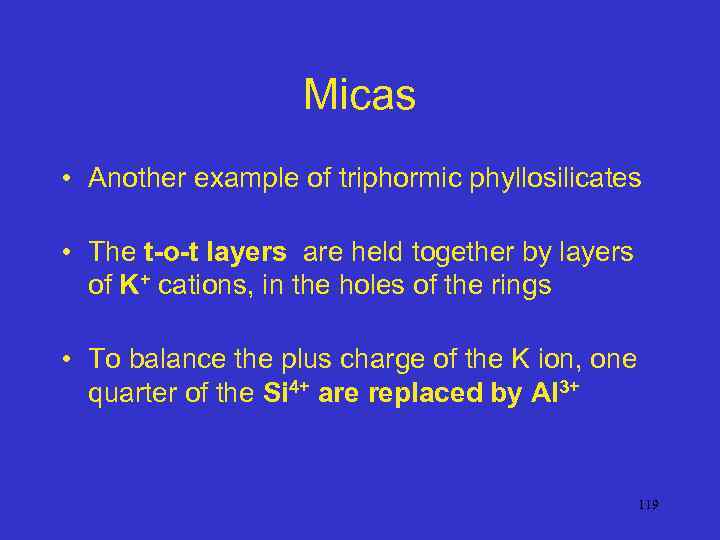 Micas • Another example of triphormic phyllosilicates • The t-o-t layers are held together by layers of K+ cations, in the holes of the rings • To balance the plus charge of the K ion, one quarter of the Si 4+ are replaced by Al 3+ 119
Micas • Another example of triphormic phyllosilicates • The t-o-t layers are held together by layers of K+ cations, in the holes of the rings • To balance the plus charge of the K ion, one quarter of the Si 4+ are replaced by Al 3+ 119
 Brittle Micas • Half of the Si 4+ ions are replaced by Al 3+ • This means the interlayer cations be divalent, like Ca 2+ • Ca 2+ bonds are stronger and consequently the cages are not flexible • Margite - dioctahedral Ca. Al 2{Al 2 Si 2 O 10}(OH)2 • Clintonite - trioctahedral Ca. Mg 3{Al 2 Si 2 O 10}(OH)2 120
Brittle Micas • Half of the Si 4+ ions are replaced by Al 3+ • This means the interlayer cations be divalent, like Ca 2+ • Ca 2+ bonds are stronger and consequently the cages are not flexible • Margite - dioctahedral Ca. Al 2{Al 2 Si 2 O 10}(OH)2 • Clintonite - trioctahedral Ca. Mg 3{Al 2 Si 2 O 10}(OH)2 120
 Swelling Clays • Building damaged by expansion and contraction of clay minerals in the soil 121
Swelling Clays • Building damaged by expansion and contraction of clay minerals in the soil 121
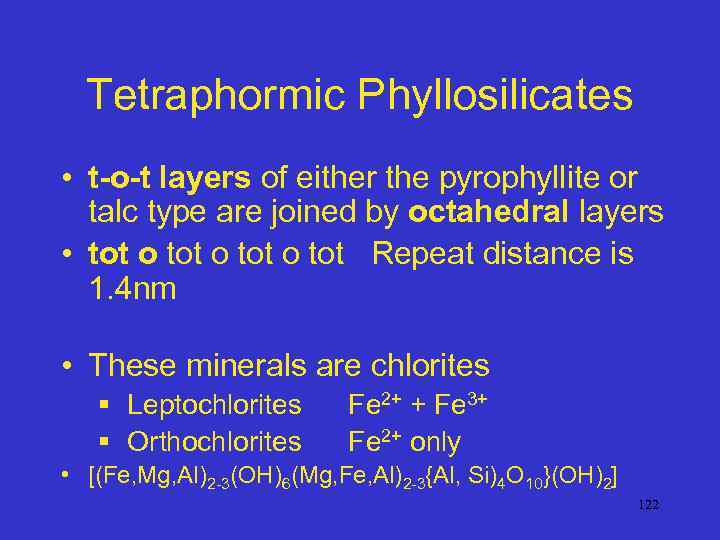 Tetraphormic Phyllosilicates • t-o-t layers of either the pyrophyllite or talc type are joined by octahedral layers • tot o tot Repeat distance is 1. 4 nm • These minerals are chlorites § Leptochlorites § Orthochlorites Fe 2+ + Fe 3+ Fe 2+ only • [(Fe, Mg, Al)2 -3(OH)6(Mg, Fe, Al)2 -3{Al, Si)4 O 10}(OH)2] 122
Tetraphormic Phyllosilicates • t-o-t layers of either the pyrophyllite or talc type are joined by octahedral layers • tot o tot Repeat distance is 1. 4 nm • These minerals are chlorites § Leptochlorites § Orthochlorites Fe 2+ + Fe 3+ Fe 2+ only • [(Fe, Mg, Al)2 -3(OH)6(Mg, Fe, Al)2 -3{Al, Si)4 O 10}(OH)2] 122
 • Chlorite –(Mg, Fe)3(Si, Al)4 O 10(OH)2 * (Mg, Fe)3(OH)6 –Greenschist facies metamorphic rocks
• Chlorite –(Mg, Fe)3(Si, Al)4 O 10(OH)2 * (Mg, Fe)3(OH)6 –Greenschist facies metamorphic rocks
 Tectosilicates • The tectosilicates are three – dimensional, or framework, silicates • They involve linkage of Si. O 4 tetrahedra through all four oxygen atoms • The resulting structure is stable and strongly bonded • Si: O ratio is 1: 2 124
Tectosilicates • The tectosilicates are three – dimensional, or framework, silicates • They involve linkage of Si. O 4 tetrahedra through all four oxygen atoms • The resulting structure is stable and strongly bonded • Si: O ratio is 1: 2 124
 • Essentially “pure” Si. O 2 • Component of many felsic and intermediate igneous rocks – Not present in: • Ultramafic igneous rocks • Alkaline (feldspathoidal) igneous rocks • Common particulate residue during bedrock weathering • Common chemical precipitate in surface through hydrothermal settings • Common component of metamorphic rocks
• Essentially “pure” Si. O 2 • Component of many felsic and intermediate igneous rocks – Not present in: • Ultramafic igneous rocks • Alkaline (feldspathoidal) igneous rocks • Common particulate residue during bedrock weathering • Common chemical precipitate in surface through hydrothermal settings • Common component of metamorphic rocks
 Varieties of Crystalline Quartz Amethyst Blue Milky Citrine Rose 126
Varieties of Crystalline Quartz Amethyst Blue Milky Citrine Rose 126
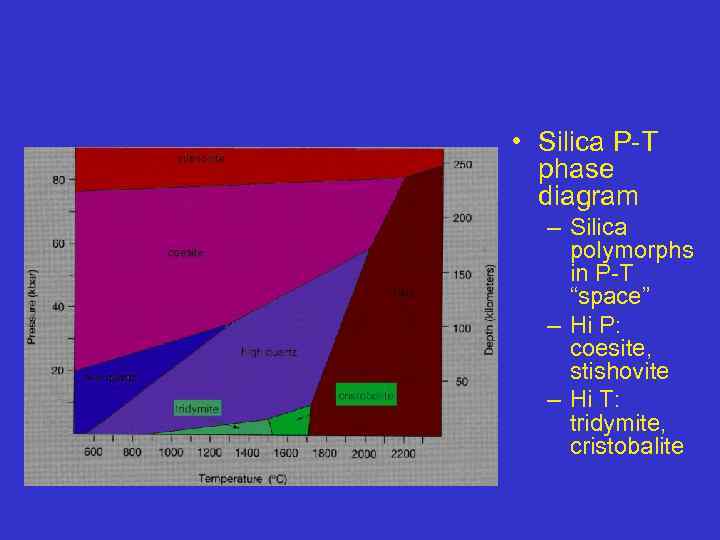 • Silica P-T phase diagram – Silica polymorphs in P-T “space” – Hi P: coesite, stishovite – Hi T: tridymite, cristobalite
• Silica P-T phase diagram – Silica polymorphs in P-T “space” – Hi P: coesite, stishovite – Hi T: tridymite, cristobalite
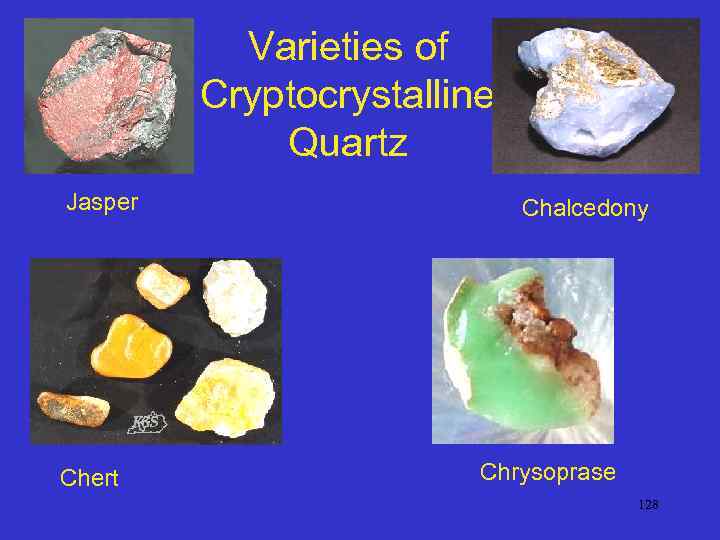 Varieties of Cryptocrystalline Quartz Jasper Chert Chalcedony Chrysoprase 128
Varieties of Cryptocrystalline Quartz Jasper Chert Chalcedony Chrysoprase 128
 • Chalcedony: a micro- (very small) to cryptocrystalline (almost amorphous {non-crystalline}) fibrous quartz • Common precipitate in surface and near-surface conditions
• Chalcedony: a micro- (very small) to cryptocrystalline (almost amorphous {non-crystalline}) fibrous quartz • Common precipitate in surface and near-surface conditions
 Feldspars • Alkali – Potassium and Ab 95 -100 • Sodi-calcic feldspars: Plagioclase An 5 -100 • Barium feldspars § Celsian Ba. Al 2 Si 2 O 8 § Hyalophane (K, Ba)(A 1, Si)2 Si 2 O 8 130
Feldspars • Alkali – Potassium and Ab 95 -100 • Sodi-calcic feldspars: Plagioclase An 5 -100 • Barium feldspars § Celsian Ba. Al 2 Si 2 O 8 § Hyalophane (K, Ba)(A 1, Si)2 Si 2 O 8 130
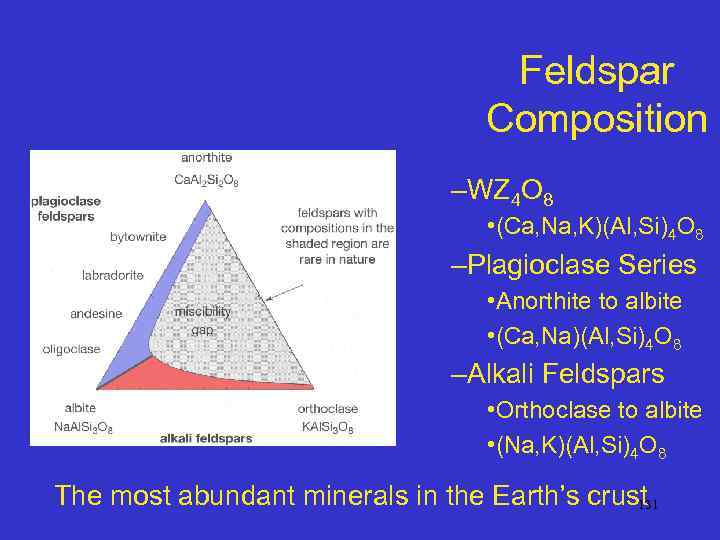 Feldspar Composition –WZ 4 O 8 • (Ca, Na, K)(Al, Si)4 O 8 –Plagioclase Series • Anorthite to albite • (Ca, Na)(Al, Si)4 O 8 –Alkali Feldspars • Orthoclase to albite • (Na, K)(Al, Si)4 O 8 The most abundant minerals in the Earth’s crust 131
Feldspar Composition –WZ 4 O 8 • (Ca, Na, K)(Al, Si)4 O 8 –Plagioclase Series • Anorthite to albite • (Ca, Na)(Al, Si)4 O 8 –Alkali Feldspars • Orthoclase to albite • (Na, K)(Al, Si)4 O 8 The most abundant minerals in the Earth’s crust 131
 Plagioclase Feldspars • Albite § An 0 -10 - Found only in very sodic rocks, hence usually metamorphic or formed in marine conditions as a sedimentary cement, or by ion exchange with more calcic plagioclase. • Oligoclase § An 10 -30 - The dominant plagioclase in granitic rocks • Andesine § An 30 -50 - Found in intermediate igneous rocks • Labradorite § An 50 -70 - The dominant plagioclase in gabbro and basalt. Also, despite their name, most anorthosites are made up of labradorite. • Bytownite § An 70 -90 - The rarest. Requires both a lot of calcium and also significant sodium. Most igneous settings have too much sodium, most calc-silicate metamorphic settings have too little sodium. • Anorthite § An 90 -100 - Generally a metamorphic mineral in calc-silicate rocks. 132
Plagioclase Feldspars • Albite § An 0 -10 - Found only in very sodic rocks, hence usually metamorphic or formed in marine conditions as a sedimentary cement, or by ion exchange with more calcic plagioclase. • Oligoclase § An 10 -30 - The dominant plagioclase in granitic rocks • Andesine § An 30 -50 - Found in intermediate igneous rocks • Labradorite § An 50 -70 - The dominant plagioclase in gabbro and basalt. Also, despite their name, most anorthosites are made up of labradorite. • Bytownite § An 70 -90 - The rarest. Requires both a lot of calcium and also significant sodium. Most igneous settings have too much sodium, most calc-silicate metamorphic settings have too little sodium. • Anorthite § An 90 -100 - Generally a metamorphic mineral in calc-silicate rocks. 132
 Plagioclase Series • Essential minerals in most igneous, • sedimentary, and metamorphic rocks • Complete (temperature dependant) • solid solution between –Albite (Na. Al. Si 3 O 8) –Anorthite (Ca. Al 2 Si 2 O 8) –Minor solid solution of K+ –increasing with increasing Ab content
Plagioclase Series • Essential minerals in most igneous, • sedimentary, and metamorphic rocks • Complete (temperature dependant) • solid solution between –Albite (Na. Al. Si 3 O 8) –Anorthite (Ca. Al 2 Si 2 O 8) –Minor solid solution of K+ –increasing with increasing Ab content
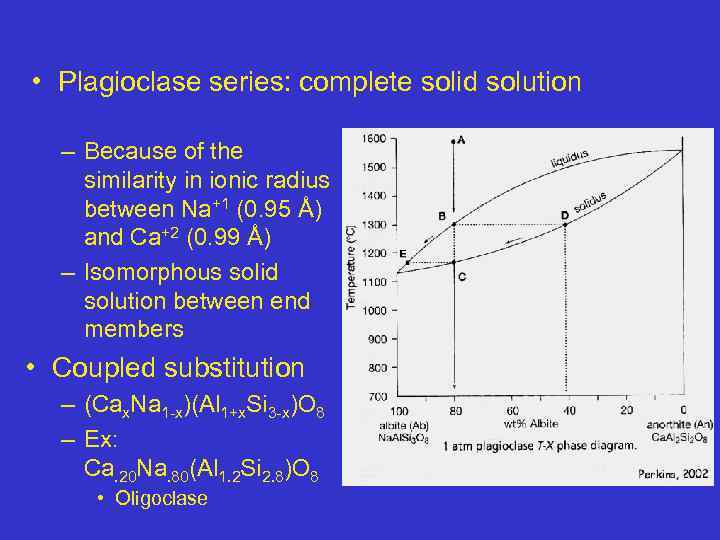 • Plagioclase series: complete solid solution – Because of the similarity in ionic radius between Na+1 (0. 95 Å) and Ca+2 (0. 99 Å) – Isomorphous solid solution between end members • Coupled substitution – (Cax. Na 1 -x)(Al 1+x. Si 3 -x)O 8 – Ex: Ca. 20 Na. 80(Al 1. 2 Si 2. 8)O 8 • Oligoclase
• Plagioclase series: complete solid solution – Because of the similarity in ionic radius between Na+1 (0. 95 Å) and Ca+2 (0. 99 Å) – Isomorphous solid solution between end members • Coupled substitution – (Cax. Na 1 -x)(Al 1+x. Si 3 -x)O 8 – Ex: Ca. 20 Na. 80(Al 1. 2 Si 2. 8)O 8 • Oligoclase
 Alkali Feldspars • K-spar shows a variety of polymorphic forms § Sanidine § Orthoclase § Microcline Orthoclase Sanidine 135
Alkali Feldspars • K-spar shows a variety of polymorphic forms § Sanidine § Orthoclase § Microcline Orthoclase Sanidine 135
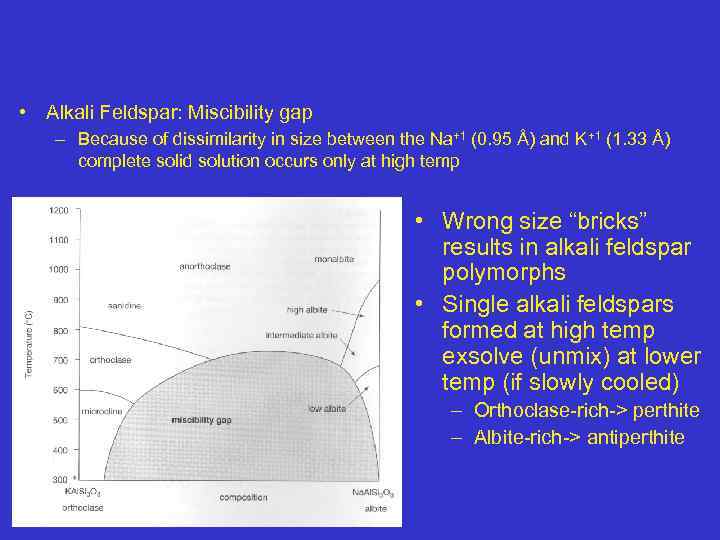 • Alkali Feldspar: Miscibility gap – Because of dissimilarity in size between the Na+1 (0. 95 Å) and K+1 (1. 33 Å) complete solid solution occurs only at high temp • Wrong size “bricks” results in alkali feldspar polymorphs • Single alkali feldspars formed at high temp exsolve (unmix) at lower temp (if slowly cooled) – Orthoclase-rich-> perthite – Albite-rich-> antiperthite
• Alkali Feldspar: Miscibility gap – Because of dissimilarity in size between the Na+1 (0. 95 Å) and K+1 (1. 33 Å) complete solid solution occurs only at high temp • Wrong size “bricks” results in alkali feldspar polymorphs • Single alkali feldspars formed at high temp exsolve (unmix) at lower temp (if slowly cooled) – Orthoclase-rich-> perthite – Albite-rich-> antiperthite
 Perthite and Antiperthite • Albite in K-spar host = perthite • K-spar in plagioclase host = antiperthite Perthite 137
Perthite and Antiperthite • Albite in K-spar host = perthite • K-spar in plagioclase host = antiperthite Perthite 137
 Plagioclase Name • Plagioclases are triclinic • Their a-b and b-c angles are a bit more oblique than microcline • Hence the name: plagio-, oblique and clase, break Albite 138
Plagioclase Name • Plagioclases are triclinic • Their a-b and b-c angles are a bit more oblique than microcline • Hence the name: plagio-, oblique and clase, break Albite 138
 Charge Balance • Since Na and Ca differ in valence, Al has to substitute for Si to compensate • The Al-Si orderings of albite and anorthite are different, and at low temperatures, plagioclases in the middle of the composition range also exsolve, but on a submicroscopic scale • These submicroscopic textures are probably responsible for the iridescence of some plagioclases 139
Charge Balance • Since Na and Ca differ in valence, Al has to substitute for Si to compensate • The Al-Si orderings of albite and anorthite are different, and at low temperatures, plagioclases in the middle of the composition range also exsolve, but on a submicroscopic scale • These submicroscopic textures are probably responsible for the iridescence of some plagioclases 139
 Feldspathoids • Alumino – silicates but contain less Si. O 2 than feldspars • They are rich in alkalis • The feldspathorids often include unusual anions such as Cl-, CO 3 -, etc. 140
Feldspathoids • Alumino – silicates but contain less Si. O 2 than feldspars • They are rich in alkalis • The feldspathorids often include unusual anions such as Cl-, CO 3 -, etc. 140
 Important Feldspathoids 141
Important Feldspathoids 141
 Scapolites • Metamorphic rock minerals probably derived from feldspars • The alumino-silicate framework forms chains in the c-direction and has large open spaces which can accommodate large anions such a Cl, CO 3, SO 4 142
Scapolites • Metamorphic rock minerals probably derived from feldspars • The alumino-silicate framework forms chains in the c-direction and has large open spaces which can accommodate large anions such a Cl, CO 3, SO 4 142
 Scapolite Minerals • Marialite Na 4(Al. Si 3 O 8)3(Cl 2, CO 3, SO 4) • Meionite Ca 4(Al 2 Si 2 O 8)3(Cl 2, CO 3, SO 4) Marialite cluster 143
Scapolite Minerals • Marialite Na 4(Al. Si 3 O 8)3(Cl 2, CO 3, SO 4) • Meionite Ca 4(Al 2 Si 2 O 8)3(Cl 2, CO 3, SO 4) Marialite cluster 143
 Zeolites • Hydrous aluminosilicates with very open structures. Stilbite • Rings of Al. O 4 and Si. O 4 tetrahedra are penetrated by open channels in the structure • Non-silicon cations hold the structure together. 144
Zeolites • Hydrous aluminosilicates with very open structures. Stilbite • Rings of Al. O 4 and Si. O 4 tetrahedra are penetrated by open channels in the structure • Non-silicon cations hold the structure together. 144
 Cation Exchange • Water can easily pass though these channels and dissolve and replace the cations present in the structure • This process in known as cation exchange and is reversible • Thus, the zeolites can serve as catalysts and water-softening agents • Petroleum companies have been particularly interested in zeolites for this reason 145
Cation Exchange • Water can easily pass though these channels and dissolve and replace the cations present in the structure • This process in known as cation exchange and is reversible • Thus, the zeolites can serve as catalysts and water-softening agents • Petroleum companies have been particularly interested in zeolites for this reason 145
 Important Natural Zeolites • Chabazite Ca 2(Al 2 Si 4 O 12)∙ 6 H 2 O • Heulandite Ca(Al 2 Si 7018)∙ 6 H 2 O • Stilbite (Na, K, Ca 0. 5)9 Na(Al 9 Si 27 O 72)∙ 28 H 2 O • Natrolite Na 2(Al 2 Si 3 O 10)∙ 2 H 2 O • Analcime. Na(Al. Si 2 O 6)∙H 2 O 146
Important Natural Zeolites • Chabazite Ca 2(Al 2 Si 4 O 12)∙ 6 H 2 O • Heulandite Ca(Al 2 Si 7018)∙ 6 H 2 O • Stilbite (Na, K, Ca 0. 5)9 Na(Al 9 Si 27 O 72)∙ 28 H 2 O • Natrolite Na 2(Al 2 Si 3 O 10)∙ 2 H 2 O • Analcime. Na(Al. Si 2 O 6)∙H 2 O 146
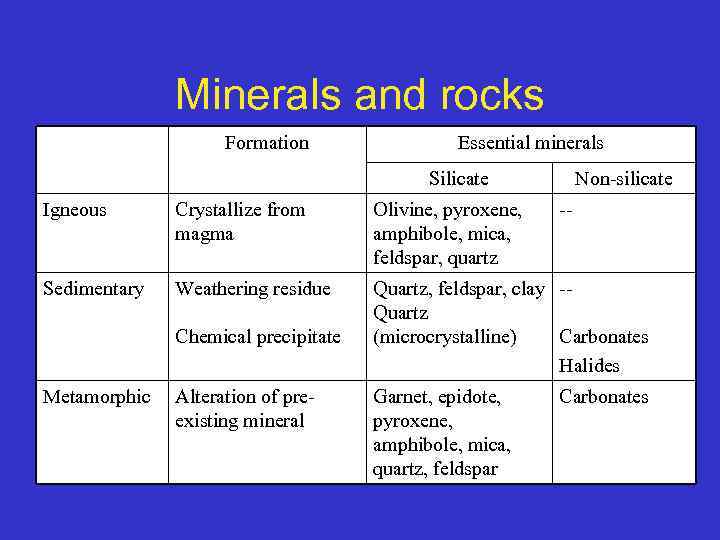 Minerals and rocks Formation Essential minerals Silicate Non-silicate Igneous Crystallize from magma Olivine, pyroxene, amphibole, mica, feldspar, quartz Sedimentary Weathering residue Quartz, feldspar, clay -Quartz (microcrystalline) Carbonates Halides Chemical precipitate Metamorphic Alteration of preexisting mineral Garnet, epidote, pyroxene, amphibole, mica, quartz, feldspar -- Carbonates
Minerals and rocks Formation Essential minerals Silicate Non-silicate Igneous Crystallize from magma Olivine, pyroxene, amphibole, mica, feldspar, quartz Sedimentary Weathering residue Quartz, feldspar, clay -Quartz (microcrystalline) Carbonates Halides Chemical precipitate Metamorphic Alteration of preexisting mineral Garnet, epidote, pyroxene, amphibole, mica, quartz, feldspar -- Carbonates


