5 - Сharacteristics of CPS.pptx
- Количество слайдов: 12
 Сharacteristics of Chemical power sources (CPS)
Сharacteristics of Chemical power sources (CPS)
 The following battery characteristics must be taken into consideration when selecting a battery: Ø Type Ø Voltage Ø Charge/Discharge curve Ø Capacity Ø Energy density Ø Temperature dependence Ø Service life Ø Self Discharge
The following battery characteristics must be taken into consideration when selecting a battery: Ø Type Ø Voltage Ø Charge/Discharge curve Ø Capacity Ø Energy density Ø Temperature dependence Ø Service life Ø Self Discharge
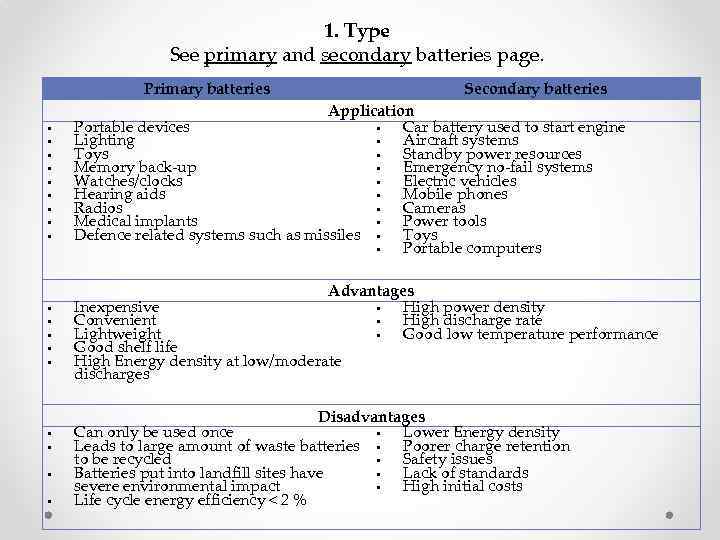 1. Type See primary and secondary batteries page. Primary batteries Secondary batteries Application Portable devices Car battery used to start engine Lighting Aircraft systems Toys Standby power resources Memory back-up Emergency no-fail systems Watches/clocks Electric vehicles Hearing aids Mobile phones Radios Cameras Medical implants Power tools Defence related systems such as missiles Toys Portable computers Advantages Inexpensive High power density Convenient High discharge rate Lightweight Good low temperature performance Good shelf life High Energy density at low/moderate discharges Disadvantages Can only be used once Lower Energy density Leads to large amount of waste batteries Poorer charge retention to be recycled Safety issues Batteries put into landfill sites have Lack of standards severe environmental impact High initial costs Life cycle energy efficiency < 2 %
1. Type See primary and secondary batteries page. Primary batteries Secondary batteries Application Portable devices Car battery used to start engine Lighting Aircraft systems Toys Standby power resources Memory back-up Emergency no-fail systems Watches/clocks Electric vehicles Hearing aids Mobile phones Radios Cameras Medical implants Power tools Defence related systems such as missiles Toys Portable computers Advantages Inexpensive High power density Convenient High discharge rate Lightweight Good low temperature performance Good shelf life High Energy density at low/moderate discharges Disadvantages Can only be used once Lower Energy density Leads to large amount of waste batteries Poorer charge retention to be recycled Safety issues Batteries put into landfill sites have Lack of standards severe environmental impact High initial costs Life cycle energy efficiency < 2 %
 2. Voltage The theoretical standard cell voltage can be determined from the electrochemical series using Eo values: Eo (cathodic) – Eo (anodic) = Eo (cell) ЭДС – электродвижущая сила (теоретиич. ) The open circuit voltage (OCV), U 0 Напряжение разомкнутой цепи (эксперим. )
2. Voltage The theoretical standard cell voltage can be determined from the electrochemical series using Eo values: Eo (cathodic) – Eo (anodic) = Eo (cell) ЭДС – электродвижущая сила (теоретиич. ) The open circuit voltage (OCV), U 0 Напряжение разомкнутой цепи (эксперим. )
 3. The Charge/Discharge Curve The measured terminal voltage of any battery will vary as it is charged and discharged (see Figure 1). The MPV (mid-point voltage) is the nominal voltage of the cell during charge or discharge. The maximum and minimum voltage excursion from the nominal value is an important design consideration: a "flatter“ discharge curve means less voltage variation that the design must tolerate. When peak charged, the actual cell voltage will be higher than the MPV. When nearing the EODV (end of discharge voltage) point, the cell voltage will be less than the MPV. The EODV is sometimes referred to as the EOL (end of life) voltage by manufacturers.
3. The Charge/Discharge Curve The measured terminal voltage of any battery will vary as it is charged and discharged (see Figure 1). The MPV (mid-point voltage) is the nominal voltage of the cell during charge or discharge. The maximum and minimum voltage excursion from the nominal value is an important design consideration: a "flatter“ discharge curve means less voltage variation that the design must tolerate. When peak charged, the actual cell voltage will be higher than the MPV. When nearing the EODV (end of discharge voltage) point, the cell voltage will be less than the MPV. The EODV is sometimes referred to as the EOL (end of life) voltage by manufacturers.
 4. Capacity The theoretical capacity of a battery is the quantity of electricity involved in the electro-chemical reaction. It is denoted Q and is given by: where x = number of moles of reaction, n = number of electrons transferred per mole of reaction and F = Faraday's constant. The capacity is usually given in terms of mass, not the number of moles: where Mr = Molecular Mass. This gives the capacity in units of Amperehours per gram (Ah/g). Емкость элемента – это количество электричества, которое химический источник тока отдает при разряде C = I. (А. ч, если элемент разряжается током I (A) в течение (ч))
4. Capacity The theoretical capacity of a battery is the quantity of electricity involved in the electro-chemical reaction. It is denoted Q and is given by: where x = number of moles of reaction, n = number of electrons transferred per mole of reaction and F = Faraday's constant. The capacity is usually given in terms of mass, not the number of moles: where Mr = Molecular Mass. This gives the capacity in units of Amperehours per gram (Ah/g). Емкость элемента – это количество электричества, которое химический источник тока отдает при разряде C = I. (А. ч, если элемент разряжается током I (A) в течение (ч))
 5. Energy Density (By Weight and Volume) The energy density of a battery is generally expressed in two ways (see Figure 2). The gravimetric energy density of a battery is a measure of how much energy a battery contains in comparison to its weight, and is typically expressed in Watt-hours/kilogram (W-hr/kg). The volumetric energy density of a battery is a measure of how much energy a battery contains in comparison to its volume, and is typically expressed in Watt-hours/liter (W-hr/l).
5. Energy Density (By Weight and Volume) The energy density of a battery is generally expressed in two ways (see Figure 2). The gravimetric energy density of a battery is a measure of how much energy a battery contains in comparison to its weight, and is typically expressed in Watt-hours/kilogram (W-hr/kg). The volumetric energy density of a battery is a measure of how much energy a battery contains in comparison to its volume, and is typically expressed in Watt-hours/liter (W-hr/l).

 6. Temperature dependence The rate of decrease of voltage with increasing discharge will also be higher at lower temperatures, as will the capacity- this is illustrated by the following graph:
6. Temperature dependence The rate of decrease of voltage with increasing discharge will also be higher at lower temperatures, as will the capacity- this is illustrated by the following graph:
 7. Service life Batteries can also be subjected to premature death by: q Over-charging q Over-discharging q Short circuiting q Drawing more current than it was designed to produce q extreme temperatures q physical shock or vibrations Metallic dendrites (battery death due to aging)
7. Service life Batteries can also be subjected to premature death by: q Over-charging q Over-discharging q Short circuiting q Drawing more current than it was designed to produce q extreme temperatures q physical shock or vibrations Metallic dendrites (battery death due to aging)
 8. Self Discharge Self-discharge (which occurs in all batteries) determines the "shelf life" of a battery. In general, Li-Ion is the best of the lot, while Ni-Cd and Ni-MH are fairly comparable to each other. Ni-Cd is typically a little better than Ni-MH, but this may even out as Ni-MH manufacturing technology matures.
8. Self Discharge Self-discharge (which occurs in all batteries) determines the "shelf life" of a battery. In general, Li-Ion is the best of the lot, while Ni-Cd and Ni-MH are fairly comparable to each other. Ni-Cd is typically a little better than Ni-MH, but this may even out as Ni-MH manufacturing technology matures.



