a5ee6ea98bb08801952716c1d23426b1.ppt
- Количество слайдов: 38
 Second WHO consultation on International Reference Preparation for Chagas Diagnostic Tests January 27 & 28 January, 2009 TESTS USED IN BLOOD SCREENING Dr Azzedine ASSAL French Blood Services (EFS)
Second WHO consultation on International Reference Preparation for Chagas Diagnostic Tests January 27 & 28 January, 2009 TESTS USED IN BLOOD SCREENING Dr Azzedine ASSAL French Blood Services (EFS)
 Background • The choice of a Chagas disease screening assay or strategy for TT prevention is far from straightforward • Recommendation of the PAHO (1994): parallel use of at least 2 different serological tests in Chagas disease screening in blood donations (Lack of sensitivity and specificity). • Recommendations of WHO, 2002: one ELISA is recommended for blood bank screening
Background • The choice of a Chagas disease screening assay or strategy for TT prevention is far from straightforward • Recommendation of the PAHO (1994): parallel use of at least 2 different serological tests in Chagas disease screening in blood donations (Lack of sensitivity and specificity). • Recommendations of WHO, 2002: one ELISA is recommended for blood bank screening
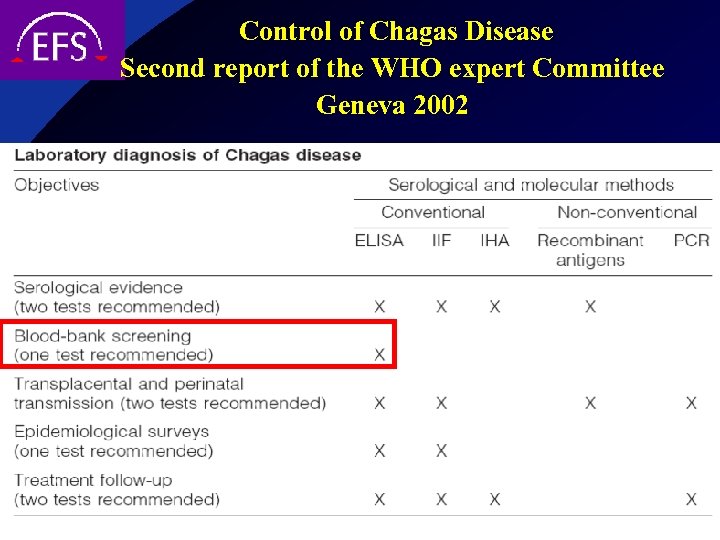 Control of Chagas Disease Second report of the WHO expert Committee Geneva 2002
Control of Chagas Disease Second report of the WHO expert Committee Geneva 2002
 Screening Strategies ØEndemic Countries : ØBrazil: until 2002: 2 tests (>70% ELISA + IHA) ØBrazil: since 2003: 1 test ELISA ØArgentina 2004: 2 tests ØCosta Rica 2006: 2 tests (ELISA rec + Lys) Ø Non endemic countries ØUK 1999 to 2005: 1 ELISA Lys, from 2006 ELISA rec Ø USA 2007: 1 test (ELISA Lys) Ø France 2007: 2 tests (ELISA rec + Lys) Ø Spain 2008: 2 tests (ELISA rec + Lys) Amadeo Sáez-Alquezar. Fondation Mérieux. May 2008.
Screening Strategies ØEndemic Countries : ØBrazil: until 2002: 2 tests (>70% ELISA + IHA) ØBrazil: since 2003: 1 test ELISA ØArgentina 2004: 2 tests ØCosta Rica 2006: 2 tests (ELISA rec + Lys) Ø Non endemic countries ØUK 1999 to 2005: 1 ELISA Lys, from 2006 ELISA rec Ø USA 2007: 1 test (ELISA Lys) Ø France 2007: 2 tests (ELISA rec + Lys) Ø Spain 2008: 2 tests (ELISA rec + Lys) Amadeo Sáez-Alquezar. Fondation Mérieux. May 2008.
 Ideal screening serological test Ø 100 % sensitivity Ø 100 % specificity Ø Reproducible Ø Easy to perform Ø Fast and automated Ø Non subjective reading Ø Not expensive The ideal test does not exist
Ideal screening serological test Ø 100 % sensitivity Ø 100 % specificity Ø Reproducible Ø Easy to perform Ø Fast and automated Ø Non subjective reading Ø Not expensive The ideal test does not exist
 Different strategies for blood banks Ø Using only one test Ø High sensitivity test (Ig. G + Ig. M) Ø Use a whole parasite Lysate test (mixture of parasite antigens) Ø Using 2 tests Ø 1 Lysate ELISA + 1 rec ELISA Ø IFI + ELISA
Different strategies for blood banks Ø Using only one test Ø High sensitivity test (Ig. G + Ig. M) Ø Use a whole parasite Lysate test (mixture of parasite antigens) Ø Using 2 tests Ø 1 Lysate ELISA + 1 rec ELISA Ø IFI + ELISA
 French Screening Strategy ü Commercial assays available : IHA or other agglutination tests, ELISA, IFA. ü French strategy: Screening based on 2 parallel ELISAs (Crude and recombinant antigens). ü IFA as an alternative test (“confirmation”) test in case of positivity or discrepancy between the 2 ELISAs.
French Screening Strategy ü Commercial assays available : IHA or other agglutination tests, ELISA, IFA. ü French strategy: Screening based on 2 parallel ELISAs (Crude and recombinant antigens). ü IFA as an alternative test (“confirmation”) test in case of positivity or discrepancy between the 2 ELISAs.
 EVALUATED ASSAYS 1) ELISAs • Recombinant ELISAs Bioelisa Chagas (Biokit, Spain). CE mark. • Crude ELISAs Ø ELISA Cruzi (Bio. Mérieux). No CE mark. Ø Chagatek Elisa (Lemos, Argentina), No CE mark. Ø T. cruzi ELISA Test System– 1 (OCD). CE mark. Ø EIAgen Trypanosoma Cruzi Ab (manufactured by Adaltis and distributed by Ingen, France). CE mark. 2) IFA § Immunofluor Chagas (Biocientifica. Argentina). CE mark.
EVALUATED ASSAYS 1) ELISAs • Recombinant ELISAs Bioelisa Chagas (Biokit, Spain). CE mark. • Crude ELISAs Ø ELISA Cruzi (Bio. Mérieux). No CE mark. Ø Chagatek Elisa (Lemos, Argentina), No CE mark. Ø T. cruzi ELISA Test System– 1 (OCD). CE mark. Ø EIAgen Trypanosoma Cruzi Ab (manufactured by Adaltis and distributed by Ingen, France). CE mark. 2) IFA § Immunofluor Chagas (Biocientifica. Argentina). CE mark.
 EVALUATED FEATURES ü FEASIBILITY ü CLINICAL SENSITIVITY üSPECIFICITY ü REPRODUCIBILITY
EVALUATED FEATURES ü FEASIBILITY ü CLINICAL SENSITIVITY üSPECIFICITY ü REPRODUCIBILITY
 Reference material for test evaluation Ideally Sensitivity evaluation Ø Strong positive samples Ø Borderline samples Ø Discordant samples Ø Samples with reactivity against main strains of the 2 lineages of T. cruzi Specificity evaluation Ø “True” negative samples Ø Potential cross-reactive samples (leishmania, T. Rangeli, other protozoans)
Reference material for test evaluation Ideally Sensitivity evaluation Ø Strong positive samples Ø Borderline samples Ø Discordant samples Ø Samples with reactivity against main strains of the 2 lineages of T. cruzi Specificity evaluation Ø “True” negative samples Ø Potential cross-reactive samples (leishmania, T. Rangeli, other protozoans)
 Material and methods Panels and samples • BBI panel : 14 positive samples + 1 negative sample • Dilutions of Positive Control (Accurun, Ingen) • Brazilian donor Panel (Blood Bank Sao Paulo): 36 samples of positive and negative donors, tested with ELISA, IHA et IFA. • Patient samples (French Guyana) 35 positive and negative samples, tested with ID Pa. Gia (Diamed), Biokit ELISA and PCR • French Blood donors for specificity study.
Material and methods Panels and samples • BBI panel : 14 positive samples + 1 negative sample • Dilutions of Positive Control (Accurun, Ingen) • Brazilian donor Panel (Blood Bank Sao Paulo): 36 samples of positive and negative donors, tested with ELISA, IHA et IFA. • Patient samples (French Guyana) 35 positive and negative samples, tested with ID Pa. Gia (Diamed), Biokit ELISA and PCR • French Blood donors for specificity study.
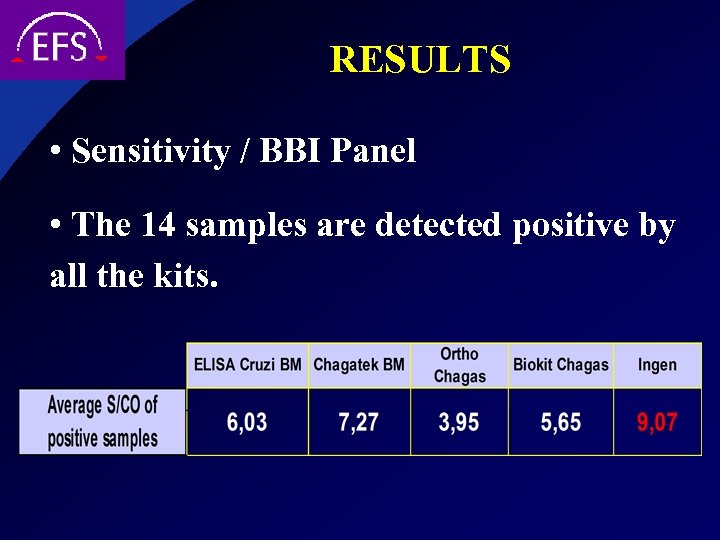 RESULTS • Sensitivity / BBI Panel • The 14 samples are detected positive by all the kits.
RESULTS • Sensitivity / BBI Panel • The 14 samples are detected positive by all the kits.
 RESULTS (2) Sensitivity / Brazilian donor panel • Negative samples: No discrepancies with Brazilian data.
RESULTS (2) Sensitivity / Brazilian donor panel • Negative samples: No discrepancies with Brazilian data.
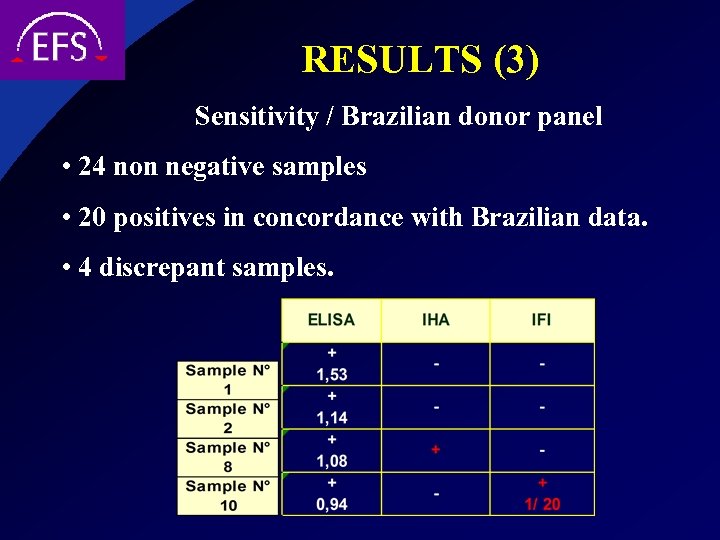 RESULTS (3) Sensitivity / Brazilian donor panel • 24 non negative samples • 20 positives in concordance with Brazilian data. • 4 discrepant samples.
RESULTS (3) Sensitivity / Brazilian donor panel • 24 non negative samples • 20 positives in concordance with Brazilian data. • 4 discrepant samples.
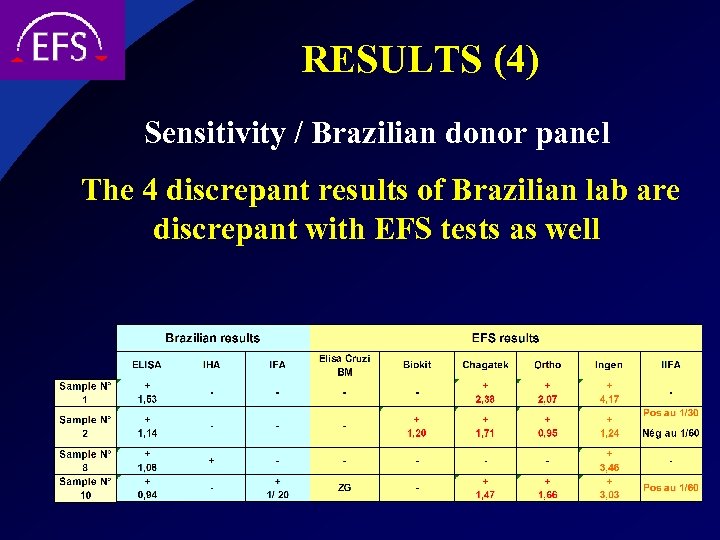 RESULTS (4) Sensitivity / Brazilian donor panel The 4 discrepant results of Brazilian lab are discrepant with EFS tests as well
RESULTS (4) Sensitivity / Brazilian donor panel The 4 discrepant results of Brazilian lab are discrepant with EFS tests as well
 RESULTS (5) Conclusion on the sensitivity of Brazilian donor panel • Good overall sensitivity of all the kits • Follow up of Brazilian discrepant samples showed that the discrepant samples were false positive samples
RESULTS (5) Conclusion on the sensitivity of Brazilian donor panel • Good overall sensitivity of all the kits • Follow up of Brazilian discrepant samples showed that the discrepant samples were false positive samples
 RESULTS (6) Guyana patient samples w A set of 35 negative and positive patient samples (Dr Christine Aznar. Laboratory of Parasitology, Cayenne Hospital, French Guyana). w Tested by 3 different assays in Guyana: • Agglutination test (ID-Pa. GIA, Diamed, France). • ELISA (Bioelisa Chagas, Biokit). • In-house PCR. w Blind testing before result comparison with Guyana data.
RESULTS (6) Guyana patient samples w A set of 35 negative and positive patient samples (Dr Christine Aznar. Laboratory of Parasitology, Cayenne Hospital, French Guyana). w Tested by 3 different assays in Guyana: • Agglutination test (ID-Pa. GIA, Diamed, France). • ELISA (Bioelisa Chagas, Biokit). • In-house PCR. w Blind testing before result comparison with Guyana data.
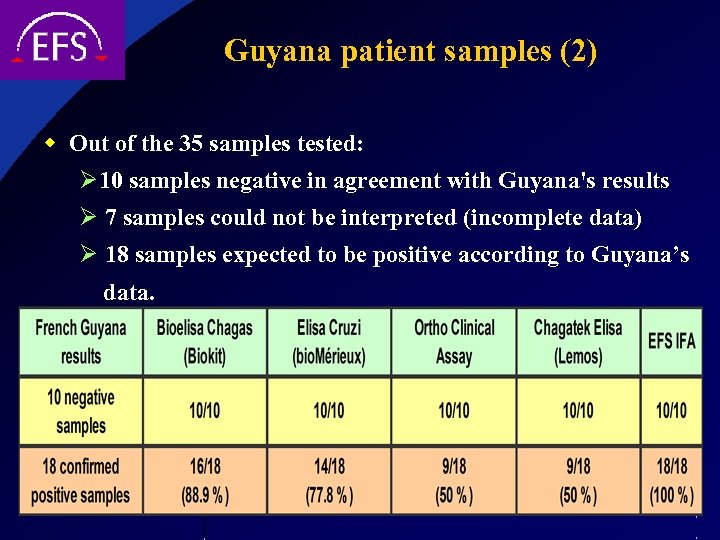 Guyana patient samples (2) w Out of the 35 samples tested: Ø 10 samples negative in agreement with Guyana's results Ø 7 samples could not be interpreted (incomplete data) Ø 18 samples expected to be positive according to Guyana’s data.
Guyana patient samples (2) w Out of the 35 samples tested: Ø 10 samples negative in agreement with Guyana's results Ø 7 samples could not be interpreted (incomplete data) Ø 18 samples expected to be positive according to Guyana’s data.
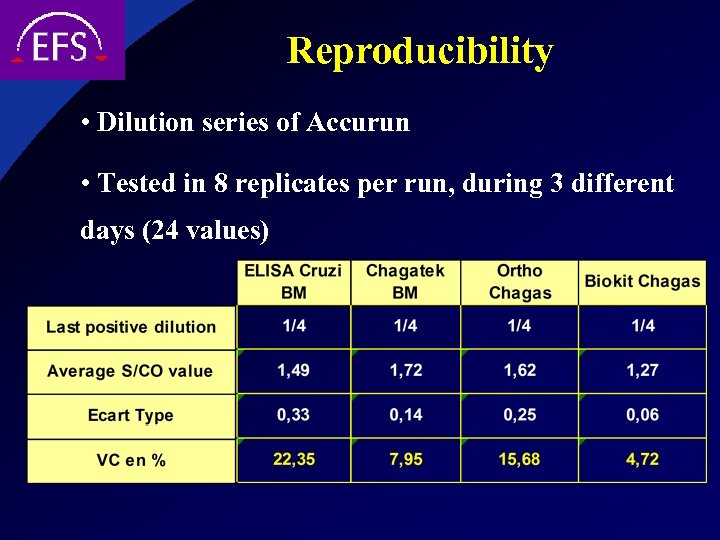 Reproducibility • Dilution series of Accurun • Tested in 8 replicates per run, during 3 different days (24 values)
Reproducibility • Dilution series of Accurun • Tested in 8 replicates per run, during 3 different days (24 values)
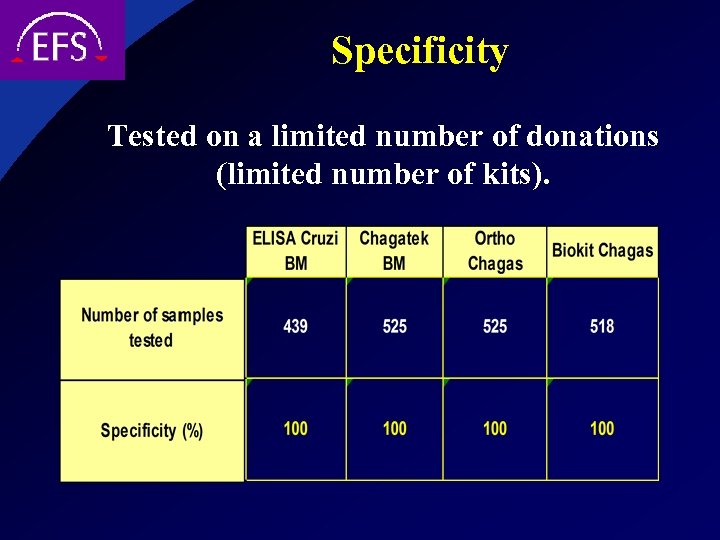 Specificity Tested on a limited number of donations (limited number of kits).
Specificity Tested on a limited number of donations (limited number of kits).
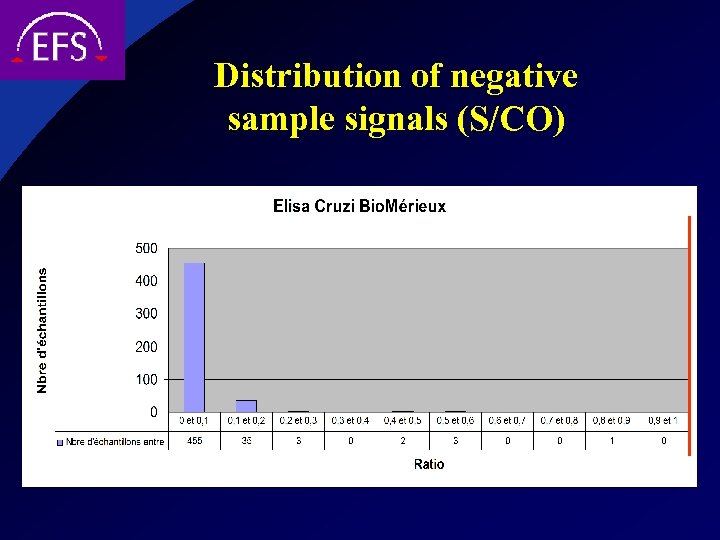 Distribution of negative sample signals (S/CO)
Distribution of negative sample signals (S/CO)
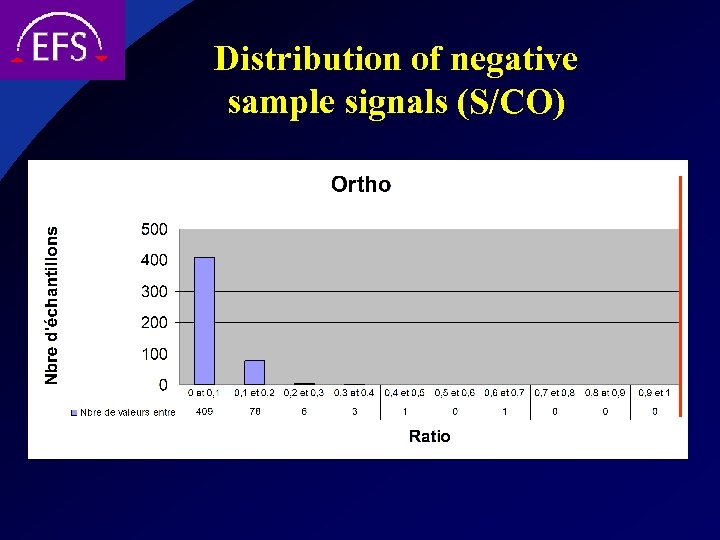 Distribution of negative sample signals (S/CO)
Distribution of negative sample signals (S/CO)
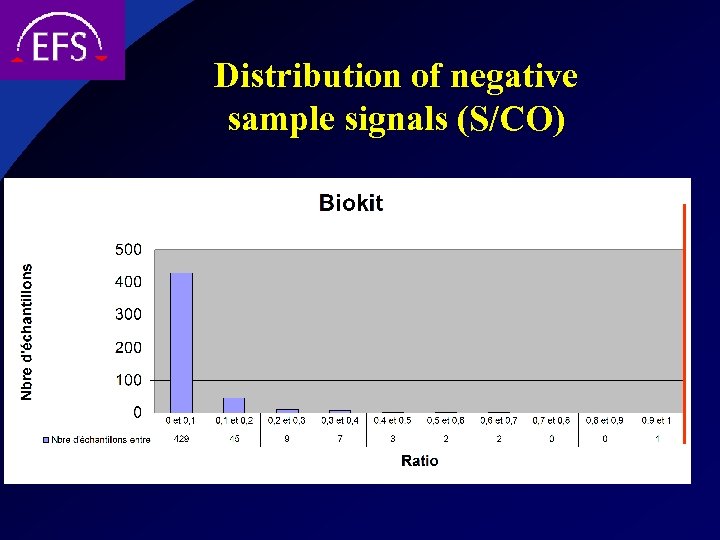 Distribution of negative sample signals (S/CO)
Distribution of negative sample signals (S/CO)
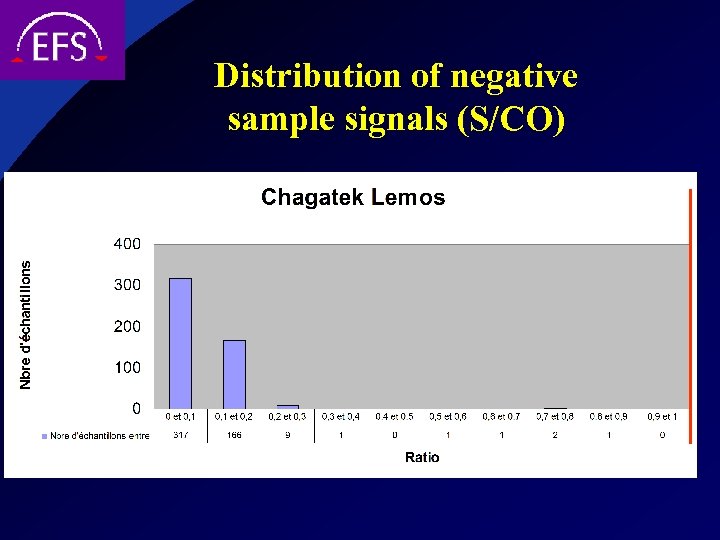 Distribution of negative sample signals (S/CO)
Distribution of negative sample signals (S/CO)
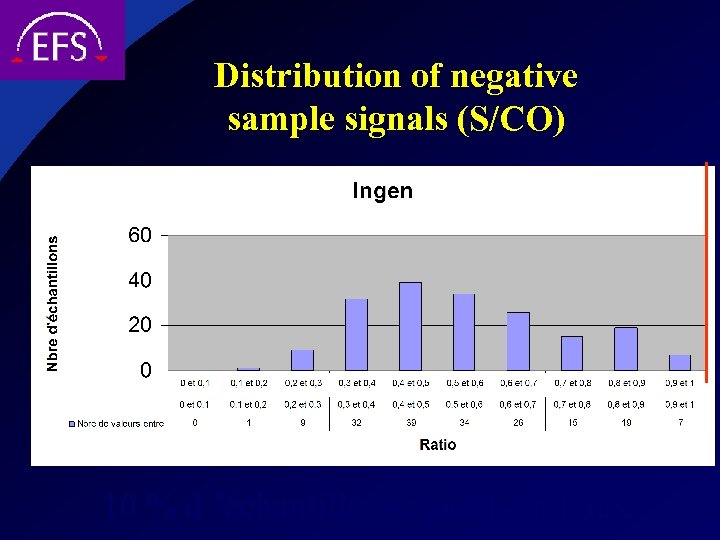 Distribution of negative sample signals (S/CO) 10 % d ’échantillons réactifs initiaux
Distribution of negative sample signals (S/CO) 10 % d ’échantillons réactifs initiaux
 SELECTED TESTS • ELISA assays • Bioelisa Chagas (Biokit, Spain). • ELISA Cruzi (Bio. Mérieux, Brazil). • Immunofluorescence Assay Immunofluor Chagas (Biocientifica, Argentine). • Implementation date: May 2 nd, 2007.
SELECTED TESTS • ELISA assays • Bioelisa Chagas (Biokit, Spain). • ELISA Cruzi (Bio. Mérieux, Brazil). • Immunofluorescence Assay Immunofluor Chagas (Biocientifica, Argentine). • Implementation date: May 2 nd, 2007.
 Measures taken to prevent T. cruzi Transfusion transmitted infections. • Temporary deferral, for 4 months of travelers or residents returning from endemic areas. • Screening for antibodies to T. cruzi in targeted at risk blood donors.
Measures taken to prevent T. cruzi Transfusion transmitted infections. • Temporary deferral, for 4 months of travelers or residents returning from endemic areas. • Screening for antibodies to T. cruzi in targeted at risk blood donors.
 At risk blood donors • Donors born in endemic areas • Travelers and residents returning from endemic areas • Donors born in France from a mother born in risk areas • Donors who underwent blood transfusion
At risk blood donors • Donors born in endemic areas • Travelers and residents returning from endemic areas • Donors born in France from a mother born in risk areas • Donors who underwent blood transfusion
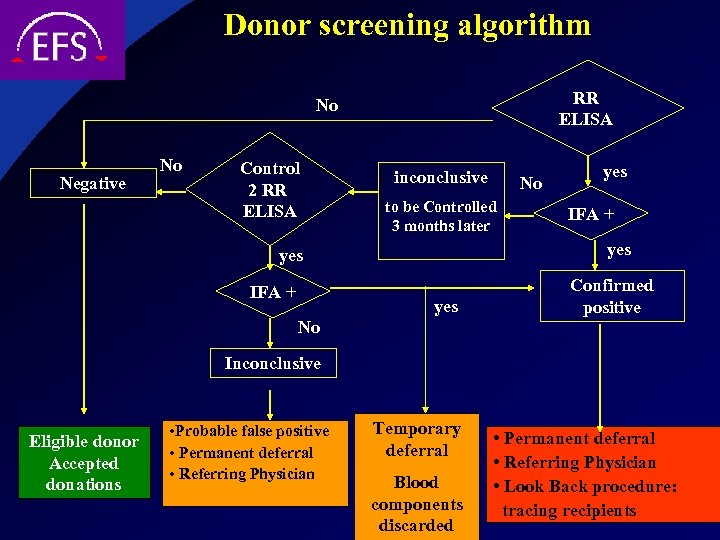 Donor screening algorithm RR ELISA No Negative No Control 2 RR ELISA inconclusive to be Controlled 3 months later yes IFA + No yes Confirmed positive No Inconclusive Eligible donor Accepted donations • Probable false positive • Permanent deferral • Referring Physician Temporary deferral Blood components discarded • Permanent deferral • Referring Physician • Look Back procedure: tracing recipients
Donor screening algorithm RR ELISA No Negative No Control 2 RR ELISA inconclusive to be Controlled 3 months later yes IFA + No yes Confirmed positive No Inconclusive Eligible donor Accepted donations • Probable false positive • Permanent deferral • Referring Physician Temporary deferral Blood components discarded • Permanent deferral • Referring Physician • Look Back procedure: tracing recipients
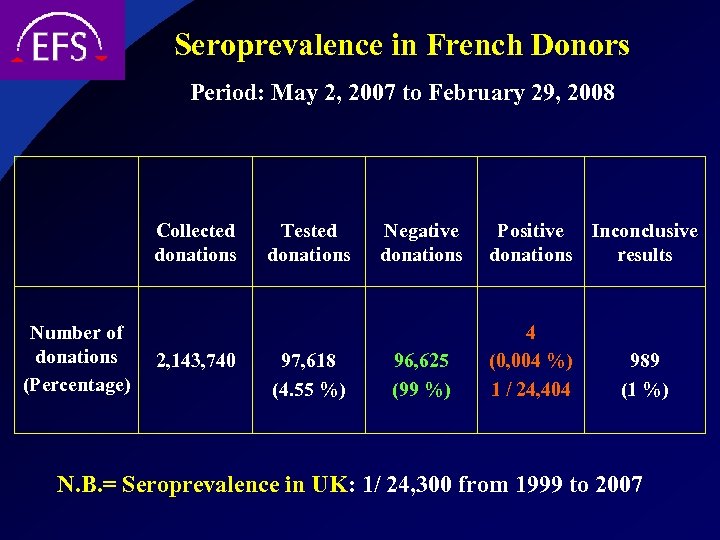 Seroprevalence in French Donors Period: May 2, 2007 to February 29, 2008 Collected donations Number of donations (Percentage) 2, 143, 740 Tested donations 97, 618 (4. 55 %) Negative donations 96, 625 (99 %) Positive Inconclusive donations results 4 (0, 004 %) 1 / 24, 404 989 (1 %) N. B. = Seroprevalence in UK: 1/ 24, 300 from 1999 to 2007
Seroprevalence in French Donors Period: May 2, 2007 to February 29, 2008 Collected donations Number of donations (Percentage) 2, 143, 740 Tested donations 97, 618 (4. 55 %) Negative donations 96, 625 (99 %) Positive Inconclusive donations results 4 (0, 004 %) 1 / 24, 404 989 (1 %) N. B. = Seroprevalence in UK: 1/ 24, 300 from 1999 to 2007
 Positive Donors in France • 2 first-time Bolivian donors • 2 donors from San Salvador Ø One first-time donor Ø One repeat donor: only 2 previous donations transfused to recipients who died from underlying diseases.
Positive Donors in France • 2 first-time Bolivian donors • 2 donors from San Salvador Ø One first-time donor Ø One repeat donor: only 2 previous donations transfused to recipients who died from underlying diseases.
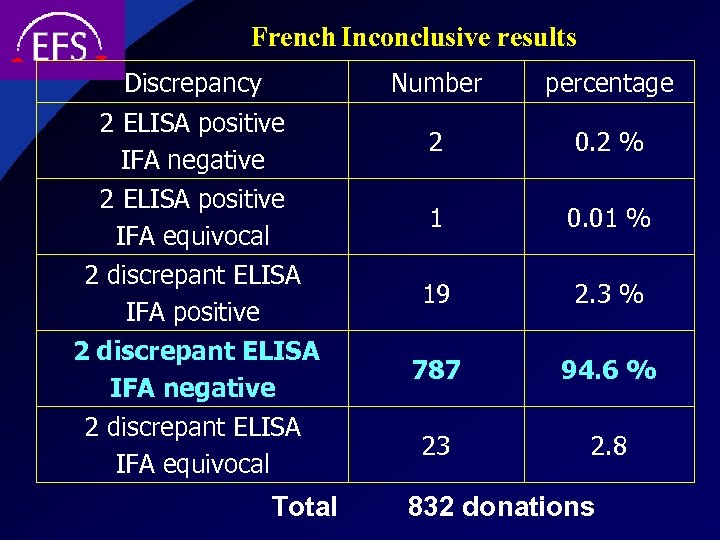 French Inconclusive results Discrepancy Number 2 ELISA positive IFA negative 2 ELISA positive IFA equivocal 2 discrepant ELISA IFA positive 2 discrepant ELISA IFA negative 2 discrepant ELISA IFA equivocal Total percentage 2 0. 2 % 1 0. 01 % 19 2. 3 % 787 94. 6 % 23 2. 8 832 donations
French Inconclusive results Discrepancy Number 2 ELISA positive IFA negative 2 ELISA positive IFA equivocal 2 discrepant ELISA IFA positive 2 discrepant ELISA IFA negative 2 discrepant ELISA IFA equivocal Total percentage 2 0. 2 % 1 0. 01 % 19 2. 3 % 787 94. 6 % 23 2. 8 832 donations
 Control of French Inconclusive results • 465 donors with inconclusive results could be controlled. • Out of these 213 (46 %) were found negative.
Control of French Inconclusive results • 465 donors with inconclusive results could be controlled. • Out of these 213 (46 %) were found negative.
 Reevaluation of Ortho test Tobler LH et al. Evaluation of a new enzyme-linked immunosorbent assay for detection of Chagas antibody in US blood donors. Transfusion January 2007; 47: 90 -96
Reevaluation of Ortho test Tobler LH et al. Evaluation of a new enzyme-linked immunosorbent assay for detection of Chagas antibody in US blood donors. Transfusion January 2007; 47: 90 -96
 Reevaluation of Ortho test • Cut off calculation of Ortho test modified: better sensitivity • Same sensitivity with BBI panel and Brazilian samples • Good sensitivity with 53 Mexican samples: higher S/CO than those obtained with Bio. Mérieux and Biokit kits • Specificity evaluated on 4000 donations: • 1 non repeated reactive sample • 2 repeat reactive samples (specificity: 99. 95 % )
Reevaluation of Ortho test • Cut off calculation of Ortho test modified: better sensitivity • Same sensitivity with BBI panel and Brazilian samples • Good sensitivity with 53 Mexican samples: higher S/CO than those obtained with Bio. Mérieux and Biokit kits • Specificity evaluated on 4000 donations: • 1 non repeated reactive sample • 2 repeat reactive samples (specificity: 99. 95 % )
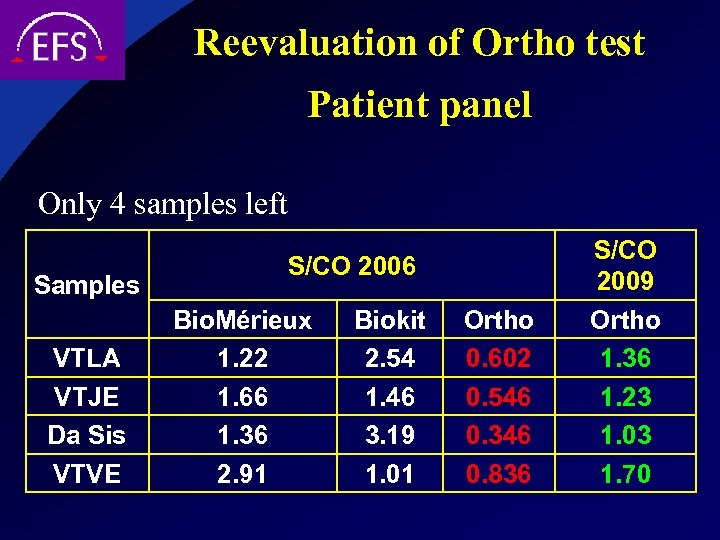 Reevaluation of Ortho test Patient panel Only 4 samples left Samples VTLA VTJE Da Sis VTVE S/CO 2006 Bio. Mérieux 1. 22 1. 66 1. 36 2. 91 Biokit 2. 54 1. 46 3. 19 1. 01 Ortho 0. 602 0. 546 0. 346 0. 836 S/CO 2009 Ortho 1. 36 1. 23 1. 03 1. 70
Reevaluation of Ortho test Patient panel Only 4 samples left Samples VTLA VTJE Da Sis VTVE S/CO 2006 Bio. Mérieux 1. 22 1. 66 1. 36 2. 91 Biokit 2. 54 1. 46 3. 19 1. 01 Ortho 0. 602 0. 546 0. 346 0. 836 S/CO 2009 Ortho 1. 36 1. 23 1. 03 1. 70
 Conclusions • Current serological tests (ELISAs) have good performance • Performance continuously improved by manufactures under stringent Quality Control procedures • Current screening strategy results in Large number of Indeterminate results (false positive ? ).
Conclusions • Current serological tests (ELISAs) have good performance • Performance continuously improved by manufactures under stringent Quality Control procedures • Current screening strategy results in Large number of Indeterminate results (false positive ? ).
 Conclusions (2) • Revision of screening strategy in France • Screening strategy should be simplified ü Screening with a single ELISA sufficient ü Replace IFA by true confirmatory assays (Western Blot, immunoblot , RIPA, …)
Conclusions (2) • Revision of screening strategy in France • Screening strategy should be simplified ü Screening with a single ELISA sufficient ü Replace IFA by true confirmatory assays (Western Blot, immunoblot , RIPA, …)


