37cfef6070eab943c773593774ecae7a.ppt
- Количество слайдов: 32
 SHAr. K Outreach ⊂ Powering the Planet (NSF CCI Solar Program) Ella Wong 2009 Summer Solar Class Professor Wamser, PSU
SHAr. K Outreach ⊂ Powering the Planet (NSF CCI Solar Program) Ella Wong 2009 Summer Solar Class Professor Wamser, PSU
 SHAr. K (Solar Hydrogen Activity Research Kit)- Mission/Goals • “Dedicated to splitting water with sunlight” • To discover stable metal oxide semiconductors that can efficiently photoelectrolyze water and that is economically viable • To engage and encourage young people to take an active role in solving the global energy problem • To provide students the opportunity to participate in real time, active scientific research • Outreach effort from Bruce Parkinson’s group (University of Wyoming) • SHAr. K is part of the NSF-funded Powering the Planet Project http: //www. thesharkproject. org/
SHAr. K (Solar Hydrogen Activity Research Kit)- Mission/Goals • “Dedicated to splitting water with sunlight” • To discover stable metal oxide semiconductors that can efficiently photoelectrolyze water and that is economically viable • To engage and encourage young people to take an active role in solving the global energy problem • To provide students the opportunity to participate in real time, active scientific research • Outreach effort from Bruce Parkinson’s group (University of Wyoming) • SHAr. K is part of the NSF-funded Powering the Planet Project http: //www. thesharkproject. org/
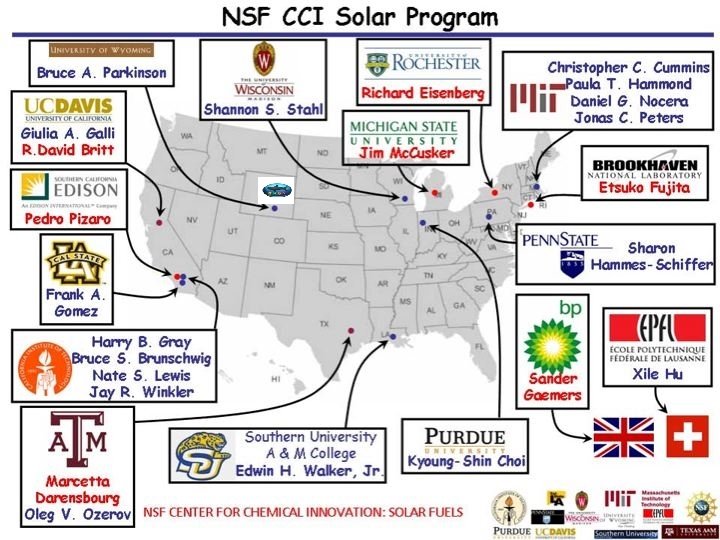
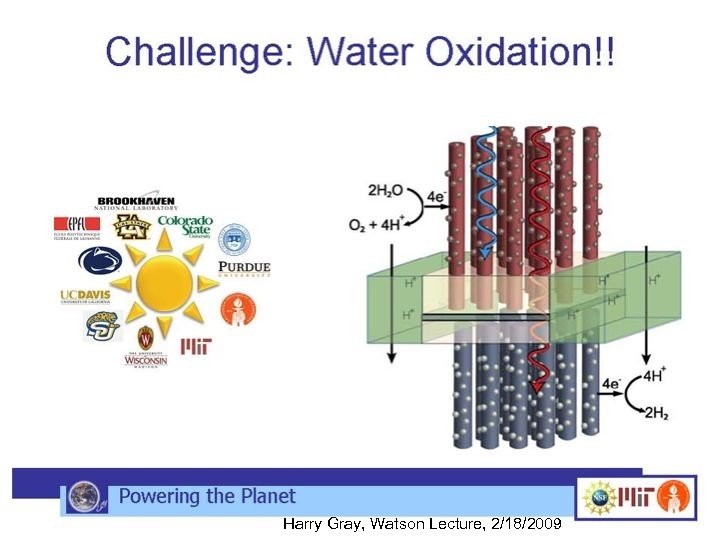
 NSF CCI (Center for Chemical Innovation) Solar Program- Powering the Planet http: //www. ccisolar. caltech. edu/ • Goal to develop efficient, inexpensive, sustainable way(s) to convert solar energy into stored chemical fuel(s) • Ideally to split water with sunlight using cheap, safe, chemically stable, easilyobtained earth-abundant materials yielding hydrogen as chemical fuel • 3 component water splitting model
NSF CCI (Center for Chemical Innovation) Solar Program- Powering the Planet http: //www. ccisolar. caltech. edu/ • Goal to develop efficient, inexpensive, sustainable way(s) to convert solar energy into stored chemical fuel(s) • Ideally to split water with sunlight using cheap, safe, chemically stable, easilyobtained earth-abundant materials yielding hydrogen as chemical fuel • 3 component water splitting model
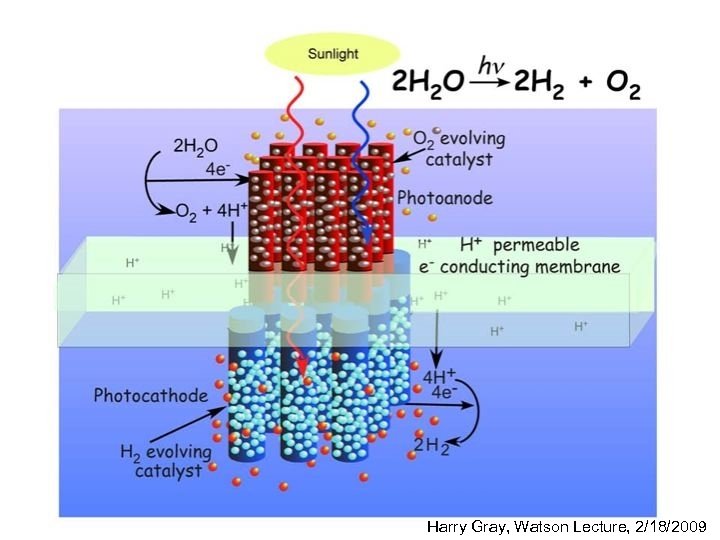
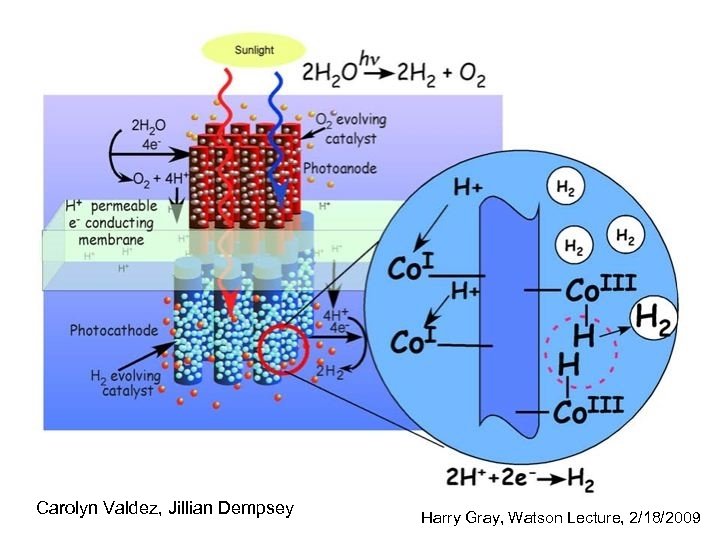
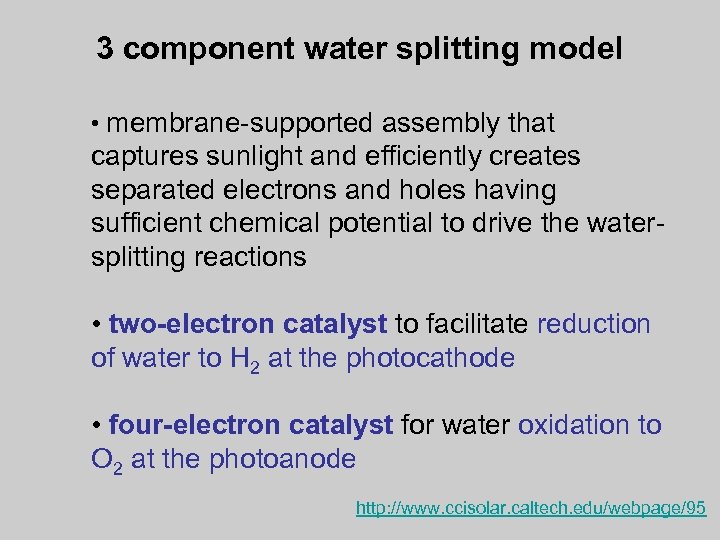 3 component water splitting model • membrane-supported assembly that captures sunlight and efficiently creates separated electrons and holes having sufficient chemical potential to drive the watersplitting reactions • two-electron catalyst to facilitate reduction of water to H 2 at the photocathode • four-electron catalyst for water oxidation to O 2 at the photoanode http: //www. ccisolar. caltech. edu/webpage/95
3 component water splitting model • membrane-supported assembly that captures sunlight and efficiently creates separated electrons and holes having sufficient chemical potential to drive the watersplitting reactions • two-electron catalyst to facilitate reduction of water to H 2 at the photocathode • four-electron catalyst for water oxidation to O 2 at the photoanode http: //www. ccisolar. caltech. edu/webpage/95
 Harry Gray, Watson Lecture, 2/18/2009
Harry Gray, Watson Lecture, 2/18/2009
 Harry Gray, Watson Lecture, 2/18/2009
Harry Gray, Watson Lecture, 2/18/2009
 Carolyn Valdez, Jillian Dempsey Harry Gray, Watson Lecture, 2/18/2009
Carolyn Valdez, Jillian Dempsey Harry Gray, Watson Lecture, 2/18/2009
 Harry Gray, Watson Lecture, 2/18/2009
Harry Gray, Watson Lecture, 2/18/2009
 MIT group’s progress on catalyst for oxygen-evolving side Tutorial Review Chem. Soc. Rev. , 2009, 38, 109 - 114, DOI: 10. 1039/b 802885 k Cobalt–phosphate oxygen-evolving compound Matthew W. Kanan, Yogesh Surendranath and Daniel G. Nocera The utilization of solar energy on a large scale requires efficient storage. Solarto-fuels has the capacity to meet large scale storage needs as demonstrated by natural photosynthesis. This process uses sunlight to rearrange the bonds of water to furnish O 2 and an H 2 -equivalent. We present a tutorial review of our efforts to develop an amorphous cobalt–phosphate catalyst that oxidizes water to O 2. The use of earth-abundant materials, operation in water at neutral p. H, and the formation of the catalyst in situ captures functional elements of the oxygen evolving complex of Photosystem II. http: //www. technologyreview. com/Energy/21155/page 1/ http: //web. mit. edu/newsoffice/2008/oxygen-0731. html • runs on electricity from outlet • still needs development work on photoanode material
MIT group’s progress on catalyst for oxygen-evolving side Tutorial Review Chem. Soc. Rev. , 2009, 38, 109 - 114, DOI: 10. 1039/b 802885 k Cobalt–phosphate oxygen-evolving compound Matthew W. Kanan, Yogesh Surendranath and Daniel G. Nocera The utilization of solar energy on a large scale requires efficient storage. Solarto-fuels has the capacity to meet large scale storage needs as demonstrated by natural photosynthesis. This process uses sunlight to rearrange the bonds of water to furnish O 2 and an H 2 -equivalent. We present a tutorial review of our efforts to develop an amorphous cobalt–phosphate catalyst that oxidizes water to O 2. The use of earth-abundant materials, operation in water at neutral p. H, and the formation of the catalyst in situ captures functional elements of the oxygen evolving complex of Photosystem II. http: //www. technologyreview. com/Energy/21155/page 1/ http: //web. mit. edu/newsoffice/2008/oxygen-0731. html • runs on electricity from outlet • still needs development work on photoanode material
 Harry Gray, Watson Lecture, 2/18/2009
Harry Gray, Watson Lecture, 2/18/2009
 Harry Gray, Watson Lecture, 2/18/2009 http: //pr. caltech. edu/periodicals/caltechnews/articles/v 42/sun. html
Harry Gray, Watson Lecture, 2/18/2009 http: //pr. caltech. edu/periodicals/caltechnews/articles/v 42/sun. html
 SHAr. K Outreach http: //www. thesharkproject. org/ Harry Gray’s Powering the Planet with Solar Fuels Lecture at Caltech’s Beckman Auditorium on 2/18/2009 features SHAr. K demonstration and a call for a “solar army” of students to work on the challenges. http: //today. caltech. edu/theater/item? story%5 fid=33982 Lectures by Nate Lewishttp: //today. caltech. edu/theater/item? story_id=8424 (5/25/05) http: //nrg. caltech. edu/index. html (11/30/2007)
SHAr. K Outreach http: //www. thesharkproject. org/ Harry Gray’s Powering the Planet with Solar Fuels Lecture at Caltech’s Beckman Auditorium on 2/18/2009 features SHAr. K demonstration and a call for a “solar army” of students to work on the challenges. http: //today. caltech. edu/theater/item? story%5 fid=33982 Lectures by Nate Lewishttp: //today. caltech. edu/theater/item? story_id=8424 (5/25/05) http: //nrg. caltech. edu/index. html (11/30/2007)
 Harry Gray, Watson Lecture, 2/18/2009
Harry Gray, Watson Lecture, 2/18/2009
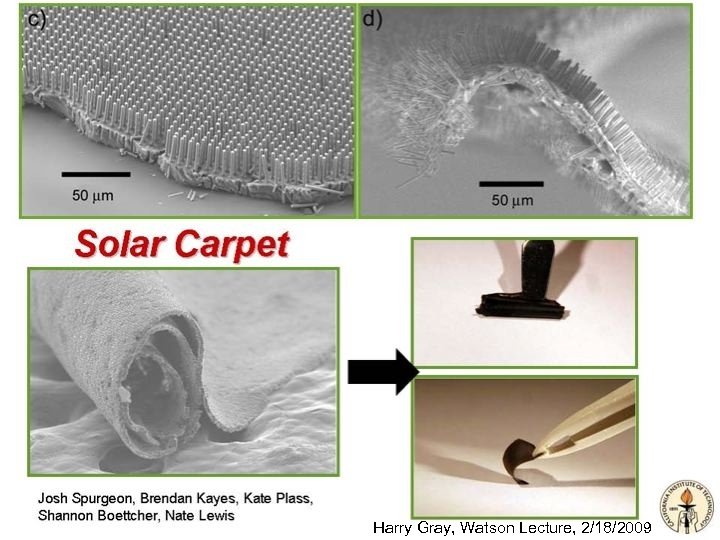
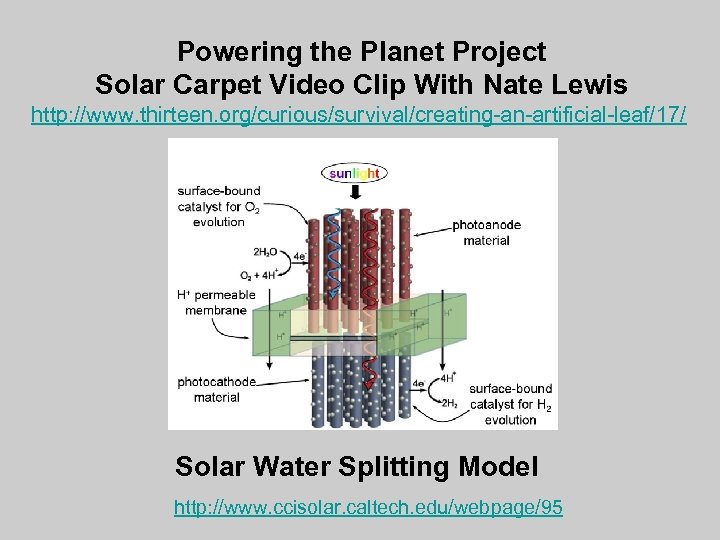 Powering the Planet Project Solar Carpet Video Clip With Nate Lewis http: //www. thirteen. org/curious/survival/creating-an-artificial-leaf/17/ Solar Water Splitting Model http: //www. ccisolar. caltech. edu/webpage/95
Powering the Planet Project Solar Carpet Video Clip With Nate Lewis http: //www. thirteen. org/curious/survival/creating-an-artificial-leaf/17/ Solar Water Splitting Model http: //www. ccisolar. caltech. edu/webpage/95
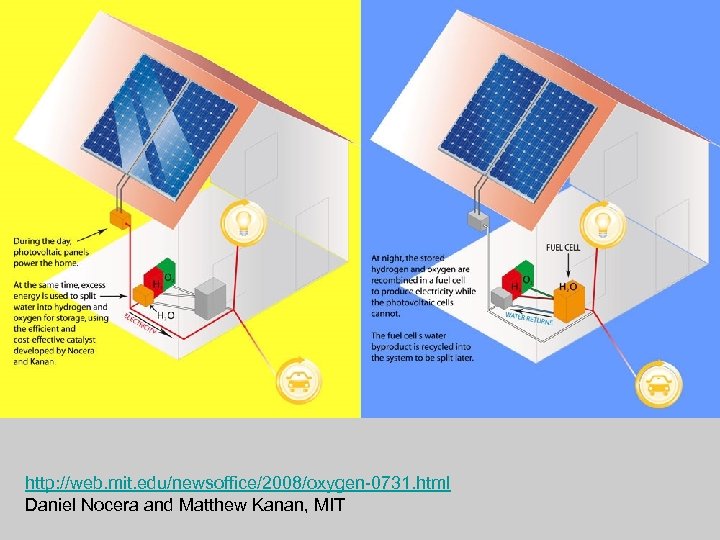 http: //web. mit. edu/newsoffice/2008/oxygen-0731. html Daniel Nocera and Matthew Kanan, MIT
http: //web. mit. edu/newsoffice/2008/oxygen-0731. html Daniel Nocera and Matthew Kanan, MIT
 SHAr. K Specifics
SHAr. K Specifics
 A SHAr. K Project Kit * LEGO Mindstorms® Kits * Extra LEGOs® Parts * Commercial Inkjet Printer * Pipettes * Commercial Green Laser Pointer * Data Acquisition Box * Conductive Glass Substrates * Etched Glass Electrochemical Cell * Alligator clips, Copper wire, and Graphite (counterelectrode) * Laser Safety Goggles * Software installed from website http: //www. thesharkproject. org/
A SHAr. K Project Kit * LEGO Mindstorms® Kits * Extra LEGOs® Parts * Commercial Inkjet Printer * Pipettes * Commercial Green Laser Pointer * Data Acquisition Box * Conductive Glass Substrates * Etched Glass Electrochemical Cell * Alligator clips, Copper wire, and Graphite (counterelectrode) * Laser Safety Goggles * Software installed from website http: //www. thesharkproject. org/
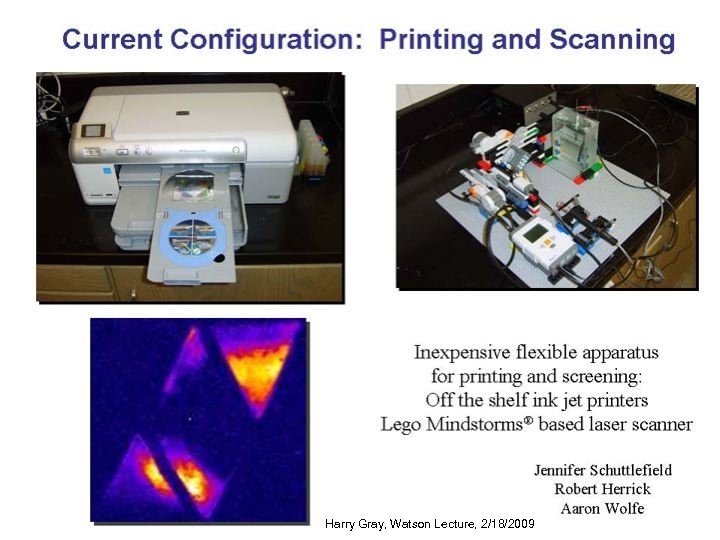
 Printing and Scanning Basics • An inkjet printer is used to print a mixture of aqueous metal nitrate solutions onto a conductive glass substrate to produce ternary metal oxides. The printed substrate is baked in an oven to decompose the nitrates salts to metal oxides. • Printer template designed to print two internal standards (Fe and Cu) with 3 or 4 other metals in a combinatorial pattern that allows for many different combinations and compositions of the metal oxides to be produced and screened at a time.
Printing and Scanning Basics • An inkjet printer is used to print a mixture of aqueous metal nitrate solutions onto a conductive glass substrate to produce ternary metal oxides. The printed substrate is baked in an oven to decompose the nitrates salts to metal oxides. • Printer template designed to print two internal standards (Fe and Cu) with 3 or 4 other metals in a combinatorial pattern that allows for many different combinations and compositions of the metal oxides to be produced and screened at a time.
 Printing and Scanning Basics • Fe 2 O 3 is n-type standard (photoanode, O 2 production) • “lights up” with positive bias • Cu. O is p-type standard (photocathode, H 2 production) • “lights up” with negative bias • Printed metal oxide arrays are scanned for solar water splitting potential using the Lego Laser Scanning Station • Material with photocurrent response at least 2 times higher than either of the standards is considered promising. SHAr. K Manual Version 2. 0 and SHAr. K Brochure
Printing and Scanning Basics • Fe 2 O 3 is n-type standard (photoanode, O 2 production) • “lights up” with positive bias • Cu. O is p-type standard (photocathode, H 2 production) • “lights up” with negative bias • Printed metal oxide arrays are scanned for solar water splitting potential using the Lego Laser Scanning Station • Material with photocurrent response at least 2 times higher than either of the standards is considered promising. SHAr. K Manual Version 2. 0 and SHAr. K Brochure
 Some material specifics • Glass substrate- fluorine-doped tin oxide (FTO) coating • Metal precursors-. 35 M metal nitrate salt with. 6 M ammonium nitrate (NH 4 NO 3) and. 015 M nitric acid (HNO 3) • 500 °C overnight to convert to metal oxide • HP D 5460 ink jet printer • Lego Mindstorms-based laser scanner ( = 532 nm, green) SHAr. K Manual Version 2. 0
Some material specifics • Glass substrate- fluorine-doped tin oxide (FTO) coating • Metal precursors-. 35 M metal nitrate salt with. 6 M ammonium nitrate (NH 4 NO 3) and. 015 M nitric acid (HNO 3) • 500 °C overnight to convert to metal oxide • HP D 5460 ink jet printer • Lego Mindstorms-based laser scanner ( = 532 nm, green) SHAr. K Manual Version 2. 0
 Metal oxides- Potential role in photoelectrode • Structural (Ti, W, Zr, Ta, Si, Mo, Nb, Hf, In, Sn, Ga, Y, Sc) • Light absorbing (Fe, Cr, V, Co, Mn, Ni, Cu, and some rare earths such as Ce) • Catalytic (Ru, Rh, Pd, Pt, Ir, Os, Re, Ni) • Ionic charge compensators (Ca, Sr, Ba, Mg, Zn, Cd, Li, Na, K, Rb, Cs) • Avoid toxic metals (Pb, Tl, Cd, Hg) Chem. Mater. 2005, 17, p. 4320
Metal oxides- Potential role in photoelectrode • Structural (Ti, W, Zr, Ta, Si, Mo, Nb, Hf, In, Sn, Ga, Y, Sc) • Light absorbing (Fe, Cr, V, Co, Mn, Ni, Cu, and some rare earths such as Ce) • Catalytic (Ru, Rh, Pd, Pt, Ir, Os, Re, Ni) • Ionic charge compensators (Ca, Sr, Ba, Mg, Zn, Cd, Li, Na, K, Rb, Cs) • Avoid toxic metals (Pb, Tl, Cd, Hg) Chem. Mater. 2005, 17, p. 4320
 CD/DVD Tray Cutoutholds substrate for printing http: //www. thesharkproject. org/node/506
CD/DVD Tray Cutoutholds substrate for printing http: //www. thesharkproject. org/node/506
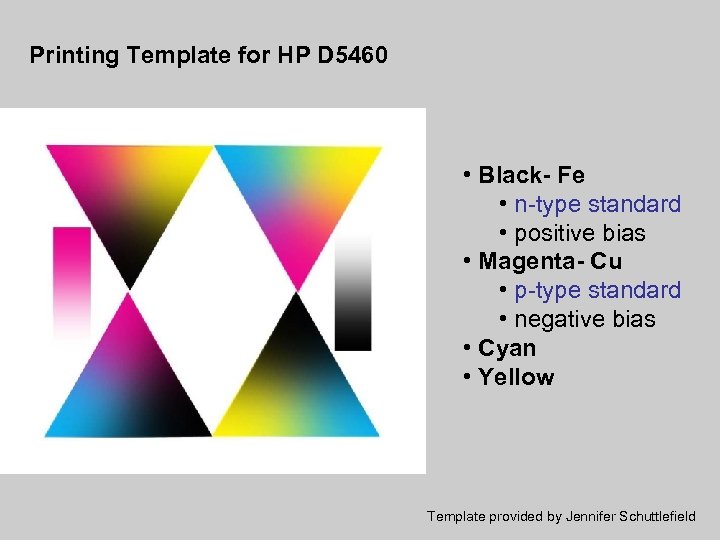 Printing Template for HP D 5460 • Black- Fe • n-type standard • positive bias • Magenta- Cu • p-type standard • negative bias • Cyan • Yellow Template provided by Jennifer Schuttlefield
Printing Template for HP D 5460 • Black- Fe • n-type standard • positive bias • Magenta- Cu • p-type standard • negative bias • Cyan • Yellow Template provided by Jennifer Schuttlefield

 Sample Scans
Sample Scans
 Cu-Cs-Fe-La From SHAr. K brochure
Cu-Cs-Fe-La From SHAr. K brochure
 Al-Co-Cs-Fe System: Template and Zero Bias Scan Chem Mater. 2008, 20, p. 2497
Al-Co-Cs-Fe System: Template and Zero Bias Scan Chem Mater. 2008, 20, p. 2497
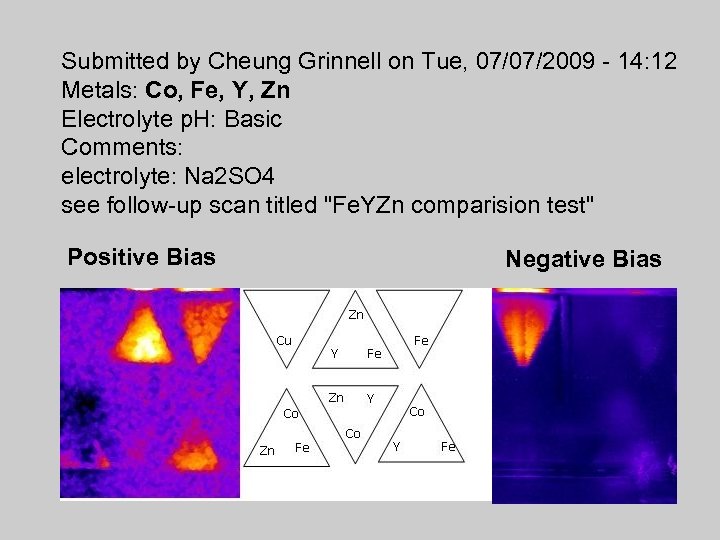 Submitted by Cheung Grinnell on Tue, 07/07/2009 - 14: 12 Metals: Co, Fe, Y, Zn Electrolyte p. H: Basic Comments: electrolyte: Na 2 SO 4 see follow-up scan titled "Fe. YZn comparision test" Positive Bias Negative Bias
Submitted by Cheung Grinnell on Tue, 07/07/2009 - 14: 12 Metals: Co, Fe, Y, Zn Electrolyte p. H: Basic Comments: electrolyte: Na 2 SO 4 see follow-up scan titled "Fe. YZn comparision test" Positive Bias Negative Bias
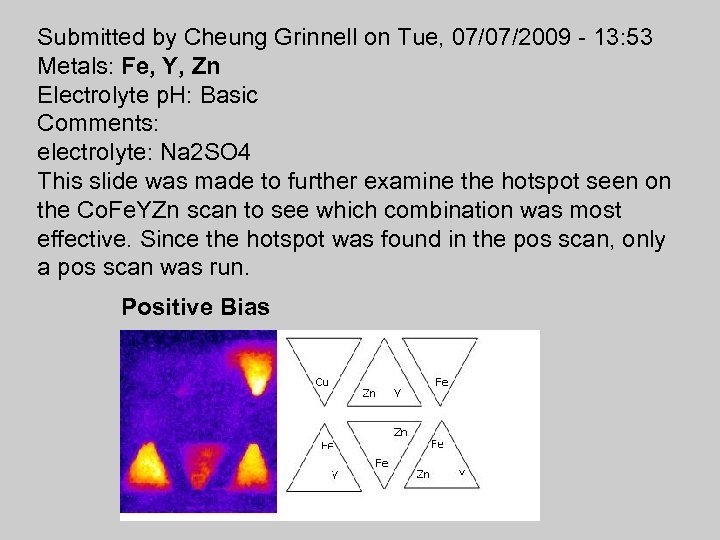 Submitted by Cheung Grinnell on Tue, 07/07/2009 - 13: 53 Metals: Fe, Y, Zn Electrolyte p. H: Basic Comments: electrolyte: Na 2 SO 4 This slide was made to further examine the hotspot seen on the Co. Fe. YZn scan to see which combination was most effective. Since the hotspot was found in the pos scan, only a pos scan was run. Positive Bias
Submitted by Cheung Grinnell on Tue, 07/07/2009 - 13: 53 Metals: Fe, Y, Zn Electrolyte p. H: Basic Comments: electrolyte: Na 2 SO 4 This slide was made to further examine the hotspot seen on the Co. Fe. YZn scan to see which combination was most effective. Since the hotspot was found in the pos scan, only a pos scan was run. Positive Bias
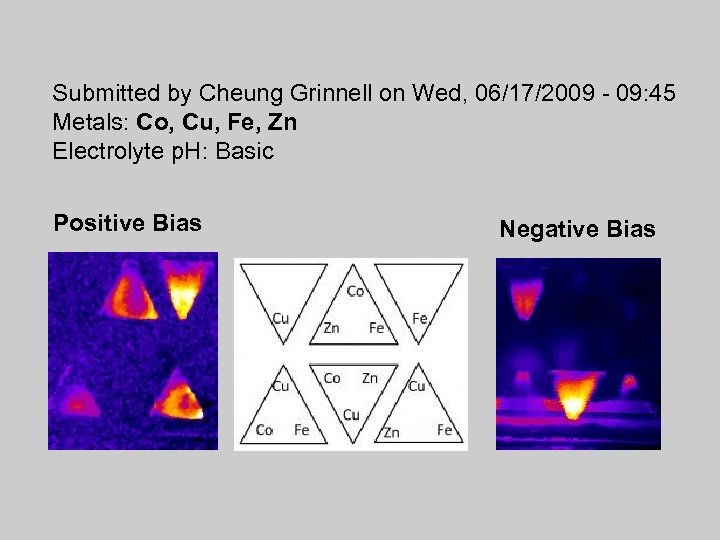 Submitted by Cheung Grinnell on Wed, 06/17/2009 - 09: 45 Metals: Co, Cu, Fe, Zn Electrolyte p. H: Basic Positive Bias Negative Bias
Submitted by Cheung Grinnell on Wed, 06/17/2009 - 09: 45 Metals: Co, Cu, Fe, Zn Electrolyte p. H: Basic Positive Bias Negative Bias
 Summary • SHAr. K engages students and provides a great opportunity to learn chemistry while participating in relevant research to help solve the global energy and climate change problems. • SHAr. K in conjunction with Powering the Planet joins students with researchers and scientists of many disciplines all over the world as we strive together to meet the Terawatt Challenge. • Special thanks to Bruce Parkinson and Jennifer Schuttlefield (University of Wyoming) for developing SHAr. K and reaching out. • And thanks to Professor Carl Wamser for bringing SHAr. K to PSU.
Summary • SHAr. K engages students and provides a great opportunity to learn chemistry while participating in relevant research to help solve the global energy and climate change problems. • SHAr. K in conjunction with Powering the Planet joins students with researchers and scientists of many disciplines all over the world as we strive together to meet the Terawatt Challenge. • Special thanks to Bruce Parkinson and Jennifer Schuttlefield (University of Wyoming) for developing SHAr. K and reaching out. • And thanks to Professor Carl Wamser for bringing SHAr. K to PSU.


