3a2e4c3f64b94962d1535452cffcf1f2.ppt
- Количество слайдов: 69
 Science Update Programme New Developments in Electrochemical Cells Education Bureau, HKSAR & Department of Chemistry The University of Hong Kong June 2002 Electrochemical Cells, K. Y. Chan, HKU
Science Update Programme New Developments in Electrochemical Cells Education Bureau, HKSAR & Department of Chemistry The University of Hong Kong June 2002 Electrochemical Cells, K. Y. Chan, HKU
 References Batteries Capacitors www. nec-tokin. net www. duracell. com www. faradnet. com Fuel Cells Green Energy www. fuelcells. com www. greenenergy. org. uk chem. . hku. hk/~fuelcell www. greenenergyohio. org Utilities www. ifc. com www. gepower. com Portable Power Sources www. nokia. com www. motorola. com Electric Vehicles Evworld. com Books: A. J. Bard, L. Faulkner, “Electrochemical Methods”, 2001, Wiley. Derek Pletcher and Frank C. Walsh, “Industrial Electrochemistry”, Chapman and Hall, 1990. C. A. Vincent and B. Scrosati, “Modern Batteries : An Introduction to Electrochemical Power Sources”, Butterworth. Heinemann, 1998. James Larminie and Andrew Dicks, “Fuel Cell Systems Explained”, Wiley, 2000. June 2002 Electrochemical Cells, K. Y. Chan, HKU 2
References Batteries Capacitors www. nec-tokin. net www. duracell. com www. faradnet. com Fuel Cells Green Energy www. fuelcells. com www. greenenergy. org. uk chem. . hku. hk/~fuelcell www. greenenergyohio. org Utilities www. ifc. com www. gepower. com Portable Power Sources www. nokia. com www. motorola. com Electric Vehicles Evworld. com Books: A. J. Bard, L. Faulkner, “Electrochemical Methods”, 2001, Wiley. Derek Pletcher and Frank C. Walsh, “Industrial Electrochemistry”, Chapman and Hall, 1990. C. A. Vincent and B. Scrosati, “Modern Batteries : An Introduction to Electrochemical Power Sources”, Butterworth. Heinemann, 1998. James Larminie and Andrew Dicks, “Fuel Cell Systems Explained”, Wiley, 2000. June 2002 Electrochemical Cells, K. Y. Chan, HKU 2
 Multidisciplinary and Integrated Science • Electrochemistry, General Chemistry • Physical Chemistry: Thermodynamics, Kinetics, Transport • Organic Chemistry • Inorganic, Solid State Chemistry • Materials Science • Basics Physics, Energy, Electricity • Environmental Science and Ecological/Biological Issues Can be discussed with different emphasis, at different levels, and platforms. June 2002 Electrochemical Cells, K. Y. Chan, HKU 3
Multidisciplinary and Integrated Science • Electrochemistry, General Chemistry • Physical Chemistry: Thermodynamics, Kinetics, Transport • Organic Chemistry • Inorganic, Solid State Chemistry • Materials Science • Basics Physics, Energy, Electricity • Environmental Science and Ecological/Biological Issues Can be discussed with different emphasis, at different levels, and platforms. June 2002 Electrochemical Cells, K. Y. Chan, HKU 3
 1. Fundamental Theories and Concepts 2. Batteries 3. Fuel Cells 4. Applications June 2002 Electrochemical Cells, K. Y. Chan, HKU 4
1. Fundamental Theories and Concepts 2. Batteries 3. Fuel Cells 4. Applications June 2002 Electrochemical Cells, K. Y. Chan, HKU 4
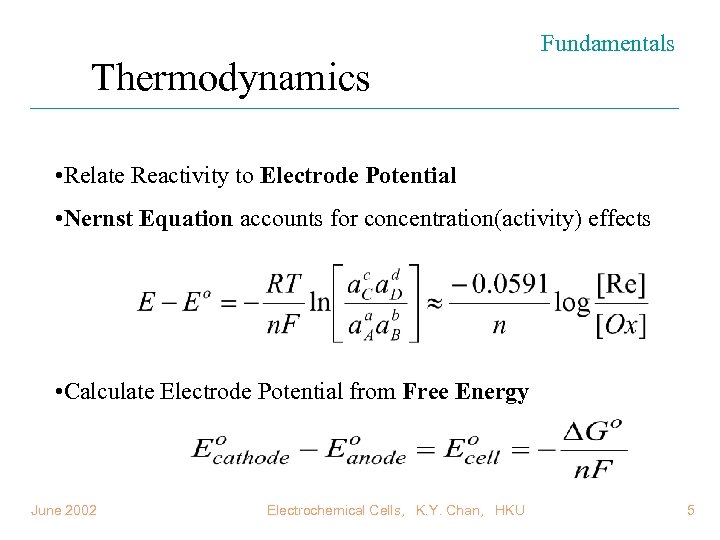 Thermodynamics Fundamentals • Relate Reactivity to Electrode Potential • Nernst Equation accounts for concentration(activity) effects • Calculate Electrode Potential from Free Energy June 2002 Electrochemical Cells, K. Y. Chan, HKU 5
Thermodynamics Fundamentals • Relate Reactivity to Electrode Potential • Nernst Equation accounts for concentration(activity) effects • Calculate Electrode Potential from Free Energy June 2002 Electrochemical Cells, K. Y. Chan, HKU 5
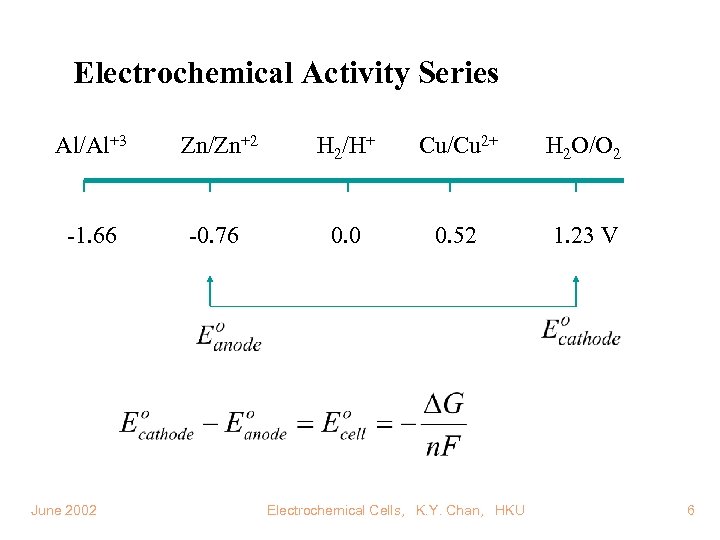 Electrochemical Activity Series Al/Al+3 Zn/Zn+2 H 2/H+ Cu/Cu 2+ H 2 O/O 2 -1. 66 -0. 76 0. 0 0. 52 1. 23 V June 2002 Electrochemical Cells, K. Y. Chan, HKU 6
Electrochemical Activity Series Al/Al+3 Zn/Zn+2 H 2/H+ Cu/Cu 2+ H 2 O/O 2 -1. 66 -0. 76 0. 0 0. 52 1. 23 V June 2002 Electrochemical Cells, K. Y. Chan, HKU 6
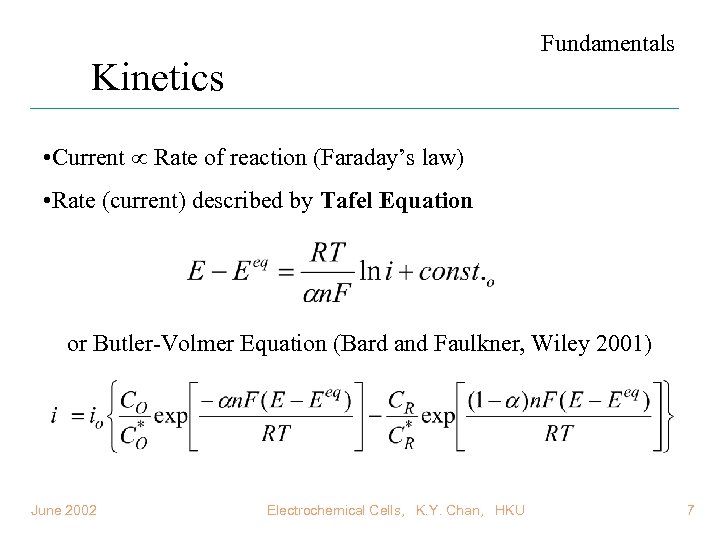 Fundamentals Kinetics • Current Rate of reaction (Faraday’s law) • Rate (current) described by Tafel Equation or Butler-Volmer Equation (Bard and Faulkner, Wiley 2001) June 2002 Electrochemical Cells, K. Y. Chan, HKU 7
Fundamentals Kinetics • Current Rate of reaction (Faraday’s law) • Rate (current) described by Tafel Equation or Butler-Volmer Equation (Bard and Faulkner, Wiley 2001) June 2002 Electrochemical Cells, K. Y. Chan, HKU 7
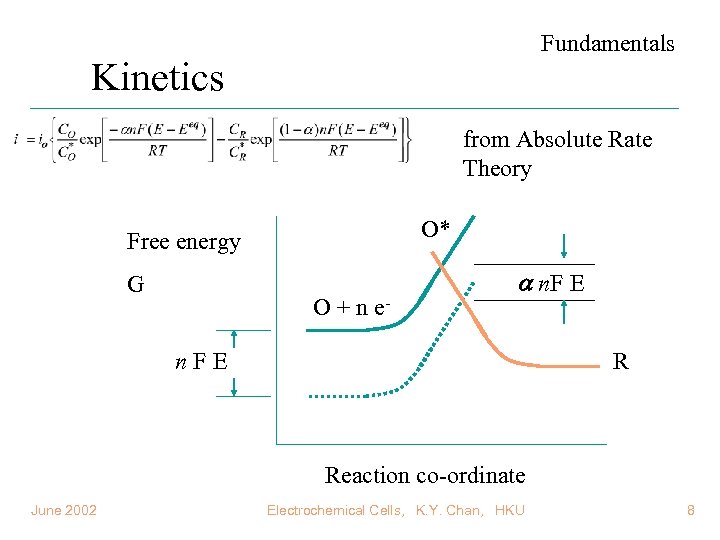 Fundamentals Kinetics from Absolute Rate Theory O* Free energy G O + n e- n. F E n. FE R Reaction co-ordinate June 2002 Electrochemical Cells, K. Y. Chan, HKU 8
Fundamentals Kinetics from Absolute Rate Theory O* Free energy G O + n e- n. F E n. FE R Reaction co-ordinate June 2002 Electrochemical Cells, K. Y. Chan, HKU 8
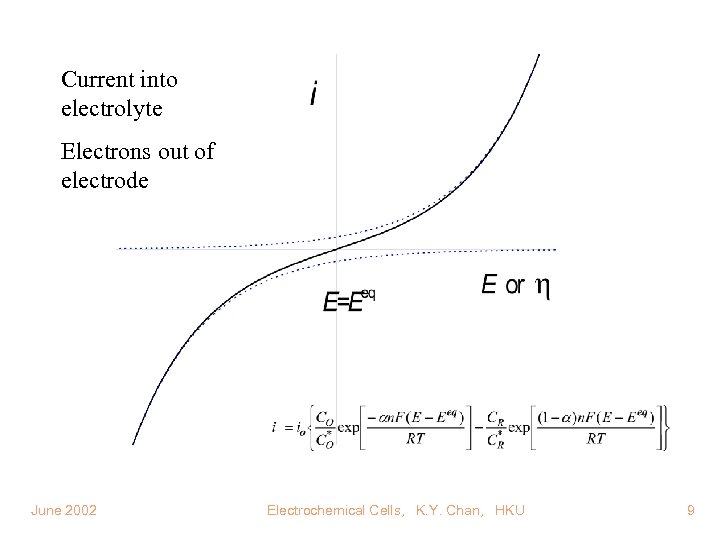 Current into electrolyte Electrons out of electrode June 2002 Electrochemical Cells, K. Y. Chan, HKU 9
Current into electrolyte Electrons out of electrode June 2002 Electrochemical Cells, K. Y. Chan, HKU 9
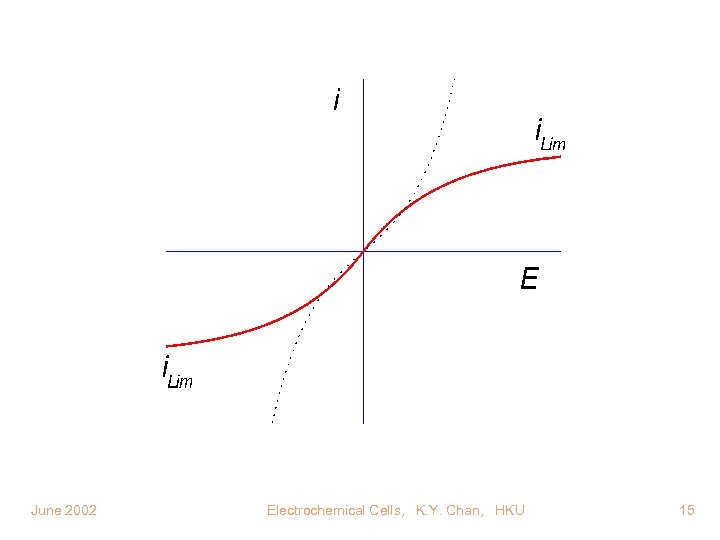
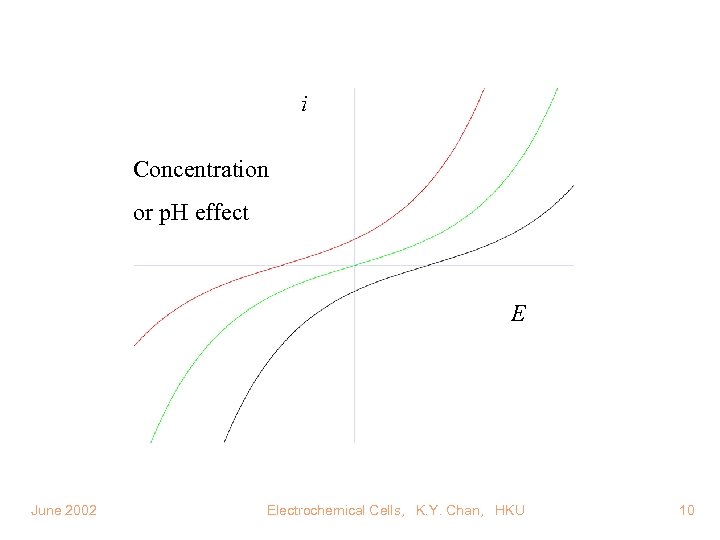 i Concentration or p. H effect E June 2002 Electrochemical Cells, K. Y. Chan, HKU 10
i Concentration or p. H effect E June 2002 Electrochemical Cells, K. Y. Chan, HKU 10
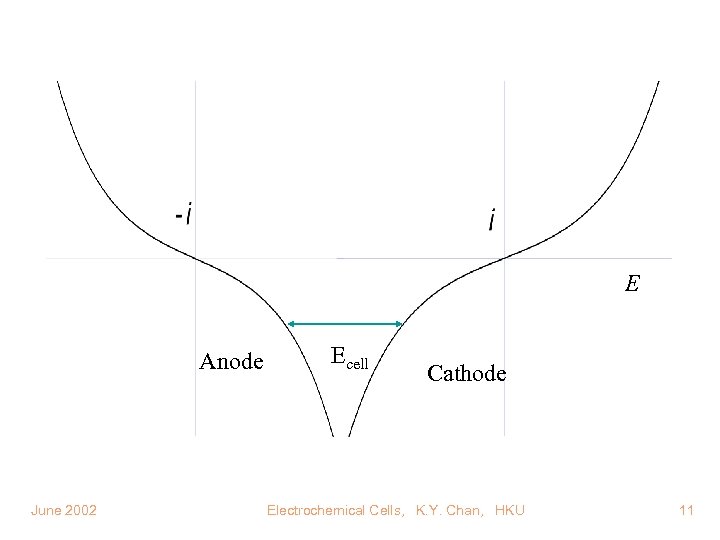 E Anode June 2002 Ecell Cathode Electrochemical Cells, K. Y. Chan, HKU 11
E Anode June 2002 Ecell Cathode Electrochemical Cells, K. Y. Chan, HKU 11
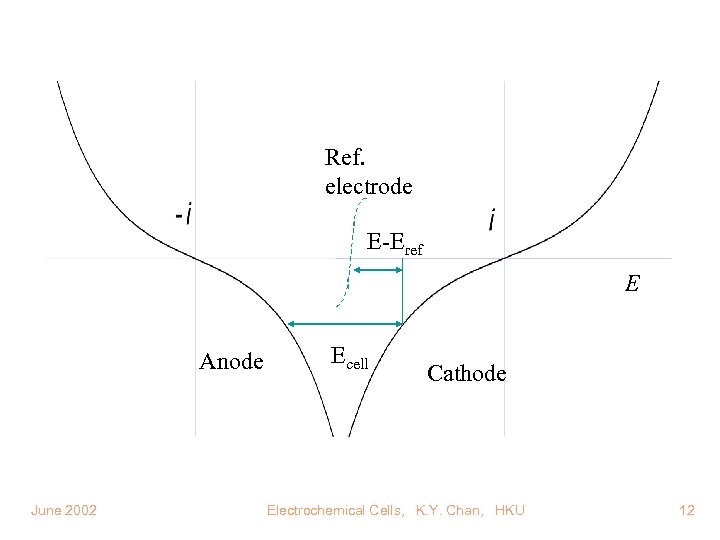 Ref. electrode E-Eref E Anode June 2002 Ecell Cathode Electrochemical Cells, K. Y. Chan, HKU 12
Ref. electrode E-Eref E Anode June 2002 Ecell Cathode Electrochemical Cells, K. Y. Chan, HKU 12
 Transport and Interfaces Fundamentals • Rate of supply of raw materials : diffusion of active materials • Rate of removal of: products including ions, electrons ionic vs ohmic resistance • Change of solid interfaces: dentritic growth • Wetting/non-wetting affects gas transport into electrolyte • Selectivity of transport, e. g. cationic membrane June 2002 Electrochemical Cells, K. Y. Chan, HKU 13
Transport and Interfaces Fundamentals • Rate of supply of raw materials : diffusion of active materials • Rate of removal of: products including ions, electrons ionic vs ohmic resistance • Change of solid interfaces: dentritic growth • Wetting/non-wetting affects gas transport into electrolyte • Selectivity of transport, e. g. cationic membrane June 2002 Electrochemical Cells, K. Y. Chan, HKU 13
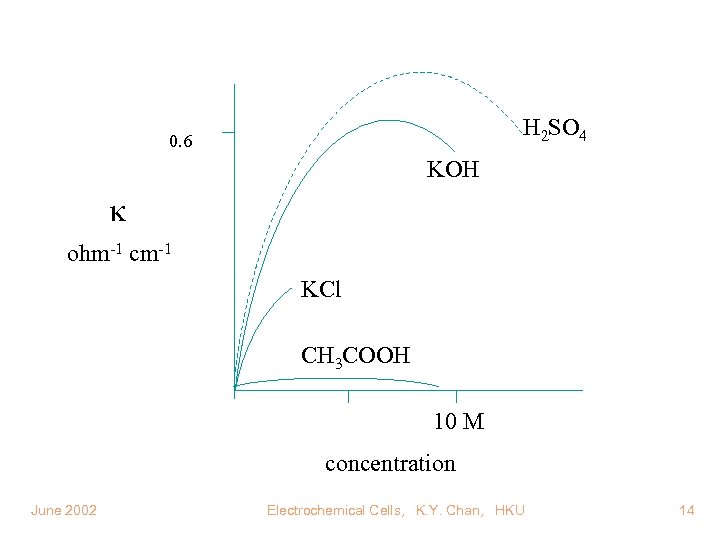 H 2 SO 4 0. 6 KOH ohm-1 cm-1 KCl CH 3 COOH 10 M concentration June 2002 Electrochemical Cells, K. Y. Chan, HKU 14
H 2 SO 4 0. 6 KOH ohm-1 cm-1 KCl CH 3 COOH 10 M concentration June 2002 Electrochemical Cells, K. Y. Chan, HKU 14
 June 2002 Electrochemical Cells, K. Y. Chan, HKU 15
June 2002 Electrochemical Cells, K. Y. Chan, HKU 15
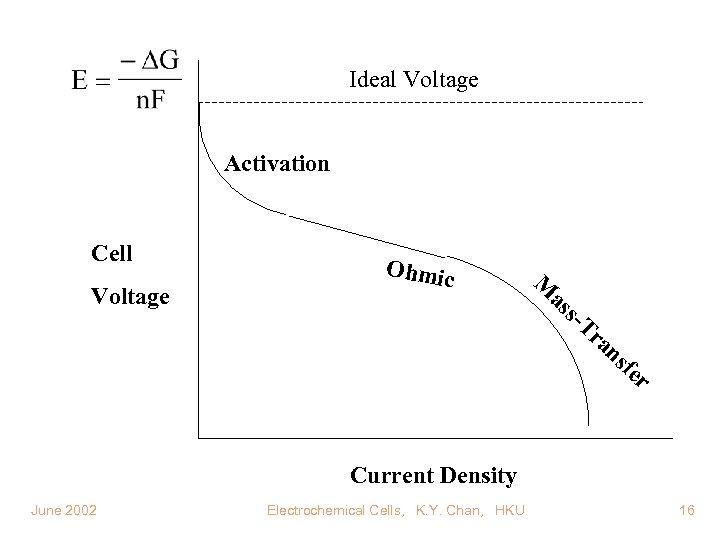 Ideal Voltage Activation Cell Voltage Ohmic M as s- Tr an sf er Current Density June 2002 Electrochemical Cells, K. Y. Chan, HKU 16
Ideal Voltage Activation Cell Voltage Ohmic M as s- Tr an sf er Current Density June 2002 Electrochemical Cells, K. Y. Chan, HKU 16
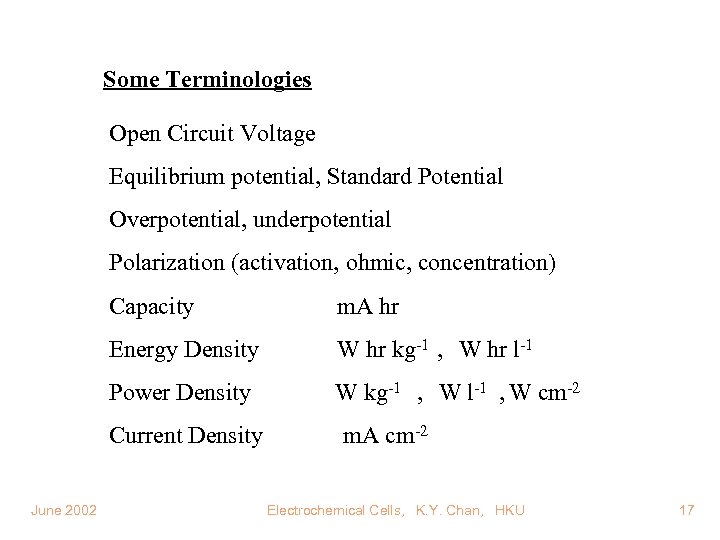 Some Terminologies Open Circuit Voltage Equilibrium potential, Standard Potential Overpotential, underpotential Polarization (activation, ohmic, concentration) Capacity m. A hr Energy Density W hr kg-1 , W hr l-1 Power Density W kg-1 , W l-1 , W cm-2 Current Density June 2002 m. A cm-2 Electrochemical Cells, K. Y. Chan, HKU 17
Some Terminologies Open Circuit Voltage Equilibrium potential, Standard Potential Overpotential, underpotential Polarization (activation, ohmic, concentration) Capacity m. A hr Energy Density W hr kg-1 , W hr l-1 Power Density W kg-1 , W l-1 , W cm-2 Current Density June 2002 m. A cm-2 Electrochemical Cells, K. Y. Chan, HKU 17
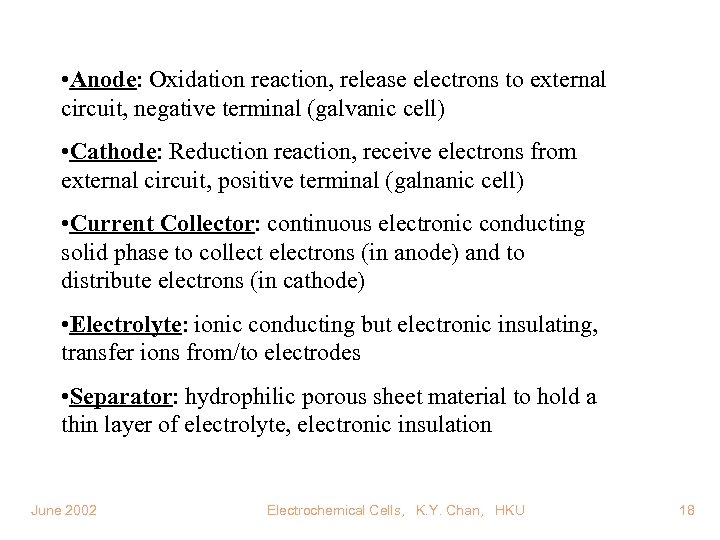 • Anode: Oxidation reaction, release electrons to external circuit, negative terminal (galvanic cell) • Cathode: Reduction reaction, receive electrons from external circuit, positive terminal (galnanic cell) • Current Collector: continuous electronic conducting solid phase to collect electrons (in anode) and to distribute electrons (in cathode) • Electrolyte: ionic conducting but electronic insulating, transfer ions from/to electrodes • Separator: hydrophilic porous sheet material to hold a thin layer of electrolyte, electronic insulation June 2002 Electrochemical Cells, K. Y. Chan, HKU 18
• Anode: Oxidation reaction, release electrons to external circuit, negative terminal (galvanic cell) • Cathode: Reduction reaction, receive electrons from external circuit, positive terminal (galnanic cell) • Current Collector: continuous electronic conducting solid phase to collect electrons (in anode) and to distribute electrons (in cathode) • Electrolyte: ionic conducting but electronic insulating, transfer ions from/to electrodes • Separator: hydrophilic porous sheet material to hold a thin layer of electrolyte, electronic insulation June 2002 Electrochemical Cells, K. Y. Chan, HKU 18
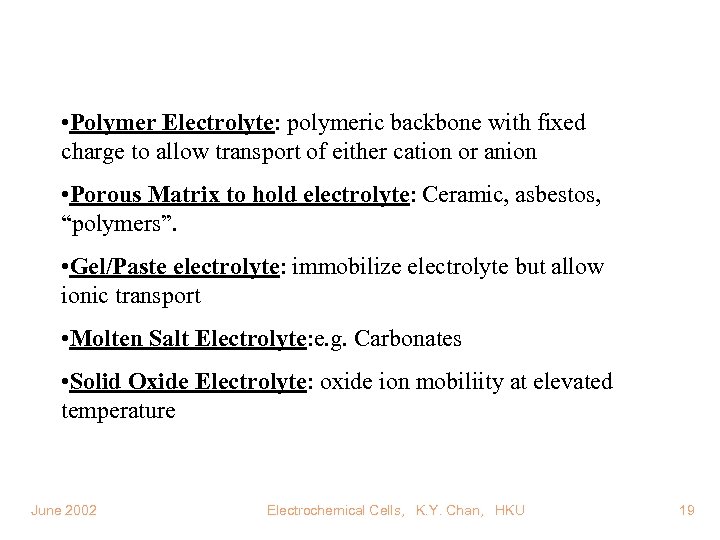 • Polymer Electrolyte: polymeric backbone with fixed charge to allow transport of either cation or anion • Porous Matrix to hold electrolyte: Ceramic, asbestos, “polymers”. • Gel/Paste electrolyte: immobilize electrolyte but allow ionic transport • Molten Salt Electrolyte: e. g. Carbonates • Solid Oxide Electrolyte: oxide ion mobiliity at elevated temperature June 2002 Electrochemical Cells, K. Y. Chan, HKU 19
• Polymer Electrolyte: polymeric backbone with fixed charge to allow transport of either cation or anion • Porous Matrix to hold electrolyte: Ceramic, asbestos, “polymers”. • Gel/Paste electrolyte: immobilize electrolyte but allow ionic transport • Molten Salt Electrolyte: e. g. Carbonates • Solid Oxide Electrolyte: oxide ion mobiliity at elevated temperature June 2002 Electrochemical Cells, K. Y. Chan, HKU 19
 Batteries A. Volta, 1880 June 2002 Electrochemical Cells, K. Y. Chan, HKU
Batteries A. Volta, 1880 June 2002 Electrochemical Cells, K. Y. Chan, HKU
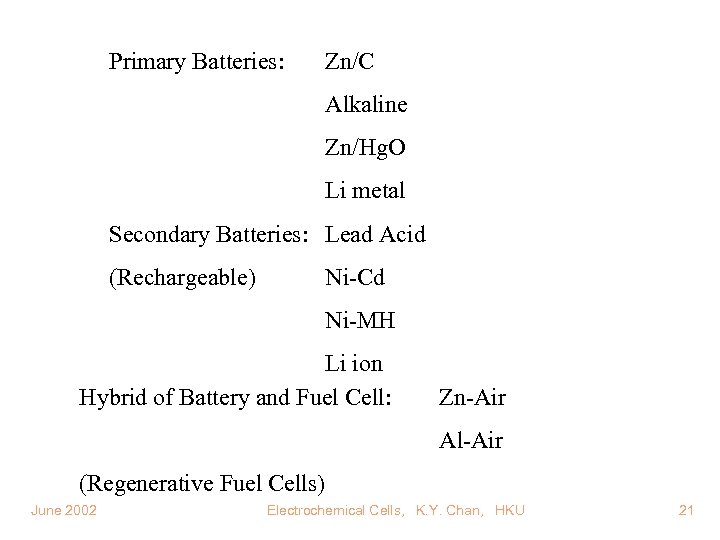 Primary Batteries: Zn/C Alkaline Zn/Hg. O Li metal Secondary Batteries: Lead Acid (Rechargeable) Ni-Cd Ni-MH Li ion Hybrid of Battery and Fuel Cell: Zn-Air Al-Air (Regenerative Fuel Cells) June 2002 Electrochemical Cells, K. Y. Chan, HKU 21
Primary Batteries: Zn/C Alkaline Zn/Hg. O Li metal Secondary Batteries: Lead Acid (Rechargeable) Ni-Cd Ni-MH Li ion Hybrid of Battery and Fuel Cell: Zn-Air Al-Air (Regenerative Fuel Cells) June 2002 Electrochemical Cells, K. Y. Chan, HKU 21
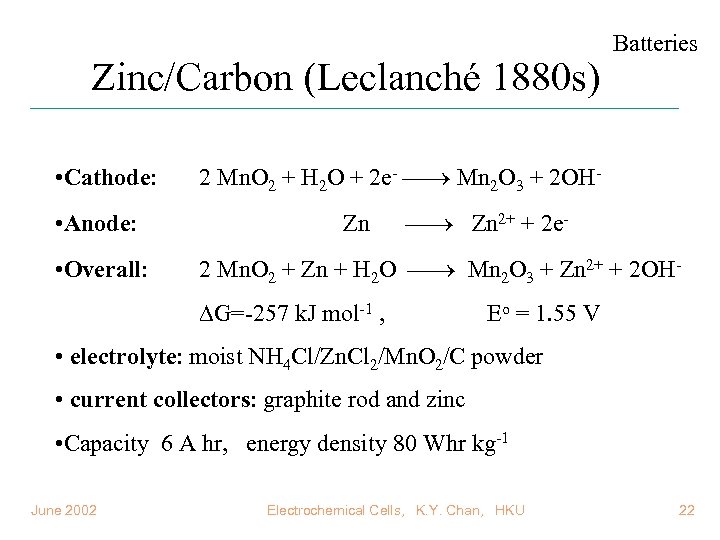 Zinc/Carbon (Leclanché 1880 s) • Cathode: • Anode: • Overall: Batteries 2 Mn. O 2 + H 2 O + 2 e- Mn 2 O 3 + 2 OHZn Zn 2+ + 2 e- 2 Mn. O 2 + Zn + H 2 O Mn 2 O 3 + Zn 2+ + 2 OH G=-257 k. J mol-1 , Eo = 1. 55 V • electrolyte: moist NH 4 Cl/Zn. Cl 2/Mn. O 2/C powder • current collectors: graphite rod and zinc • Capacity 6 A hr, energy density 80 Whr kg-1 June 2002 Electrochemical Cells, K. Y. Chan, HKU 22
Zinc/Carbon (Leclanché 1880 s) • Cathode: • Anode: • Overall: Batteries 2 Mn. O 2 + H 2 O + 2 e- Mn 2 O 3 + 2 OHZn Zn 2+ + 2 e- 2 Mn. O 2 + Zn + H 2 O Mn 2 O 3 + Zn 2+ + 2 OH G=-257 k. J mol-1 , Eo = 1. 55 V • electrolyte: moist NH 4 Cl/Zn. Cl 2/Mn. O 2/C powder • current collectors: graphite rod and zinc • Capacity 6 A hr, energy density 80 Whr kg-1 June 2002 Electrochemical Cells, K. Y. Chan, HKU 22
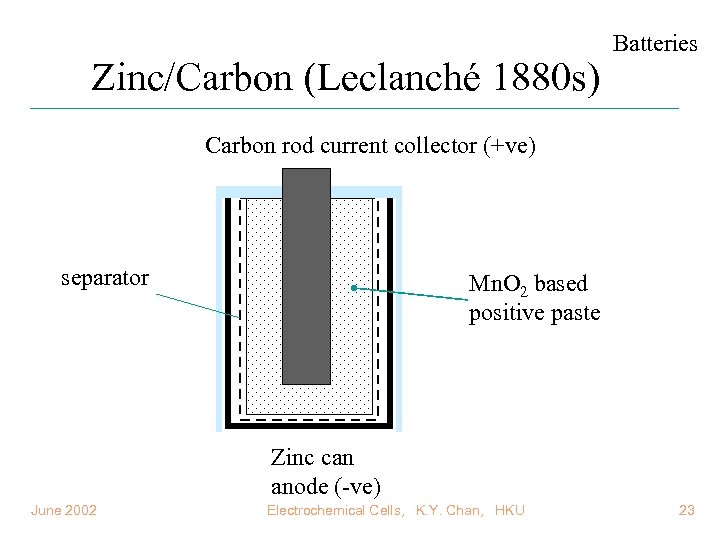 Zinc/Carbon (Leclanché 1880 s) Batteries Carbon rod current collector (+ve) separator Mn. O 2 based positive paste Zinc can anode (-ve) June 2002 Electrochemical Cells, K. Y. Chan, HKU 23
Zinc/Carbon (Leclanché 1880 s) Batteries Carbon rod current collector (+ve) separator Mn. O 2 based positive paste Zinc can anode (-ve) June 2002 Electrochemical Cells, K. Y. Chan, HKU 23
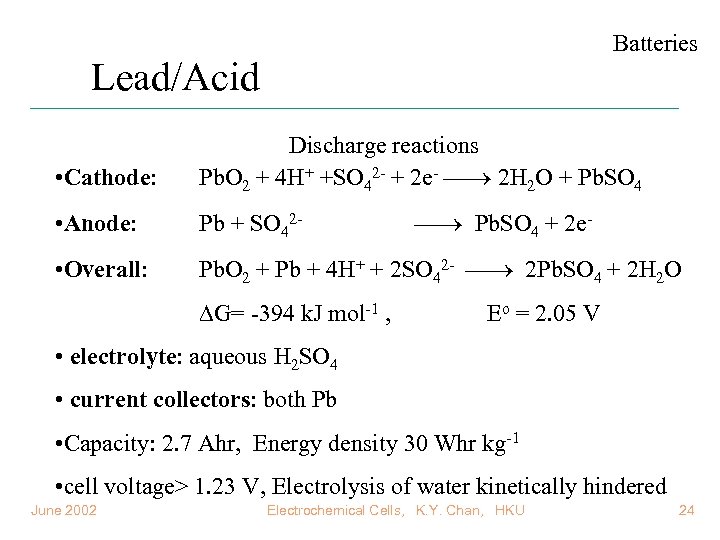 Batteries Lead/Acid • Cathode: Discharge reactions Pb. O 2 + 4 H+ +SO 42 - + 2 e- 2 H 2 O + Pb. SO 4 • Anode: Pb + SO 42 - • Overall: Pb. O 2 + Pb + 4 H+ + 2 SO 42 - 2 Pb. SO 4 + 2 H 2 O G= -394 k. J mol-1 , Pb. SO 4 + 2 e. Eo = 2. 05 V • electrolyte: aqueous H 2 SO 4 • current collectors: both Pb • Capacity: 2. 7 Ahr, Energy density 30 Whr kg-1 • cell voltage> 1. 23 V, Electrolysis of water kinetically hindered June 2002 Electrochemical Cells, K. Y. Chan, HKU 24
Batteries Lead/Acid • Cathode: Discharge reactions Pb. O 2 + 4 H+ +SO 42 - + 2 e- 2 H 2 O + Pb. SO 4 • Anode: Pb + SO 42 - • Overall: Pb. O 2 + Pb + 4 H+ + 2 SO 42 - 2 Pb. SO 4 + 2 H 2 O G= -394 k. J mol-1 , Pb. SO 4 + 2 e. Eo = 2. 05 V • electrolyte: aqueous H 2 SO 4 • current collectors: both Pb • Capacity: 2. 7 Ahr, Energy density 30 Whr kg-1 • cell voltage> 1. 23 V, Electrolysis of water kinetically hindered June 2002 Electrochemical Cells, K. Y. Chan, HKU 24
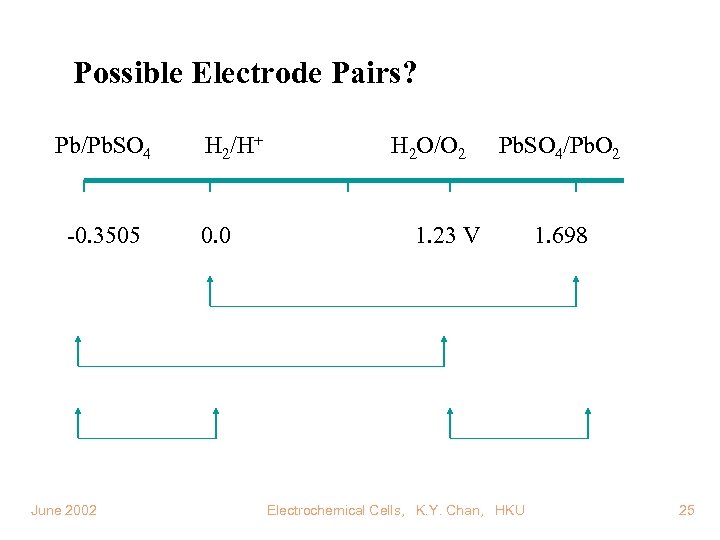 Possible Electrode Pairs? Pb/Pb. SO 4 -0. 3505 June 2002 H 2/H+ 0. 0 H 2 O/O 2 Pb. SO 4/Pb. O 2 1. 23 V Electrochemical Cells, K. Y. Chan, HKU 1. 698 25
Possible Electrode Pairs? Pb/Pb. SO 4 -0. 3505 June 2002 H 2/H+ 0. 0 H 2 O/O 2 Pb. SO 4/Pb. O 2 1. 23 V Electrochemical Cells, K. Y. Chan, HKU 1. 698 25
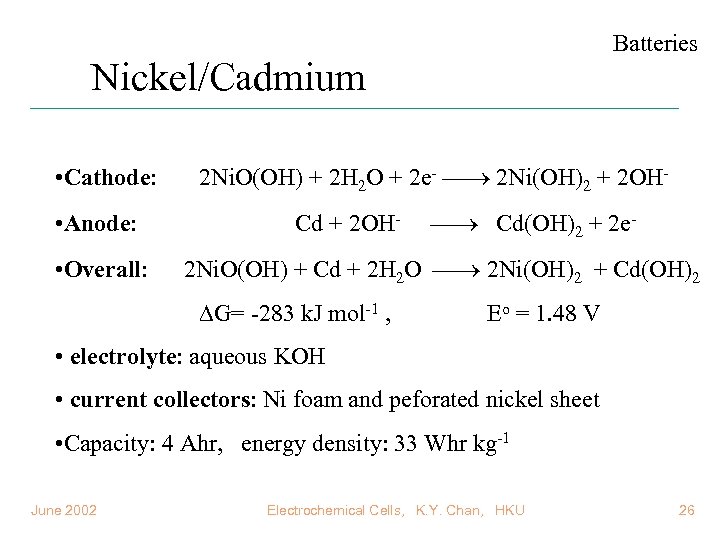 Batteries Nickel/Cadmium • Cathode: • Anode: • Overall: 2 Ni. O(OH) + 2 H 2 O + 2 e- 2 Ni(OH)2 + 2 OHCd + 2 OH- Cd(OH)2 + 2 e- 2 Ni. O(OH) + Cd + 2 H 2 O 2 Ni(OH)2 + Cd(OH)2 G= -283 k. J mol-1 , Eo = 1. 48 V • electrolyte: aqueous KOH • current collectors: Ni foam and peforated nickel sheet • Capacity: 4 Ahr, energy density: 33 Whr kg-1 June 2002 Electrochemical Cells, K. Y. Chan, HKU 26
Batteries Nickel/Cadmium • Cathode: • Anode: • Overall: 2 Ni. O(OH) + 2 H 2 O + 2 e- 2 Ni(OH)2 + 2 OHCd + 2 OH- Cd(OH)2 + 2 e- 2 Ni. O(OH) + Cd + 2 H 2 O 2 Ni(OH)2 + Cd(OH)2 G= -283 k. J mol-1 , Eo = 1. 48 V • electrolyte: aqueous KOH • current collectors: Ni foam and peforated nickel sheet • Capacity: 4 Ahr, energy density: 33 Whr kg-1 June 2002 Electrochemical Cells, K. Y. Chan, HKU 26
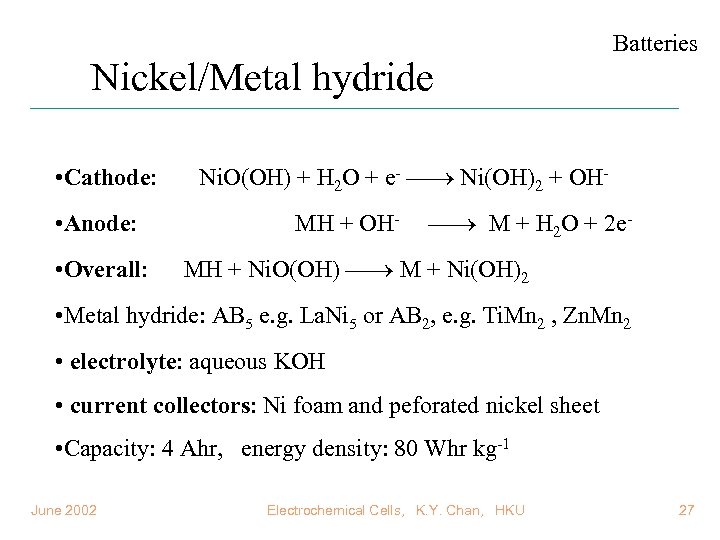 Nickel/Metal hydride • Cathode: • Anode: • Overall: Batteries Ni. O(OH) + H 2 O + e- Ni(OH)2 + OHMH + OH- M + H 2 O + 2 e- MH + Ni. O(OH) M + Ni(OH)2 • Metal hydride: AB 5 e. g. La. Ni 5 or AB 2, e. g. Ti. Mn 2 , Zn. Mn 2 • electrolyte: aqueous KOH • current collectors: Ni foam and peforated nickel sheet • Capacity: 4 Ahr, energy density: 80 Whr kg-1 June 2002 Electrochemical Cells, K. Y. Chan, HKU 27
Nickel/Metal hydride • Cathode: • Anode: • Overall: Batteries Ni. O(OH) + H 2 O + e- Ni(OH)2 + OHMH + OH- M + H 2 O + 2 e- MH + Ni. O(OH) M + Ni(OH)2 • Metal hydride: AB 5 e. g. La. Ni 5 or AB 2, e. g. Ti. Mn 2 , Zn. Mn 2 • electrolyte: aqueous KOH • current collectors: Ni foam and peforated nickel sheet • Capacity: 4 Ahr, energy density: 80 Whr kg-1 June 2002 Electrochemical Cells, K. Y. Chan, HKU 27
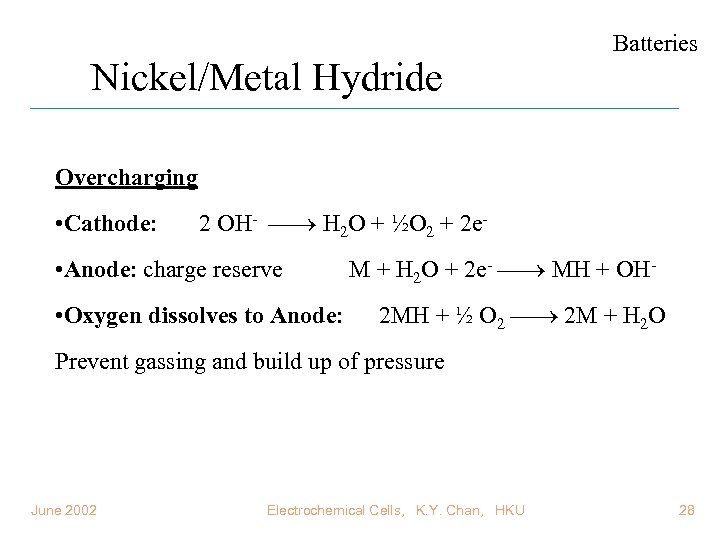 Nickel/Metal Hydride Batteries Overcharging • Cathode: 2 OH- H 2 O + ½O 2 + 2 e- • Anode: charge reserve • Oxygen dissolves to Anode: M + H 2 O + 2 e- MH + OH 2 MH + ½ O 2 2 M + H 2 O Prevent gassing and build up of pressure June 2002 Electrochemical Cells, K. Y. Chan, HKU 28
Nickel/Metal Hydride Batteries Overcharging • Cathode: 2 OH- H 2 O + ½O 2 + 2 e- • Anode: charge reserve • Oxygen dissolves to Anode: M + H 2 O + 2 e- MH + OH 2 MH + ½ O 2 2 M + H 2 O Prevent gassing and build up of pressure June 2002 Electrochemical Cells, K. Y. Chan, HKU 28
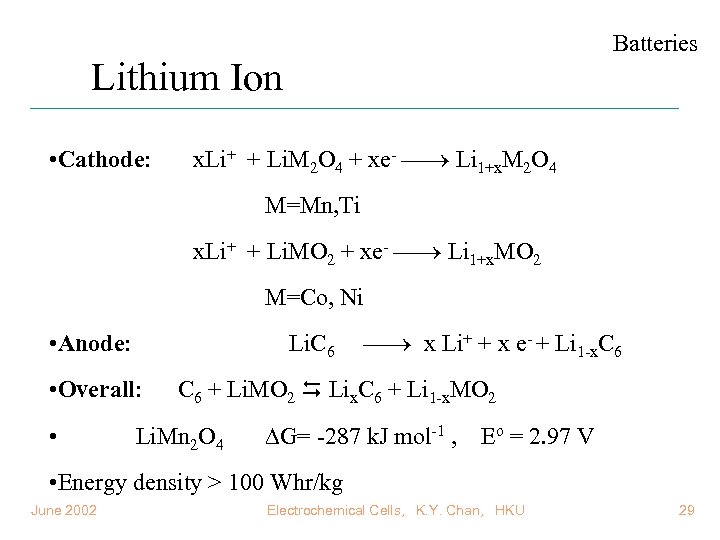 Batteries Lithium Ion • Cathode: x. Li+ + Li. M 2 O 4 + xe- Li 1+x. M 2 O 4 M=Mn, Ti x. Li+ + Li. MO 2 + xe- Li 1+x. MO 2 M=Co, Ni • Anode: Li. C 6 • Overall: • x Li+ + x e- + Li 1 -x. C 6 + Li. MO 2 Lix. C 6 + Li 1 -x. MO 2 Li. Mn 2 O 4 G= -287 k. J mol-1 , Eo = 2. 97 V • Energy density > 100 Whr/kg June 2002 Electrochemical Cells, K. Y. Chan, HKU 29
Batteries Lithium Ion • Cathode: x. Li+ + Li. M 2 O 4 + xe- Li 1+x. M 2 O 4 M=Mn, Ti x. Li+ + Li. MO 2 + xe- Li 1+x. MO 2 M=Co, Ni • Anode: Li. C 6 • Overall: • x Li+ + x e- + Li 1 -x. C 6 + Li. MO 2 Lix. C 6 + Li 1 -x. MO 2 Li. Mn 2 O 4 G= -287 k. J mol-1 , Eo = 2. 97 V • Energy density > 100 Whr/kg June 2002 Electrochemical Cells, K. Y. Chan, HKU 29
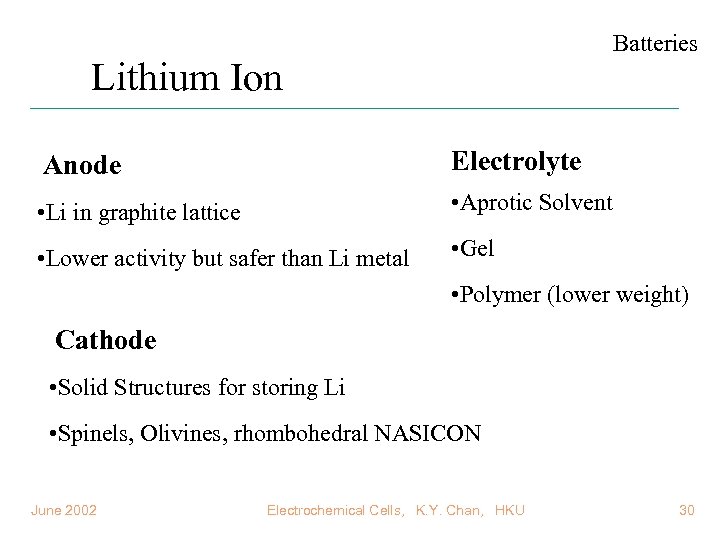 Batteries Lithium Ion Anode Electrolyte • Li in graphite lattice • Aprotic Solvent • Lower activity but safer than Li metal • Gel • Polymer (lower weight) Cathode • Solid Structures for storing Li • Spinels, Olivines, rhombohedral NASICON June 2002 Electrochemical Cells, K. Y. Chan, HKU 30
Batteries Lithium Ion Anode Electrolyte • Li in graphite lattice • Aprotic Solvent • Lower activity but safer than Li metal • Gel • Polymer (lower weight) Cathode • Solid Structures for storing Li • Spinels, Olivines, rhombohedral NASICON June 2002 Electrochemical Cells, K. Y. Chan, HKU 30
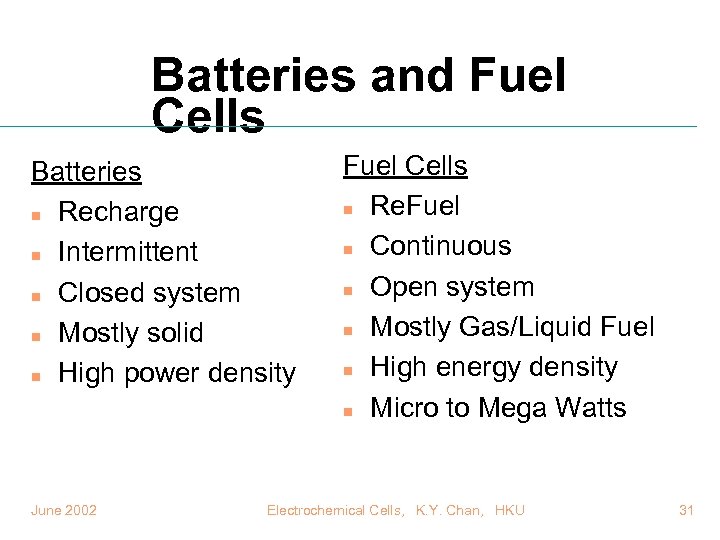 Batteries and Fuel Cells Batteries n Recharge n Intermittent n Closed system n Mostly solid n High power density June 2002 Fuel Cells n Re. Fuel n Continuous n Open system n Mostly Gas/Liquid Fuel n High energy density n Micro to Mega Watts Electrochemical Cells, K. Y. Chan, HKU 31
Batteries and Fuel Cells Batteries n Recharge n Intermittent n Closed system n Mostly solid n High power density June 2002 Fuel Cells n Re. Fuel n Continuous n Open system n Mostly Gas/Liquid Fuel n High energy density n Micro to Mega Watts Electrochemical Cells, K. Y. Chan, HKU 31
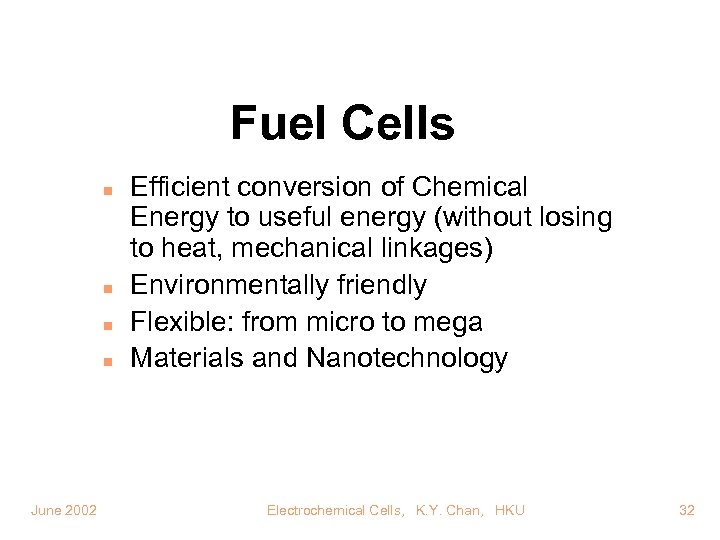 Fuel Cells n n June 2002 Efficient conversion of Chemical Energy to useful energy (without losing to heat, mechanical linkages) Environmentally friendly Flexible: from micro to mega Materials and Nanotechnology Electrochemical Cells, K. Y. Chan, HKU 32
Fuel Cells n n June 2002 Efficient conversion of Chemical Energy to useful energy (without losing to heat, mechanical linkages) Environmentally friendly Flexible: from micro to mega Materials and Nanotechnology Electrochemical Cells, K. Y. Chan, HKU 32
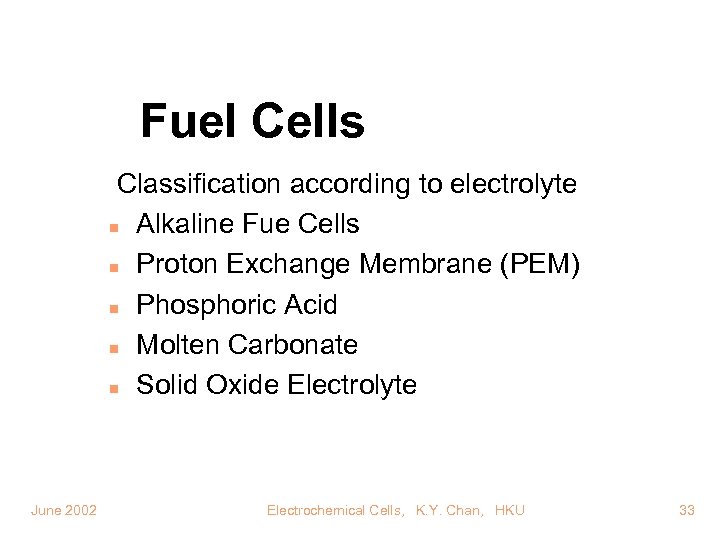 Fuel Cells Classification according to electrolyte n Alkaline Fue Cells n Proton Exchange Membrane (PEM) n Phosphoric Acid n Molten Carbonate n Solid Oxide Electrolyte June 2002 Electrochemical Cells, K. Y. Chan, HKU 33
Fuel Cells Classification according to electrolyte n Alkaline Fue Cells n Proton Exchange Membrane (PEM) n Phosphoric Acid n Molten Carbonate n Solid Oxide Electrolyte June 2002 Electrochemical Cells, K. Y. Chan, HKU 33
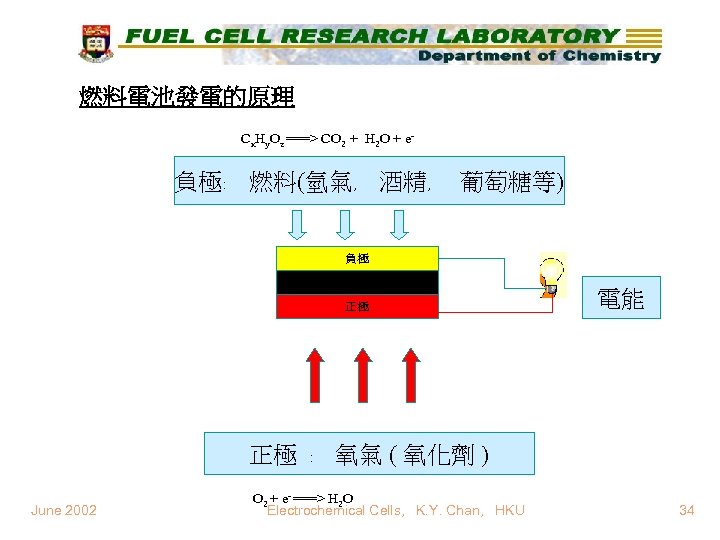 燃料電池發電的原理 Cx. Hy. Oz ===> CO 2 + H 2 O + e- 負極﹕ 燃料(氫氣﹐ 酒精﹐ 葡萄糖等) 負極 電解液 正極 電能 正極 ﹕ 氧氣 ( 氧化劑 ) June 2002 O 2 + e- ===> H 2 O Electrochemical Cells, K. Y. Chan, HKU 34
燃料電池發電的原理 Cx. Hy. Oz ===> CO 2 + H 2 O + e- 負極﹕ 燃料(氫氣﹐ 酒精﹐ 葡萄糖等) 負極 電解液 正極 電能 正極 ﹕ 氧氣 ( 氧化劑 ) June 2002 O 2 + e- ===> H 2 O Electrochemical Cells, K. Y. Chan, HKU 34
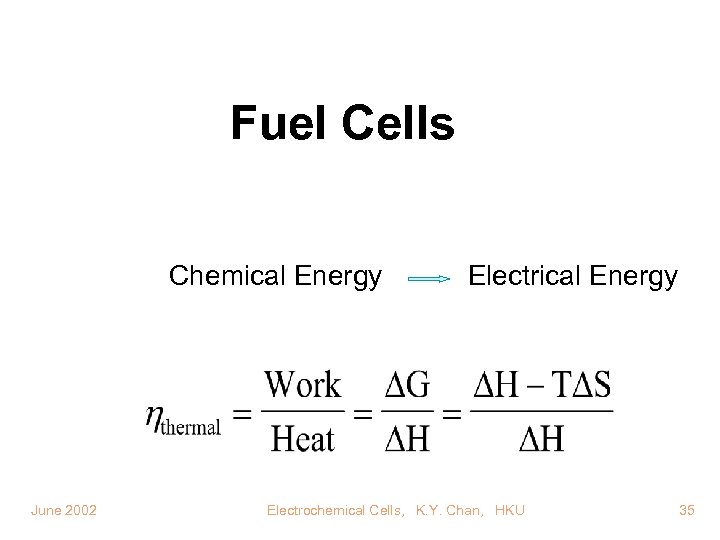 Fuel Cells Chemical Energy June 2002 Electrical Energy Electrochemical Cells, K. Y. Chan, HKU 35
Fuel Cells Chemical Energy June 2002 Electrical Energy Electrochemical Cells, K. Y. Chan, HKU 35
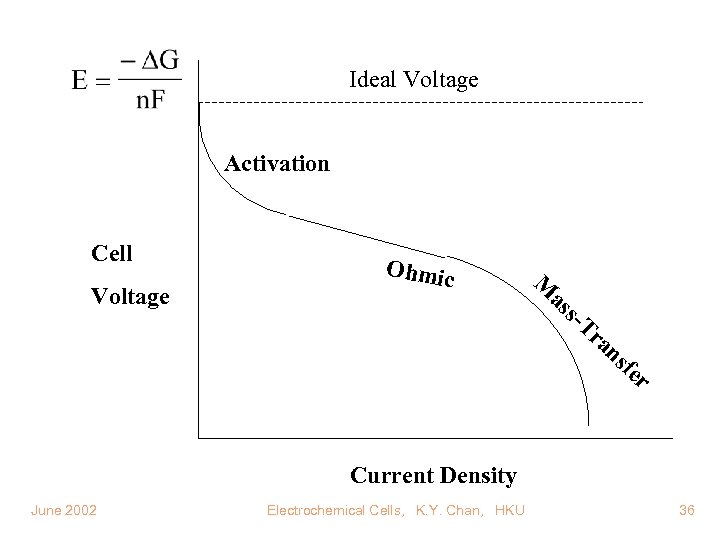 Ideal Voltage Activation Cell Voltage Ohmic M as s- Tr an sf er Current Density June 2002 Electrochemical Cells, K. Y. Chan, HKU 36
Ideal Voltage Activation Cell Voltage Ohmic M as s- Tr an sf er Current Density June 2002 Electrochemical Cells, K. Y. Chan, HKU 36
 Diversity of Technology and Materials Problems in Fuel Cells n n n n June 2002 Fuel Oxidant Catalyst Container Control Transport Storage Electrochemical Cells, K. Y. Chan, HKU 37
Diversity of Technology and Materials Problems in Fuel Cells n n n n June 2002 Fuel Oxidant Catalyst Container Control Transport Storage Electrochemical Cells, K. Y. Chan, HKU 37
 Fuels: Hydrogen Catalyst Support: Metals Porous Carbon Natural Gas Ceramic Matrix Small Hydrocarbons Molecular Sieves (methanol, glucose) Polymer Oxidant: air oxygen halides oxides Catalysts: platinum metals metal oxides macrocycles June 2002 Container and Movable Parts: Alloys Ceramic Polymers Transport/Electrolyte: Proton Exchange Membranes PTFE (Teflon) Solid Electrolyte Storage: Metal Hydride Electrochemical Cells, K. Y. Chan, HKU 38
Fuels: Hydrogen Catalyst Support: Metals Porous Carbon Natural Gas Ceramic Matrix Small Hydrocarbons Molecular Sieves (methanol, glucose) Polymer Oxidant: air oxygen halides oxides Catalysts: platinum metals metal oxides macrocycles June 2002 Container and Movable Parts: Alloys Ceramic Polymers Transport/Electrolyte: Proton Exchange Membranes PTFE (Teflon) Solid Electrolyte Storage: Metal Hydride Electrochemical Cells, K. Y. Chan, HKU 38
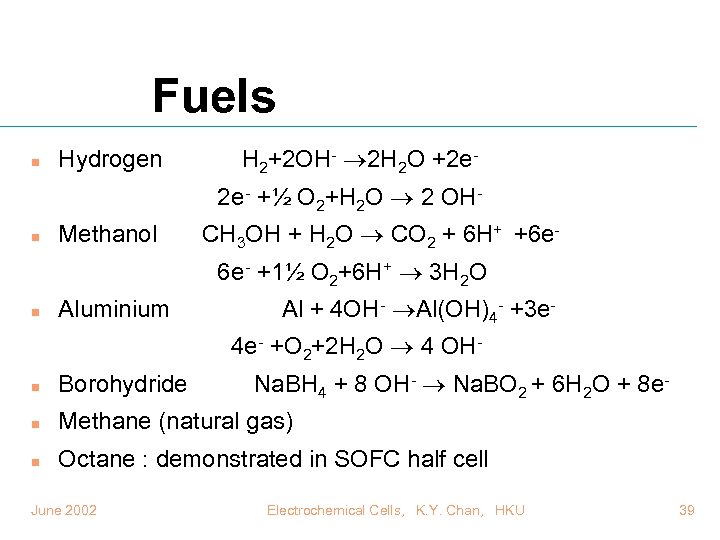 Fuels n Hydrogen H 2+2 OH- 2 H 2 O +2 e 2 e- +½ O 2+H 2 O 2 OH- n Methanol CH 3 OH + H 2 O CO 2 + 6 H+ +6 e 6 e- +1½ O 2+6 H+ 3 H 2 O n Aluminium Al + 4 OH- Al(OH)4 - +3 e 4 e- +O 2+2 H 2 O 4 OHNa. BH 4 + 8 OH- Na. BO 2 + 6 H 2 O + 8 e- n Borohydride n Methane (natural gas) n Octane : demonstrated in SOFC half cell June 2002 Electrochemical Cells, K. Y. Chan, HKU 39
Fuels n Hydrogen H 2+2 OH- 2 H 2 O +2 e 2 e- +½ O 2+H 2 O 2 OH- n Methanol CH 3 OH + H 2 O CO 2 + 6 H+ +6 e 6 e- +1½ O 2+6 H+ 3 H 2 O n Aluminium Al + 4 OH- Al(OH)4 - +3 e 4 e- +O 2+2 H 2 O 4 OHNa. BH 4 + 8 OH- Na. BO 2 + 6 H 2 O + 8 e- n Borohydride n Methane (natural gas) n Octane : demonstrated in SOFC half cell June 2002 Electrochemical Cells, K. Y. Chan, HKU 39
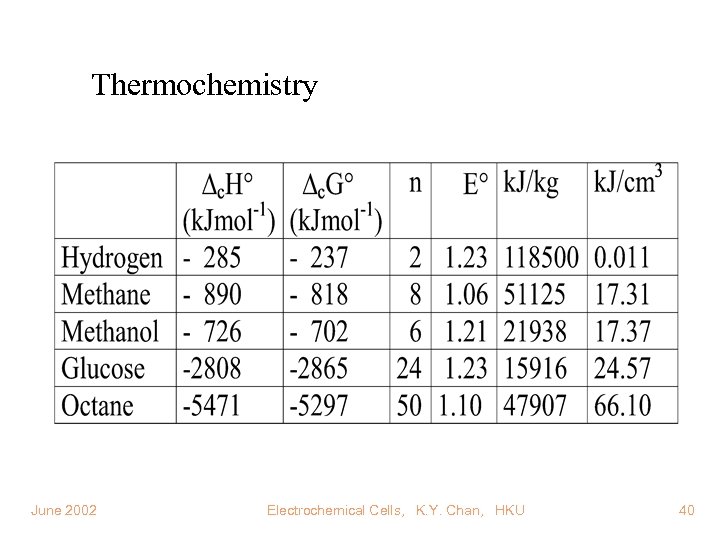 Thermochemistry June 2002 Electrochemical Cells, K. Y. Chan, HKU 40
Thermochemistry June 2002 Electrochemical Cells, K. Y. Chan, HKU 40
 Micro and Nanostructured Electrodes: n n n n June 2002 Catalyst Support: High Surface Carbon Size Effects of Catalysts Controlled Porosity Controlled Wetting Maxinum Gas-Liquid-Solid Interface Minimize ohmic resistance Minimize ionic resistance Electrochemical Cells, K. Y. Chan, HKU 41
Micro and Nanostructured Electrodes: n n n n June 2002 Catalyst Support: High Surface Carbon Size Effects of Catalysts Controlled Porosity Controlled Wetting Maxinum Gas-Liquid-Solid Interface Minimize ohmic resistance Minimize ionic resistance Electrochemical Cells, K. Y. Chan, HKU 41
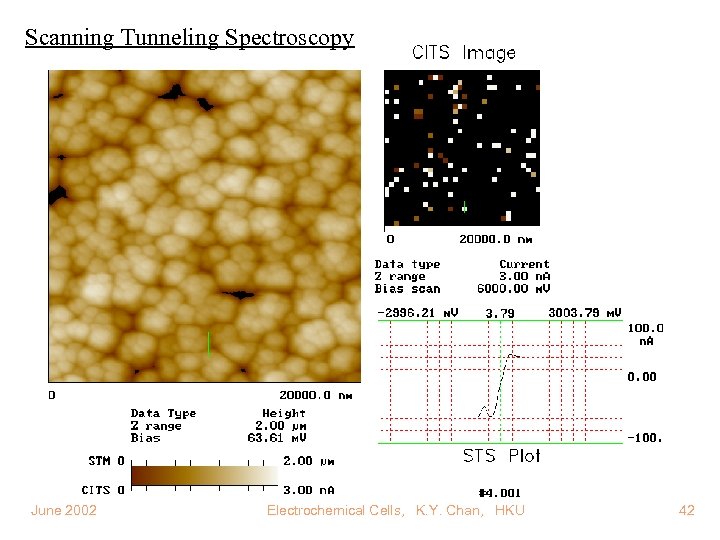 Scanning Tunneling Spectroscopy June 2002 Electrochemical Cells, K. Y. Chan, HKU 42
Scanning Tunneling Spectroscopy June 2002 Electrochemical Cells, K. Y. Chan, HKU 42
 Catalysts n n n Platinum is the most important for both anode and cathode Platinum can be replaced by Ag, Mn, Co, only for oxygen reduction in alkaline medium Platinum subject to CO poisoning (impure H 2) Binary/Ternary system, macrocycle, bifunctional Stability/Life of nanometals June 2002 Electrochemical Cells, K. Y. Chan, HKU 43
Catalysts n n n Platinum is the most important for both anode and cathode Platinum can be replaced by Ag, Mn, Co, only for oxygen reduction in alkaline medium Platinum subject to CO poisoning (impure H 2) Binary/Ternary system, macrocycle, bifunctional Stability/Life of nanometals June 2002 Electrochemical Cells, K. Y. Chan, HKU 43
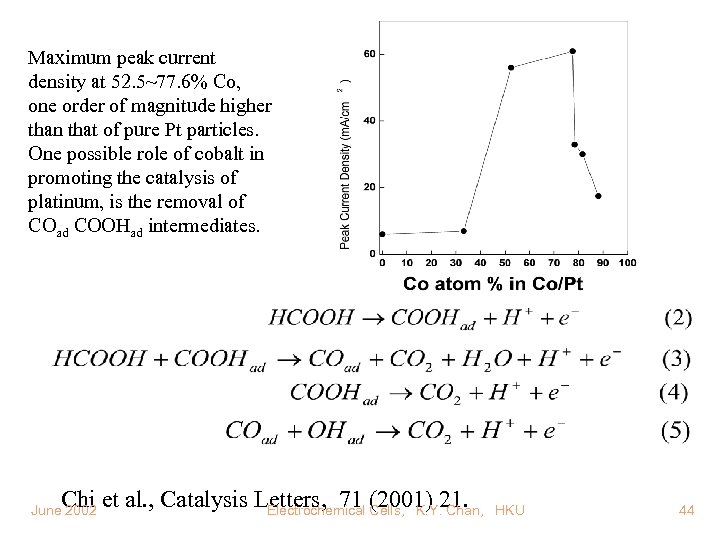 Maximum peak current density at 52. 5~77. 6% Co, one order of magnitude higher than that of pure Pt particles. One possible role of cobalt in promoting the catalysis of platinum, is the removal of COad COOHad intermediates. Chi et al. , Catalysis Letters, 71 (2001) 21. Electrochemical Cells, K. Y. Chan, June 2002 HKU 44
Maximum peak current density at 52. 5~77. 6% Co, one order of magnitude higher than that of pure Pt particles. One possible role of cobalt in promoting the catalysis of platinum, is the removal of COad COOHad intermediates. Chi et al. , Catalysis Letters, 71 (2001) 21. Electrochemical Cells, K. Y. Chan, June 2002 HKU 44
 Catalysts n n n Oxygen Cathode is most limiting and is present in most fuel cells Non-platinum cathode catalyst can tolerant cross over effect. At high temperature, no precious metal or no catalysts is needed in MCFC and SOFC June 2002 Electrochemical Cells, K. Y. Chan, HKU 45
Catalysts n n n Oxygen Cathode is most limiting and is present in most fuel cells Non-platinum cathode catalyst can tolerant cross over effect. At high temperature, no precious metal or no catalysts is needed in MCFC and SOFC June 2002 Electrochemical Cells, K. Y. Chan, HKU 45
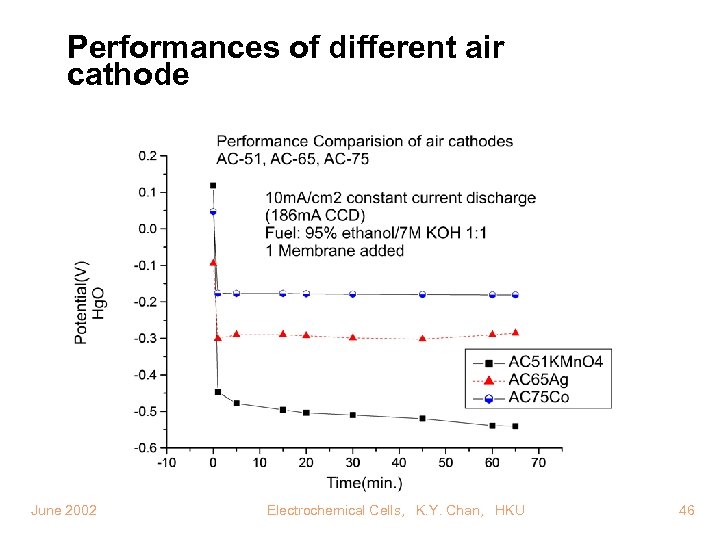 Performances of different air cathode June 2002 Electrochemical Cells, K. Y. Chan, HKU 46
Performances of different air cathode June 2002 Electrochemical Cells, K. Y. Chan, HKU 46
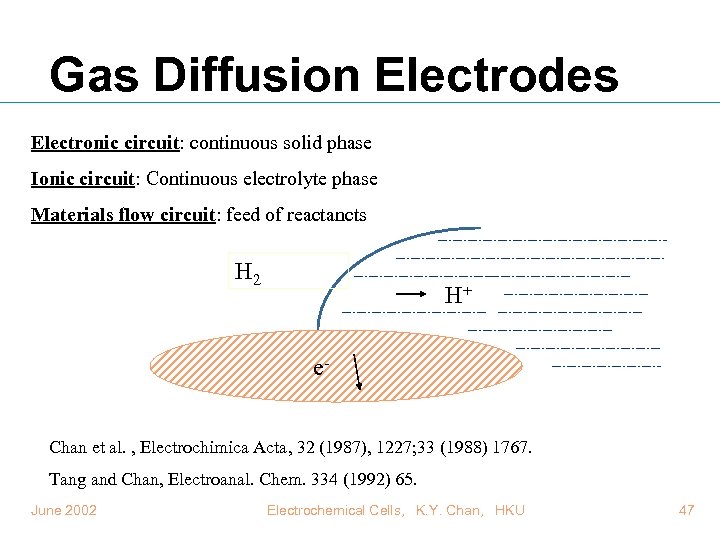 Gas Diffusion Electrodes Electronic circuit: continuous solid phase Ionic circuit: Continuous electrolyte phase Materials flow circuit: feed of reactancts H 2 H+ e- Chan et al. , Electrochimica Acta, 32 (1987), 1227; 33 (1988) 1767. Tang and Chan, Electroanal. Chem. 334 (1992) 65. June 2002 Electrochemical Cells, K. Y. Chan, HKU 47
Gas Diffusion Electrodes Electronic circuit: continuous solid phase Ionic circuit: Continuous electrolyte phase Materials flow circuit: feed of reactancts H 2 H+ e- Chan et al. , Electrochimica Acta, 32 (1987), 1227; 33 (1988) 1767. Tang and Chan, Electroanal. Chem. 334 (1992) 65. June 2002 Electrochemical Cells, K. Y. Chan, HKU 47
 Single air cathode June 2002 Electrochemical Cells, K. Y. Chan, HKU 48
Single air cathode June 2002 Electrochemical Cells, K. Y. Chan, HKU 48
 Electrolyte n n n n June 2002 Alkaline electrolyte (first deployed for Apollo mission) Phosphoric Acid 180 C Polymer Electrolyte Cross Over Stability (CO 2 removal in alkaline) Solid Oxide (YSZ, doped Ceria) Shunt Current / Leak Current Electrochemical Cells, K. Y. Chan, HKU 49
Electrolyte n n n n June 2002 Alkaline electrolyte (first deployed for Apollo mission) Phosphoric Acid 180 C Polymer Electrolyte Cross Over Stability (CO 2 removal in alkaline) Solid Oxide (YSZ, doped Ceria) Shunt Current / Leak Current Electrochemical Cells, K. Y. Chan, HKU 49
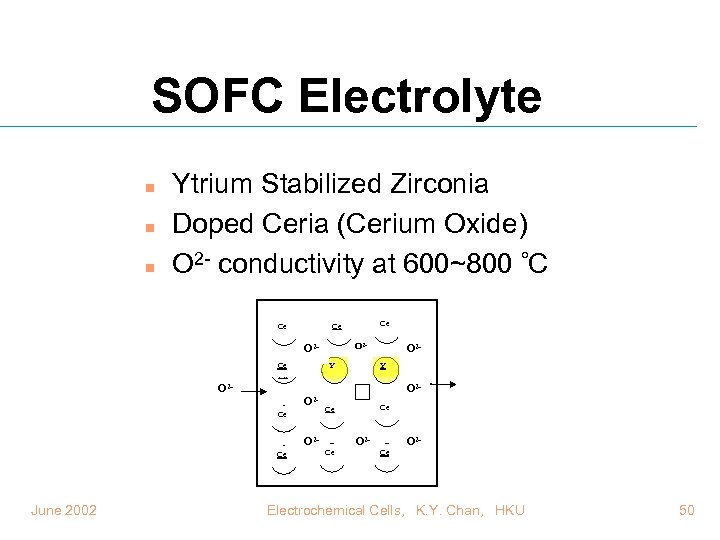 SOFC Electrolyte n n n Ytrium Stabilized Zirconia Doped Ceria (Cerium Oxide) O 2 - conductivity at 600~800 C Ce O 2 - O 2 Ce O 2 - Y Zr Ce Y Ce Ce O 2 - June 2002 O 2 O 2 - Ce Ce Ce O 2 Ce Electrochemical Cells, K. Y. Chan, HKU 50
SOFC Electrolyte n n n Ytrium Stabilized Zirconia Doped Ceria (Cerium Oxide) O 2 - conductivity at 600~800 C Ce O 2 - O 2 Ce O 2 - Y Zr Ce Y Ce Ce O 2 - June 2002 O 2 O 2 - Ce Ce Ce O 2 Ce Electrochemical Cells, K. Y. Chan, HKU 50
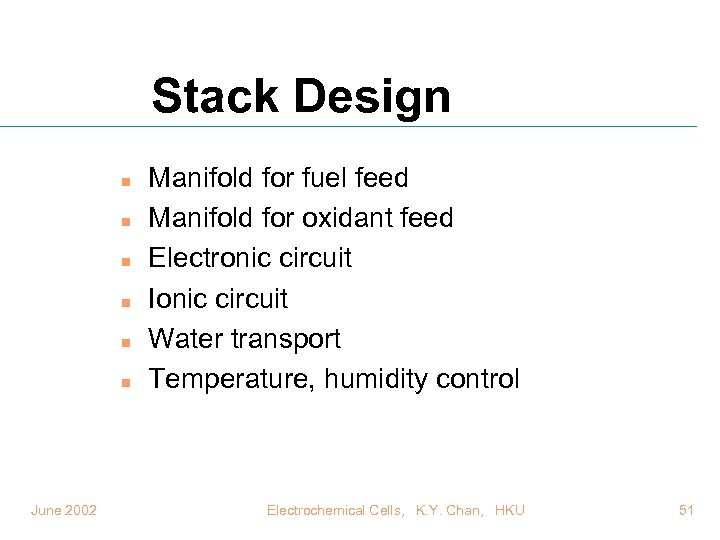 Stack Design n n n June 2002 Manifold for fuel feed Manifold for oxidant feed Electronic circuit Ionic circuit Water transport Temperature, humidity control Electrochemical Cells, K. Y. Chan, HKU 51
Stack Design n n n June 2002 Manifold for fuel feed Manifold for oxidant feed Electronic circuit Ionic circuit Water transport Temperature, humidity control Electrochemical Cells, K. Y. Chan, HKU 51
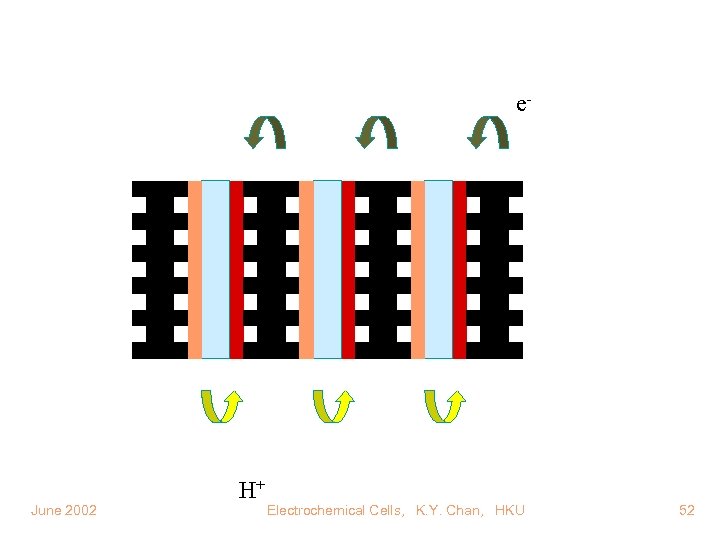 e- June 2002 H+ Electrochemical Cells, K. Y. Chan, HKU 52
e- June 2002 H+ Electrochemical Cells, K. Y. Chan, HKU 52
 Stack Design Applications Demonstrated: Radio(Voice of Glucose); Portable CD player; Mobile Phone(GSM). Product Name: Fuel Cell Stack Fuels usable: Glucose, methanol, Na. BH 4 No. of Fuel Cells: 10 in Serial Open Circuit Voltage: 4. 0 -9. 0 V Power Output: 0. 5 -1. 0 W Application: Stationary or Portable ( Mobile phone or toy cars ) June 2002 Electrochemical Cells, K. Y. Chan, HKU 53
Stack Design Applications Demonstrated: Radio(Voice of Glucose); Portable CD player; Mobile Phone(GSM). Product Name: Fuel Cell Stack Fuels usable: Glucose, methanol, Na. BH 4 No. of Fuel Cells: 10 in Serial Open Circuit Voltage: 4. 0 -9. 0 V Power Output: 0. 5 -1. 0 W Application: Stationary or Portable ( Mobile phone or toy cars ) June 2002 Electrochemical Cells, K. Y. Chan, HKU 53
 Electronic circuit: continuous solid phase with minimum electrical resistance to electronically connect anode and cathode through external circuit. • Ionic circuit: to complete the other half of the “charge circuit”. Continuous electrolyte phase connecting cathode and anode, but electronic insulating. Maintain balance of ions for anodic, cathodic reactions. • Materials flow circuit: feed of reactancts to and removal of products from anode/cathode. • Avoid shunt current, leak current in multiple cells • Avoid short circuit of cathode and anode • Avoid breaking electrochemical window of electrolyte June 2002 Electrochemical Cells, K. Y. Chan, HKU 54
Electronic circuit: continuous solid phase with minimum electrical resistance to electronically connect anode and cathode through external circuit. • Ionic circuit: to complete the other half of the “charge circuit”. Continuous electrolyte phase connecting cathode and anode, but electronic insulating. Maintain balance of ions for anodic, cathodic reactions. • Materials flow circuit: feed of reactancts to and removal of products from anode/cathode. • Avoid shunt current, leak current in multiple cells • Avoid short circuit of cathode and anode • Avoid breaking electrochemical window of electrolyte June 2002 Electrochemical Cells, K. Y. Chan, HKU 54
 Stationery Power Utilities 10~100 k. W 100~500 k. Whr ONSY (IFC), Fiji SOFC (Westing House, Honey Well) Load Levelling Power Distribution Life
Stationery Power Utilities 10~100 k. W 100~500 k. Whr ONSY (IFC), Fiji SOFC (Westing House, Honey Well) Load Levelling Power Distribution Life
 June 2002 Electrochemical Cells, K. Y. Chan, HKU 56
June 2002 Electrochemical Cells, K. Y. Chan, HKU 56
 Electric Vehicles 10~100 k. W 100~500 k. Whr Battery vs Fuel Cells Hybrid with ICE and capacitor Costs: 7 times normal costs Startup time Direct/Reformer Fueling Station Infrastructure
Electric Vehicles 10~100 k. W 100~500 k. Whr Battery vs Fuel Cells Hybrid with ICE and capacitor Costs: 7 times normal costs Startup time Direct/Reformer Fueling Station Infrastructure
 Transportation Fuel Cell Ballard Power Systems 1 st Generation Fuel Cell Transit Bus 2 nd Generation Fuel Cell Transit Bus Chrysler Fuel Cell Vehicle Model. Coval H 2 Parteners T-1000 Neighborhood Truck. Daimler-Benz The NECAR 3 Three Generations of NECAR Vehicles The NEBUS Daimler. Chrysler Jeep Commander Hybrid Fuel Cell Concept Energy Partners The "Gator" Utility Vehicle The "Genesis" Golf Cart June 2002 The "Green Electrochemical Cells, K. Y. Chan, HKU Car" 58
Transportation Fuel Cell Ballard Power Systems 1 st Generation Fuel Cell Transit Bus 2 nd Generation Fuel Cell Transit Bus Chrysler Fuel Cell Vehicle Model. Coval H 2 Parteners T-1000 Neighborhood Truck. Daimler-Benz The NECAR 3 Three Generations of NECAR Vehicles The NEBUS Daimler. Chrysler Jeep Commander Hybrid Fuel Cell Concept Energy Partners The "Gator" Utility Vehicle The "Genesis" Golf Cart June 2002 The "Green Electrochemical Cells, K. Y. Chan, HKU Car" 58
 Ford Motor Company The P 2000 Prodigy Hydrogen Fuel Cell Vehicle The P 2000 - Platform for a Fuel Cell Vehicle. General Motors Fuel Cell Engine Model H Power Corporation Fuel Cell Bus 50 w PEM Fuel Cell Bicycle Fuel Cell Wheelchair Humboldt State University's Schatz Energy Research Center The Kewet (Danish 2 -Seater) Fuel Cell Golf Carts International Fuel Cells The Georgetown University Fuel Cell Bus June 2002 Electrochemical Cells, K. Y. Chan, HKU 59
Ford Motor Company The P 2000 Prodigy Hydrogen Fuel Cell Vehicle The P 2000 - Platform for a Fuel Cell Vehicle. General Motors Fuel Cell Engine Model H Power Corporation Fuel Cell Bus 50 w PEM Fuel Cell Bicycle Fuel Cell Wheelchair Humboldt State University's Schatz Energy Research Center The Kewet (Danish 2 -Seater) Fuel Cell Golf Carts International Fuel Cells The Georgetown University Fuel Cell Bus June 2002 Electrochemical Cells, K. Y. Chan, HKU 59
 Mazda The Fuel Cell Demio The Demio - On the Road Under the Hood of the Demio Opel The Fuel Cell Sintra The Fuel Cell Zafira Siemens AG PEM Fuel Cell Powered Forklift Toyota The Fuel Cell RAV 4 Volkswagen/Volvo The Fuel Cell Golf (coming soon) Zevco The Fuel Cell Taxi Cab (London) June 2002 Updated January 8, 1999 Electrochemical Cells, K. Y. Chan, HKU 60
Mazda The Fuel Cell Demio The Demio - On the Road Under the Hood of the Demio Opel The Fuel Cell Sintra The Fuel Cell Zafira Siemens AG PEM Fuel Cell Powered Forklift Toyota The Fuel Cell RAV 4 Volkswagen/Volvo The Fuel Cell Golf (coming soon) Zevco The Fuel Cell Taxi Cab (London) June 2002 Updated January 8, 1999 Electrochemical Cells, K. Y. Chan, HKU 60
 June 2002 Electrochemical Cells, K. Y. Chan, HKU 61
June 2002 Electrochemical Cells, K. Y. Chan, HKU 61
 Portable Power Sources 10~100 k. W 100~500 k. Whr Battery vs Fuel Cells Safety (H 2 , Me. OH, caustic electrolyte), Open vs Closed System Volume vs Weight Refueling Vs Recharging
Portable Power Sources 10~100 k. W 100~500 k. Whr Battery vs Fuel Cells Safety (H 2 , Me. OH, caustic electrolyte), Open vs Closed System Volume vs Weight Refueling Vs Recharging
 Special Applications Space/Defence Communication Energy Storage for Solar Energy Vector Biomedical Enery Recovery from Waste Marine and Remote Power Sources
Special Applications Space/Defence Communication Energy Storage for Solar Energy Vector Biomedical Enery Recovery from Waste Marine and Remote Power Sources
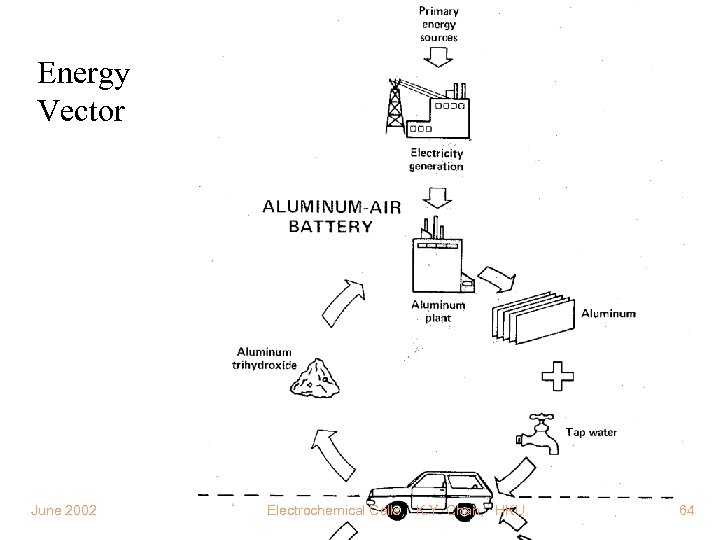 Energy Vector June 2002 Electrochemical Cells, K. Y. Chan, HKU 64
Energy Vector June 2002 Electrochemical Cells, K. Y. Chan, HKU 64
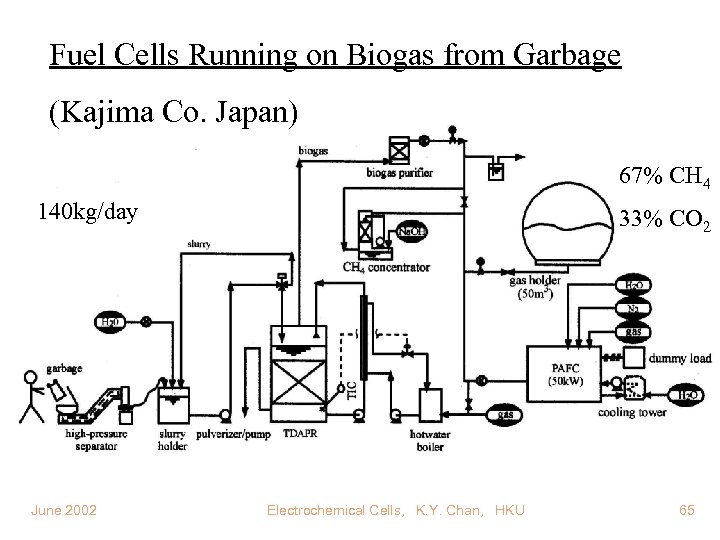 Fuel Cells Running on Biogas from Garbage (Kajima Co. Japan) 67% CH 4 140 kg/day June 2002 33% CO 2 Electrochemical Cells, K. Y. Chan, HKU 65
Fuel Cells Running on Biogas from Garbage (Kajima Co. Japan) 67% CH 4 140 kg/day June 2002 33% CO 2 Electrochemical Cells, K. Y. Chan, HKU 65
 June 2002 Electrochemical Cells, K. Y. Chan, HKU 66
June 2002 Electrochemical Cells, K. Y. Chan, HKU 66
 Demo Fuel Cells 0. 02 ~ 10 W H 2 , Me. OH, Glucose, alcohols, Na. BH 4 PEM, Alkaline
Demo Fuel Cells 0. 02 ~ 10 W H 2 , Me. OH, Glucose, alcohols, Na. BH 4 PEM, Alkaline
 World’s first glucose FC Demonstration Kit (HKU-002, Version 3) June 2002 Electrochemical Cells, K. Y. Chan, HKU 68
World’s first glucose FC Demonstration Kit (HKU-002, Version 3) June 2002 Electrochemical Cells, K. Y. Chan, HKU 68
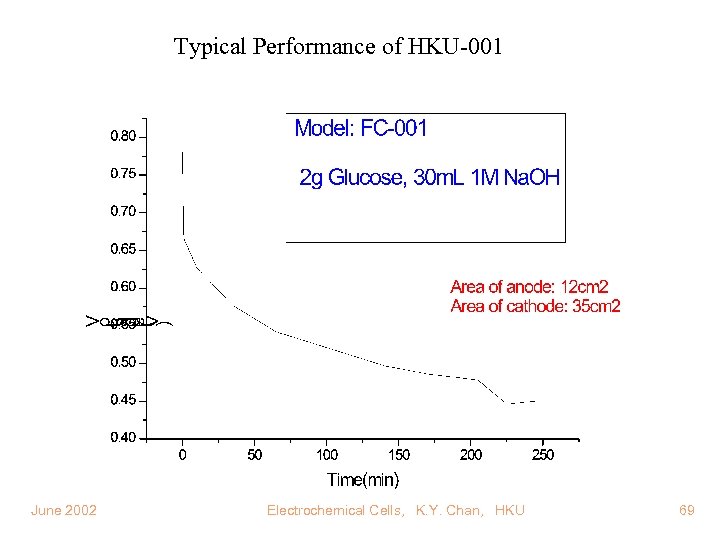 Typical Performance of HKU-001 June 2002 Electrochemical Cells, K. Y. Chan, HKU 69
Typical Performance of HKU-001 June 2002 Electrochemical Cells, K. Y. Chan, HKU 69


