9ba83ca831da71db403bf8145aee634e.ppt
- Количество слайдов: 21
 • • Select Agents and Toxins National Select Agent Registry Established in 1996 after the OKC bombing Antiterrorism and Effective Death Penalty Act of 1996 42 CFR 72. 6 Responsibility of the Department of Health and Human Services 2001 - USA PATRIOT Act (42 CFR 73) Agents published Federal Register on March 18, 2005 HHS - Human-related agents USDA - Agricultural-related agents http: //edocket. access. gpo. gov/2005/pdf/055216. pdf • • •
• • Select Agents and Toxins National Select Agent Registry Established in 1996 after the OKC bombing Antiterrorism and Effective Death Penalty Act of 1996 42 CFR 72. 6 Responsibility of the Department of Health and Human Services 2001 - USA PATRIOT Act (42 CFR 73) Agents published Federal Register on March 18, 2005 HHS - Human-related agents USDA - Agricultural-related agents http: //edocket. access. gpo. gov/2005/pdf/055216. pdf • • •
 • • • Select Agents and Toxins 7 CFR Part 331, 9 CFR Part 121, and 42 CFR Part 73 Work with these agents require: • • • FBI background investigation Mental health assessment Laboratory certification Training certification(s) Transfer and shipping documentation CDC’s role • • Laboratory Registration and Select Agents Tracking Program Designated by the HHS http: //www. selectagents. gov/Select%20 Agents%20 and%20 Toxins%20 List. html
• • • Select Agents and Toxins 7 CFR Part 331, 9 CFR Part 121, and 42 CFR Part 73 Work with these agents require: • • • FBI background investigation Mental health assessment Laboratory certification Training certification(s) Transfer and shipping documentation CDC’s role • • Laboratory Registration and Select Agents Tracking Program Designated by the HHS http: //www. selectagents. gov/Select%20 Agents%20 and%20 Toxins%20 List. html
 Select Agent Viruses • • • • Cercopithecine herpesvirus 1 (Herpes B virus) Crimean-Congo haemorrhagic fever virus Eastern Equine Encephalitis virus Ebola virus Lassa fever virus Lujo virus Marburg virus Hendra virus. Nipah virus. Rift Valley fever Monkeypox virus. Venezuelan Equine Encephalitis virus Reconstructed replication competent forms of the 1918 pandemic influenza virus containing any USDA Select Agents AND TOXINSAfrican horse sickness portion of the coding regions of all eight gene virus. African swine fever virus. Akabane virus. Avian segments (Reconstructed 1918 Influenza virus) influenza virus (highly pathogenic)Bluetongue virus South American Haemorrhagic Fever viruses (exotic)Bovine spongiform encephalopathy agent. Camel Flexal pox virus. Classical swine fever virus. Foot-and-mouth Guanarito disease virus. Goat pox virus. Japanese encephalitis Junin virus. Lumpy skin disease virus. Malignant catarrhal fever virus(Alcelaphine herpesvirus type 1)Menangle Machupo virus. Peste des petits ruminants virus. Rinderpest Sabia virus. Sheep pox virus. Swine vesicular disease Chapare virus. Vesicular stomatitis virus Indiana subtypes IN 2, Variola major virus (Smallpox virus) IN 3 Virulent Newcastle disease virus 1 Variola minor virus (Alastrim) Tick-borne encephalitis complex (flavi) viruses Central European Tick-borne encephalitis Far Eastern Tick-borne encephalitis Kyasanur Forest disease Omsk Hemorrhagic Fever Russian Spring and Summer encephalitis • • •
Select Agent Viruses • • • • Cercopithecine herpesvirus 1 (Herpes B virus) Crimean-Congo haemorrhagic fever virus Eastern Equine Encephalitis virus Ebola virus Lassa fever virus Lujo virus Marburg virus Hendra virus. Nipah virus. Rift Valley fever Monkeypox virus. Venezuelan Equine Encephalitis virus Reconstructed replication competent forms of the 1918 pandemic influenza virus containing any USDA Select Agents AND TOXINSAfrican horse sickness portion of the coding regions of all eight gene virus. African swine fever virus. Akabane virus. Avian segments (Reconstructed 1918 Influenza virus) influenza virus (highly pathogenic)Bluetongue virus South American Haemorrhagic Fever viruses (exotic)Bovine spongiform encephalopathy agent. Camel Flexal pox virus. Classical swine fever virus. Foot-and-mouth Guanarito disease virus. Goat pox virus. Japanese encephalitis Junin virus. Lumpy skin disease virus. Malignant catarrhal fever virus(Alcelaphine herpesvirus type 1)Menangle Machupo virus. Peste des petits ruminants virus. Rinderpest Sabia virus. Sheep pox virus. Swine vesicular disease Chapare virus. Vesicular stomatitis virus Indiana subtypes IN 2, Variola major virus (Smallpox virus) IN 3 Virulent Newcastle disease virus 1 Variola minor virus (Alastrim) Tick-borne encephalitis complex (flavi) viruses Central European Tick-borne encephalitis Far Eastern Tick-borne encephalitis Kyasanur Forest disease Omsk Hemorrhagic Fever Russian Spring and Summer encephalitis • • •
 Arthropod-Borne Viral Diseases Chapter 38
Arthropod-Borne Viral Diseases Chapter 38
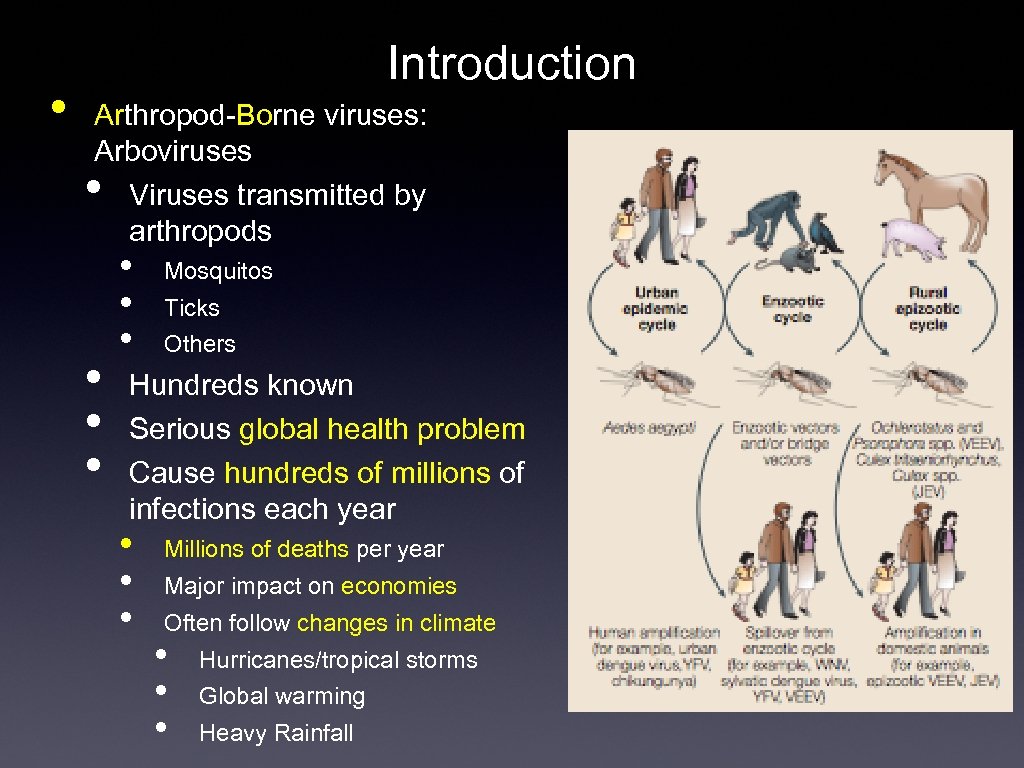 • Introduction Arthropod-Borne viruses: Arboviruses Viruses transmitted by arthropods • • Mosquitos Ticks Others Hundreds known Serious global health problem Cause hundreds of millions of infections each year • • • Millions of deaths per year Major impact on economies Often follow changes in climate • • • Hurricanes/tropical storms Global warming Heavy Rainfall
• Introduction Arthropod-Borne viruses: Arboviruses Viruses transmitted by arthropods • • Mosquitos Ticks Others Hundreds known Serious global health problem Cause hundreds of millions of infections each year • • • Millions of deaths per year Major impact on economies Often follow changes in climate • • • Hurricanes/tropical storms Global warming Heavy Rainfall
 Zoonoses • • • A zoonotic agent is one that normally persists in animals in a non- or low-pathogenic state, but can cause disease in humans In most instances, transmission to humans is incidental It is also a dead-end for the virus The diseases are termed zoonoses (sing: zoonosis) Outbreaks of zoonoses are termed epizootic Persistence of a disease agent in a particular environment is termed endemic An epidemic of animal infections is termed enzootic • •
Zoonoses • • • A zoonotic agent is one that normally persists in animals in a non- or low-pathogenic state, but can cause disease in humans In most instances, transmission to humans is incidental It is also a dead-end for the virus The diseases are termed zoonoses (sing: zoonosis) Outbreaks of zoonoses are termed epizootic Persistence of a disease agent in a particular environment is termed endemic An epidemic of animal infections is termed enzootic • •
 Togaviruses • • • Family Togaviridae, genus Alphavirus Plus-strand RNA Can undergo recombination with other alphaviruses More than 30 viruses Enveloped Notable members Venezuelan equine encephalitis virus group Eastern equine encephalitis virus group Western equine encephalitis virus group Chikungunya virus O'nyong-nyong virus Ross River virus Semliki forest virus • •
Togaviruses • • • Family Togaviridae, genus Alphavirus Plus-strand RNA Can undergo recombination with other alphaviruses More than 30 viruses Enveloped Notable members Venezuelan equine encephalitis virus group Eastern equine encephalitis virus group Western equine encephalitis virus group Chikungunya virus O'nyong-nyong virus Ross River virus Semliki forest virus • •
 Flaviviruses • • • Family Flaviviridae Plus-strand RNA Enveloped Virus maturation on cytoplasmic membranes Dozens of known species Notable members Dengue virus group Japanese encephalitis virus group • • JE virus West nile virus St. Louis encephalitis virus Tick-borne encephalitis virus group Yellow fever virus group Russian Spring-Summer encephalitis virus
Flaviviruses • • • Family Flaviviridae Plus-strand RNA Enveloped Virus maturation on cytoplasmic membranes Dozens of known species Notable members Dengue virus group Japanese encephalitis virus group • • JE virus West nile virus St. Louis encephalitis virus Tick-borne encephalitis virus group Yellow fever virus group Russian Spring-Summer encephalitis virus
 • • • Bunyaviruses Family Bunyaviridae Negative-strand or ambisense RNA Tripartite genomes Three gene segments S - small (nucleocapsid) M - medium (glycoproteins G 1 and G 2) L - large (RNA polymerase) Enveloped Dozens of known species Five genera Nairovirus Orthobunyavirus Phlebovirus Hantavirus (rodent-borne) Tospovirus (plant viruses) • • •
• • • Bunyaviruses Family Bunyaviridae Negative-strand or ambisense RNA Tripartite genomes Three gene segments S - small (nucleocapsid) M - medium (glycoproteins G 1 and G 2) L - large (RNA polymerase) Enveloped Dozens of known species Five genera Nairovirus Orthobunyavirus Phlebovirus Hantavirus (rodent-borne) Tospovirus (plant viruses) • • •
 Bunyaviruses • Notable members (arthropod-borne) Phleboviruses Toscana and Tehran viruses (Sandfly fever) Punto Toro virus (hemorrhagic fever) Rift Valley fever virus (hemorrhagic fever) Coltivirus Colorado tick fever Orthobunyavirus La Crosse virus (encephalitis) • •
Bunyaviruses • Notable members (arthropod-borne) Phleboviruses Toscana and Tehran viruses (Sandfly fever) Punto Toro virus (hemorrhagic fever) Rift Valley fever virus (hemorrhagic fever) Coltivirus Colorado tick fever Orthobunyavirus La Crosse virus (encephalitis) • •
 Venezuelan Equine Encephalitis • • • First recognized as South American equine disease (1930 s) Highly fatal Subsequently identified as human disease (1950 s) Rarely fatal; instances of permanent neurological damage Until 1995, sporadic epizootic outbreaks of human and equine cases in SA Sometimes hundreds of thousands Mid 1990 s outbreaks occurred in Central America and Mexico Similar alphaviruses in the VEE group were found in Florida (e. g. Everglades virus) Ecology Endemic vector: Culex species mosquitos Amplification hosts: rodents Epidemic vectors: Ochlerotatus and Psorophora spp. mosquitos • • •
Venezuelan Equine Encephalitis • • • First recognized as South American equine disease (1930 s) Highly fatal Subsequently identified as human disease (1950 s) Rarely fatal; instances of permanent neurological damage Until 1995, sporadic epizootic outbreaks of human and equine cases in SA Sometimes hundreds of thousands Mid 1990 s outbreaks occurred in Central America and Mexico Similar alphaviruses in the VEE group were found in Florida (e. g. Everglades virus) Ecology Endemic vector: Culex species mosquitos Amplification hosts: rodents Epidemic vectors: Ochlerotatus and Psorophora spp. mosquitos • • •
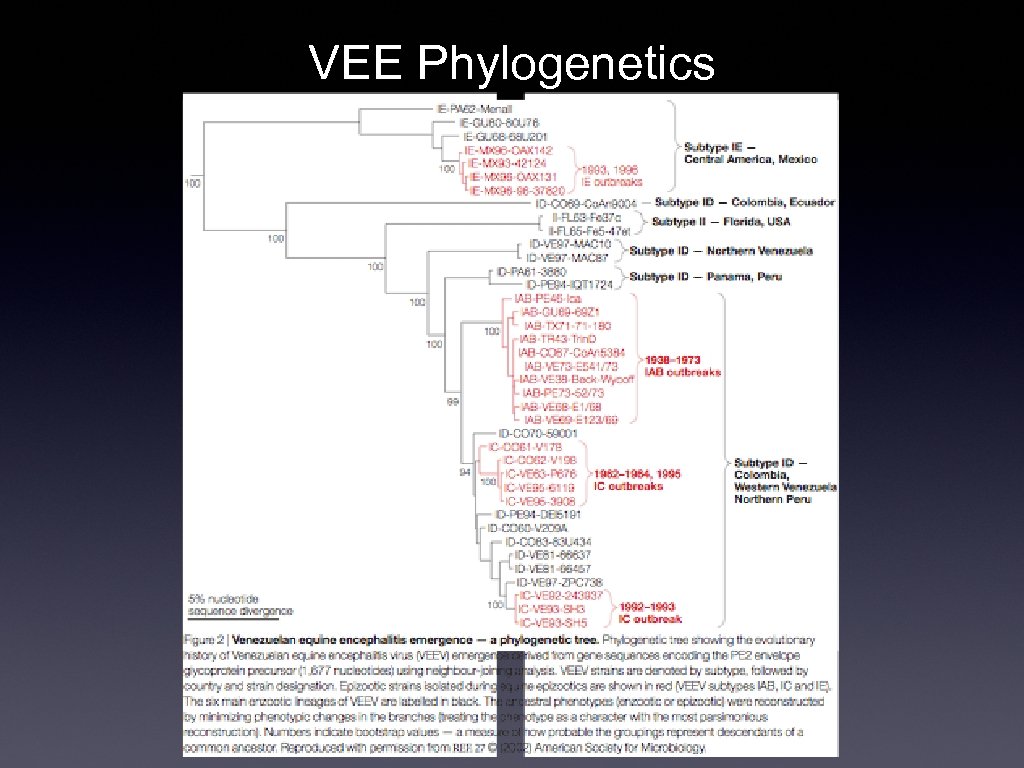 VEE Phylogenetics
VEE Phylogenetics
 • • Dengue Four serotypes: 1 -4 Most important human arboviral pathogen About 50 million infections each year 2. 5 billion people at risk Humans and other primates are amplifying hosts Endemic in Texas Peridomestic vectors Aedes aegypti Aedes albopictus Pathogenesis: Immunopathology Infection with first dengue virus results in dengue fever Second infection with another serotype can result in dengue hemorrhagic fever Inflammatory immune response Cytokine storm • • •
• • Dengue Four serotypes: 1 -4 Most important human arboviral pathogen About 50 million infections each year 2. 5 billion people at risk Humans and other primates are amplifying hosts Endemic in Texas Peridomestic vectors Aedes aegypti Aedes albopictus Pathogenesis: Immunopathology Infection with first dengue virus results in dengue fever Second infection with another serotype can result in dengue hemorrhagic fever Inflammatory immune response Cytokine storm • • •
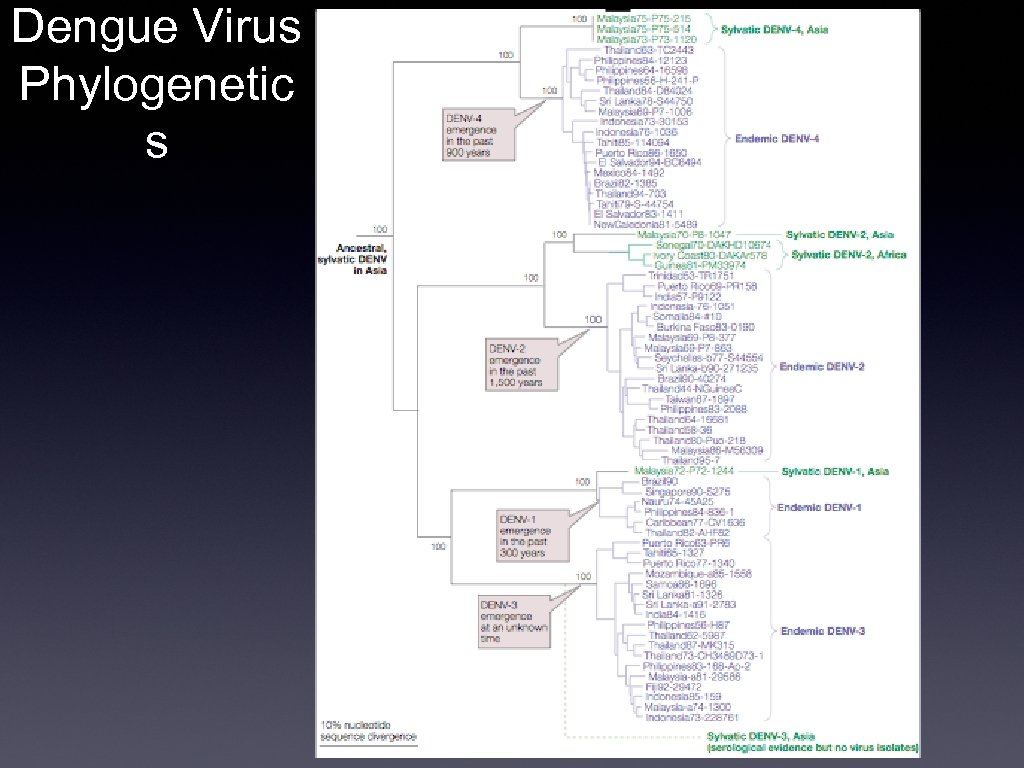 Dengue Virus Phylogenetic s
Dengue Virus Phylogenetic s
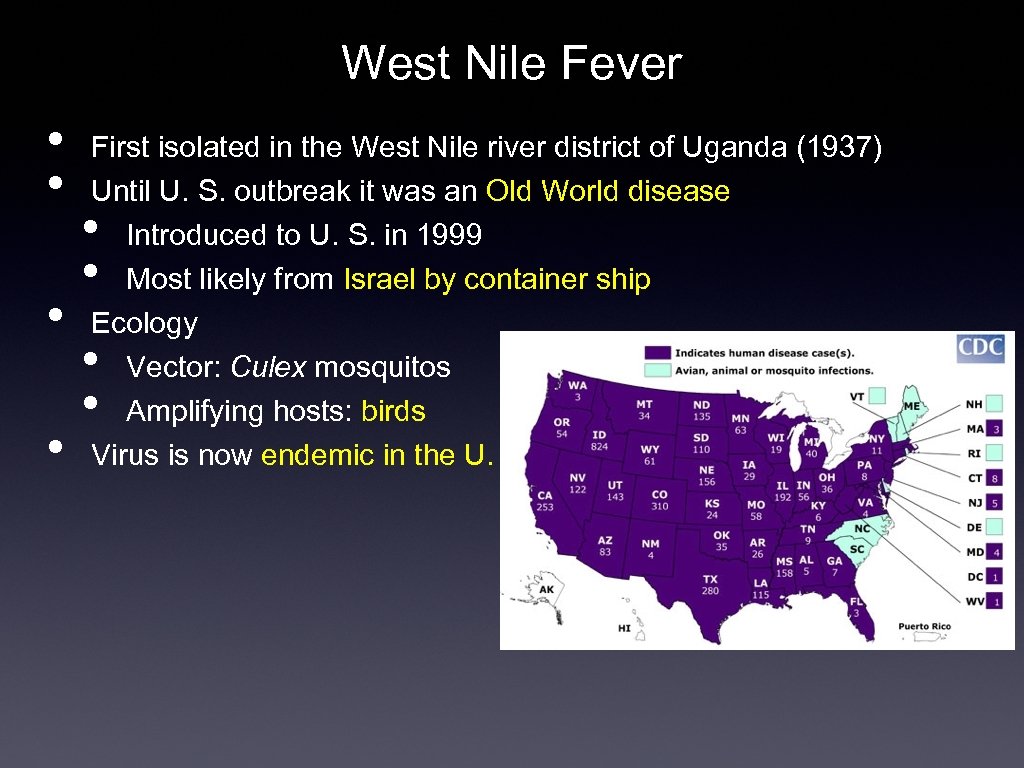 West Nile Fever • • First isolated in the West Nile river district of Uganda (1937) Until U. S. outbreak it was an Old World disease Introduced to U. S. in 1999 Most likely from Israel by container ship Ecology Vector: Culex mosquitos Amplifying hosts: birds Virus is now endemic in the U. S. • •
West Nile Fever • • First isolated in the West Nile river district of Uganda (1937) Until U. S. outbreak it was an Old World disease Introduced to U. S. in 1999 Most likely from Israel by container ship Ecology Vector: Culex mosquitos Amplifying hosts: birds Virus is now endemic in the U. S. • •
 West Nile Fever • Pathogenesis Historically, only known to cause a febrile illness 1996 Romanian outbreak resulted in unusually higher mortality and encephalitis 1998 Russian outbreak also had high incidence of neurological manifestations Arrival in U. S. resulted in tens of thousands of infections • • Naive population Blood bank studies suggest only 1 in 5 show symptoms Molecular mechanisms are largely unknown • • Animal studies suggest a chemokine response in the CNS may recruit inflammatory cells CTL infiltrates kill neurons in mice infected with WNV
West Nile Fever • Pathogenesis Historically, only known to cause a febrile illness 1996 Romanian outbreak resulted in unusually higher mortality and encephalitis 1998 Russian outbreak also had high incidence of neurological manifestations Arrival in U. S. resulted in tens of thousands of infections • • Naive population Blood bank studies suggest only 1 in 5 show symptoms Molecular mechanisms are largely unknown • • Animal studies suggest a chemokine response in the CNS may recruit inflammatory cells CTL infiltrates kill neurons in mice infected with WNV
 West Nile Virus • Clinical outcomes • • • Completely asymptomatic (80%) West Nile fever - headache, confusion, complete recovery West Nile meningitis • • • meningeal inflammation photophobia phonophobia Kernig sign (inability to straighten leg without pain) Brudzinski's sign (lift head and hips and knees flex) West Nile encephalitis • • • encephalopathy (altered level of consciousness, lethargy, personality change) seizures CNS inflammation West Nile meningoencephalitis Acute flaccid paralysis • May have long-term or indefinite weakness
West Nile Virus • Clinical outcomes • • • Completely asymptomatic (80%) West Nile fever - headache, confusion, complete recovery West Nile meningitis • • • meningeal inflammation photophobia phonophobia Kernig sign (inability to straighten leg without pain) Brudzinski's sign (lift head and hips and knees flex) West Nile encephalitis • • • encephalopathy (altered level of consciousness, lethargy, personality change) seizures CNS inflammation West Nile meningoencephalitis Acute flaccid paralysis • May have long-term or indefinite weakness
 • Yellow Fever Principally a disease of Africa and South America It is thought that YFV arrived in the Americas during the slave trade Infected slaves, who had some genetic resistance, carried the virus, which then entered the local ecosystem after mosquito bites Two forms Urban Yellow Fever • • Mosquito-human transmission cycle Aedes aegypti Jungle Yellow Fever • • • Mosquito-primate transmission cycle with spillover to humans Aedes spp. in Africa Haemagogus and Sabethes spp. in
• Yellow Fever Principally a disease of Africa and South America It is thought that YFV arrived in the Americas during the slave trade Infected slaves, who had some genetic resistance, carried the virus, which then entered the local ecosystem after mosquito bites Two forms Urban Yellow Fever • • Mosquito-human transmission cycle Aedes aegypti Jungle Yellow Fever • • • Mosquito-primate transmission cycle with spillover to humans Aedes spp. in Africa Haemagogus and Sabethes spp. in
 Yellow Fever Virus • Genome 10. 8 kb RNA Single ORF of 10. 2 kb • • Structural proteins • • • nucleocapsid (C) Premembrane/membrane (pr. M/M) - involved in membrane fusion Envelope (E) - spike protein, HA activity, neutralizing antibody target Nonstructural proteins • • NS 1 - expressed on cell membranes, including surface; involved in RNA synthesis NS 2 A - Interacts with NS 3 and NS 5 to regulate viral gene expression NS 2 B - Interacts with NS 3 to form protease for viral polypeptide NS 3 NS 4 A - Involved with RNA synthesis NS 4 B - Involved with RNA synthesis NS 5 - RNA polymerase and methyltransferase for 5’ Met-G cap formation
Yellow Fever Virus • Genome 10. 8 kb RNA Single ORF of 10. 2 kb • • Structural proteins • • • nucleocapsid (C) Premembrane/membrane (pr. M/M) - involved in membrane fusion Envelope (E) - spike protein, HA activity, neutralizing antibody target Nonstructural proteins • • NS 1 - expressed on cell membranes, including surface; involved in RNA synthesis NS 2 A - Interacts with NS 3 and NS 5 to regulate viral gene expression NS 2 B - Interacts with NS 3 to form protease for viral polypeptide NS 3 NS 4 A - Involved with RNA synthesis NS 4 B - Involved with RNA synthesis NS 5 - RNA polymerase and methyltransferase for 5’ Met-G cap formation
 • Yellow Fever Pathogenesis Onset 3 -6 days Sometimes a brief (48 hr) recovery period occurs, followed by jaundice Primary replication in lymph nodes Systemic infection • • • Liver, spleen, kidneys, bone marrow, lymph nodes Acute liver failure Apoptosis of hepatocytes Inflammation Immune infiltrates (immunopathology? ) 10% mortality Control Virus first isolated in 1928 YFV 17 D live attenuated vaccine strain derived 1937 31 amino acid differences between vaccine strain and wild-type • • •
• Yellow Fever Pathogenesis Onset 3 -6 days Sometimes a brief (48 hr) recovery period occurs, followed by jaundice Primary replication in lymph nodes Systemic infection • • • Liver, spleen, kidneys, bone marrow, lymph nodes Acute liver failure Apoptosis of hepatocytes Inflammation Immune infiltrates (immunopathology? ) 10% mortality Control Virus first isolated in 1928 YFV 17 D live attenuated vaccine strain derived 1937 31 amino acid differences between vaccine strain and wild-type • • •
 Yellow Fever Vaccine • • Rare adverse events following vaccination Recent report of a death (Doblas et al. 2006. J Clin Virol. 36: 156) • • • 26 -year old Spanish woman planning to travel to Africa Developed yellow fever a few days after vaccination Died 10 days after vaccination Genome of recovered virus sequenced • Only two silent mutations were found Chimeri. Vax Yellow Fever Vaccine Backbone Proprietary (Acambis, Inc) Reverse genetics system of 17 D Replace E gene with E gene of choice Vaccine Candidates • • West Nile Chimeri. Vax candidate is in clinical trials JEV Chimerivax DENV four serotype Chimerivax
Yellow Fever Vaccine • • Rare adverse events following vaccination Recent report of a death (Doblas et al. 2006. J Clin Virol. 36: 156) • • • 26 -year old Spanish woman planning to travel to Africa Developed yellow fever a few days after vaccination Died 10 days after vaccination Genome of recovered virus sequenced • Only two silent mutations were found Chimeri. Vax Yellow Fever Vaccine Backbone Proprietary (Acambis, Inc) Reverse genetics system of 17 D Replace E gene with E gene of choice Vaccine Candidates • • West Nile Chimeri. Vax candidate is in clinical trials JEV Chimerivax DENV four serotype Chimerivax


