85a4629762fdfc53d4cdc4a63ca0c5b3.ppt
- Количество слайдов: 83
 Session 3 Optical Spectroscopy: Introduction/Fundamentals Atomic and molecular spectroscopies Instrumentation 1
Session 3 Optical Spectroscopy: Introduction/Fundamentals Atomic and molecular spectroscopies Instrumentation 1
 Overview n n Physical basis of absorption and emission n Atomic spectra n Molecular spectra Instrumentation: components of optical systems for spectrometers Common techniques in atomic spectroscopy: AAS and ICP-OES Calibration 2
Overview n n Physical basis of absorption and emission n Atomic spectra n Molecular spectra Instrumentation: components of optical systems for spectrometers Common techniques in atomic spectroscopy: AAS and ICP-OES Calibration 2
 Useful websites for spectroscopy n n http: //www. shsu. edu/~chm_tgc/sounds/fl ashfiles/ICPw. CCD. swf http: //www. thespectroscopynet. com/Inde x. html? / http: //teaching. shu. ac. uk/hwb/chemistry/t utorials/molspec/ http: //www. chemguide. co. uk/analysis/uvvi siblemenu. html See also individual citations on slides 3
Useful websites for spectroscopy n n http: //www. shsu. edu/~chm_tgc/sounds/fl ashfiles/ICPw. CCD. swf http: //www. thespectroscopynet. com/Inde x. html? / http: //teaching. shu. ac. uk/hwb/chemistry/t utorials/molspec/ http: //www. chemguide. co. uk/analysis/uvvi siblemenu. html See also individual citations on slides 3
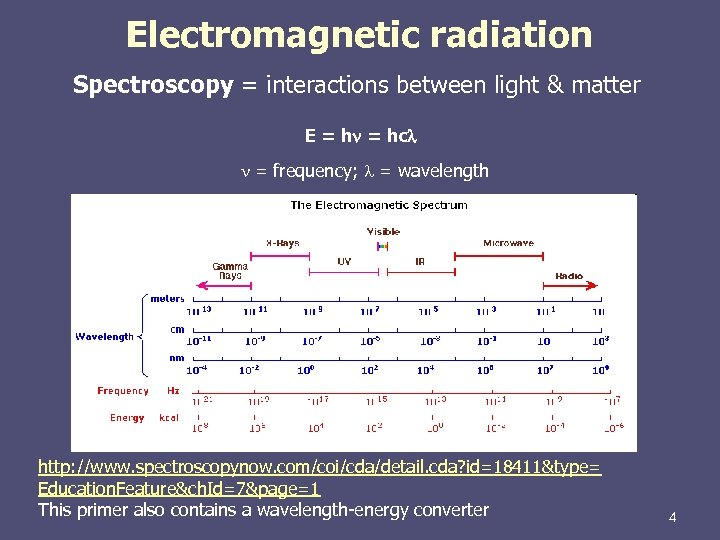 Electromagnetic radiation Spectroscopy = interactions between light & matter E = hn = hc n = frequency; = wavelength http: //www. spectroscopynow. com/coi/cda/detail. cda? id=18411&type= Education. Feature&ch. Id=7&page=1 This primer also contains a wavelength-energy converter 4
Electromagnetic radiation Spectroscopy = interactions between light & matter E = hn = hc n = frequency; = wavelength http: //www. spectroscopynow. com/coi/cda/detail. cda? id=18411&type= Education. Feature&ch. Id=7&page=1 This primer also contains a wavelength-energy converter 4
 Fundamentals n Absorption and emission of light by compounds is generally associated with transitions of electrons between different energy levels E 2 DE 2 excited states DE 2 DE = hn = hc/ E 1 DE 1 E 0 ground state Emission: Sample (in an excited state) produces light/looses energy DE 1 E 0 Absorption: sample takes up energy Consumes light of appropriate wavelength Atomic spectra: line spectra provide specificity: each element has its own pattern, as each element has its own electronic configuration http: //physics. nist. gov/Phys. Ref. Data/ASD/lines_form. html 5
Fundamentals n Absorption and emission of light by compounds is generally associated with transitions of electrons between different energy levels E 2 DE 2 excited states DE 2 DE = hn = hc/ E 1 DE 1 E 0 ground state Emission: Sample (in an excited state) produces light/looses energy DE 1 E 0 Absorption: sample takes up energy Consumes light of appropriate wavelength Atomic spectra: line spectra provide specificity: each element has its own pattern, as each element has its own electronic configuration http: //physics. nist. gov/Phys. Ref. Data/ASD/lines_form. html 5
 Fundamentals n The population of different states is given by the Boltzmann equation: N 0: number of atoms in ground state N 1: number of atoms in excited state g 1/g 0 : weighting factors Note: Equation contains temperature: Excitation can be achieved by providing thermal energy 6
Fundamentals n The population of different states is given by the Boltzmann equation: N 0: number of atoms in ground state N 1: number of atoms in excited state g 1/g 0 : weighting factors Note: Equation contains temperature: Excitation can be achieved by providing thermal energy 6
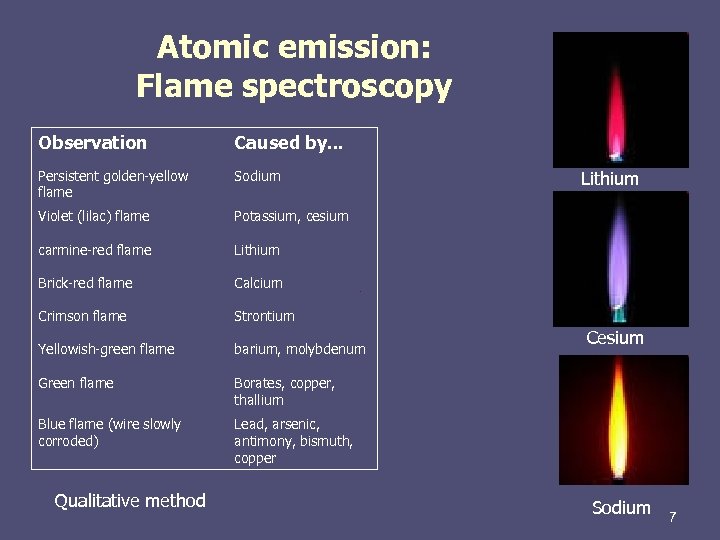 Atomic emission: Flame spectroscopy Observation Caused by. . . Persistent golden-yellow flame Sodium Violet (lilac) flame Potassium, cesium carmine-red flame Lithium Brick-red flame Calcium Crimson flame Strontium Yellowish-green flame barium, molybdenum Green flame Borates, copper, thallium Blue flame (wire slowly corroded) Lead, arsenic, antimony, bismuth, copper Qualitative method Lithium Cesium Sodium 7
Atomic emission: Flame spectroscopy Observation Caused by. . . Persistent golden-yellow flame Sodium Violet (lilac) flame Potassium, cesium carmine-red flame Lithium Brick-red flame Calcium Crimson flame Strontium Yellowish-green flame barium, molybdenum Green flame Borates, copper, thallium Blue flame (wire slowly corroded) Lead, arsenic, antimony, bismuth, copper Qualitative method Lithium Cesium Sodium 7
 A simple spectroscope n n n Spectroscope: Device for qualitative assessment of a sample E. g. used in flame analysis E. g. used in gemmology 8
A simple spectroscope n n n Spectroscope: Device for qualitative assessment of a sample E. g. used in flame analysis E. g. used in gemmology 8
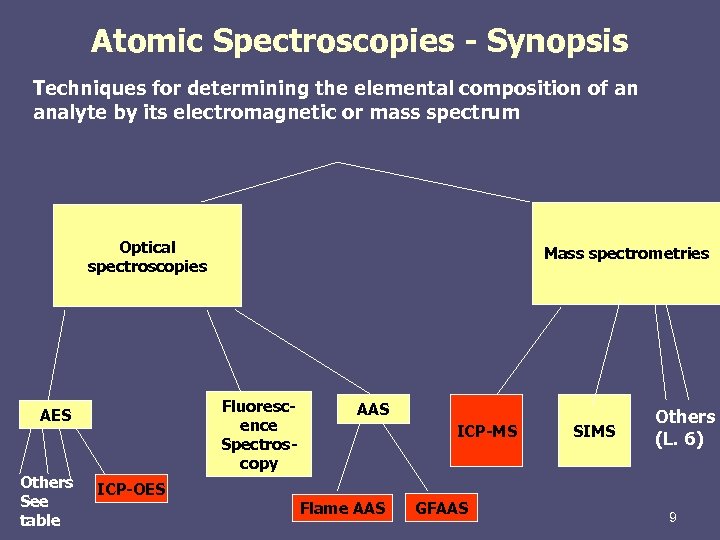 Atomic Spectroscopies - Synopsis Techniques for determining the elemental composition of an analyte by its electromagnetic or mass spectrum Optical spectroscopies Fluorescence Spectroscopy AES Others See table Mass spectrometries AAS ICP-MS ICP-OES Flame AAS GFAAS SIMS Others (L. 6) 9
Atomic Spectroscopies - Synopsis Techniques for determining the elemental composition of an analyte by its electromagnetic or mass spectrum Optical spectroscopies Fluorescence Spectroscopy AES Others See table Mass spectrometries AAS ICP-MS ICP-OES Flame AAS GFAAS SIMS Others (L. 6) 9
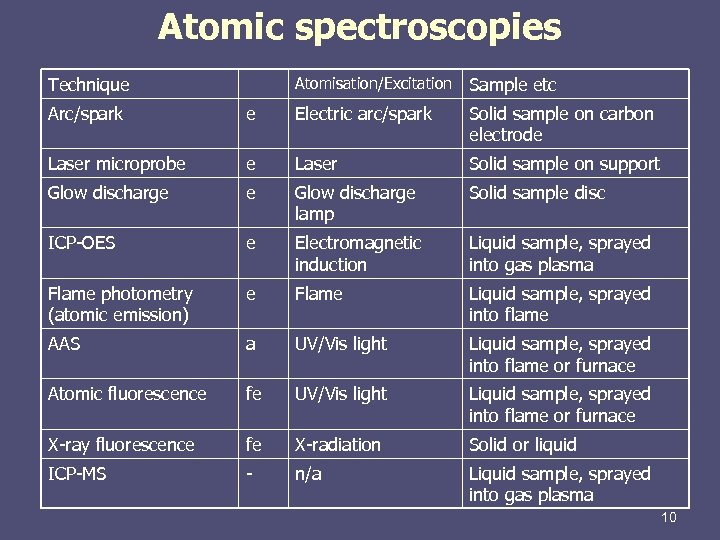 Atomic spectroscopies Technique Atomisation/Excitation Sample etc Arc/spark e Electric arc/spark Solid sample on carbon electrode Laser microprobe e Laser Solid sample on support Glow discharge e Glow discharge lamp Solid sample disc ICP-OES e Electromagnetic induction Liquid sample, sprayed into gas plasma Flame photometry (atomic emission) e Flame Liquid sample, sprayed into flame AAS a UV/Vis light Liquid sample, sprayed into flame or furnace Atomic fluorescence fe UV/Vis light Liquid sample, sprayed into flame or furnace X-ray fluorescence fe X-radiation Solid or liquid ICP-MS - n/a Liquid sample, sprayed into gas plasma 10
Atomic spectroscopies Technique Atomisation/Excitation Sample etc Arc/spark e Electric arc/spark Solid sample on carbon electrode Laser microprobe e Laser Solid sample on support Glow discharge e Glow discharge lamp Solid sample disc ICP-OES e Electromagnetic induction Liquid sample, sprayed into gas plasma Flame photometry (atomic emission) e Flame Liquid sample, sprayed into flame AAS a UV/Vis light Liquid sample, sprayed into flame or furnace Atomic fluorescence fe UV/Vis light Liquid sample, sprayed into flame or furnace X-ray fluorescence fe X-radiation Solid or liquid ICP-MS - n/a Liquid sample, sprayed into gas plasma 10
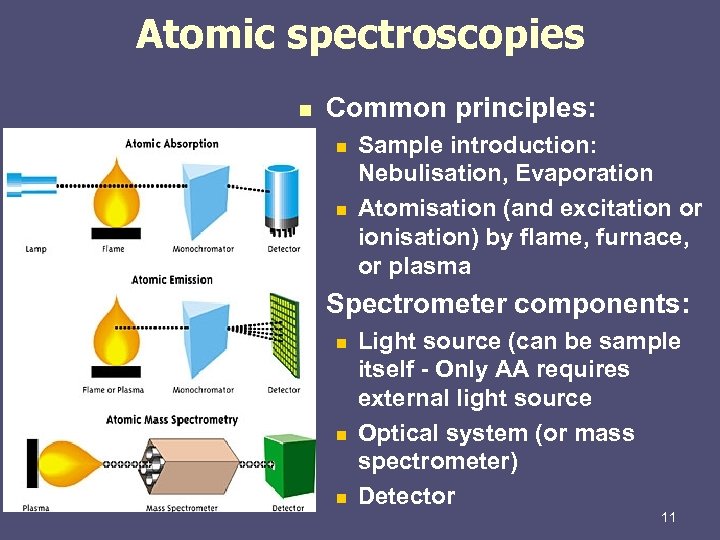 Atomic spectroscopies n Common principles: n n n Sample introduction: Nebulisation, Evaporation Atomisation (and excitation or ionisation) by flame, furnace, or plasma Spectrometer components: n n n Light source (can be sample itself - Only AA requires external light source Optical system (or mass spectrometer) Detector 11
Atomic spectroscopies n Common principles: n n n Sample introduction: Nebulisation, Evaporation Atomisation (and excitation or ionisation) by flame, furnace, or plasma Spectrometer components: n n n Light source (can be sample itself - Only AA requires external light source Optical system (or mass spectrometer) Detector 11
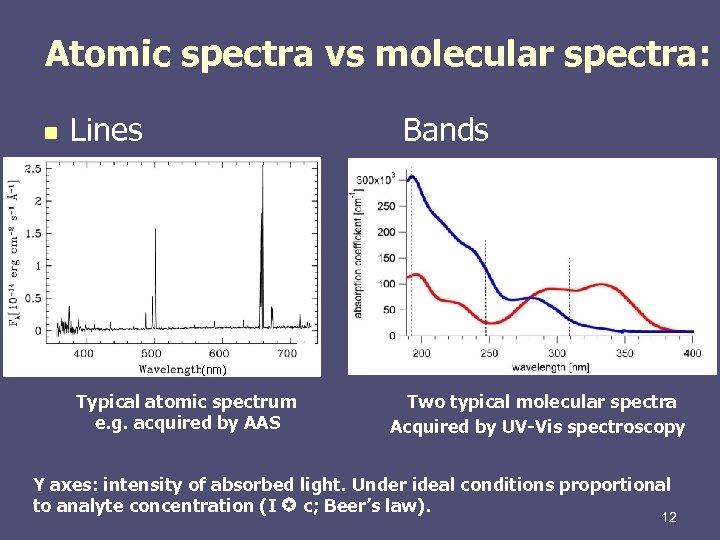 Atomic spectra vs molecular spectra: n Lines Bands (nm) Typical atomic spectrum e. g. acquired by AAS Two typical molecular spectra Acquired by UV-Vis spectroscopy Y axes: intensity of absorbed light. Under ideal conditions proportional to analyte concentration (I c; Beer’s law). 12
Atomic spectra vs molecular spectra: n Lines Bands (nm) Typical atomic spectrum e. g. acquired by AAS Two typical molecular spectra Acquired by UV-Vis spectroscopy Y axes: intensity of absorbed light. Under ideal conditions proportional to analyte concentration (I c; Beer’s law). 12
 Origin of bands in molecular spectra Molecules have chemical bonds n Electrons are in molecular orbitals n Absorption of light causes electron transitions between HOMO and LUMO n Molecules undergo bond rotations and vibrations: different energy sub-states occupied at RT and accessible through absorption: many transitions possible: èA band is the sum of many lines n LUMO Vibrational substates rotational substates HOMO 13
Origin of bands in molecular spectra Molecules have chemical bonds n Electrons are in molecular orbitals n Absorption of light causes electron transitions between HOMO and LUMO n Molecules undergo bond rotations and vibrations: different energy sub-states occupied at RT and accessible through absorption: many transitions possible: èA band is the sum of many lines n LUMO Vibrational substates rotational substates HOMO 13
 Quantitative analysis by molecular absorption: Colorimetry n Because absorption spectroscopy is widely applicable, sensitive (10 -5 -10 -7 M), selective, accurate (0. 1 -3% typically), and easy: n n n 95% of quantitative analyses in field of health performed with UV/Vis tests Hemoglobin in blood First step in analysis: establish working conditions n n n Selection, cleaning and handling of cells Calibration: determine relationship between absorbance and 14 concentration
Quantitative analysis by molecular absorption: Colorimetry n Because absorption spectroscopy is widely applicable, sensitive (10 -5 -10 -7 M), selective, accurate (0. 1 -3% typically), and easy: n n n 95% of quantitative analyses in field of health performed with UV/Vis tests Hemoglobin in blood First step in analysis: establish working conditions n n n Selection, cleaning and handling of cells Calibration: determine relationship between absorbance and 14 concentration
 Instrument components AAS Spectrometer Light Source Monochromator Sample Detector Readout/Data system ICP-OES Spectrometer Sample = light source Monochromator Detector Readout/Data system UV-Vis Spectrometer: Light Source Monochromator Sample Detector Readout/Data system 15
Instrument components AAS Spectrometer Light Source Monochromator Sample Detector Readout/Data system ICP-OES Spectrometer Sample = light source Monochromator Detector Readout/Data system UV-Vis Spectrometer: Light Source Monochromator Sample Detector Readout/Data system 15
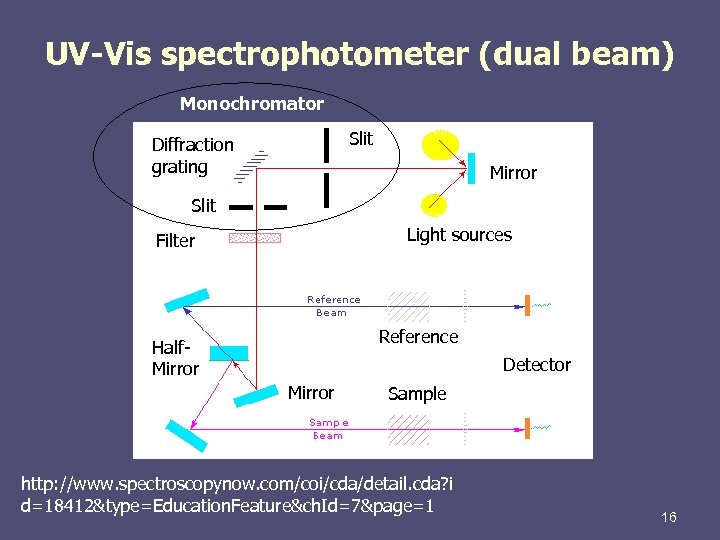 UV-Vis spectrophotometer (dual beam) Monochromator Slit Diffraction grating Mirror Slit Light sources Filter Reference Half. Mirror Detector Mirror Sample http: //www. spectroscopynow. com/coi/cda/detail. cda? i d=18412&type=Education. Feature&ch. Id=7&page=1 16
UV-Vis spectrophotometer (dual beam) Monochromator Slit Diffraction grating Mirror Slit Light sources Filter Reference Half. Mirror Detector Mirror Sample http: //www. spectroscopynow. com/coi/cda/detail. cda? i d=18412&type=Education. Feature&ch. Id=7&page=1 16
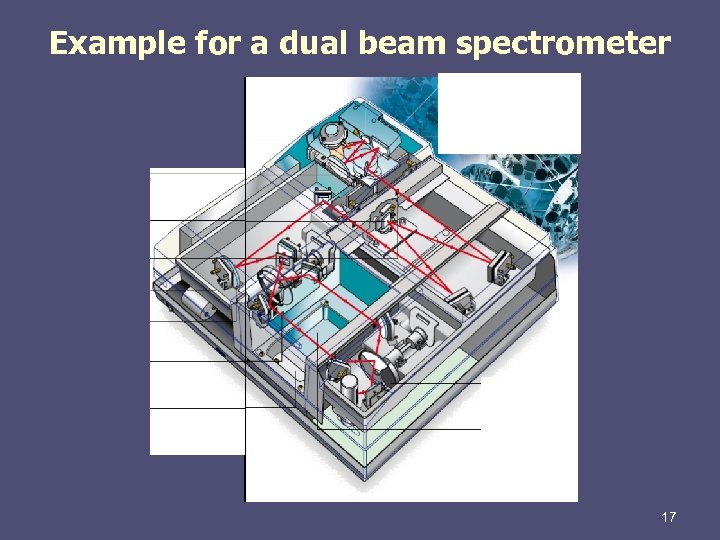 Example for a dual beam spectrometer 17
Example for a dual beam spectrometer 17
 Single beam 18
Single beam 18
 UV-Vis spectroscopy practicalities: Referencing n n Matrix (solvent, buffer etc) might also have absorbance: Must be taken care of In dual beam: n n n Simultaneous measurement of reference cell eliminates absorbance of background Recording of baseline recommended Single beam: n n Requires measurement of reference spectrum, can be subtracted from sample spectrum Preferentially in same cuvette 19
UV-Vis spectroscopy practicalities: Referencing n n Matrix (solvent, buffer etc) might also have absorbance: Must be taken care of In dual beam: n n n Simultaneous measurement of reference cell eliminates absorbance of background Recording of baseline recommended Single beam: n n Requires measurement of reference spectrum, can be subtracted from sample spectrum Preferentially in same cuvette 19
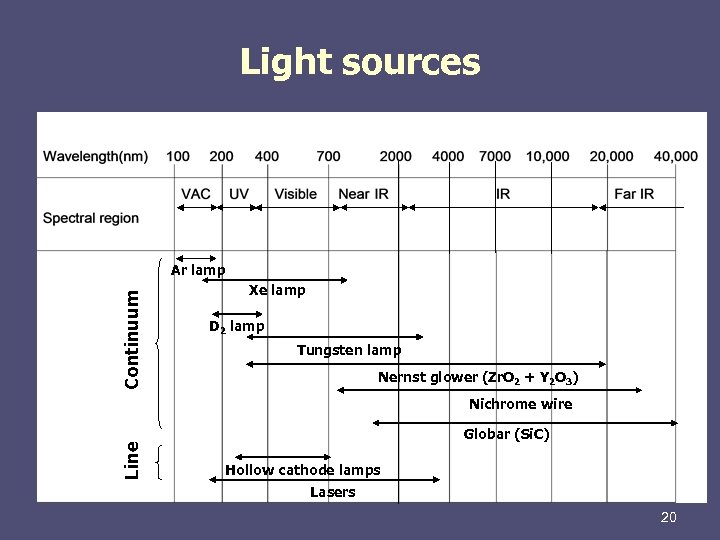 Light sources Continuum Ar lamp Xe lamp D 2 lamp Tungsten lamp Nernst glower (Zr. O 2 + Y 2 O 3) Line Nichrome wire Globar (Si. C) Hollow cathode lamps Lasers 20
Light sources Continuum Ar lamp Xe lamp D 2 lamp Tungsten lamp Nernst glower (Zr. O 2 + Y 2 O 3) Line Nichrome wire Globar (Si. C) Hollow cathode lamps Lasers 20
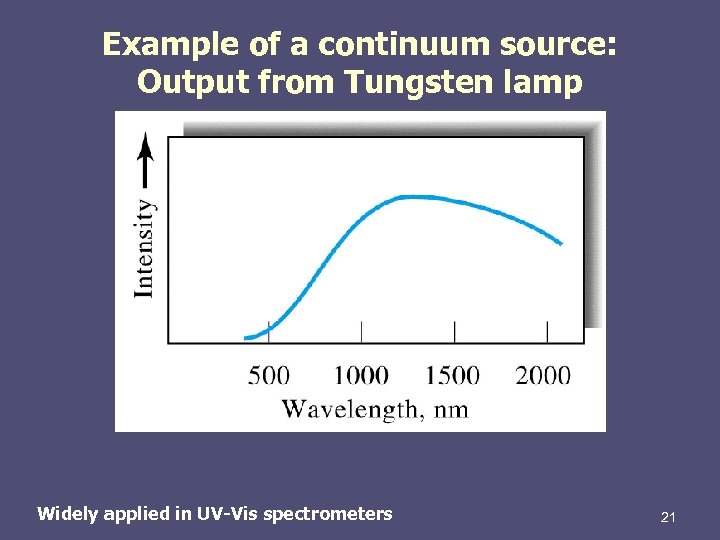 Example of a continuum source: Output from Tungsten lamp Widely applied in UV-Vis spectrometers 21
Example of a continuum source: Output from Tungsten lamp Widely applied in UV-Vis spectrometers 21
 Hollow cathode lamp Used in AAS n Filled with Ne or Ar at a pressure of 130 -700 Pa (1 -5 Torr). n When high voltage is applied between anode and cathode, filler gas becomes ionised n Positive ions accelerated toward cathode n Strike cathode with enough energy to "sputter" metal atoms from the cathode to yield cloud with excited atoms • Atoms emit line spectra n 22
Hollow cathode lamp Used in AAS n Filled with Ne or Ar at a pressure of 130 -700 Pa (1 -5 Torr). n When high voltage is applied between anode and cathode, filler gas becomes ionised n Positive ions accelerated toward cathode n Strike cathode with enough energy to "sputter" metal atoms from the cathode to yield cloud with excited atoms • Atoms emit line spectra n 22
 Example: Output from iron hollow cathode lamp n n n Small portion of spectrum from Fe hollow cathode lamp Shows sharp lines characteristic of gaseous atoms Linewidths are artificially broadened by monochromator (bandwidth = 0. 08 nm) 23
Example: Output from iron hollow cathode lamp n n n Small portion of spectrum from Fe hollow cathode lamp Shows sharp lines characteristic of gaseous atoms Linewidths are artificially broadened by monochromator (bandwidth = 0. 08 nm) 23
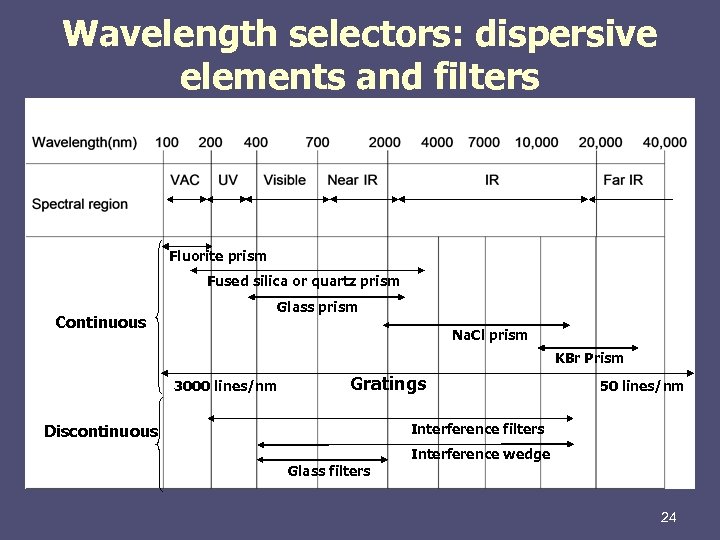 Wavelength selectors: dispersive elements and filters Fluorite prism Fused silica or quartz prism Continuous Glass prism Na. Cl prism KBr Prism 3000 lines/nm Gratings 50 lines/nm Interference filters Discontinuous Interference wedge Glass filters 24
Wavelength selectors: dispersive elements and filters Fluorite prism Fused silica or quartz prism Continuous Glass prism Na. Cl prism KBr Prism 3000 lines/nm Gratings 50 lines/nm Interference filters Discontinuous Interference wedge Glass filters 24
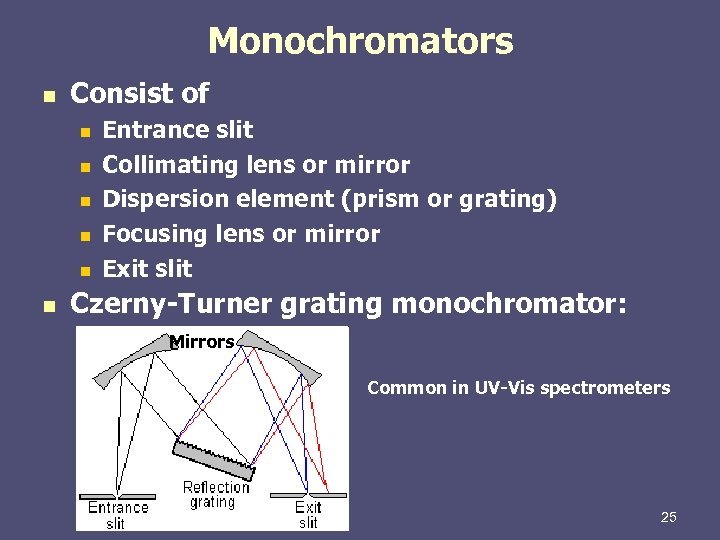 Monochromators n Consist of n n n Entrance slit Collimating lens or mirror Dispersion element (prism or grating) Focusing lens or mirror Exit slit Czerny-Turner grating monochromator: Mirrors Common in UV-Vis spectrometers 25
Monochromators n Consist of n n n Entrance slit Collimating lens or mirror Dispersion element (prism or grating) Focusing lens or mirror Exit slit Czerny-Turner grating monochromator: Mirrors Common in UV-Vis spectrometers 25
 Dispersers n Separate polychromatic light into its components n n Prism Diffraction grating: patterned surface which diffracts light Prisms Blazed diffraction grating Holographic grating 26
Dispersers n Separate polychromatic light into its components n n Prism Diffraction grating: patterned surface which diffracts light Prisms Blazed diffraction grating Holographic grating 26
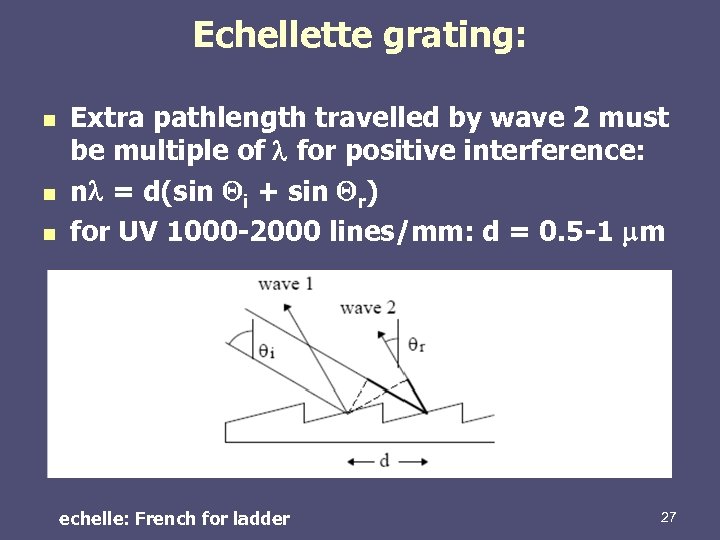 Echellette grating: n n n Extra pathlength travelled by wave 2 must be multiple of for positive interference: n = d(sin i + sin r) for UV 1000 -2000 lines/mm: d = 0. 5 -1 mm echelle: French for ladder 27
Echellette grating: n n n Extra pathlength travelled by wave 2 must be multiple of for positive interference: n = d(sin i + sin r) for UV 1000 -2000 lines/mm: d = 0. 5 -1 mm echelle: French for ladder 27
 Bandwidth of a monochromator n n n Spectral bandwidth: range of wavelengths exiting the monochromator Related to dispersion and slit widths Defines resolution of spectra: 2 features can only be distinguished if effective bandwidth is less than half the difference between the of features 28
Bandwidth of a monochromator n n n Spectral bandwidth: range of wavelengths exiting the monochromator Related to dispersion and slit widths Defines resolution of spectra: 2 features can only be distinguished if effective bandwidth is less than half the difference between the of features 28
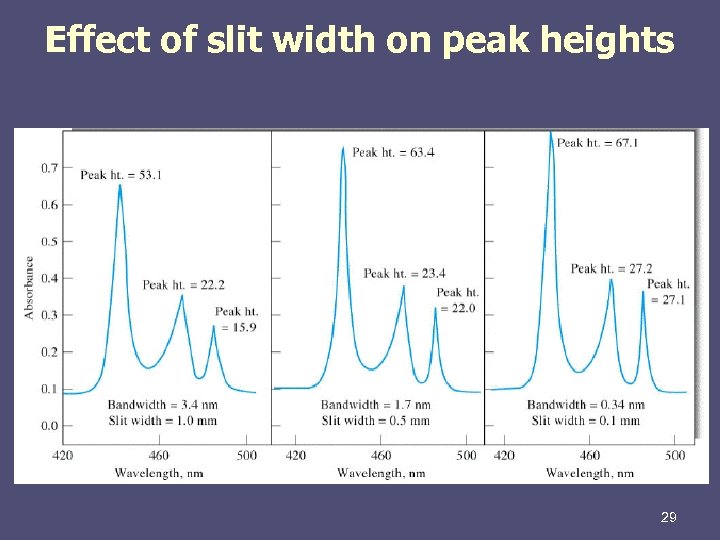 Effect of slit width on peak heights 29
Effect of slit width on peak heights 29
 Components of optical system in an ICP-OES spectrometer n n n spherical and cylindrical lenses flat and spherical mirrors parallel planes optical path under vacuum or controlled nitrogen atmosphere (necessary for wavelengths <200 nm; air absorbs far UV light) Disperser(s) 30
Components of optical system in an ICP-OES spectrometer n n n spherical and cylindrical lenses flat and spherical mirrors parallel planes optical path under vacuum or controlled nitrogen atmosphere (necessary for wavelengths <200 nm; air absorbs far UV light) Disperser(s) 30
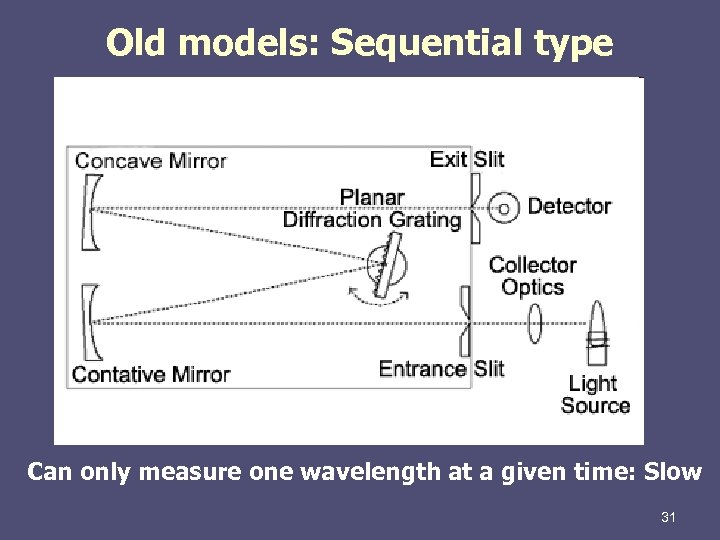 Old models: Sequential type Can only measure one wavelength at a given time: Slow 31
Old models: Sequential type Can only measure one wavelength at a given time: Slow 31
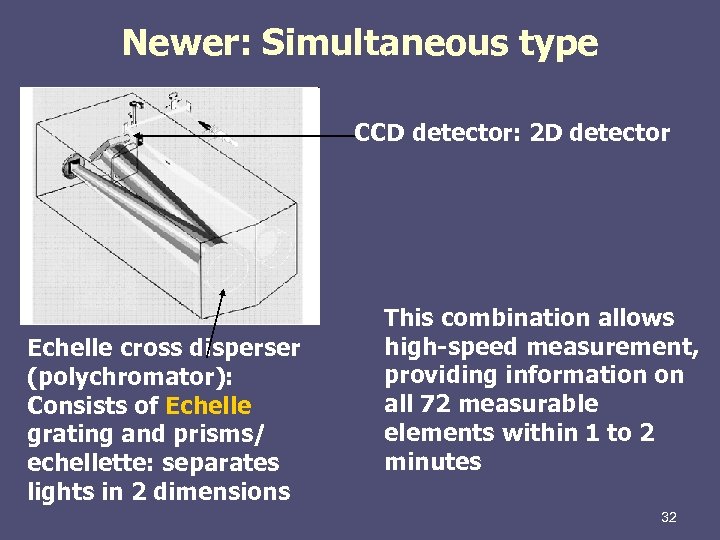 Newer: Simultaneous type CCD detector: 2 D detector Echelle cross disperser (polychromator): Consists of Echelle grating and prisms/ echellette: separates lights in 2 dimensions This combination allows high-speed measurement, providing information on all 72 measurable elements within 1 to 2 minutes 32
Newer: Simultaneous type CCD detector: 2 D detector Echelle cross disperser (polychromator): Consists of Echelle grating and prisms/ echellette: separates lights in 2 dimensions This combination allows high-speed measurement, providing information on all 72 measurable elements within 1 to 2 minutes 32
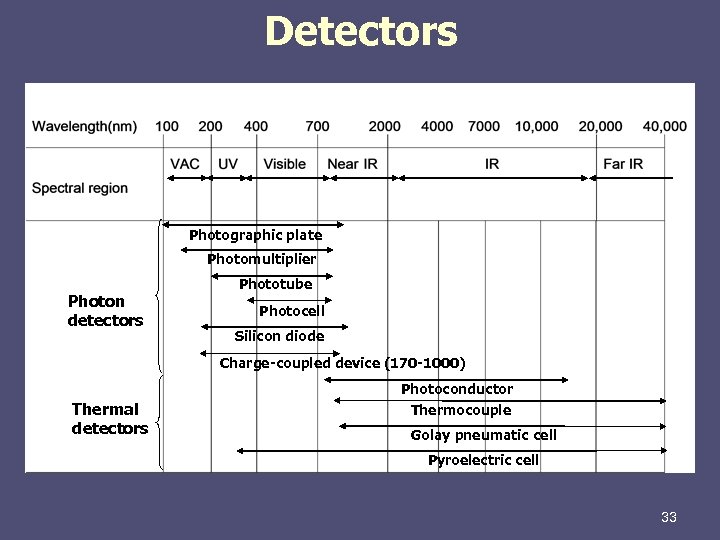 Detectors Photographic plate Photomultiplier Photon detectors Phototube Photocell Silicon diode Charge-coupled device (170 -1000) Thermal detectors Photoconductor Thermocouple Golay pneumatic cell Pyroelectric cell 33
Detectors Photographic plate Photomultiplier Photon detectors Phototube Photocell Silicon diode Charge-coupled device (170 -1000) Thermal detectors Photoconductor Thermocouple Golay pneumatic cell Pyroelectric cell 33
 Photomultiplier: detects one wavelength at a time n n n Based on photoelectric effect Photocathode and series of dynodes in an evacuated glass enclosure Photons strike cathode and electrons are emitted Electrons are accelerated towards a series of dynodes by increasing voltages Additional electrons are generated at each dynode Amplified signal is finally collected and measured at anode 34
Photomultiplier: detects one wavelength at a time n n n Based on photoelectric effect Photocathode and series of dynodes in an evacuated glass enclosure Photons strike cathode and electrons are emitted Electrons are accelerated towards a series of dynodes by increasing voltages Additional electrons are generated at each dynode Amplified signal is finally collected and measured at anode 34
 Photodiode arrays: measure several wavelengths at once n n linear array of discrete photodiodes on an integrated circuit (IC) chip Photodiode: Consists of 2 semiconductors (n-type and p-type) n n n Light promotes electrons into conducting band: generates electron-hole pair “Concentration” of these electron-hole pairs directly proportional to incident light a voltage bias is present and the concentration of lightinduced electron-hole pairs determines the current through 35 semiconductor
Photodiode arrays: measure several wavelengths at once n n linear array of discrete photodiodes on an integrated circuit (IC) chip Photodiode: Consists of 2 semiconductors (n-type and p-type) n n n Light promotes electrons into conducting band: generates electron-hole pair “Concentration” of these electron-hole pairs directly proportional to incident light a voltage bias is present and the concentration of lightinduced electron-hole pairs determines the current through 35 semiconductor
 Detection in simultaneous ICP-OES: CCD: Charge-coupled device • Also integrated-circuit chip • Contains an array of capacitors that store charge when light creates electron-hole pairs • Accumulated charge is read out at given time interval • Each wavelength is detected at a different spot • Much more sensitive than photodiode array detectors http: //www. chemistry. adelaide. edu. au/external/soc-rel/content/ccd. htm 36
Detection in simultaneous ICP-OES: CCD: Charge-coupled device • Also integrated-circuit chip • Contains an array of capacitors that store charge when light creates electron-hole pairs • Accumulated charge is read out at given time interval • Each wavelength is detected at a different spot • Much more sensitive than photodiode array detectors http: //www. chemistry. adelaide. edu. au/external/soc-rel/content/ccd. htm 36
 Lecture 4 AAS and ICP-OES Sample preparation Interferences Calibration 37
Lecture 4 AAS and ICP-OES Sample preparation Interferences Calibration 37
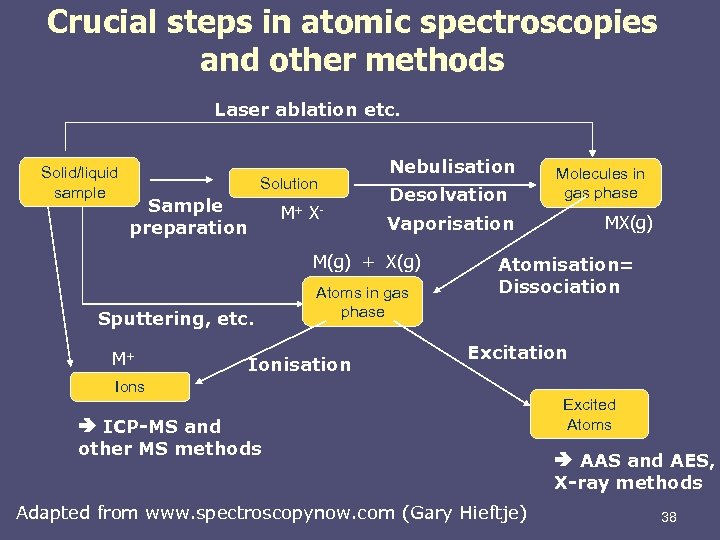 Crucial steps in atomic spectroscopies and other methods Laser ablation etc. Solid/liquid sample Solution Sample preparation M+ X- Nebulisation Desolvation M+ Atoms in gas phase Ionisation MX(g) Vaporisation M(g) + X(g) Sputtering, etc. Molecules in gas phase Atomisation= Dissociation Excitation Ions ICP-MS and other MS methods Adapted from www. spectroscopynow. com (Gary Hieftje) Excited Atoms AAS and AES, X-ray methods 38
Crucial steps in atomic spectroscopies and other methods Laser ablation etc. Solid/liquid sample Solution Sample preparation M+ X- Nebulisation Desolvation M+ Atoms in gas phase Ionisation MX(g) Vaporisation M(g) + X(g) Sputtering, etc. Molecules in gas phase Atomisation= Dissociation Excitation Ions ICP-MS and other MS methods Adapted from www. spectroscopynow. com (Gary Hieftje) Excited Atoms AAS and AES, X-ray methods 38
 Sample Introduction: liquid samples n n n Often the largest source of noise Sample is carried into flame or plasma as aerosol, vapour or fine powder Liquid samples introduced using nebuliser 39
Sample Introduction: liquid samples n n n Often the largest source of noise Sample is carried into flame or plasma as aerosol, vapour or fine powder Liquid samples introduced using nebuliser 39
 Sample preparation for analysis in solution: Digestion n n Digestion in conc. HNO 3 and mixtures thereof (e. g. aqua regia) Br 2 or H 2 O 2 can be added to conc. acids to give a more oxidising medium and increase solubility Certain materials require digestion in conc. HF Common to use microwave digestion 40
Sample preparation for analysis in solution: Digestion n n Digestion in conc. HNO 3 and mixtures thereof (e. g. aqua regia) Br 2 or H 2 O 2 can be added to conc. acids to give a more oxidising medium and increase solubility Certain materials require digestion in conc. HF Common to use microwave digestion 40
 Microwave digestion Rotor Supplied with dedicated vessels (e. g. PTFE) Closed vessel digestion minimises sample contamination Faster, more reproducible, and safer than conventional 41 methods
Microwave digestion Rotor Supplied with dedicated vessels (e. g. PTFE) Closed vessel digestion minimises sample contamination Faster, more reproducible, and safer than conventional 41 methods
 Sample preparation and sample handling for trace analysis n n n As always – sample preparation is key Ultra-trace: Contaminations introduced during sample processing can seriously limit performance characteristics Points to consider: n n n Purity of reagents Chemical inertness of reaction vessels and any other material samples come into contact with Working environment Preparation of standards and blanks crucial Also measure a “process blank”: n Important for determination of LOD and LOQ 42
Sample preparation and sample handling for trace analysis n n n As always – sample preparation is key Ultra-trace: Contaminations introduced during sample processing can seriously limit performance characteristics Points to consider: n n n Purity of reagents Chemical inertness of reaction vessels and any other material samples come into contact with Working environment Preparation of standards and blanks crucial Also measure a “process blank”: n Important for determination of LOD and LOQ 42
 Common Units in trace analysis n n n ppm, ppb, ppt, ppq…. . : parts per million etc. ppm: mg/kg; often also used as mg/L ppb: mg/kg ppt: ng/kg ppq: pg/kg 43
Common Units in trace analysis n n n ppm, ppb, ppt, ppq…. . : parts per million etc. ppm: mg/kg; often also used as mg/L ppb: mg/kg ppt: ng/kg ppq: pg/kg 43
 Atomic absorption spectroscopy 44
Atomic absorption spectroscopy 44
 Atomic Absorption Spectroscopy n n n Flame AAS has been the most widely used of all atomic methods due to its simplicity, effectiveness and low cost First introduced in 1955, commercially available since 1959 Qualitative and quantitative analysis of >70 elements n Quantitative: Can detect ppm, ppb or even less n Rapid, convenient, selective, inexpensive 45
Atomic Absorption Spectroscopy n n n Flame AAS has been the most widely used of all atomic methods due to its simplicity, effectiveness and low cost First introduced in 1955, commercially available since 1959 Qualitative and quantitative analysis of >70 elements n Quantitative: Can detect ppm, ppb or even less n Rapid, convenient, selective, inexpensive 45
 Flame AA Spectrometer Hollow cathode lamps with characteristic emissions Burner Flame fuelled by (e. g. ) acetylene and air Nebuliser and Spray chamber Hollow cathode lamps available for over 70 elements Can get lamps containing > 1 element for determination of multiple species 46
Flame AA Spectrometer Hollow cathode lamps with characteristic emissions Burner Flame fuelled by (e. g. ) acetylene and air Nebuliser and Spray chamber Hollow cathode lamps available for over 70 elements Can get lamps containing > 1 element for determination of multiple species 46
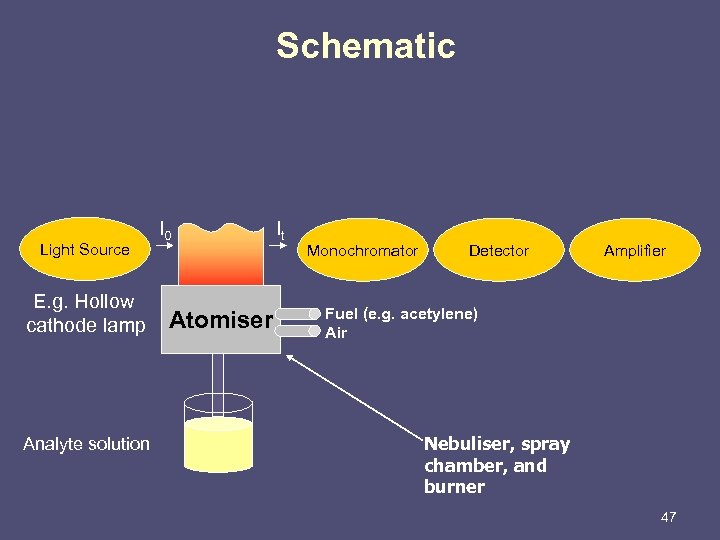 Schematic Light Source E. g. Hollow cathode lamp Analyte solution I 0 Atomiser It Monochromator Detector Amplifier Fuel (e. g. acetylene) Air Nebuliser, spray chamber, and burner 47
Schematic Light Source E. g. Hollow cathode lamp Analyte solution I 0 Atomiser It Monochromator Detector Amplifier Fuel (e. g. acetylene) Air Nebuliser, spray chamber, and burner 47
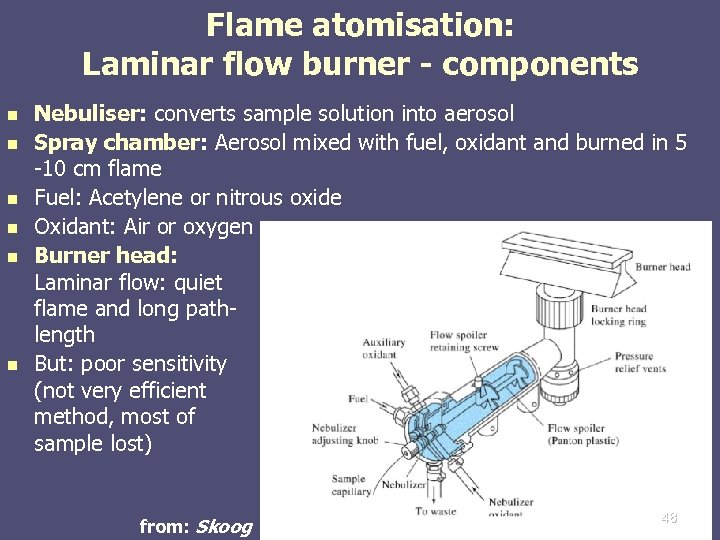 Flame atomisation: Laminar flow burner - components n n n Nebuliser: converts sample solution into aerosol Spray chamber: Aerosol mixed with fuel, oxidant and burned in 5 -10 cm flame Fuel: Acetylene or nitrous oxide Oxidant: Air or oxygen Burner head: Laminar flow: quiet flame and long pathlength But: poor sensitivity (not very efficient method, most of sample lost) from: Skoog 48
Flame atomisation: Laminar flow burner - components n n n Nebuliser: converts sample solution into aerosol Spray chamber: Aerosol mixed with fuel, oxidant and burned in 5 -10 cm flame Fuel: Acetylene or nitrous oxide Oxidant: Air or oxygen Burner head: Laminar flow: quiet flame and long pathlength But: poor sensitivity (not very efficient method, most of sample lost) from: Skoog 48
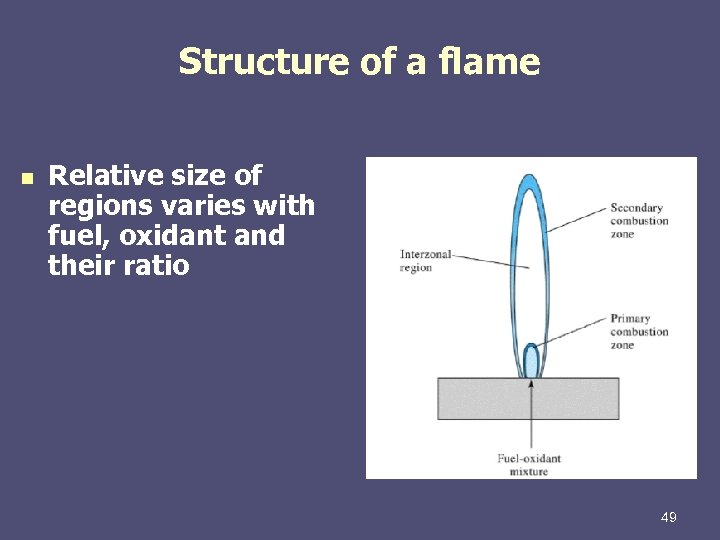 Structure of a flame n Relative size of regions varies with fuel, oxidant and their ratio 49
Structure of a flame n Relative size of regions varies with fuel, oxidant and their ratio 49
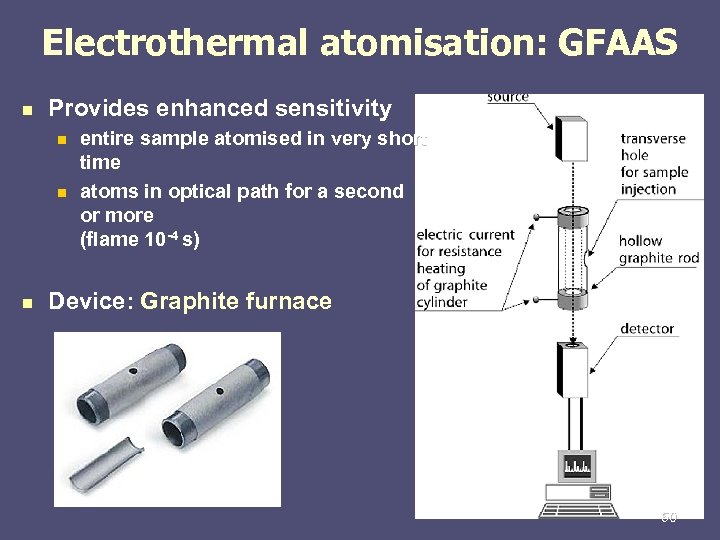 Electrothermal atomisation: GFAAS n Provides enhanced sensitivity n n n entire sample atomised in very short time atoms in optical path for a second or more (flame 10 -4 s) Device: Graphite furnace 50
Electrothermal atomisation: GFAAS n Provides enhanced sensitivity n n n entire sample atomised in very short time atoms in optical path for a second or more (flame 10 -4 s) Device: Graphite furnace 50
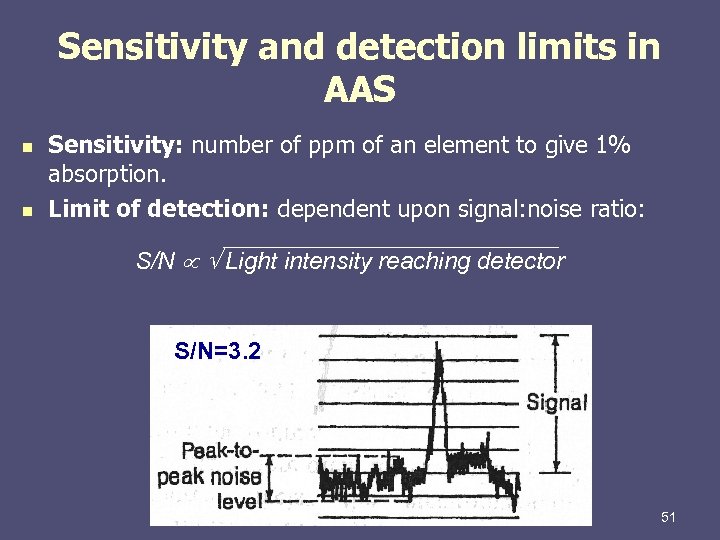 Sensitivity and detection limits in AAS n n Sensitivity: number of ppm of an element to give 1% absorption. Limit of detection: dependent upon signal: noise ratio: S/N Light intensity reaching detector S/N=3. 2 51
Sensitivity and detection limits in AAS n n Sensitivity: number of ppm of an element to give 1% absorption. Limit of detection: dependent upon signal: noise ratio: S/N Light intensity reaching detector S/N=3. 2 51
 Interferences in AAS n n Broadening of a spectral line, which can occur due to a number of factors (Physical) Spectral: emission line of another element or compound, or general background radiation from the flame, solvent, or analytical sample n Background correction can be applied Chemical: Formation of compounds that do not dissociate in the flame Ionisation of the analyte can reduce the signal Matrix interferences due to differences between surface tension and viscosity of test solutions and standards Another caveat: Non-linear response common in AAS 52
Interferences in AAS n n Broadening of a spectral line, which can occur due to a number of factors (Physical) Spectral: emission line of another element or compound, or general background radiation from the flame, solvent, or analytical sample n Background correction can be applied Chemical: Formation of compounds that do not dissociate in the flame Ionisation of the analyte can reduce the signal Matrix interferences due to differences between surface tension and viscosity of test solutions and standards Another caveat: Non-linear response common in AAS 52
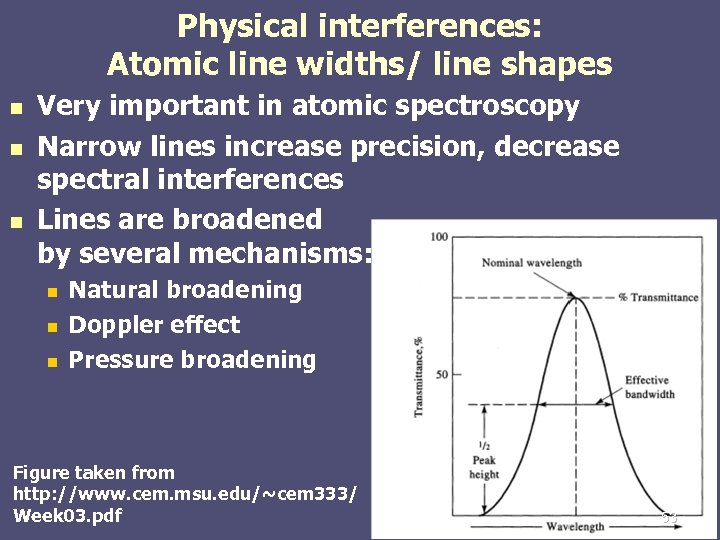 Physical interferences: Atomic line widths/ line shapes n n n Very important in atomic spectroscopy Narrow lines increase precision, decrease spectral interferences Lines are broadened by several mechanisms: n n n Natural broadening Doppler effect Pressure broadening Figure taken from http: //www. cem. msu. edu/~cem 333/ Week 03. pdf 53
Physical interferences: Atomic line widths/ line shapes n n n Very important in atomic spectroscopy Narrow lines increase precision, decrease spectral interferences Lines are broadened by several mechanisms: n n n Natural broadening Doppler effect Pressure broadening Figure taken from http: //www. cem. msu. edu/~cem 333/ Week 03. pdf 53
 Natural linewidths n n n Width of an atomic spectral line is determined by the lifetime of the excited state Consequence of the Heisenberg uncertainty principle For example, lifetime of 10 -8 seconds (10 ns) yields peak widths of 10 -5 nm 54
Natural linewidths n n n Width of an atomic spectral line is determined by the lifetime of the excited state Consequence of the Heisenberg uncertainty principle For example, lifetime of 10 -8 seconds (10 ns) yields peak widths of 10 -5 nm 54
 Doppler Effect Photon detector n n n Due to rapid motion of atoms in gas phase Atom moving toward the detector absorbs / emits radiation of shorter than atom moving perpendicular to detector. Atom moving away from the detector absorbs / emits radiation of longer : detector perceives fewer oscillations 55
Doppler Effect Photon detector n n n Due to rapid motion of atoms in gas phase Atom moving toward the detector absorbs / emits radiation of shorter than atom moving perpendicular to detector. Atom moving away from the detector absorbs / emits radiation of longer : detector perceives fewer oscillations 55
 Pressure broadening n Results from collisions of absorbing/emitting species n n n With analyte atoms or combustion products of fuel Deactivates the excited state – shorter lifetime - broader spectral lines Increases with concentration and temperature E. g. in flame, Na absorbance lines broadened up to 10 -3 nm. Doppler and pressure effects broaden atomic lines by 1 -2 orders of magnitude as compared with their natural linewidths 56
Pressure broadening n Results from collisions of absorbing/emitting species n n n With analyte atoms or combustion products of fuel Deactivates the excited state – shorter lifetime - broader spectral lines Increases with concentration and temperature E. g. in flame, Na absorbance lines broadened up to 10 -3 nm. Doppler and pressure effects broaden atomic lines by 1 -2 orders of magnitude as compared with their natural linewidths 56
 Background correction in AAS n n particularly important in GFAAS Use beam chopper to distinguish the signal due to flame from desired atomic line at the same wavelength (old method) Lamp and flame emission reach detector Only flame emission reaches detector Resulting signal 57
Background correction in AAS n n particularly important in GFAAS Use beam chopper to distinguish the signal due to flame from desired atomic line at the same wavelength (old method) Lamp and flame emission reach detector Only flame emission reaches detector Resulting signal 57
 Background correction in AAS n High energy Deuterium background corrector Detector Hollow cathode lamp Lamps are pulsed out of phase with each other Beam combiner Sample Deuterium lamp 58
Background correction in AAS n High energy Deuterium background corrector Detector Hollow cathode lamp Lamps are pulsed out of phase with each other Beam combiner Sample Deuterium lamp 58
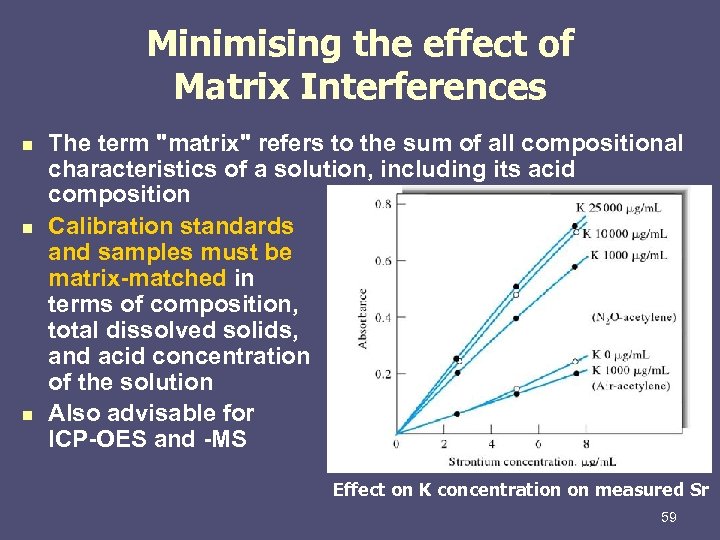 Minimising the effect of Matrix Interferences n n n The term "matrix" refers to the sum of all compositional characteristics of a solution, including its acid composition Calibration standards and samples must be matrix-matched in terms of composition, total dissolved solids, and acid concentration of the solution Also advisable for ICP-OES and -MS Effect on K concentration on measured Sr 59
Minimising the effect of Matrix Interferences n n n The term "matrix" refers to the sum of all compositional characteristics of a solution, including its acid composition Calibration standards and samples must be matrix-matched in terms of composition, total dissolved solids, and acid concentration of the solution Also advisable for ICP-OES and -MS Effect on K concentration on measured Sr 59
 Specialised applications in AAS: Flameless cold vapour methods n n Mercury: has sufficient vapour pressure at RT Hydride generation technique for determination of As, Sb, Bi, Se, Te, Ge, Pb, and Sn n n Generation of volatile metal hydrides (As, Sb, Bi, Se, Te, Ge, Pb, and Sn) Reduction by Na. BH 4 to form volatile hydride (e. g. Sn. H 4) Hydrides carried into light path by argon gas Decomposed into elemental vapour by injection into (electrothermally) heated silica cell 60
Specialised applications in AAS: Flameless cold vapour methods n n Mercury: has sufficient vapour pressure at RT Hydride generation technique for determination of As, Sb, Bi, Se, Te, Ge, Pb, and Sn n n Generation of volatile metal hydrides (As, Sb, Bi, Se, Te, Ge, Pb, and Sn) Reduction by Na. BH 4 to form volatile hydride (e. g. Sn. H 4) Hydrides carried into light path by argon gas Decomposed into elemental vapour by injection into (electrothermally) heated silica cell 60
 Calibration – some practical aspects 61
Calibration – some practical aspects 61
 Principles n n n Recap: Measured quantity must change with analyte concentration in systematic and defined way Can be determined by calibration, using defined standards Stock solutions of standards can either be prepared or purchased Working solutions are best prepared by weighing the amounts of stock solution and matrix (rather than using volumetric ware) NEVER extrapolate: concentration of sample must be in same range as standards 62
Principles n n n Recap: Measured quantity must change with analyte concentration in systematic and defined way Can be determined by calibration, using defined standards Stock solutions of standards can either be prepared or purchased Working solutions are best prepared by weighing the amounts of stock solution and matrix (rather than using volumetric ware) NEVER extrapolate: concentration of sample must be in same range as standards 62
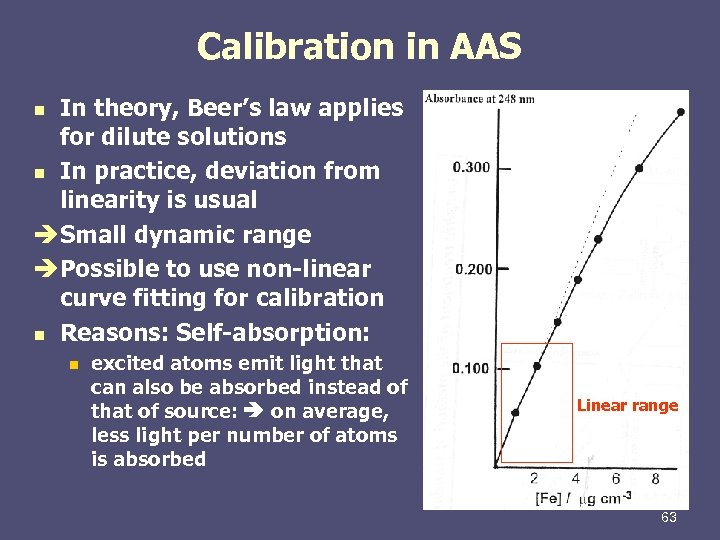 Calibration in AAS In theory, Beer’s law applies for dilute solutions n In practice, deviation from linearity is usual è Small dynamic range è Possible to use non-linear curve fitting for calibration n Reasons: Self-absorption: n n excited atoms emit light that can also be absorbed instead of that of source: on average, less light per number of atoms is absorbed Linear range 63
Calibration in AAS In theory, Beer’s law applies for dilute solutions n In practice, deviation from linearity is usual è Small dynamic range è Possible to use non-linear curve fitting for calibration n Reasons: Self-absorption: n n excited atoms emit light that can also be absorbed instead of that of source: on average, less light per number of atoms is absorbed Linear range 63
 Alternative to matrix-matching: Method of standard additions Extensively used in absorption spectroscopy, accounts for matrix effects n Several aliquots of sample n Sample (1): diluted to volume directly n Samples (2, 3, 4, 5…): known amounts of analyte added before dilution to volume n n BUT: Only makes sense if the added standard closely matches the analyte present in the samples chemically and physically n if simple, dissolved ions are analysed 64
Alternative to matrix-matching: Method of standard additions Extensively used in absorption spectroscopy, accounts for matrix effects n Several aliquots of sample n Sample (1): diluted to volume directly n Samples (2, 3, 4, 5…): known amounts of analyte added before dilution to volume n n BUT: Only makes sense if the added standard closely matches the analyte present in the samples chemically and physically n if simple, dissolved ions are analysed 64
 Method of standard additions n If linear relationship exists between measured quantity and concentration (must be verified experimentally) then: n n n Vx, Cx: volume and concentration of analyte Vs: variable volume of added standard Cs: concentration of added standard VT: total volume of volumetric flask k: proportionality constant (= єl) Ax, AT: absorbances of standard alone and sample + standard addition, respectively. 65
Method of standard additions n If linear relationship exists between measured quantity and concentration (must be verified experimentally) then: n n n Vx, Cx: volume and concentration of analyte Vs: variable volume of added standard Cs: concentration of added standard VT: total volume of volumetric flask k: proportionality constant (= єl) Ax, AT: absorbances of standard alone and sample + standard addition, respectively. 65
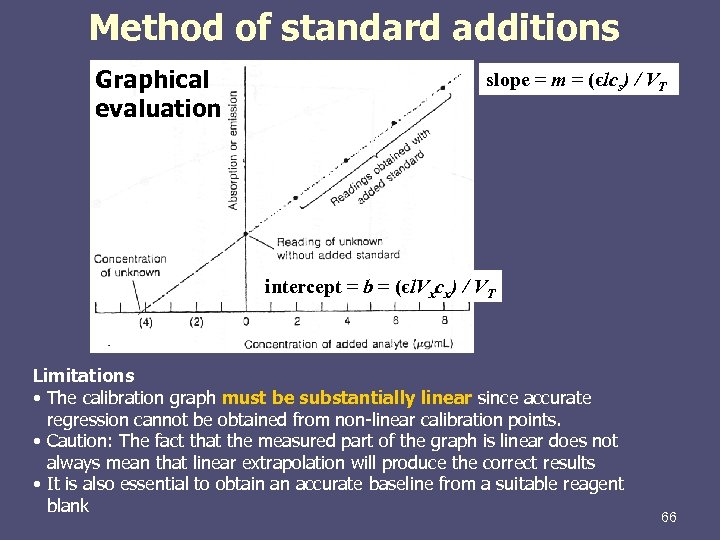 Method of standard additions Graphical evaluation slope = m = (єlcs) / VT intercept = b = (єl. Vxcx) / VT Limitations • The calibration graph must be substantially linear since accurate regression cannot be obtained from non-linear calibration points. • Caution: The fact that the measured part of the graph is linear does not always mean that linear extrapolation will produce the correct results • It is also essential to obtain an accurate baseline from a suitable reagent blank 66
Method of standard additions Graphical evaluation slope = m = (єlcs) / VT intercept = b = (єl. Vxcx) / VT Limitations • The calibration graph must be substantially linear since accurate regression cannot be obtained from non-linear calibration points. • Caution: The fact that the measured part of the graph is linear does not always mean that linear extrapolation will produce the correct results • It is also essential to obtain an accurate baseline from a suitable reagent blank 66
 Most simple version of standard addition: spiking n n Spiking means deliberately adding analyte to an unknown sample Involves: n n n preparation of sample and measurement of absorbance Addition of standard with known concentration, measurement of absorbance From difference in absorbance, calculate e From reading of sample alone, calculate amount of analyte (use Beer’s law for calculations) 67
Most simple version of standard addition: spiking n n Spiking means deliberately adding analyte to an unknown sample Involves: n n n preparation of sample and measurement of absorbance Addition of standard with known concentration, measurement of absorbance From difference in absorbance, calculate e From reading of sample alone, calculate amount of analyte (use Beer’s law for calculations) 67
 Other uses for spiking n n Add spike at beginning of sample preparation Process sample with and without spike Difference should correspond to amount spiked Deviation allows to calculate recovery factor 68
Other uses for spiking n n Add spike at beginning of sample preparation Process sample with and without spike Difference should correspond to amount spiked Deviation allows to calculate recovery factor 68
 Atomic emission spectroscopy 69
Atomic emission spectroscopy 69
 Atomic emission spectroscopy n n Historically, many techniques based on emission have been used (See Table on p. 4) Flame and electrothermal methods now widely superseded by Inductively-Coupled Plasma (ICP) method n n Developed in the 1970 s Higher energy sources than flame or electrothermal methods 70
Atomic emission spectroscopy n n Historically, many techniques based on emission have been used (See Table on p. 4) Flame and electrothermal methods now widely superseded by Inductively-Coupled Plasma (ICP) method n n Developed in the 1970 s Higher energy sources than flame or electrothermal methods 70
 ICP-AES/OES Inductively coupled plasma-atomic emission spectroscopy (or optical emission spectroscopy) n Offer several advantages over flame/electrothermal: n n n Lower inter-element interference (higher temperatures) With a single set of conditions signals for dozens of elements can be recorded simultaneously Lower LOD for elements resistant to decomposition Permit determination of non-metals (Cl, Br, I, S) Can analyse concentration ranges over several decades (vs 1 or 2 decades for other methods) Disadvantages: n n More complicated and expensive to run Require higher degree of operator skill 71
ICP-AES/OES Inductively coupled plasma-atomic emission spectroscopy (or optical emission spectroscopy) n Offer several advantages over flame/electrothermal: n n n Lower inter-element interference (higher temperatures) With a single set of conditions signals for dozens of elements can be recorded simultaneously Lower LOD for elements resistant to decomposition Permit determination of non-metals (Cl, Br, I, S) Can analyse concentration ranges over several decades (vs 1 or 2 decades for other methods) Disadvantages: n n More complicated and expensive to run Require higher degree of operator skill 71
 Modern ICP-OES spectrometer n n Over 70 elements (in principle simultaneously) Including non-metals such as sulfur, phosphorus, and halogens (not possible with AAS) ppm to ppb range Principle: Argon plasma generates excited atoms and ions; these emit characteristic radiation 72
Modern ICP-OES spectrometer n n Over 70 elements (in principle simultaneously) Including non-metals such as sulfur, phosphorus, and halogens (not possible with AAS) ppm to ppb range Principle: Argon plasma generates excited atoms and ions; these emit characteristic radiation 72
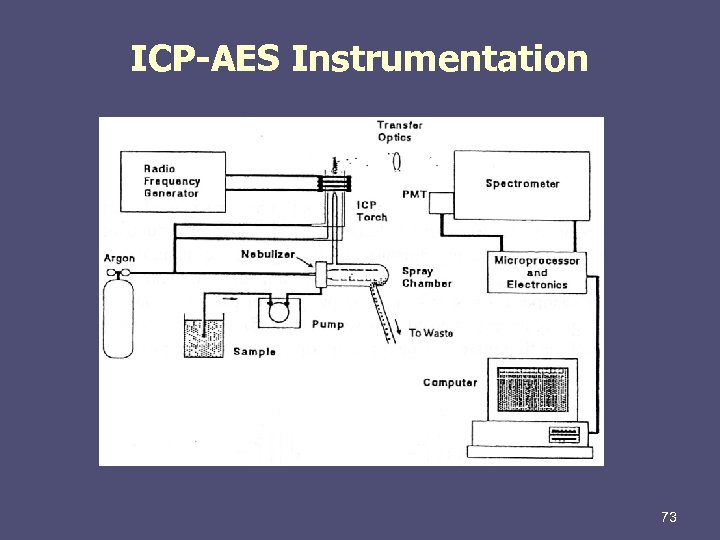 ICP-AES Instrumentation 73
ICP-AES Instrumentation 73
 Components for sample injection and the ICP torch Up to 7000°C www. cleanwatertesting. com/news_ NR 149. htm www. midwestrefineries. com /refiningandassaying. htm 74
Components for sample injection and the ICP torch Up to 7000°C www. cleanwatertesting. com/news_ NR 149. htm www. midwestrefineries. com /refiningandassaying. htm 74
 Meinhard nebuliser Caution: The capillary is easy to block and difficult to unblock 75
Meinhard nebuliser Caution: The capillary is easy to block and difficult to unblock 75
 ICP torch water cooled induction coil powered by RF generator (2 k. W power at 27 MHz) concentric quartz tubes 11 -17 L/min d=2. 5 cm 76
ICP torch water cooled induction coil powered by RF generator (2 k. W power at 27 MHz) concentric quartz tubes 11 -17 L/min d=2. 5 cm 76
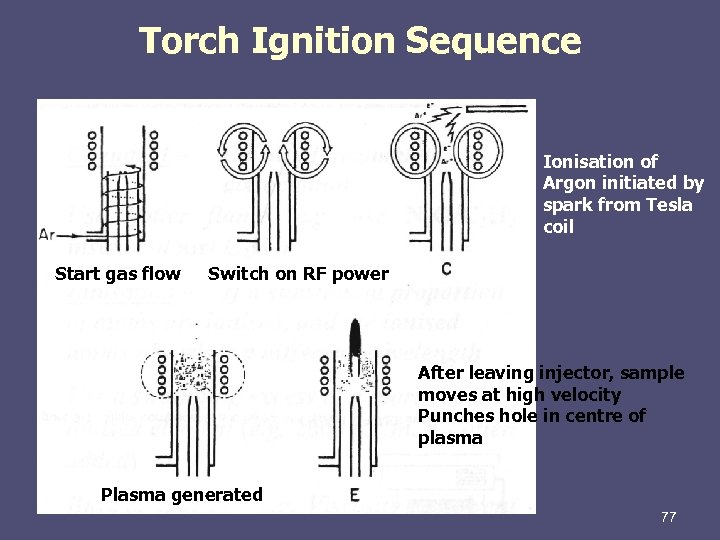 Torch Ignition Sequence Ionisation of Argon initiated by spark from Tesla coil Start gas flow Switch on RF power After leaving injector, sample moves at high velocity Punches hole in centre of plasma Plasma generated 77
Torch Ignition Sequence Ionisation of Argon initiated by spark from Tesla coil Start gas flow Switch on RF power After leaving injector, sample moves at high velocity Punches hole in centre of plasma Plasma generated 77
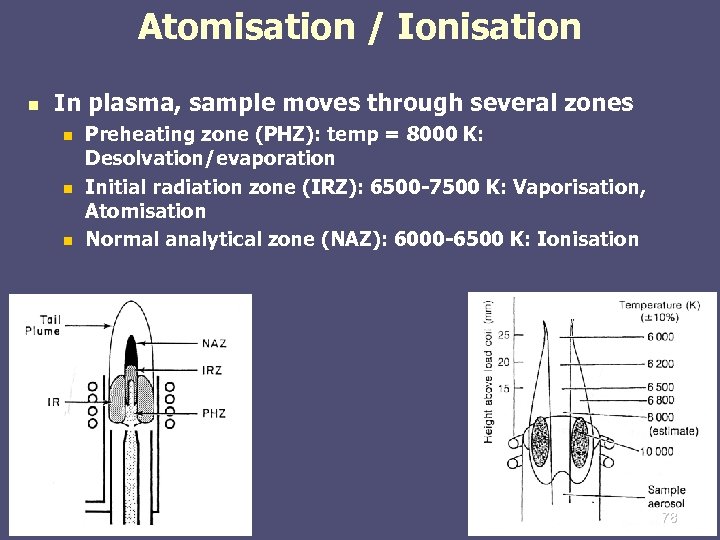 Atomisation / Ionisation n In plasma, sample moves through several zones n n n Preheating zone (PHZ): temp = 8000 K: Desolvation/evaporation Initial radiation zone (IRZ): 6500 -7500 K: Vaporisation, Atomisation Normal analytical zone (NAZ): 6000 -6500 K: Ionisation 78
Atomisation / Ionisation n In plasma, sample moves through several zones n n n Preheating zone (PHZ): temp = 8000 K: Desolvation/evaporation Initial radiation zone (IRZ): 6500 -7500 K: Vaporisation, Atomisation Normal analytical zone (NAZ): 6000 -6500 K: Ionisation 78
 Advantages of plasma n Prior to observation, atoms spend ~ 2 sec at 4000 -8000 K (about 2 -3 times that of hottest combustion flame) n n n Chemically inert environment for atomisation n n Atomisation and ionisation is more complete Fewer chemical interferences Prevents side-product (e. g. oxide) formation Temperature cross-section is uniform (no cool spots) n n Prevents self-absorption Get linear calibration curves over several orders of magnitude 79
Advantages of plasma n Prior to observation, atoms spend ~ 2 sec at 4000 -8000 K (about 2 -3 times that of hottest combustion flame) n n n Chemically inert environment for atomisation n n Atomisation and ionisation is more complete Fewer chemical interferences Prevents side-product (e. g. oxide) formation Temperature cross-section is uniform (no cool spots) n n Prevents self-absorption Get linear calibration curves over several orders of magnitude 79
 Radial and axial observation Axial Radial. Can achieve higher sensitivity Combined viewing expands dynamic range http: //las. perkinelmer. com/content/relatedmaterials/brochures/bro 80 _atomicspectroscopytechniqueguide. pdf
Radial and axial observation Axial Radial. Can achieve higher sensitivity Combined viewing expands dynamic range http: //las. perkinelmer. com/content/relatedmaterials/brochures/bro 80 _atomicspectroscopytechniqueguide. pdf
 Applications n ICP-OES used for quantitative analysis of: n Soil, sediment, rocks, minerals, air n n n n Geochemistry Mineralogy Agriculture Forestry Fornensics Environmental sciences Food industry Elements not accessible using AAS n Sulfur, Boron, Phosphorus, Titanium, and Zirconium 81
Applications n ICP-OES used for quantitative analysis of: n Soil, sediment, rocks, minerals, air n n n n Geochemistry Mineralogy Agriculture Forestry Fornensics Environmental sciences Food industry Elements not accessible using AAS n Sulfur, Boron, Phosphorus, Titanium, and Zirconium 81
 Homework for revision n Read http: //las. perkinelmer. com/content/ relatedmaterials/brochures/bro_ato micspectroscopytechniqueguide. pdf 82
Homework for revision n Read http: //las. perkinelmer. com/content/ relatedmaterials/brochures/bro_ato micspectroscopytechniqueguide. pdf 82
![Lab Experiment 3 n Analyse a Chromium complex for [Cr] in three ways: n Lab Experiment 3 n Analyse a Chromium complex for [Cr] in three ways: n](https://present5.com/presentation/85a4629762fdfc53d4cdc4a63ca0c5b3/image-83.jpg) Lab Experiment 3 n Analyse a Chromium complex for [Cr] in three ways: n n UV (absorbance & extinction coefficient) Titration (moles Cr and charge) AAS (Cr standard curve and unknown concentration) AAS data analysis n n Fit standards to quadratic equation n A=a[Cr]2 + b[Cr] + c Use a, b, and c to calculate unknown concentration 83
Lab Experiment 3 n Analyse a Chromium complex for [Cr] in three ways: n n UV (absorbance & extinction coefficient) Titration (moles Cr and charge) AAS (Cr standard curve and unknown concentration) AAS data analysis n n Fit standards to quadratic equation n A=a[Cr]2 + b[Cr] + c Use a, b, and c to calculate unknown concentration 83


