fda062559d5756cfe37b6b5cb8e8fb19.ppt
- Количество слайдов: 32
SELECTION OF A CLINICAL CHEMISTRY INSTRUMENT Prof. Abdus Sattar MBBS, MCPS, M. Phil, FCPS Dept of Pathology CMH Lahore Medical College Lahore

Introduction In the past most lab tests performed manually by endogenous reagent preparation for most of lab procedures u Now ready made reagents kits are available u Manual work replaced by automated lab instruments u

Clinical Chemistry Analyzers

Immunoassay Systems

Introduction u u u Instrument Selection Instrument Evaluation Guidelines and scheme of selection and evaluation: • • Barnett and Youden 1960 s IFCC Westgard – concepts and principles of evaluation CLIA ‘ 1988

Instrument Selection Because instrument purchases are some of the largest laboratory expenditures, the selection process should allow time for extensive research and evaluation.
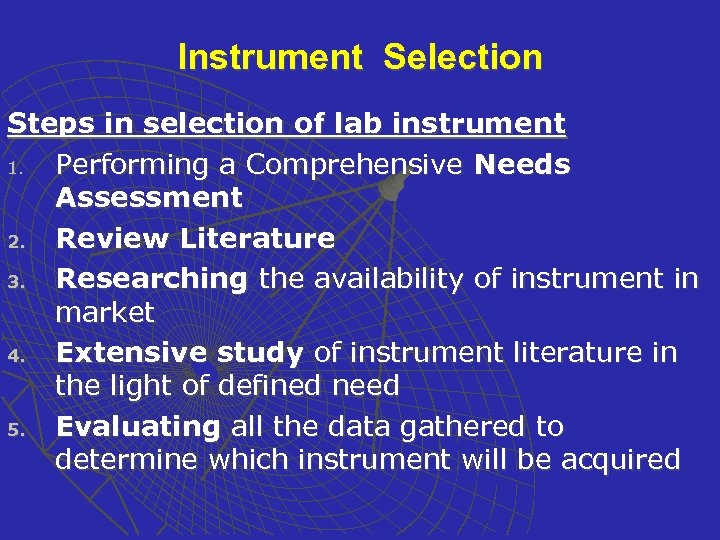
Instrument Selection Steps in selection of lab instrument 1. Performing a Comprehensive Needs Assessment 2. Review Literature 3. Researching the availability of instrument in market 4. Extensive study of instrument literature in the light of defined need 5. Evaluating all the data gathered to determine which instrument will be acquired

Selecting an Equipment u Define/ evaluate need: The key question why a new equipment is required? 1. Need may be related to clinicians request or input. 2. Requirement to replace an older, labor intensive equip with a more efficient system. 3. Need also encompasses the work load, Type of work load, running cost/test and capital money available 4. The need helps to define the characteristics required of a potential equip. 5. Pertinent properties relate to medical usefulness and include diagnostic sensitivity and specificity as well as the precision and accuracy required for effective performance.

Needs Assessment u Features & Characteristics • Should satisfy the need /requirements of lab • Should attempt to define both present & future needs • Team approach (assessment team includes reps. of personnel on all shifts, management, lab information specialist, QA officer, safety officer & others with special expertise)
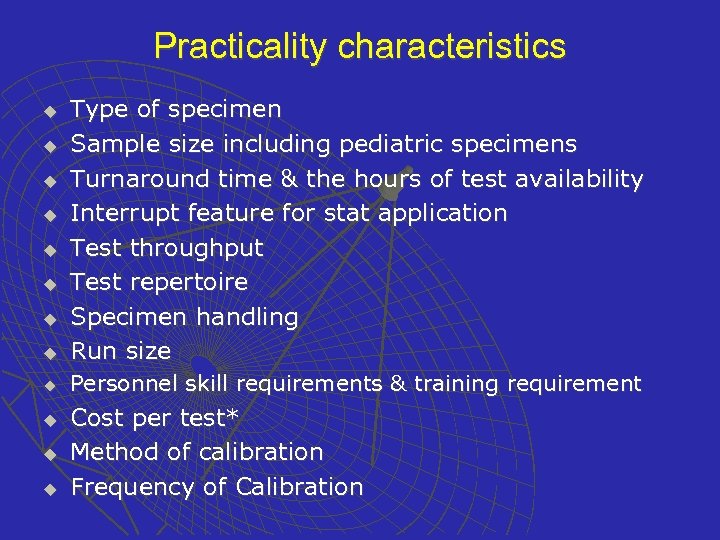
Practicality characteristics u Type of specimen Sample size including pediatric specimens Turnaround time & the hours of test availability Interrupt feature for stat application Test throughput Test repertoire Specimen handling Run size u Personnel skill requirements & training requirement u u u u u Cost per test* Method of calibration Frequency of Calibration
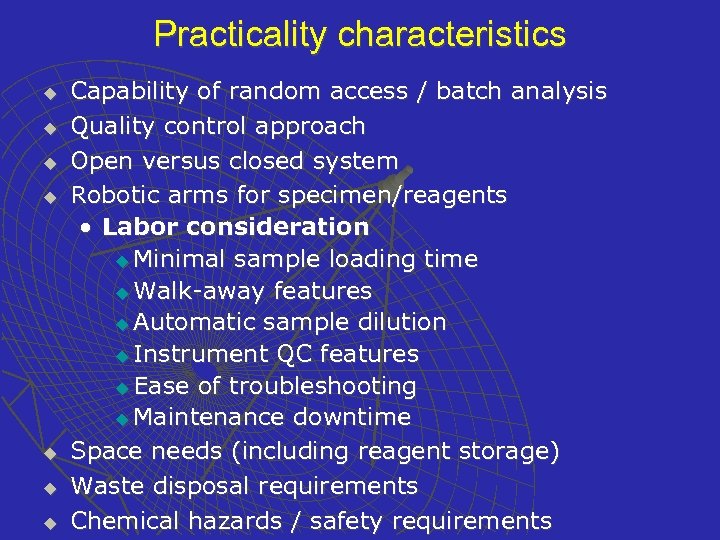
Practicality characteristics u u u u Capability of random access / batch analysis Quality control approach Open versus closed system Robotic arms for specimen/reagents • Labor consideration u Minimal sample loading time u Walk-away features u Automatic sample dilution u Instrument QC features u Ease of troubleshooting u Maintenance downtime Space needs (including reagent storage) Waste disposal requirements Chemical hazards / safety requirements

Practicality characteristics u Cost per test: • It has been defined as application characteristic due to compelling economic pressure. • The cost include; u Depreciation capital cost u Reagent cost (including water and accessories) u Cost of consumables( including daily, weekly, monthly and yearly maintenance cost) u Bar code reading facility expenses u Service and repair cost u Computer interface cost u Labor cost u Future increment in prices Open vs Closed System

Needs Assessment u Quality of performance (setting minimum standards for acceptable instrument performance) • • • Linearity Sensitivity Specificity Accuracy Precision

Research u u u Determine what is available in the market Meeting with sales personnel Review the literature supplied by the supplier Research of product from manufacturer web sites Contact colleague having experience of using the under consideration equip. Some professional organizations maintain information related to locating sources for instruments, reagents and other products (e. g. AABB Buyer’s Guide at www. aabb. org)

Research Compare the collected information u Short list the acceptable instruments u Final selection u • Net-working with other facilities • Satisfaction of other consumers regarding Manufacturer support services u Timeliness of repairs u Reagent problems u Actual instrument downtime u • Compare the actual user findings to company claims

Research u Develop a Request For Proposal (RFP) • Team to develop RFP • List of questions each vender replies • Venders may assist team by providing sample RFP questions and formats • Compare the instruments from venders answers

Evaluation & Decision Making u u Based on the developed need list and comparison data developed through the research process. Eliminate unacceptable instruments. Develop consensus decision between management and users. Worker’s input makes it “our” instrument rather than management’s new instrument.
Needs Assessment u Characteristics of the individual Lab. • Lab test menu needed in future • Current and future lab workload • Work flow situation (need of STAT capability) • Labor consideration Minimal sample loading time u Walk-away features u Automatic sample dilution u Instrument QC features u Data generation features u Ease of troubleshooting u Maintenance downtime u
Selecting An Analytical Method u u u The principle of the assay. The composition of reagents and reference materials including their storage requirements (e. g. , space, temperature, light and humidity restrictions) applicable both before and after the opening of the original containers. The stability of reagents and reference materials. Possible hazards, appropriate safety precautions. The type, quantity, and disposal of waste generated. Specimen requirements – that is, conditions for collection, specimen volume requirements, the need for anticoagulants and preservatives, and necessary storage conditions.

Selecting An Analytical Method u u u Anticipated analytical performance – that is, accuracy, precision, reportable range, and specificity using biological samples. The reference interval, including information on how it was derived; typical values obtained in health and disease. The detailed protocol for performing the test. Instrumental requirements and limitations. The availability of technical support, supplies, and service.

Selecting An Analytical Method u u u Is the requisite measure equipment available? If not, is sufficient space available for new equipment? How much of a technologist’s time is required, and what skill level should he or she have? If training the entire in a new technique is required, is such training worth the possible benefit? What is the estimated cost of performing an assay using the proposed method, including the cost of calibrators, quality control specimens, and a technologist’s time? Are the data generated compatible with the data processing equipment already available?

Research u u u Meeting with sales personnel Contacts to determine what is available in market Review the literature supplied by the supplier Research of product from manufacturer web sites Some professional organizations maintain information related to locating sources for instruments, reagents and other products (e. g. AABB Buyer’s Guide at www. aabb. org)
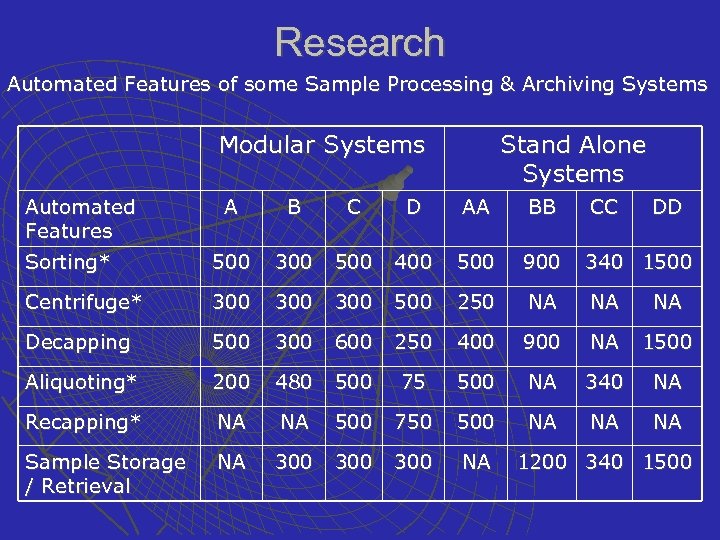
Research Automated Features of some Sample Processing & Archiving Systems Modular Systems Automated Features Stand Alone Systems A B C D AA BB CC DD Sorting* 500 300 500 400 500 900 340 1500 Centrifuge* 300 300 500 250 NA NA NA Decapping 500 300 600 250 400 900 NA 1500 Aliquoting* 200 480 500 75 500 NA 340 NA Recapping* NA NA 500 750 500 NA NA NA Sample Storage / Retrieval NA 300 300 NA 1200 340 1500
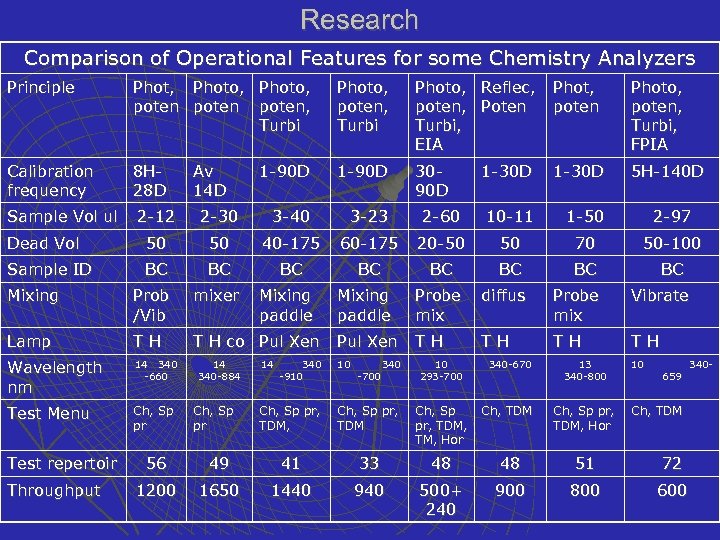
Research Comparison of Operational Features for some Chemistry Analyzers Principle Phot, Photo, poten, Turbi Photo, Reflec, poten, Poten Turbi, EIA Phot, poten Photo, poten, Turbi, FPIA Calibration frequency 8 H 28 D 1 -90 D 3090 D 1 -30 D 5 H-140 D Sample Vol ul 2 -12 2 -30 3 -40 3 -23 2 -60 10 -11 1 -50 2 -97 Dead Vol 50 50 40 -175 60 -175 20 -50 50 70 50 -100 Sample ID BC BC Mixing Prob /Vib mixer Lamp TH T H co Pul Xen Wavelength nm 14 340 -660 Test Menu Ch, Sp pr Test repertoir Throughput Av 14 D 14 340 -884 Ch, Sp pr Mixing paddle 14 340 -910 Mixing paddle Probe mix diffus Probe mix Vibrate Pul Xen TH TH 10 -700 340 10 293 -700 340 -670 13 340 -800 Ch, Sp pr, TDM, TM, Hor Ch, TDM Ch, Sp pr, TDM, Hor 10 659 Ch, TDM 56 49 41 33 48 48 51 72 1200 1650 1440 940 500+ 240 900 800 600 340 -

Needs Assessment u Additional instrument features • Instrument throughput • Sample processing method Sequential vs simultaneous u Random vs fixed ordering u Batching capabilities u • • • Bar coding Sample identification system Safety features (primary tube sampling) Methodologies (photometric vs electrochemical) Compatibility with LIS Data storage capabilities

u Cost • • • u u u Research Instrument Reagents Consumables Service contracts Spares Labor Cost is the limiting factor in determining, how many desired features are realistic. Cost may determine what is really “needed” versus what is “wanted”. Thorough research into all the details of cost will avoid surprises in the future.
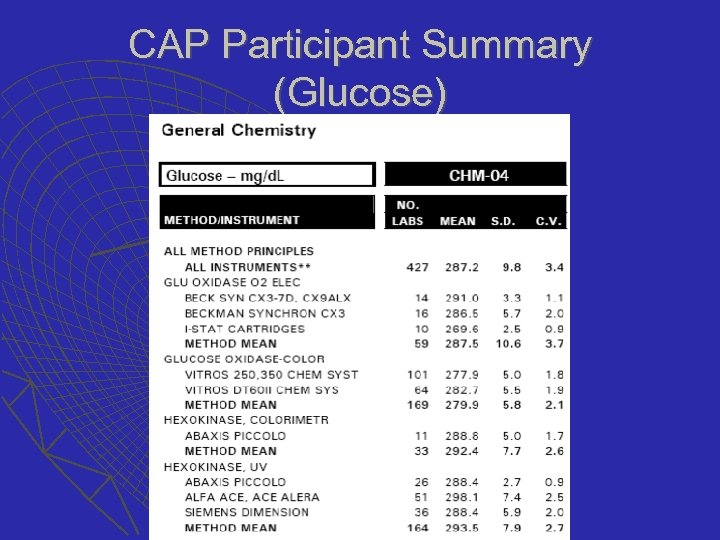
CAP Participant Summary (Glucose)
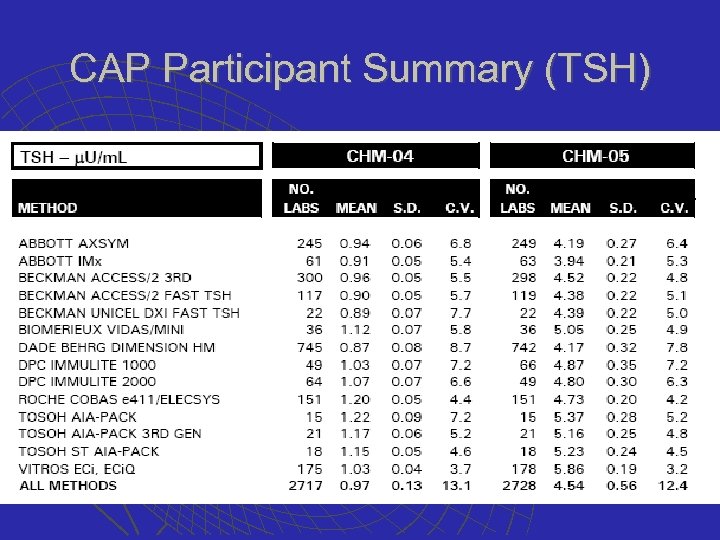
CAP Participant Summary (TSH)

Hematology Analyzer

Needs Assessment u Lab Environment • Size • Special electrical & plumbing needs • Heat generation during operation
fda062559d5756cfe37b6b5cb8e8fb19.ppt