acd3d304a5bf4ff06c7f65ba1e5d0b32.ppt
- Количество слайдов: 26
 SEC 598 F 17 Photovoltaic Systems Engineering Session 10 Storage for PV Systems Batteries – Part 1 September 21, 2017
SEC 598 F 17 Photovoltaic Systems Engineering Session 10 Storage for PV Systems Batteries – Part 1 September 21, 2017
 Session 10 content PV System Storage Options • New Approaches o o Possibilities Operation, reliability • Batteries o o Construction, types Operation, reliability, failure mechanisms 2
Session 10 content PV System Storage Options • New Approaches o o Possibilities Operation, reliability • Batteries o o Construction, types Operation, reliability, failure mechanisms 2
 Learning Outcomes • Introduction to storage science and technology • Recognition of value of batteries in PV system design and operation 3
Learning Outcomes • Introduction to storage science and technology • Recognition of value of batteries in PV system design and operation 3
 PV Systems – Storage Technologies • Electromechanical Approaches o o o Pumped water storage Flywheel Other potential energy systems • Chemical Approaches o Hydrogen generation (Solar electrolysis) • Electrical Approaches o o Capacitors Inductors 4
PV Systems – Storage Technologies • Electromechanical Approaches o o o Pumped water storage Flywheel Other potential energy systems • Chemical Approaches o Hydrogen generation (Solar electrolysis) • Electrical Approaches o o Capacitors Inductors 4
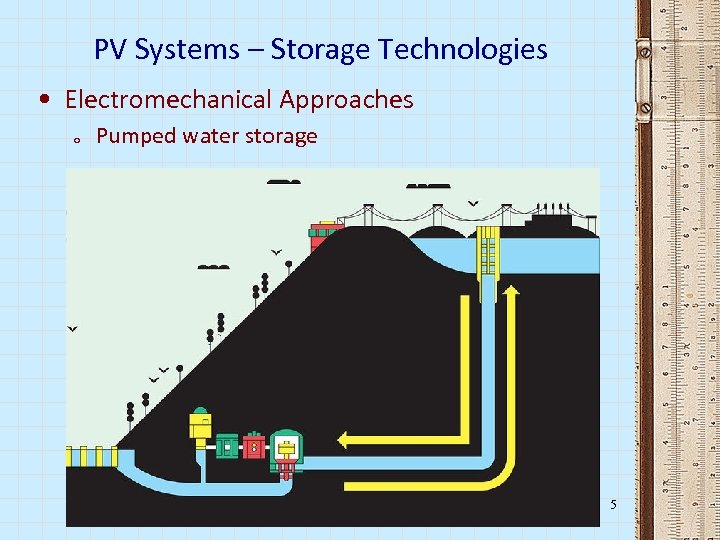 PV Systems – Storage Technologies • Electromechanical Approaches o Pumped water storage 5
PV Systems – Storage Technologies • Electromechanical Approaches o Pumped water storage 5
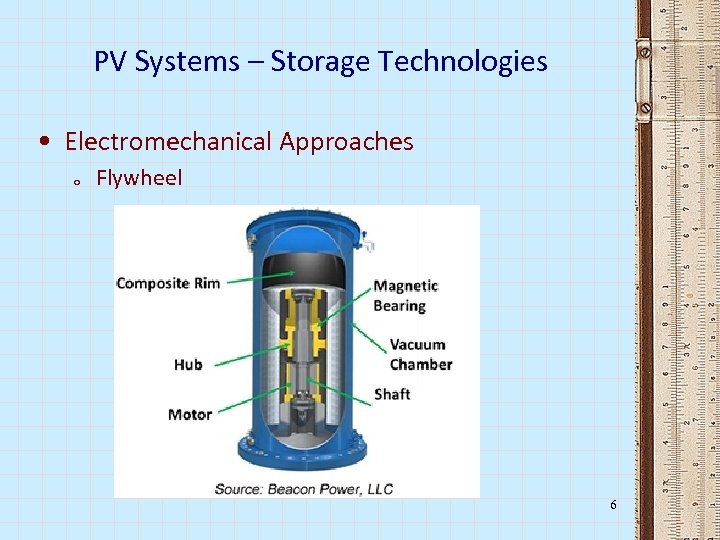 PV Systems – Storage Technologies • Electromechanical Approaches o Flywheel 6
PV Systems – Storage Technologies • Electromechanical Approaches o Flywheel 6
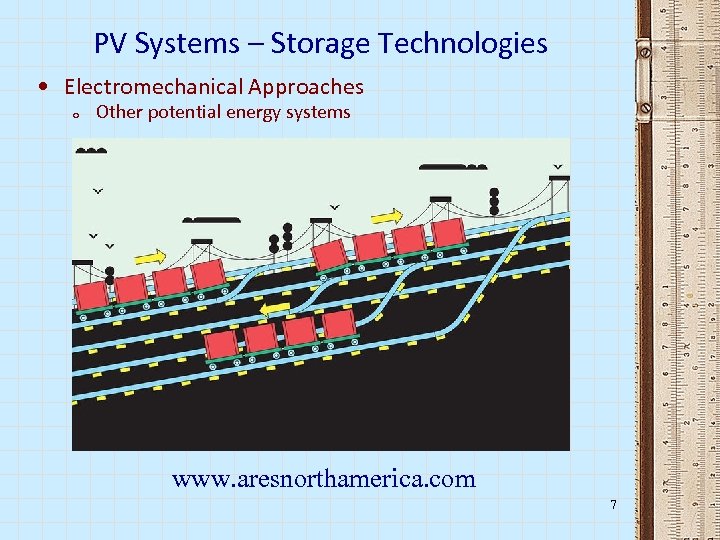 PV Systems – Storage Technologies • Electromechanical Approaches o Other potential energy systems www. aresnorthamerica. com 7
PV Systems – Storage Technologies • Electromechanical Approaches o Other potential energy systems www. aresnorthamerica. com 7
 PV Systems – Storage Technologies • Electromechanical Approaches o Other potential energy systems www. aresnorthamerica. com 8
PV Systems – Storage Technologies • Electromechanical Approaches o Other potential energy systems www. aresnorthamerica. com 8
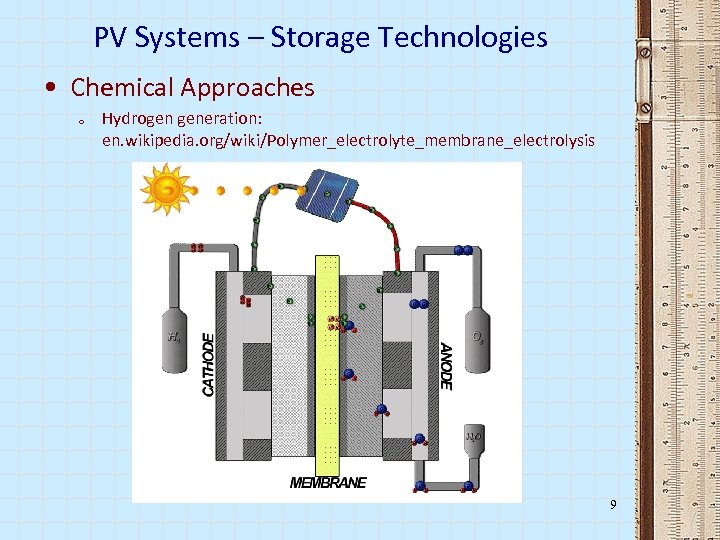 PV Systems – Storage Technologies • Chemical Approaches o Hydrogen generation: en. wikipedia. org/wiki/Polymer_electrolyte_membrane_electrolysis 9
PV Systems – Storage Technologies • Chemical Approaches o Hydrogen generation: en. wikipedia. org/wiki/Polymer_electrolyte_membrane_electrolysis 9
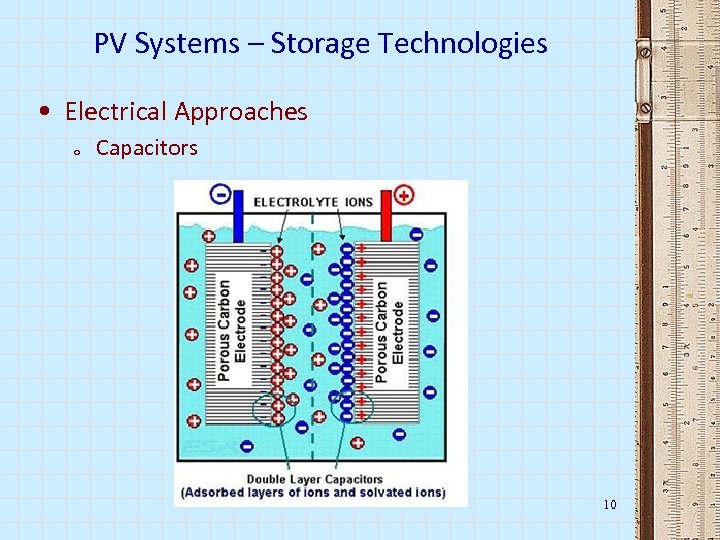 PV Systems – Storage Technologies • Electrical Approaches o Capacitors 10
PV Systems – Storage Technologies • Electrical Approaches o Capacitors 10
 PV Systems – Storage Technologies 11
PV Systems – Storage Technologies 11
 PV Systems - Batteries • The battery remains the most common technological approach for storing energy in PV and other electrical systems. It is by no means an ideal solution, but in the absence of a true electricity storage technology, it is a viable solution • A battery is a transducer – it converts electrical energy to chemical energy, or chemical energy into electrical energy • The driving force in the battery is the chemistry of reduction-oxidation (redox) reactions 12
PV Systems - Batteries • The battery remains the most common technological approach for storing energy in PV and other electrical systems. It is by no means an ideal solution, but in the absence of a true electricity storage technology, it is a viable solution • A battery is a transducer – it converts electrical energy to chemical energy, or chemical energy into electrical energy • The driving force in the battery is the chemistry of reduction-oxidation (redox) reactions 12
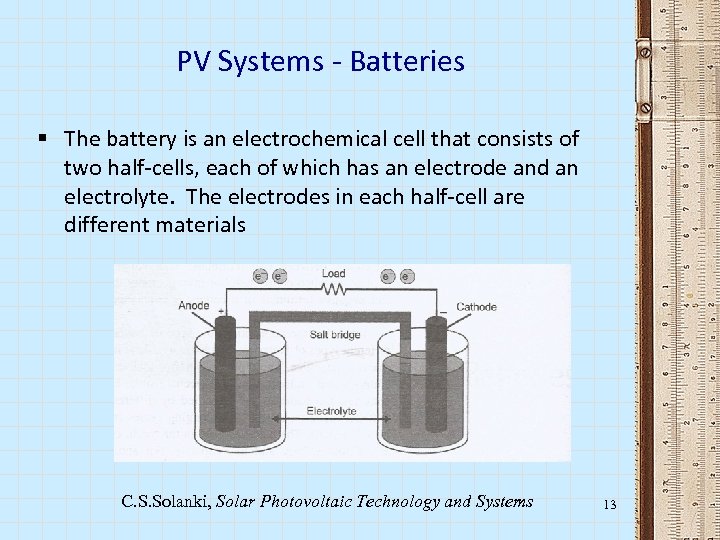 PV Systems - Batteries § The battery is an electrochemical cell that consists of two half-cells, each of which has an electrode and an electrolyte. The electrodes in each half-cell are different materials C. S. Solanki, Solar Photovoltaic Technology and Systems 13
PV Systems - Batteries § The battery is an electrochemical cell that consists of two half-cells, each of which has an electrode and an electrolyte. The electrodes in each half-cell are different materials C. S. Solanki, Solar Photovoltaic Technology and Systems 13
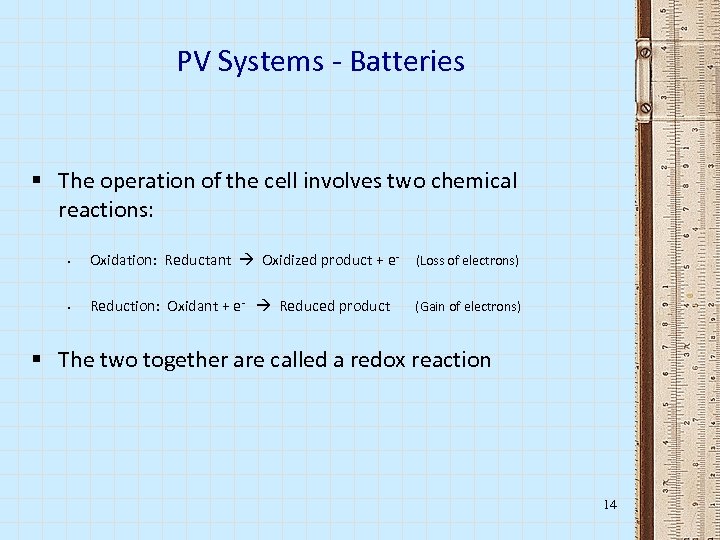 PV Systems - Batteries § The operation of the cell involves two chemical reactions: § Oxidation: Reductant Oxidized product + e- (Loss of electrons) § Reduction: Oxidant + e- Reduced product (Gain of electrons) § The two together are called a redox reaction 14
PV Systems - Batteries § The operation of the cell involves two chemical reactions: § Oxidation: Reductant Oxidized product + e- (Loss of electrons) § Reduction: Oxidant + e- Reduced product (Gain of electrons) § The two together are called a redox reaction 14
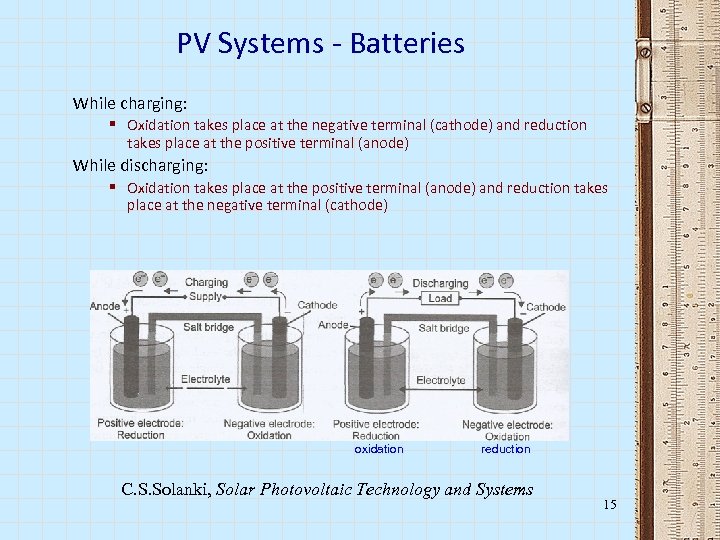 PV Systems - Batteries While charging: § Oxidation takes place at the negative terminal (cathode) and reduction takes place at the positive terminal (anode) While discharging: § Oxidation takes place at the positive terminal (anode) and reduction takes place at the negative terminal (cathode) oxidation reduction C. S. Solanki, Solar Photovoltaic Technology and Systems 15
PV Systems - Batteries While charging: § Oxidation takes place at the negative terminal (cathode) and reduction takes place at the positive terminal (anode) While discharging: § Oxidation takes place at the positive terminal (anode) and reduction takes place at the negative terminal (cathode) oxidation reduction C. S. Solanki, Solar Photovoltaic Technology and Systems 15
 PV Systems - Batteries Types of batteries • Non-rechargable, or primary, batteries • Zinc-Chloride (common AAA, C, D) • Rechargable, or secondary, batteries • • • Lead-Acid Nickel Cadmium (Ni. Cd) Nickel Metal Hydride (Ni. MH) Lithium Ion Polymer 16
PV Systems - Batteries Types of batteries • Non-rechargable, or primary, batteries • Zinc-Chloride (common AAA, C, D) • Rechargable, or secondary, batteries • • • Lead-Acid Nickel Cadmium (Ni. Cd) Nickel Metal Hydride (Ni. MH) Lithium Ion Polymer 16
 PV Systems - Batteries Parameters of batteries • • Battery terminal voltage (V) Charge storage capacity (Ah) State of charge (%) Depth of discharge (%) Number of charge-discharge cycles Life cycle Self discharge 17
PV Systems - Batteries Parameters of batteries • • Battery terminal voltage (V) Charge storage capacity (Ah) State of charge (%) Depth of discharge (%) Number of charge-discharge cycles Life cycle Self discharge 17
 PV Systems - Batteries Lead-Acid Battery • The battery that has seen the widest application in PV systems is the tried-and-true lead-acid embodiment • It consists of two (lead-based) electrodes separated physically, but chemically connected by means of a liquid electrolyte (dilute sulfuric acid) that allows conduction of ions and chemical reactions at the electrodes 18
PV Systems - Batteries Lead-Acid Battery • The battery that has seen the widest application in PV systems is the tried-and-true lead-acid embodiment • It consists of two (lead-based) electrodes separated physically, but chemically connected by means of a liquid electrolyte (dilute sulfuric acid) that allows conduction of ions and chemical reactions at the electrodes 18
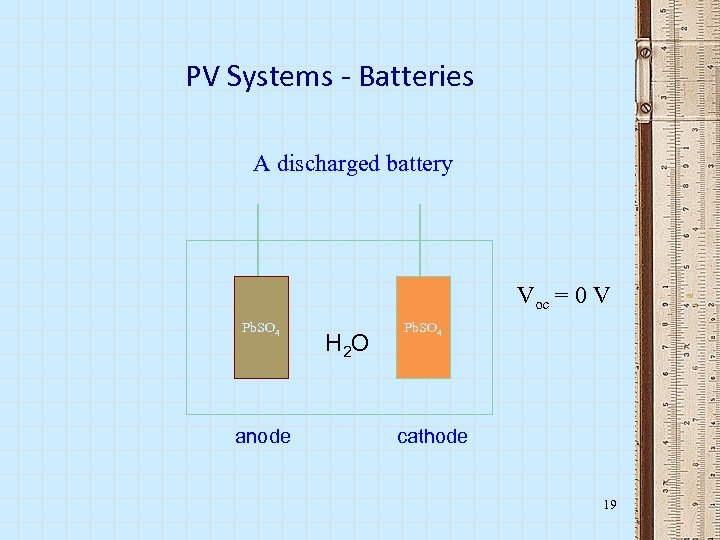 PV Systems - Batteries A discharged battery Voc = 0 V Pb. SO 4 anode H 2 O Pb. SO 4 cathode 19
PV Systems - Batteries A discharged battery Voc = 0 V Pb. SO 4 anode H 2 O Pb. SO 4 cathode 19
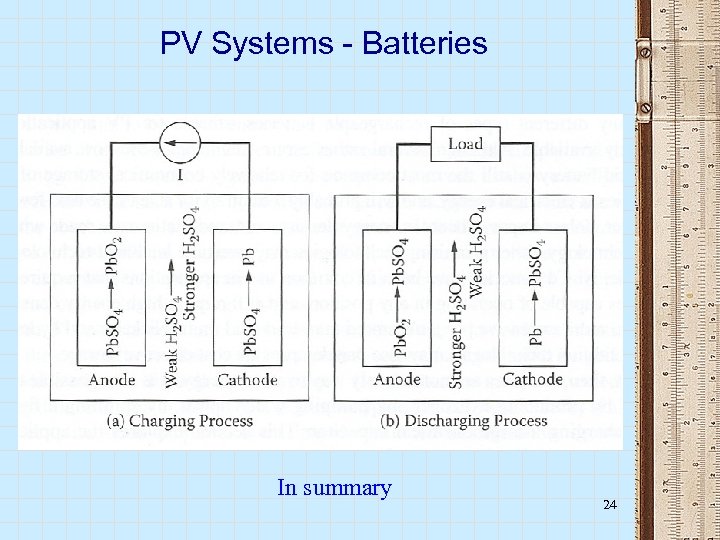
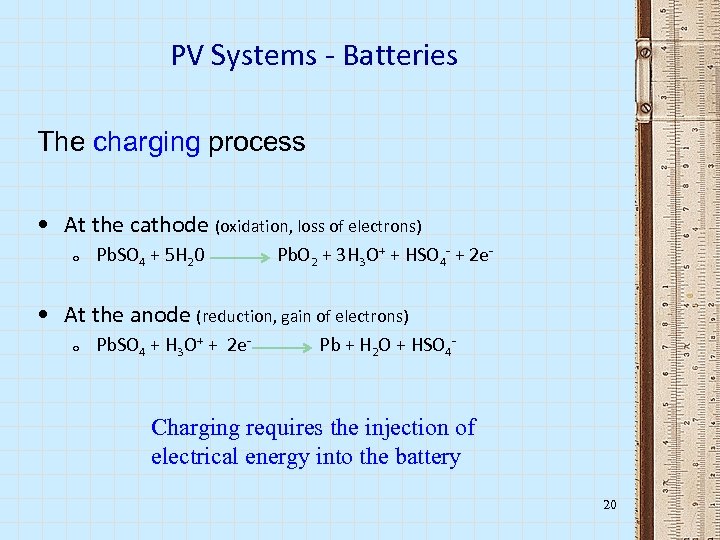 PV Systems - Batteries The charging process • At the cathode (oxidation, loss of electrons) o Pb. SO 4 + 5 H 20 Pb. O 2 + 3 H 3 O+ + HSO 4 - + 2 e- • At the anode (reduction, gain of electrons) o Pb. SO 4 + H 3 O+ + 2 e- Pb + H 2 O + HSO 4 - Charging requires the injection of electrical energy into the battery 20
PV Systems - Batteries The charging process • At the cathode (oxidation, loss of electrons) o Pb. SO 4 + 5 H 20 Pb. O 2 + 3 H 3 O+ + HSO 4 - + 2 e- • At the anode (reduction, gain of electrons) o Pb. SO 4 + H 3 O+ + 2 e- Pb + H 2 O + HSO 4 - Charging requires the injection of electrical energy into the battery 20
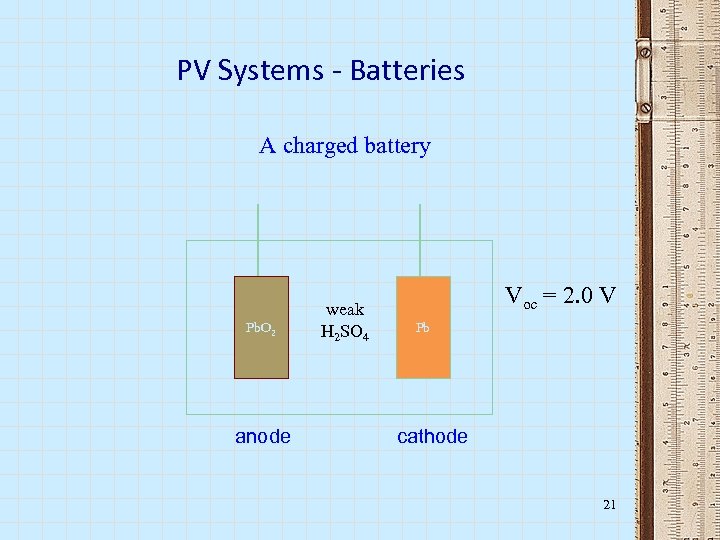 PV Systems - Batteries A charged battery Pb. O 2 anode weak H 2 SO 4 Voc = 2. 0 V Pb cathode 21
PV Systems - Batteries A charged battery Pb. O 2 anode weak H 2 SO 4 Voc = 2. 0 V Pb cathode 21
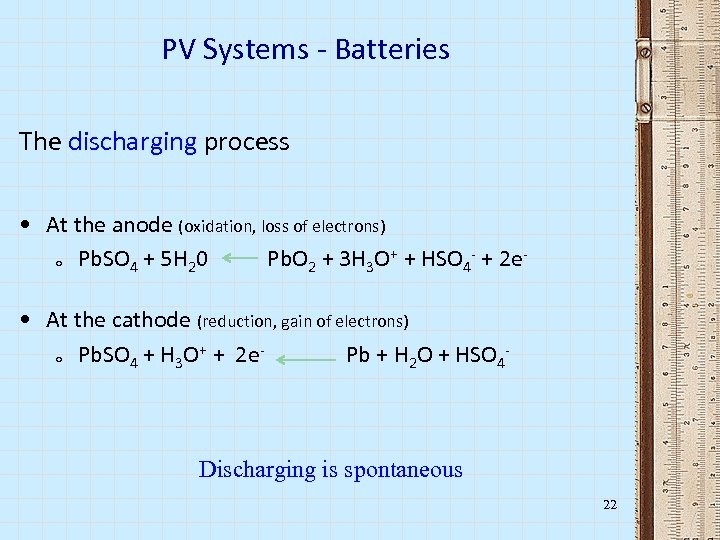 PV Systems - Batteries The discharging process • At the anode (oxidation, loss of electrons) o Pb. SO 4 + 5 H 20 Pb. O 2 + 3 H 3 O+ + HSO 4 - + 2 e • At the cathode (reduction, gain of electrons) + o Pb. SO 4 + H 3 O + 2 e Pb + H 2 O + HSO 4 - Discharging is spontaneous 22
PV Systems - Batteries The discharging process • At the anode (oxidation, loss of electrons) o Pb. SO 4 + 5 H 20 Pb. O 2 + 3 H 3 O+ + HSO 4 - + 2 e • At the cathode (reduction, gain of electrons) + o Pb. SO 4 + H 3 O + 2 e Pb + H 2 O + HSO 4 - Discharging is spontaneous 22
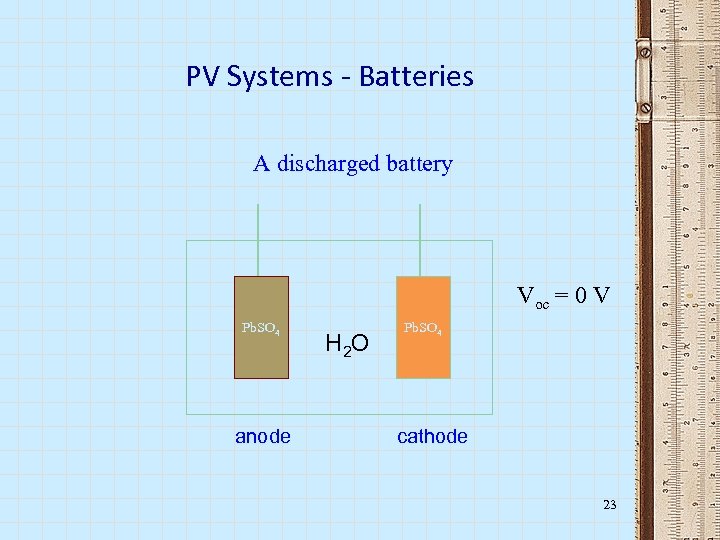 PV Systems - Batteries A discharged battery Voc = 0 V Pb. SO 4 anode H 2 O Pb. SO 4 cathode 23
PV Systems - Batteries A discharged battery Voc = 0 V Pb. SO 4 anode H 2 O Pb. SO 4 cathode 23
 PV Systems - Batteries Step 3: Battery Selection In summary 24
PV Systems - Batteries Step 3: Battery Selection In summary 24
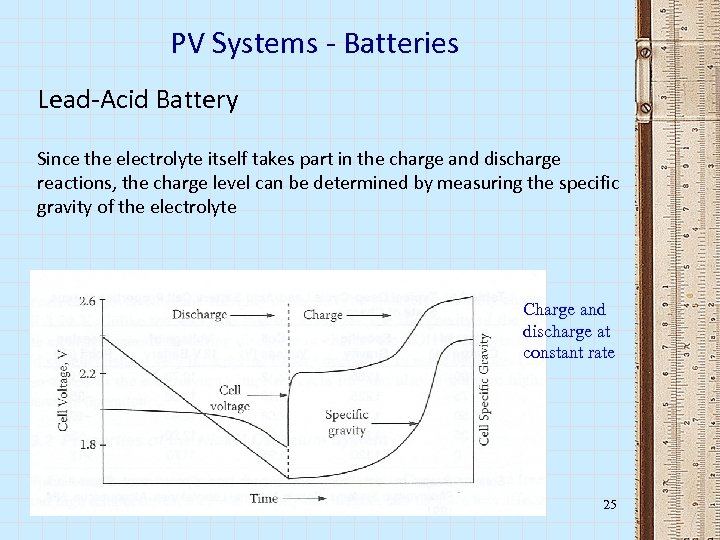 PV Systems - Batteries Lead-Acid Battery Since the electrolyte itself takes part in the charge and discharge reactions, the charge level can be determined by measuring the specific gravity of the electrolyte Charge and discharge at constant rate 25
PV Systems - Batteries Lead-Acid Battery Since the electrolyte itself takes part in the charge and discharge reactions, the charge level can be determined by measuring the specific gravity of the electrolyte Charge and discharge at constant rate 25
 References for Batteries 1. R. Messenger and A. Abtahi, Photovoltaic Systems Engineering, 4 th Ed. , CRC Press, Boca Raton, 2017 2. J. Jung, L. Zhang, J. Zhang, Lead-Acid Battery Technologies, CRC Press, Boca Raton, 2016 3. X. Yuan, H. Liu, J. Zhang, Lithium-Ion Batteries, CRC Press, Boca Raton, 2012 4. C. S. Solanki, Solar Photovoltaic Technology and Systems, PHI Publishing, Bombay, India, 2015
References for Batteries 1. R. Messenger and A. Abtahi, Photovoltaic Systems Engineering, 4 th Ed. , CRC Press, Boca Raton, 2017 2. J. Jung, L. Zhang, J. Zhang, Lead-Acid Battery Technologies, CRC Press, Boca Raton, 2016 3. X. Yuan, H. Liu, J. Zhang, Lithium-Ion Batteries, CRC Press, Boca Raton, 2012 4. C. S. Solanki, Solar Photovoltaic Technology and Systems, PHI Publishing, Bombay, India, 2015


