89700d176b724c92079e542cac6741b2.ppt
- Количество слайдов: 33
 Second WHO consultation: Development of a WHO reference panel for the control of Chagas diagnostic tests Geneva, 27 – 28 January 2009 Chagas disease in Spain: Experience from a reference laboratory Teresa Gárate Servicio de Parasitología Centro Nacional de Microbiología
Second WHO consultation: Development of a WHO reference panel for the control of Chagas diagnostic tests Geneva, 27 – 28 January 2009 Chagas disease in Spain: Experience from a reference laboratory Teresa Gárate Servicio de Parasitología Centro Nacional de Microbiología
 Experience from a Parasitology Reference Laboratory v General Role of the Parasitology Lab v Parasitology Lab and Chagas’ disease in Spain
Experience from a Parasitology Reference Laboratory v General Role of the Parasitology Lab v Parasitology Lab and Chagas’ disease in Spain
 Parasitic diseases AUTOCTHONOUS IMPORTED v Leishmaniasis v Malaria v Toxoplasmosis v Other Leishmaniasis v Cryptosporidiasis v Chagas disease v Giardiasis v Sleeping sickness v Amebiasis v Cysticercosis v Fasciolosis v Schistosomiasis v Hydatidosis v Filariasis/Oncho v Anisakiasis v Intestinal parasites v Toxocariosis v Trichinellosis National Microbiology
Parasitic diseases AUTOCTHONOUS IMPORTED v Leishmaniasis v Malaria v Toxoplasmosis v Other Leishmaniasis v Cryptosporidiasis v Chagas disease v Giardiasis v Sleeping sickness v Amebiasis v Cysticercosis v Fasciolosis v Schistosomiasis v Hydatidosis v Filariasis/Oncho v Anisakiasis v Intestinal parasites v Toxocariosis v Trichinellosis National Microbiology
 The Parasitology Lab works with Spanish hospitals and blood donor centers on Chagas disease diagnosis
The Parasitology Lab works with Spanish hospitals and blood donor centers on Chagas disease diagnosis
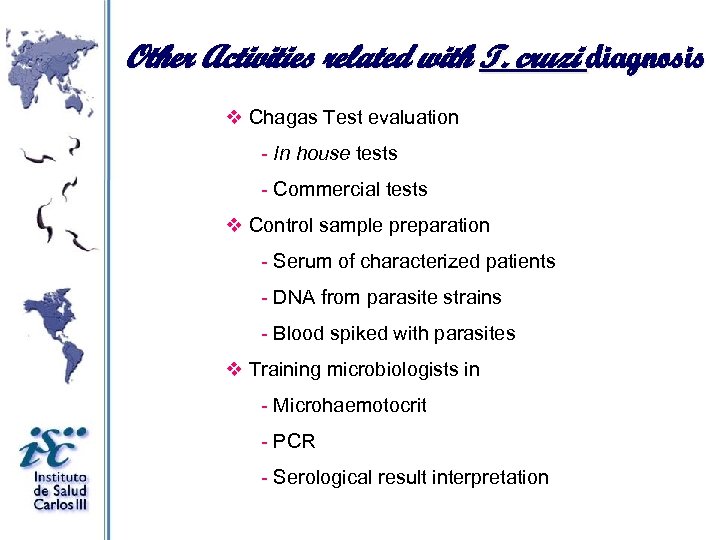 Other Activities related with T. cruzi diagnosis v Chagas Test evaluation - In house tests - Commercial tests v Control sample preparation - Serum of characterized patients - DNA from parasite strains - Blood spiked with parasites v Training microbiologists in - Microhaemotocrit - PCR - Serological result interpretation
Other Activities related with T. cruzi diagnosis v Chagas Test evaluation - In house tests - Commercial tests v Control sample preparation - Serum of characterized patients - DNA from parasite strains - Blood spiked with parasites v Training microbiologists in - Microhaemotocrit - PCR - Serological result interpretation
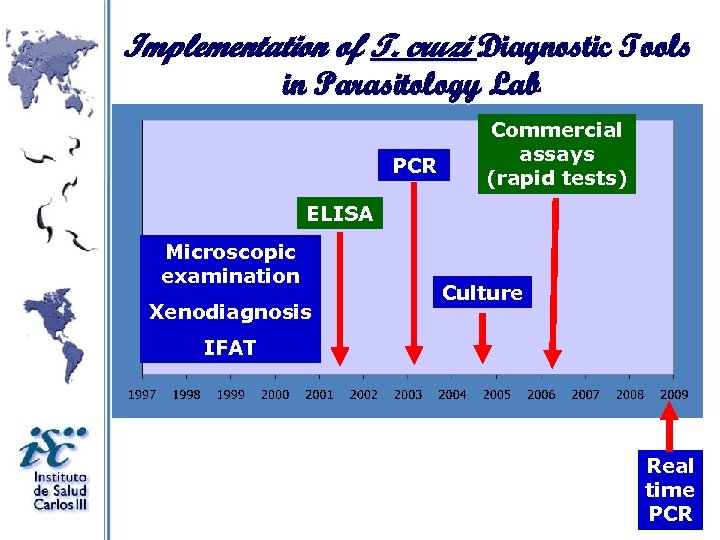 Implementation of T. cruzi Diagnostic Tools in Parasitology Lab PCR Commercial assays (rapid tests) ELISA Microscopic examination Xenodiagnosis Culture IFAT Real time PCR
Implementation of T. cruzi Diagnostic Tools in Parasitology Lab PCR Commercial assays (rapid tests) ELISA Microscopic examination Xenodiagnosis Culture IFAT Real time PCR
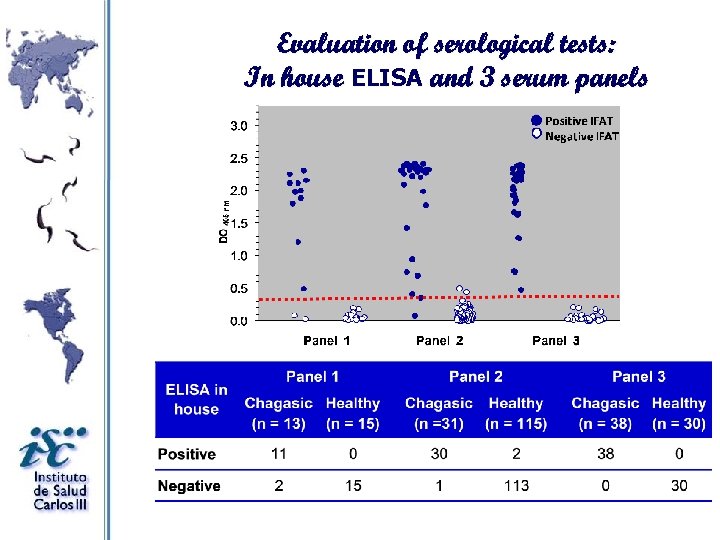 Evaluation of serological tests: In house ELISA and 3 serum panels
Evaluation of serological tests: In house ELISA and 3 serum panels
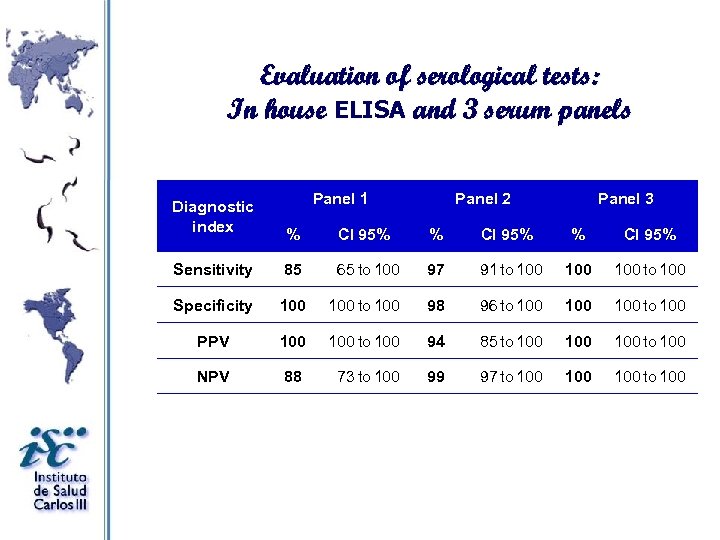 Evaluation of serological tests: In house ELISA and 3 serum panels Diagnostic index Panel 1 Panel 2 Panel 3 % CI 95% Sensitivity 85 65 to 100 97 91 to 100 100 to 100 Specificity 100 to 100 98 96 to 100 100 to 100 PPV 100 to 100 94 85 to 100 100 to 100 NPV 88 73 to 100 99 97 to 100 100 to 100
Evaluation of serological tests: In house ELISA and 3 serum panels Diagnostic index Panel 1 Panel 2 Panel 3 % CI 95% Sensitivity 85 65 to 100 97 91 to 100 100 to 100 Specificity 100 to 100 98 96 to 100 100 to 100 PPV 100 to 100 94 85 to 100 100 to 100 NPV 88 73 to 100 99 97 to 100 100 to 100
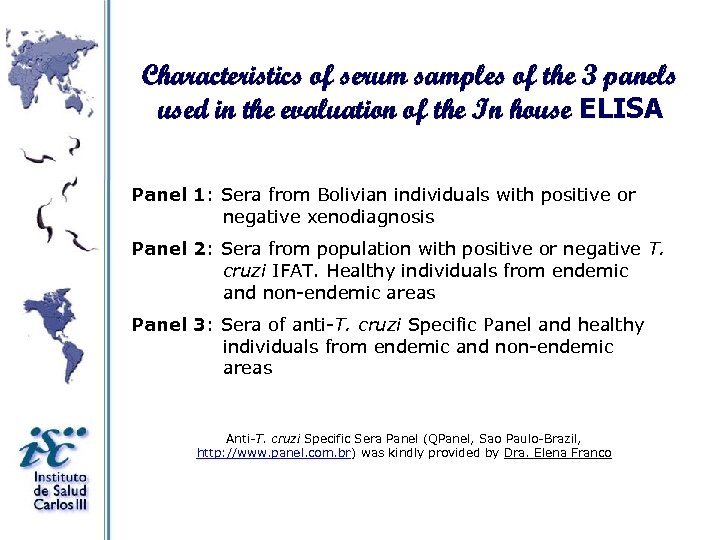 Characteristics of serum samples of the 3 panels used in the evaluation of the In house ELISA Panel 1: Sera from Bolivian individuals with positive or negative xenodiagnosis Panel 2: Sera from population with positive or negative T. cruzi IFAT. Healthy individuals from endemic and non-endemic areas Panel 3: Sera of anti-T. cruzi Specific Panel and healthy individuals from endemic and non-endemic areas Anti-T. cruzi Specific Sera Panel (QPanel, Sao Paulo-Brazil, http: //www. panel. com. br) was kindly provided by Dra. Elena Franco
Characteristics of serum samples of the 3 panels used in the evaluation of the In house ELISA Panel 1: Sera from Bolivian individuals with positive or negative xenodiagnosis Panel 2: Sera from population with positive or negative T. cruzi IFAT. Healthy individuals from endemic and non-endemic areas Panel 3: Sera of anti-T. cruzi Specific Panel and healthy individuals from endemic and non-endemic areas Anti-T. cruzi Specific Sera Panel (QPanel, Sao Paulo-Brazil, http: //www. panel. com. br) was kindly provided by Dra. Elena Franco
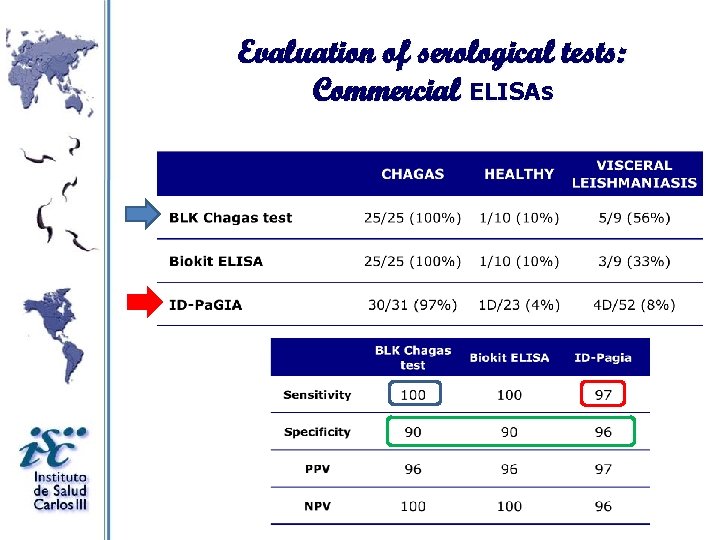 Evaluation of serological tests: Commercial ELISAs
Evaluation of serological tests: Commercial ELISAs
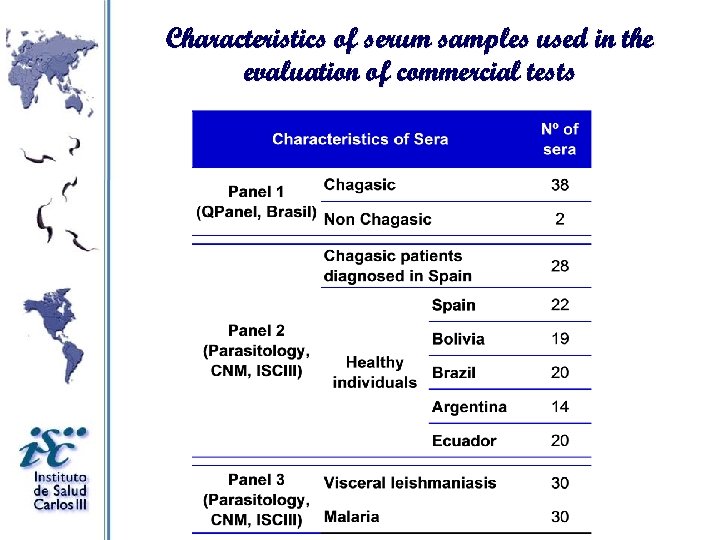 Characteristics of serum samples used in the evaluation of commercial tests
Characteristics of serum samples used in the evaluation of commercial tests
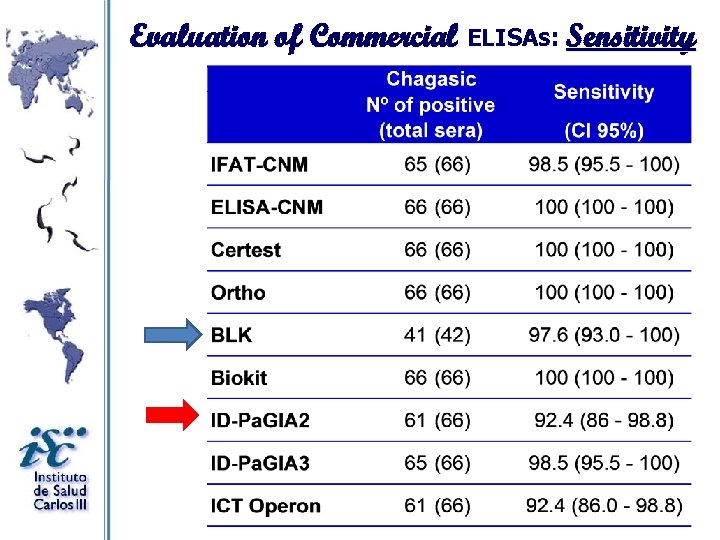 Evaluation of Commercial ELISAs: Sensitivity
Evaluation of Commercial ELISAs: Sensitivity
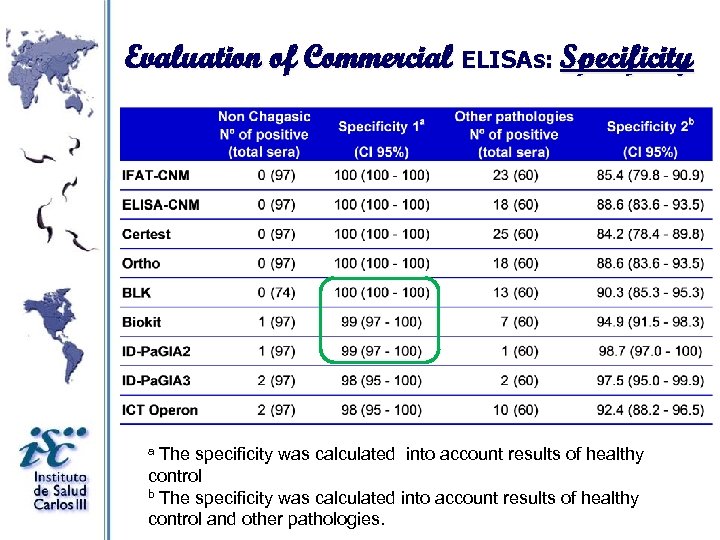 Evaluation of Commercial ELISAs: Specificity The specificity was calculated into account results of healthy control b The specificity was calculated into account results of healthy control and other pathologies. a
Evaluation of Commercial ELISAs: Specificity The specificity was calculated into account results of healthy control b The specificity was calculated into account results of healthy control and other pathologies. a
 Molecular diagnosis of T. cruzi Control assay of limit detection Samples of population at-risk k. DNA-PCR modified (121 -122 / HUF-REV) Britto et al 1993; Cruz et al 2002; Walsh et al 1991; Dorn et al 1997; Gomes et al 1998; Wincker et al 1994; Rubio et al 2002
Molecular diagnosis of T. cruzi Control assay of limit detection Samples of population at-risk k. DNA-PCR modified (121 -122 / HUF-REV) Britto et al 1993; Cruz et al 2002; Walsh et al 1991; Dorn et al 1997; Gomes et al 1998; Wincker et al 1994; Rubio et al 2002
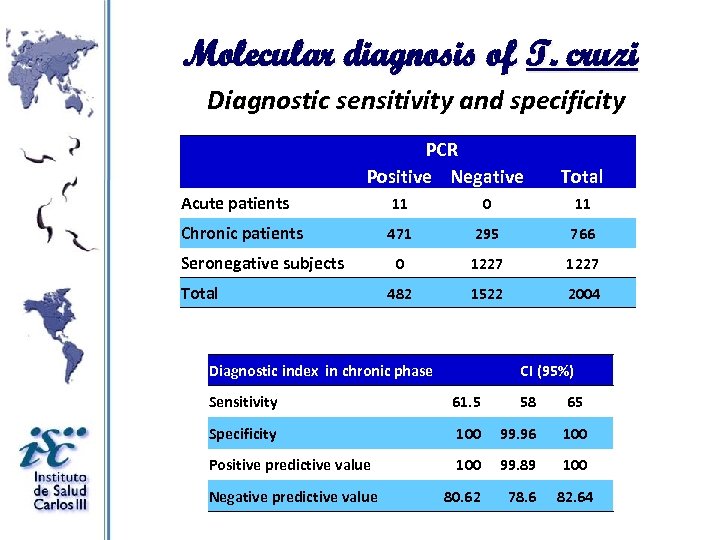 Molecular diagnosis of T. cruzi Diagnostic sensitivity and specificity PCR Positive Negative Total Acute patients 11 0 11 Chronic patients 471 295 766 0 1227 482 1522 2004 Seronegative subjects Total Diagnostic index in chronic phase CI (95%) Sensitivity 61. 5 58 65 Specificity 100 99. 96 100 Positive predictive value 100 99. 89 100 80. 62 78. 6 82. 64 Negative predictive value
Molecular diagnosis of T. cruzi Diagnostic sensitivity and specificity PCR Positive Negative Total Acute patients 11 0 11 Chronic patients 471 295 766 0 1227 482 1522 2004 Seronegative subjects Total Diagnostic index in chronic phase CI (95%) Sensitivity 61. 5 58 65 Specificity 100 99. 96 100 Positive predictive value 100 99. 89 100 80. 62 78. 6 82. 64 Negative predictive value
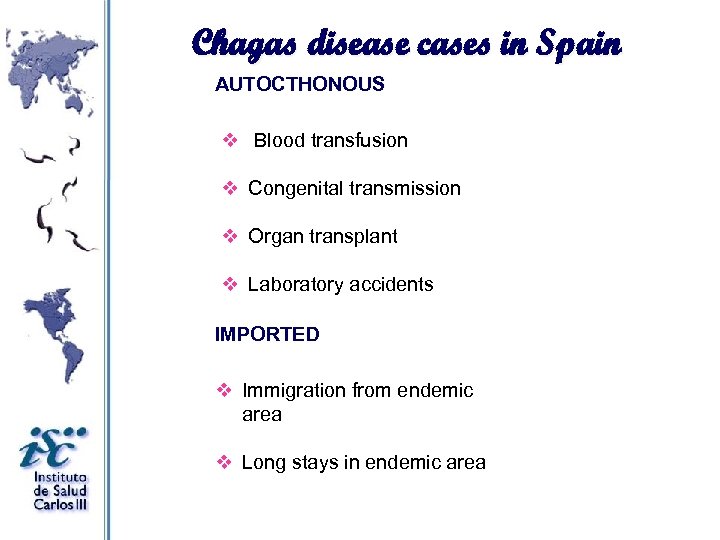 Chagas disease cases in Spain AUTOCTHONOUS v Blood transfusion v Congenital transmission v Organ transplant v Laboratory accidents IMPORTED v Immigration from endemic area v Long stays in endemic area
Chagas disease cases in Spain AUTOCTHONOUS v Blood transfusion v Congenital transmission v Organ transplant v Laboratory accidents IMPORTED v Immigration from endemic area v Long stays in endemic area
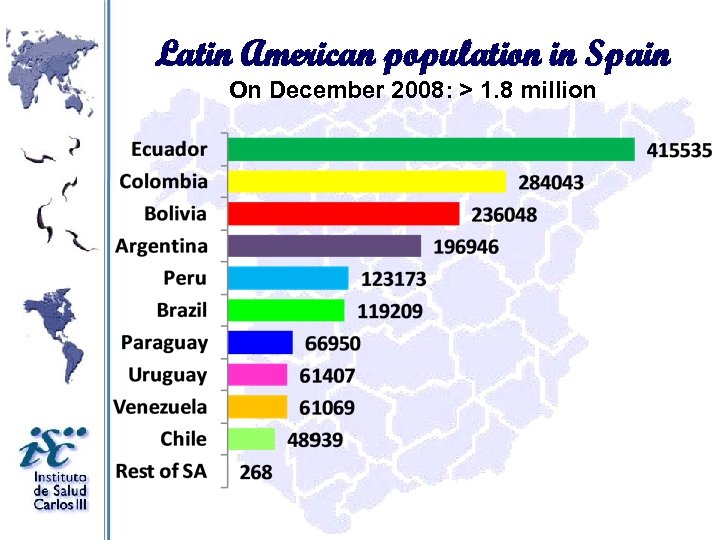 Latin American population in Spain On December 2008: > 1. 8 million
Latin American population in Spain On December 2008: > 1. 8 million
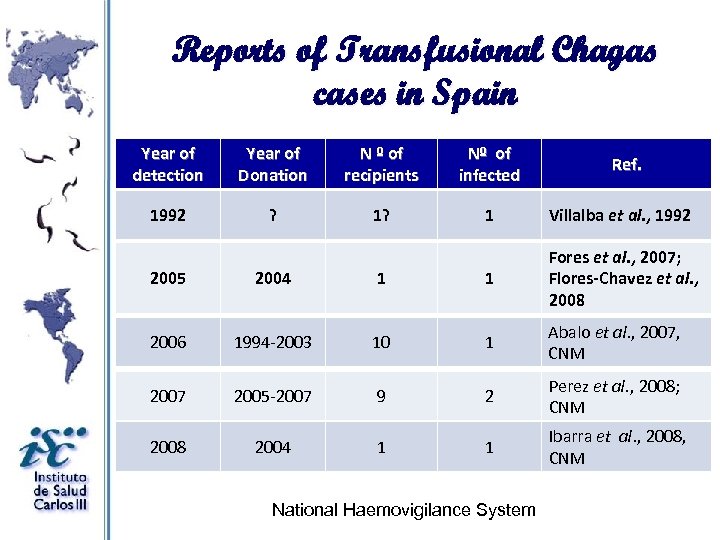 Reports of Transfusional Chagas cases in Spain Year of detection Year of Donation N º of recipients Nº of infected 1992 ? 1 Villalba et al. , 1992 Ref. 2005 2004 1 1 Fores et al. , 2007; Flores-Chavez et al. , 2008 2006 1994 -2003 10 1 Abalo et al. , 2007, CNM 2007 2005 -2007 9 2 Perez et al. , 2008; CNM 2008 2004 1 1 Ibarra et al. , 2008, CNM National Haemovigilance System
Reports of Transfusional Chagas cases in Spain Year of detection Year of Donation N º of recipients Nº of infected 1992 ? 1 Villalba et al. , 1992 Ref. 2005 2004 1 1 Fores et al. , 2007; Flores-Chavez et al. , 2008 2006 1994 -2003 10 1 Abalo et al. , 2007, CNM 2007 2005 -2007 9 2 Perez et al. , 2008; CNM 2008 2004 1 1 Ibarra et al. , 2008, CNM National Haemovigilance System
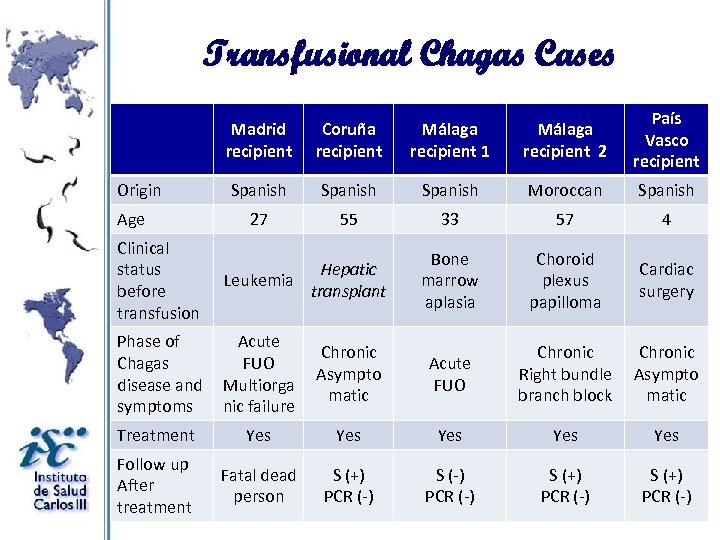 Transfusional Chagas Cases Madrid recipient Origin Age Coruña recipient Málaga recipient 1 Málaga recipient 2 País Vasco recipient Spanish Moroccan Spanish 27 55 33 57 4 Bone marrow aplasia Choroid plexus papilloma Cardiac surgery Clinical status before transfusion Hepatic Leukemia transplant Phase of Chagas disease and symptoms Acute FUO Multiorga nic failure Chronic Asympto matic Acute FUO Chronic Right bundle branch block Chronic Asympto matic Treatment Yes Yes Yes Follow up After treatment Fatal dead person S (+) PCR (-) S (-) PCR (-) S (+) PCR (-)
Transfusional Chagas Cases Madrid recipient Origin Age Coruña recipient Málaga recipient 1 Málaga recipient 2 País Vasco recipient Spanish Moroccan Spanish 27 55 33 57 4 Bone marrow aplasia Choroid plexus papilloma Cardiac surgery Clinical status before transfusion Hepatic Leukemia transplant Phase of Chagas disease and symptoms Acute FUO Multiorga nic failure Chronic Asympto matic Acute FUO Chronic Right bundle branch block Chronic Asympto matic Treatment Yes Yes Yes Follow up After treatment Fatal dead person S (+) PCR (-) S (-) PCR (-) S (+) PCR (-)
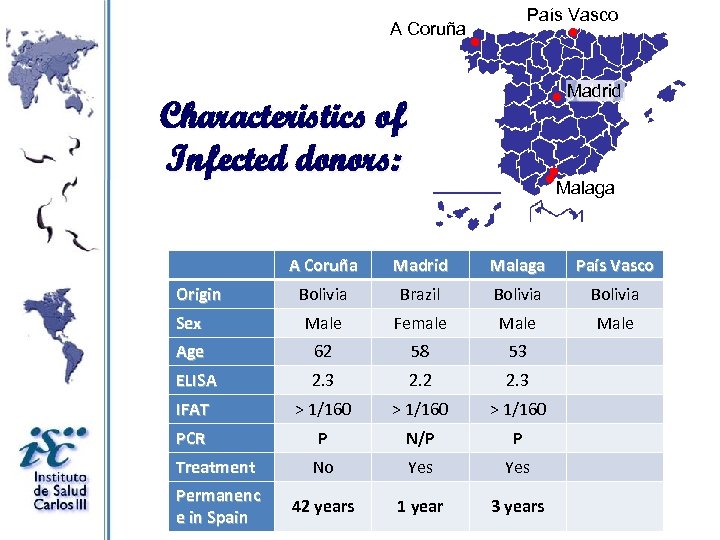 País Vasco A Coruña Madrid Characteristics of Infected donors: Malaga A Coruña Madrid Malaga País Vasco Bolivia Brazil Bolivia Sex Male Female Male Age 62 58 53 ELISA 2. 3 2. 2 2. 3 IFAT > 1/160 PCR P N/P P Treatment No Yes Permanenc e in Spain 42 years 1 year 3 years Origin
País Vasco A Coruña Madrid Characteristics of Infected donors: Malaga A Coruña Madrid Malaga País Vasco Bolivia Brazil Bolivia Sex Male Female Male Age 62 58 53 ELISA 2. 3 2. 2 2. 3 IFAT > 1/160 PCR P N/P P Treatment No Yes Permanenc e in Spain 42 years 1 year 3 years Origin
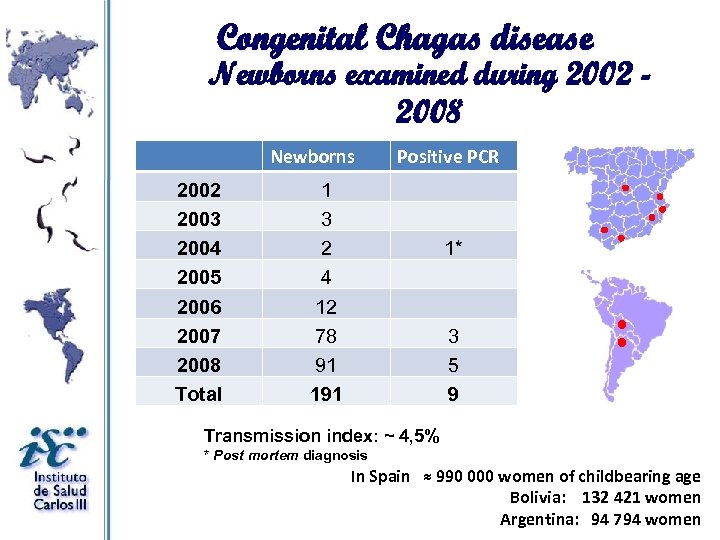 Congenital Chagas disease Newborns examined during 2002 2008 Newborns 2002 2003 2004 2005 2006 2007 2008 Total Positive PCR 1 3 2 4 12 78 91 1* 3 5 9 Transmission index: ~ 4, 5% * Post mortem diagnosis In Spain ≈ 990 000 women of childbearing age Bolivia: 132 421 women Argentina: 94 794 women
Congenital Chagas disease Newborns examined during 2002 2008 Newborns 2002 2003 2004 2005 2006 2007 2008 Total Positive PCR 1 3 2 4 12 78 91 1* 3 5 9 Transmission index: ~ 4, 5% * Post mortem diagnosis In Spain ≈ 990 000 women of childbearing age Bolivia: 132 421 women Argentina: 94 794 women
 Laboratory Accidents and Chagas disease Alvar J. 1983 Laboratorio 76(456): 645 -648
Laboratory Accidents and Chagas disease Alvar J. 1983 Laboratorio 76(456): 645 -648
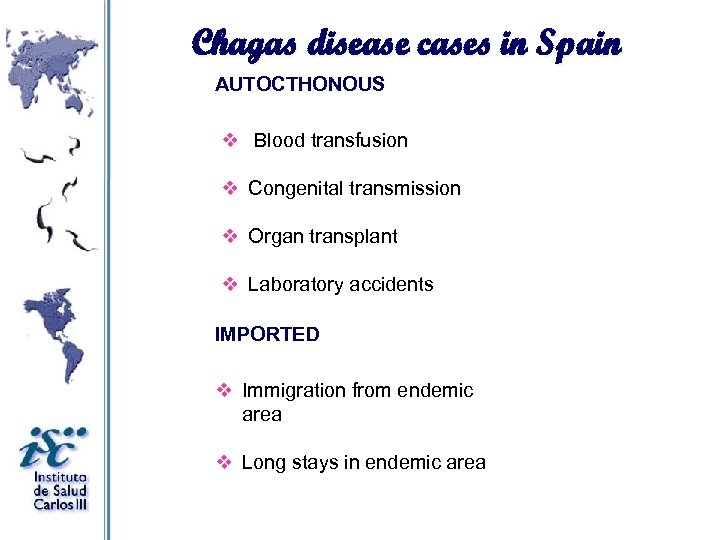 Chagas disease cases in Spain AUTOCTHONOUS v Blood transfusion v Congenital transmission v Organ transplant v Laboratory accidents IMPORTED v Immigration from endemic area v Long stays in endemic area
Chagas disease cases in Spain AUTOCTHONOUS v Blood transfusion v Congenital transmission v Organ transplant v Laboratory accidents IMPORTED v Immigration from endemic area v Long stays in endemic area
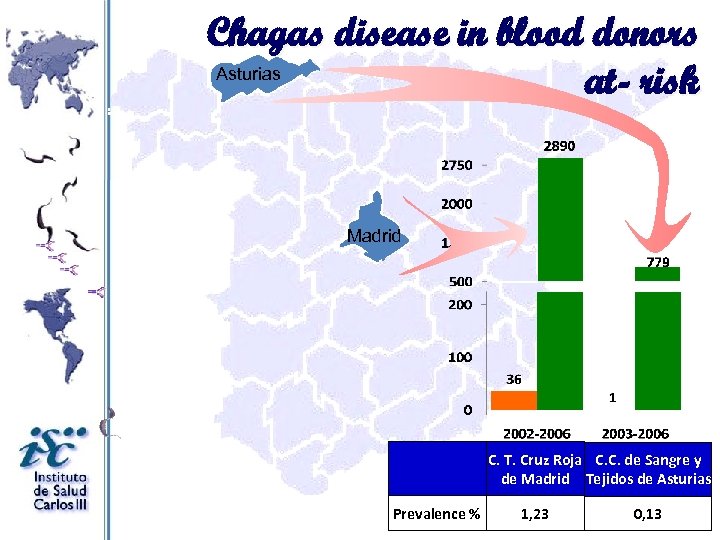 Chagas disease in blood donors Asturias at- risk Madrid Prevalence % C. T. Cruz Roja C. C. de Sangre y de Madrid Tejidos de Asturias 1, 23 0, 13
Chagas disease in blood donors Asturias at- risk Madrid Prevalence % C. T. Cruz Roja C. C. de Sangre y de Madrid Tejidos de Asturias 1, 23 0, 13
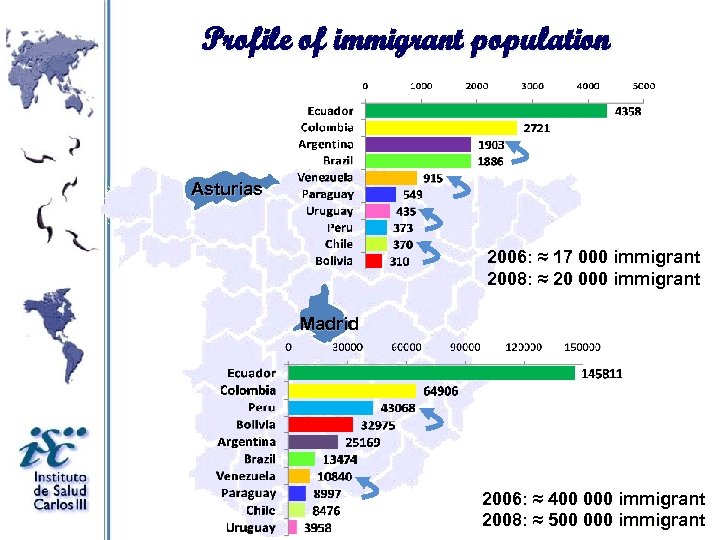 Profile of immigrant population Asturias 2006: ≈ 17 000 immigrant 2008: ≈ 20 000 immigrant Madrid 2006: ≈ 400 000 immigrant 2008: ≈ 500 000 immigrant
Profile of immigrant population Asturias 2006: ≈ 17 000 immigrant 2008: ≈ 20 000 immigrant Madrid 2006: ≈ 400 000 immigrant 2008: ≈ 500 000 immigrant
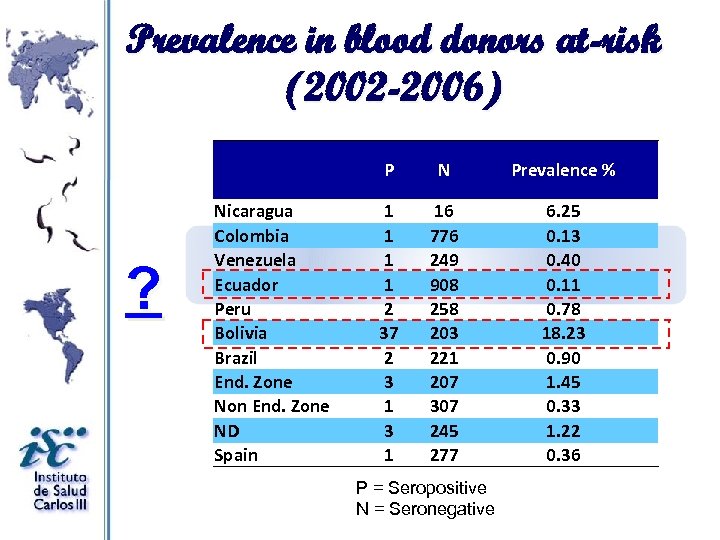 Prevalence in blood donors at-risk (2002 -2006) ? Nicaragua Colombia Venezuela Ecuador Peru Bolivia Brazil End. Zone Non End. Zone ND Spain P N Prevalence % 1 1 2 37 2 3 1 16 776 249 908 258 203 221 207 307 245 277 6. 25 0. 13 0. 40 0. 11 0. 78 18. 23 0. 90 1. 45 0. 33 1. 22 0. 36 P = Seropositive N = Seronegative
Prevalence in blood donors at-risk (2002 -2006) ? Nicaragua Colombia Venezuela Ecuador Peru Bolivia Brazil End. Zone Non End. Zone ND Spain P N Prevalence % 1 1 2 37 2 3 1 16 776 249 908 258 203 221 207 307 245 277 6. 25 0. 13 0. 40 0. 11 0. 78 18. 23 0. 90 1. 45 0. 33 1. 22 0. 36 P = Seropositive N = Seronegative
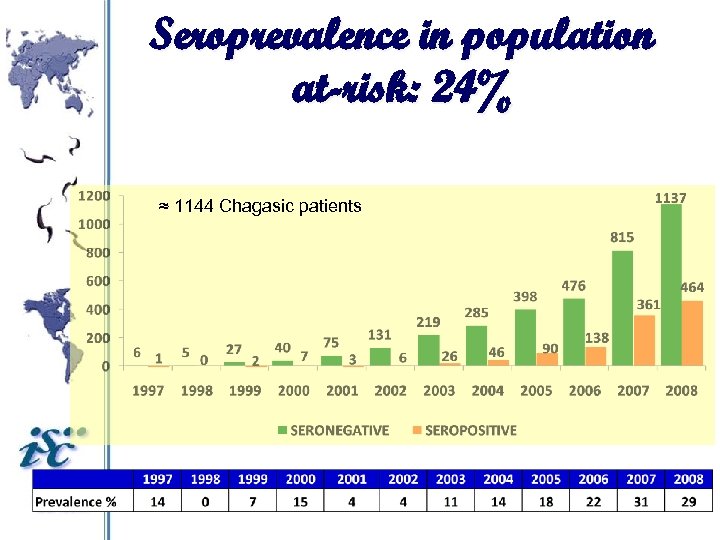 Seroprevalence in population at-risk: 24% ≈ 1144 Chagasic patients
Seroprevalence in population at-risk: 24% ≈ 1144 Chagasic patients
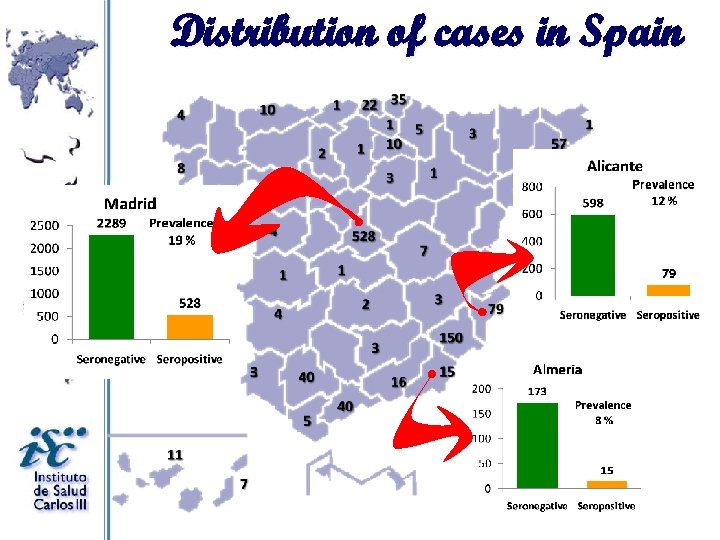 Distribution of cases in Spain
Distribution of cases in Spain
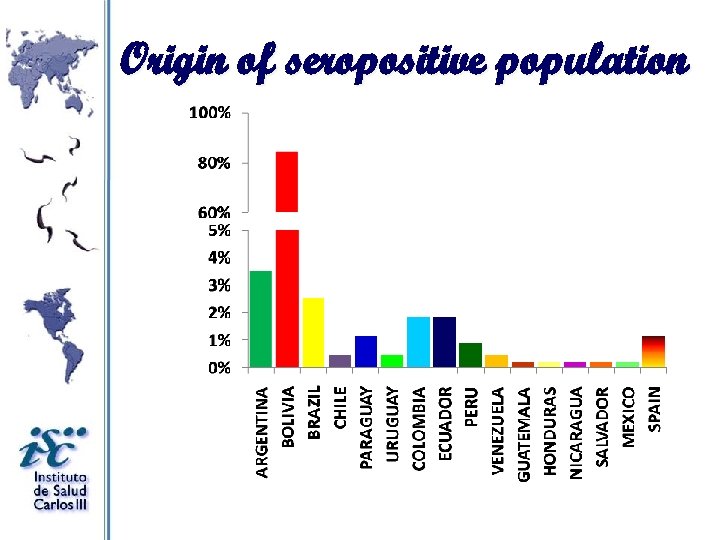 Origin of seropositive population
Origin of seropositive population
 Conclusions v Panel of Reference - Positive samples by different serological tests, and - Positive samples from chagasic individuals with parasitological, epidemiological and clinical evaluation. v Number of positive sera/panel - 3 serum samples for kit manufacturers - 100 serum samples for evaluation of tests - 3 serum samples for quality control. v Chagas in Spain - Imported and autochonous types. - Bolivian group, mainly from Santa Cruz region, shows the highest seropositivity rates and represents the highest risk for T. cruzi infection transmission.
Conclusions v Panel of Reference - Positive samples by different serological tests, and - Positive samples from chagasic individuals with parasitological, epidemiological and clinical evaluation. v Number of positive sera/panel - 3 serum samples for kit manufacturers - 100 serum samples for evaluation of tests - 3 serum samples for quality control. v Chagas in Spain - Imported and autochonous types. - Bolivian group, mainly from Santa Cruz region, shows the highest seropositivity rates and represents the highest risk for T. cruzi infection transmission.
 Thank you UNIDAD LEISHMANIA Y CHAGAS Carmen Cañavate Javier Nieto Mercedes Rodriguez Israel Cruz Elena Bodas Marta Hernández Rubén González Emilia García Carmen Chicharro María Flores
Thank you UNIDAD LEISHMANIA Y CHAGAS Carmen Cañavate Javier Nieto Mercedes Rodriguez Israel Cruz Elena Bodas Marta Hernández Rubén González Emilia García Carmen Chicharro María Flores
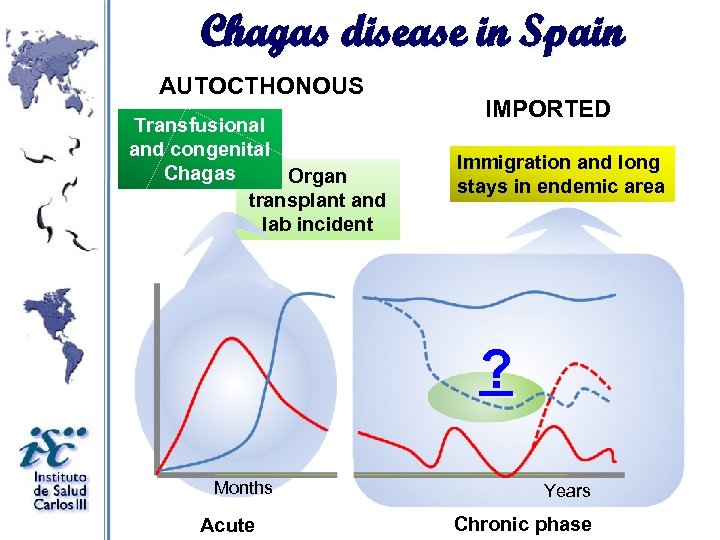 Chagas disease in Spain AUTOCTHONOUS Transfusional and congenital Chagas Organ transplant and lab incident IMPORTED Immigration and long stays in endemic area ? Months Acute Years Chronic phase
Chagas disease in Spain AUTOCTHONOUS Transfusional and congenital Chagas Organ transplant and lab incident IMPORTED Immigration and long stays in endemic area ? Months Acute Years Chronic phase
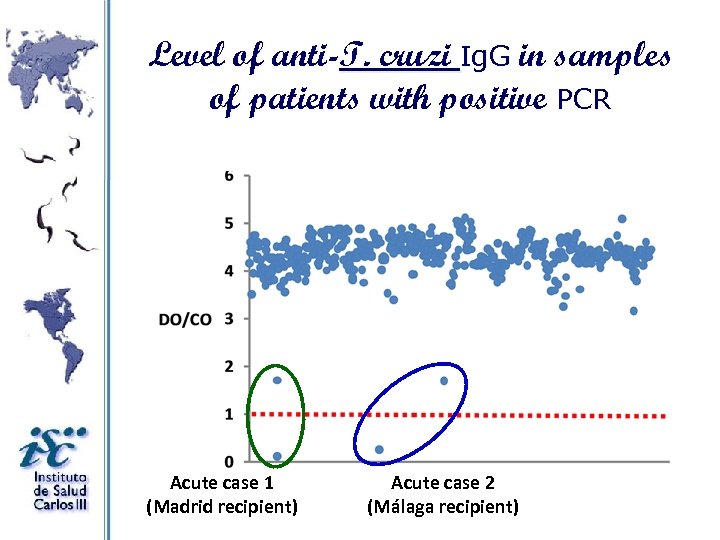 Level of anti-T. cruzi Ig. G in samples of patients with positive PCR Acute case 1 (Madrid recipient) Acute case 2 (Málaga recipient)
Level of anti-T. cruzi Ig. G in samples of patients with positive PCR Acute case 1 (Madrid recipient) Acute case 2 (Málaga recipient)


