lesson1.pptx
- Количество слайдов: 48

Separation of biopolymers Analysis Isolation and purification of biopolymers

Literature Laboratory techniques in biochemistry and molecular biology. Vol. 4, Part 2 / Ed. by T. S. Work and E. Work. North-Holland Publishing Company, 1976. Guide to protein purification, 2 nd edition / Ed. by R. D. Burgess and M. P. Deutscher. Elsevier, 2009

BIOPOLYMERS (Polymers produced by living and biological organisms) Proteins Polymers of amino acids with possible addition of other compounds Polynucleotides Polymers of nucleotides – adducts of purine or pyrimidine bases with a sugar and a phosphate group Polysaccharides Polymers of natural carbohydrates
Proteins Nomenclature based on the chemical composition of polymer chains Peptides = polymers of amino acids Glycoprotein = polypeptides that contain polysaccharides chains Lipoproteins = polypeptides containing covalently attached lipids et cetera

Proteins Four levels of structural organization

Proteins Secondary structure is the localized organization of parts of a polypeptide chain due to hydrogen bonds Three main types of the secondary structure are distinguished: a-helixes, b-sheets, and random coils

The a-helix is a coiled secondary structure due to a hydrogen bond every fourth amino acid


The b-pleated sheet is formed by hydrogen bonds between parallel parts of the protein



The tertiary structure • Gives a specific overall shape to a protein • Involves interactions and crosslinks between different parts of a protein chains • Is stabilized by van der Waals interactions Hydrogen bonds Ionic interactions (Salt bridges) Disulphide bridges

Interactions among the “side chains” of amino acids involved in the tertiary structure

Interactions among the “side chains” of amino acids involved in the tertiary structure

Interactions among the “side chains” of amino acids involved in the tertiary structure

Interactions among the “side chains” of amino acids involved in the tertiary structure

The quaternary structure results from interaction of two or more tertiary subunits Globular Polypeptide chains folded in a globular shape Hemoglobin Fibrous Polypeptides arranged in long strands or sheets Collagen

Denaturation Renaturation (Unfolding) (Folding) Only the native folded conformations of proteins are biologically active. These conformations are stable in a certain range of conditions ensuring a positive balance of forces maintaining the folded structure. Denaturation is a process of loosing the native conformation A reverse process is called renaturation or refolding

Denaturation is a process of loosing the native conformation. It results in disruption of the secondary, tertiary, or quaternary structure of the protein A reverse process is called renaturation or refolding

Denaturation is induced by − High temperature − Extreme p. H (acid and bases) − Organic solvents − Reducing agents − Heavy metals

Denaturation • Disrupt Hydrogen Bonding - alcohol

Denaturation • Disrupt salt bridges - acids and bases

Denaturation • Disrupt disulfide bonds - reducing agents, heavy metals.

Refolding a process of returning back to the native conformation Once the conditions that are favorable for the native conformation of a protein are created, the protein may spontaneously fold to the native state (Anfinsen, 1973). This process can be directed and facilitated by the experimenter. Necessary conditions: ü Room temperature ü Moderate p. H ≠ p. I ü Concentrations of denaturants < the level of denaturation ü Diluted solution

Refolding A danger of aggregation at this step, therefore the necessity of dilution

NUCLEIC ACIDS Polymers of nucleotides 3 elements of a nucleotide: § Sugar residue § Phosphate residue § Organic base DNA RN RNA A

DNA: Three levels of structure organization Primary Structure • nucleotide sequences
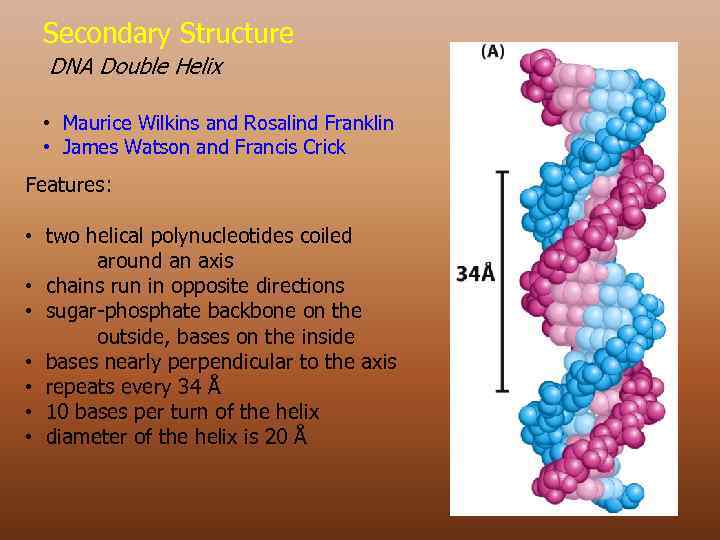
Secondary Structure DNA Double Helix • Maurice Wilkins and Rosalind Franklin • James Watson and Francis Crick Features: • two helical polynucleotides coiled around an axis • chains run in opposite directions • sugar-phosphate backbone on the outside, bases on the inside • bases nearly perpendicular to the axis • repeats every 34 Å • 10 bases per turn of the helix • diameter of the helix is 20 Å

Double helix is stabilized by hydrogen bondings

The double helix exists in multiple conformations

The double helix exists in multiple conformations Standard DNA double helix under physiological conditions

The double helix exists in multiple conformations Left-handed laboratory anomaly


Tertiary Structure Supercoiling supercoiled DNA Topoisomeraze relaxed DNA

Structure of Single-stranded DNA Stem Loop

Denaturation of DNA • A process by which double stranded DNA unwinds and separates into single stranded strands through the breaking of hydrogen bonding between the bases • Can be achieved through heating the DNA in solution • Complete denaturation- ~94°C • Temperature needed depends on the base content of DNA; High G-C content will need a higher temperature. And why? 36

Denaturation of DNA 37

Ethidium ions cause DNA to unwind

RNA: STRUCTURE

RNA chains fold back on themselves to form local regions of double helix similar to A-form DNA

Pseudoknot

Secondary Structure transfer RNA (t. RNA) : Brings amino acids to ribosomes during translation

ribosomal RNA (r. RNA) : Makes up the ribosomes, together with ribosomal proteins. Ribosomes synthesize proteins All ribosomes contain large and small subunits r. RNA molecules make up about 2/3 of ribosome Secondary structure features seem to be conserved, whereas sequence is not There must be common designs and functions that must be conserved

messenger RNA (m. RNA) : Encodes amino acid sequence of a polypeptide

small nuclear RNA (sn. RNA) : With proteins, forms complexes that are used in RNA processing in eukaryotes. (Not found in prokaryotes. )

Sources Body liquids Human Animal Tissues/cells Plants Fungi/Yeast Microorganisms Human Animal Chemical Hazards Biohazards Mutagens Infectious agents Carcinogens Environmental Biotoxins Hazards Safety issues Ethical issues

Ethical Guidelines EXPERIMENTS with HUMANS EXPERIMENTS with ANIMALS Voluntary and informed Justified importance Respectful Controlled As harmless as possible As painless as possible See special guidelines (Ethics codes) for details

General Rules in Handling Biopolymers in Lab • Long storage -20 o. C • Storage during experiment, transportation – on ice (ice boxes) • Experimental manipulations at 4 o. C unless other conditions specified • Use gloves (protect from hand grease) • Control purity always
lesson1.pptx