2ac616a0a2b7109a9d789bb5a655157a.ppt
- Количество слайдов: 68
 Sarcomas David Walterhouse, MD
Sarcomas David Walterhouse, MD
 Disclosure Information • No financial interests • Discussion of unlabeled uses of chemotherapeutic agents
Disclosure Information • No financial interests • Discussion of unlabeled uses of chemotherapeutic agents
 Objectives • To review the ABP Content Outline in detail for rhabdomyosarcoma (RMS), osteosarcoma (OS) and Ewing sarcoma (EWS) • To describe the set of tumors referred to as non-rhabdomyosarcoma soft tissue sarcomas (NRSTS) and review general principles specified in the ABP Content Outline, applying to these tumors • To contrast the clinical presentation, therapeutic approach, and response to
Objectives • To review the ABP Content Outline in detail for rhabdomyosarcoma (RMS), osteosarcoma (OS) and Ewing sarcoma (EWS) • To describe the set of tumors referred to as non-rhabdomyosarcoma soft tissue sarcomas (NRSTS) and review general principles specified in the ABP Content Outline, applying to these tumors • To contrast the clinical presentation, therapeutic approach, and response to
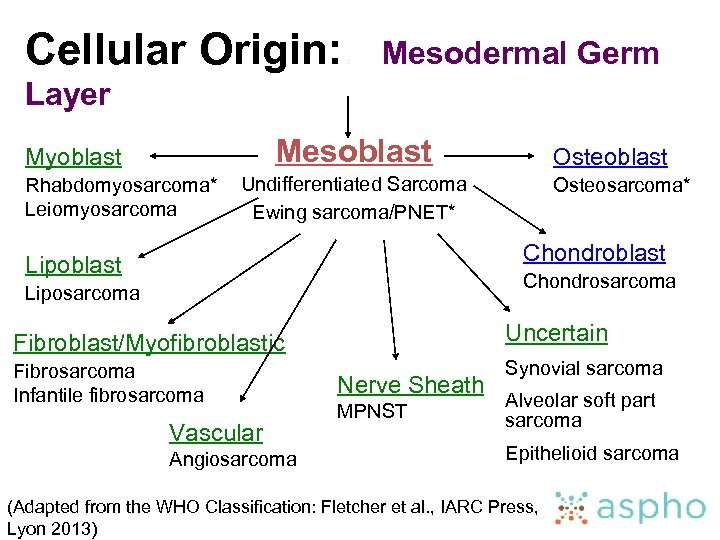 Cellular Origin: : Mesodermal Germ Layer Mesoblast Myoblast Rhabdomyosarcoma* Leiomyosarcoma Osteoblast Undifferentiated Sarcoma Ewing sarcoma/PNET* Osteosarcoma* Chondroblast Lipoblast Chondrosarcoma Liposarcoma Fibroblast/Myofibroblastic Uncertain Fibrosarcoma Infantile fibrosarcoma Synovial sarcoma Vascular Angiosarcoma Nerve Sheath MPNST Alveolar soft part sarcoma Epithelioid sarcoma (Adapted from the WHO Classification: Fletcher et al. , IARC Press, Lyon 2013)
Cellular Origin: : Mesodermal Germ Layer Mesoblast Myoblast Rhabdomyosarcoma* Leiomyosarcoma Osteoblast Undifferentiated Sarcoma Ewing sarcoma/PNET* Osteosarcoma* Chondroblast Lipoblast Chondrosarcoma Liposarcoma Fibroblast/Myofibroblastic Uncertain Fibrosarcoma Infantile fibrosarcoma Synovial sarcoma Vascular Angiosarcoma Nerve Sheath MPNST Alveolar soft part sarcoma Epithelioid sarcoma (Adapted from the WHO Classification: Fletcher et al. , IARC Press, Lyon 2013)
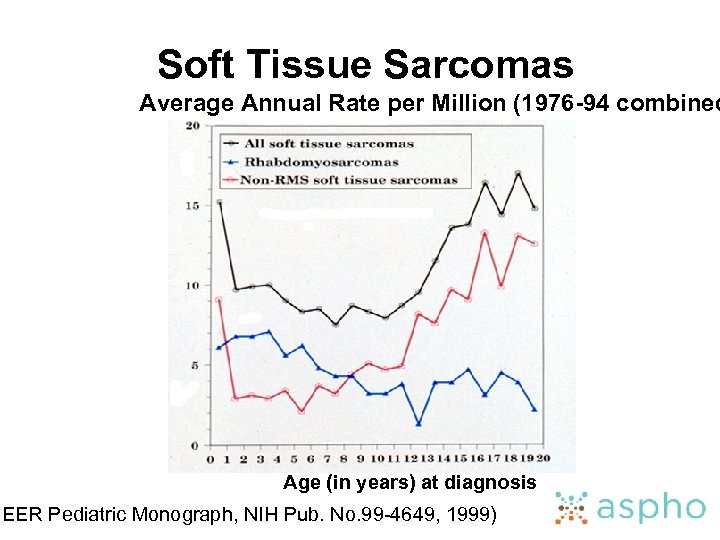 Soft Tissue Sarcomas Average Annual Rate per Million (1976 -94 combined Age (in years) at diagnosis SEER Pediatric Monograph, NIH Pub. No. 99 -4649, 1999)
Soft Tissue Sarcomas Average Annual Rate per Million (1976 -94 combined Age (in years) at diagnosis SEER Pediatric Monograph, NIH Pub. No. 99 -4649, 1999)
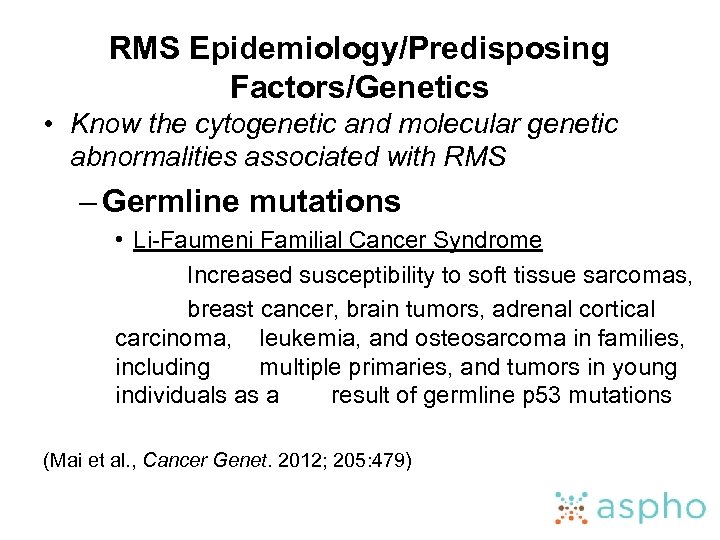 RMS Epidemiology/Predisposing Factors/Genetics • Know the cytogenetic and molecular genetic abnormalities associated with RMS – Germline mutations • Li-Faumeni Familial Cancer Syndrome Increased susceptibility to soft tissue sarcomas, breast cancer, brain tumors, adrenal cortical carcinoma, leukemia, and osteosarcoma in families, including multiple primaries, and tumors in young individuals as a result of germline p 53 mutations (Mai et al. , Cancer Genet. 2012; 205: 479)
RMS Epidemiology/Predisposing Factors/Genetics • Know the cytogenetic and molecular genetic abnormalities associated with RMS – Germline mutations • Li-Faumeni Familial Cancer Syndrome Increased susceptibility to soft tissue sarcomas, breast cancer, brain tumors, adrenal cortical carcinoma, leukemia, and osteosarcoma in families, including multiple primaries, and tumors in young individuals as a result of germline p 53 mutations (Mai et al. , Cancer Genet. 2012; 205: 479)
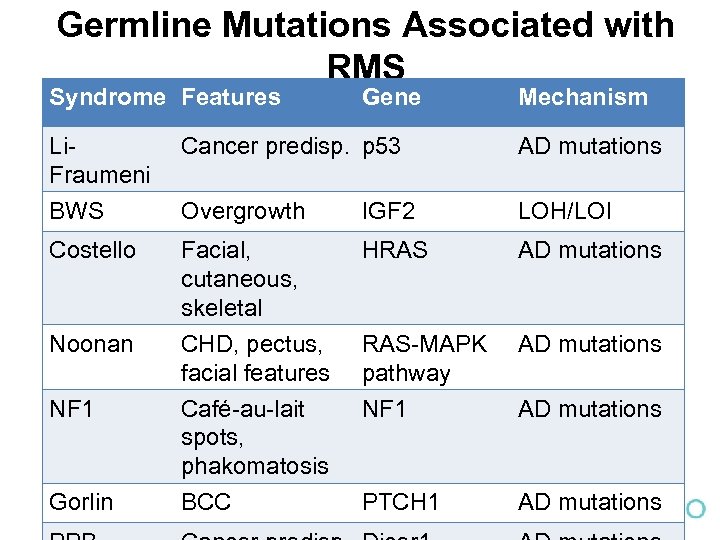 Germline Mutations Associated with RMS Syndrome Features Gene Mechanism Li. Fraumeni Cancer predisp. p 53 AD mutations BWS Overgrowth IGF 2 LOH/LOI Costello Facial, cutaneous, skeletal CHD, pectus, facial features Café-au-lait spots, phakomatosis HRAS AD mutations RAS-MAPK pathway NF 1 AD mutations BCC PTCH 1 AD mutations Noonan NF 1 Gorlin AD mutations
Germline Mutations Associated with RMS Syndrome Features Gene Mechanism Li. Fraumeni Cancer predisp. p 53 AD mutations BWS Overgrowth IGF 2 LOH/LOI Costello Facial, cutaneous, skeletal CHD, pectus, facial features Café-au-lait spots, phakomatosis HRAS AD mutations RAS-MAPK pathway NF 1 AD mutations BCC PTCH 1 AD mutations Noonan NF 1 Gorlin AD mutations
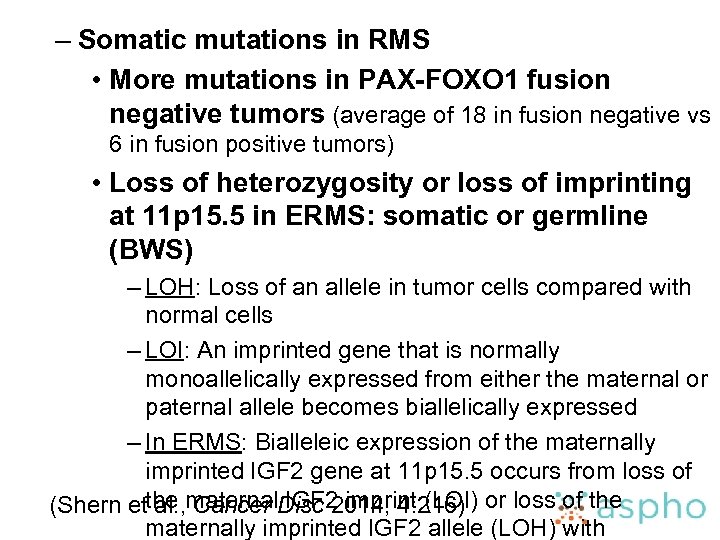 – Somatic mutations in RMS • More mutations in PAX-FOXO 1 fusion negative tumors (average of 18 in fusion negative vs 6 in fusion positive tumors) • Loss of heterozygosity or loss of imprinting at 11 p 15. 5 in ERMS: somatic or germline (BWS) – LOH: Loss of an allele in tumor cells compared with normal cells – LOI: An imprinted gene that is normally monoallelically expressed from either the maternal or paternal allele becomes biallelically expressed – In ERMS: Bialleleic expression of the maternally imprinted IGF 2 gene at 11 p 15. 5 occurs from loss of (Shern etthe maternal. Disc 2014; 4: 216) or loss of the al. , Cancer IGF 2 imprint (LOI) maternally imprinted IGF 2 allele (LOH) with
– Somatic mutations in RMS • More mutations in PAX-FOXO 1 fusion negative tumors (average of 18 in fusion negative vs 6 in fusion positive tumors) • Loss of heterozygosity or loss of imprinting at 11 p 15. 5 in ERMS: somatic or germline (BWS) – LOH: Loss of an allele in tumor cells compared with normal cells – LOI: An imprinted gene that is normally monoallelically expressed from either the maternal or paternal allele becomes biallelically expressed – In ERMS: Bialleleic expression of the maternally imprinted IGF 2 gene at 11 p 15. 5 occurs from loss of (Shern etthe maternal. Disc 2014; 4: 216) or loss of the al. , Cancer IGF 2 imprint (LOI) maternally imprinted IGF 2 allele (LOH) with
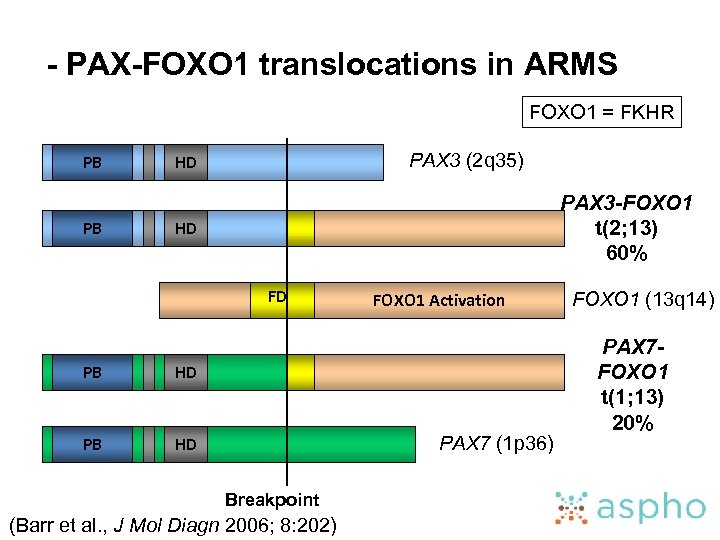 - PAX-FOXO 1 translocations in ARMS FOXO 1 = FKHR PB PB PAX 3 (2 q 35) HD PAX 3 -FOXO 1 t(2; 13) 60% HD FD PB HD FOXO 1 Activation PAX 7 (1 p 36) Breakpoint (Barr et al. , J Mol Diagn 2006; 8: 202) FOXO 1 (13 q 14) PAX 7 FOXO 1 t(1; 13) 20%
- PAX-FOXO 1 translocations in ARMS FOXO 1 = FKHR PB PB PAX 3 (2 q 35) HD PAX 3 -FOXO 1 t(2; 13) 60% HD FD PB HD FOXO 1 Activation PAX 7 (1 p 36) Breakpoint (Barr et al. , J Mol Diagn 2006; 8: 202) FOXO 1 (13 q 14) PAX 7 FOXO 1 t(1; 13) 20%
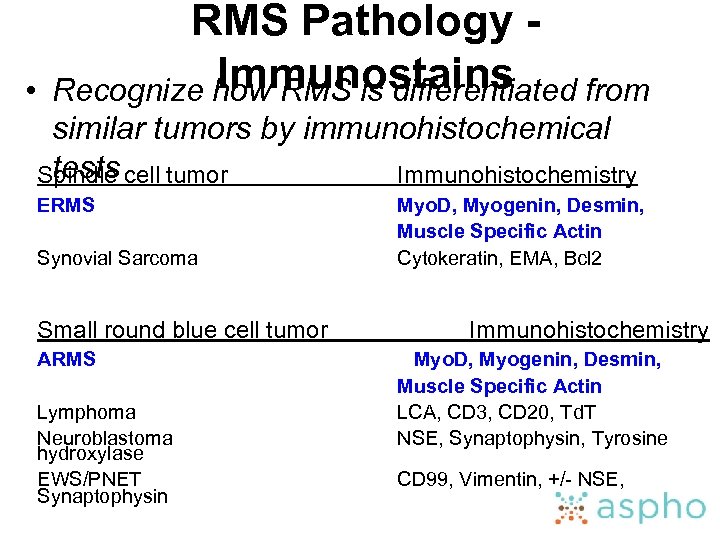 • RMS Pathology Immunostains Recognize how RMS is differentiated from similar tumors by immunohistochemical tests Spindle cell tumor Immunohistochemistry ERMS Synovial Sarcoma Small round blue cell tumor ARMS Lymphoma Neuroblastoma hydroxylase EWS/PNET Synaptophysin Myo. D, Myogenin, Desmin, Muscle Specific Actin Cytokeratin, EMA, Bcl 2 Immunohistochemistry Myo. D, Myogenin, Desmin, Muscle Specific Actin LCA, CD 3, CD 20, Td. T NSE, Synaptophysin, Tyrosine CD 99, Vimentin, +/- NSE,
• RMS Pathology Immunostains Recognize how RMS is differentiated from similar tumors by immunohistochemical tests Spindle cell tumor Immunohistochemistry ERMS Synovial Sarcoma Small round blue cell tumor ARMS Lymphoma Neuroblastoma hydroxylase EWS/PNET Synaptophysin Myo. D, Myogenin, Desmin, Muscle Specific Actin Cytokeratin, EMA, Bcl 2 Immunohistochemistry Myo. D, Myogenin, Desmin, Muscle Specific Actin LCA, CD 3, CD 20, Td. T NSE, Synaptophysin, Tyrosine CD 99, Vimentin, +/- NSE,
 RMS Pathology - Subtypes • Recognize the pathologic subtypes of RMS relative to prognosis and patterns of presentation and spread – ERMS • 60 -70% of cases • Simulates immature skeletal muscle • Associated with younger age, favorable site, localized disease, favorable prognosis • Embryonal Variants: – Embryonal, now includes botryoid; favorable to very favorable (WHO Classification: Fletcher et al. , IARC Press, Lyon 2013)
RMS Pathology - Subtypes • Recognize the pathologic subtypes of RMS relative to prognosis and patterns of presentation and spread – ERMS • 60 -70% of cases • Simulates immature skeletal muscle • Associated with younger age, favorable site, localized disease, favorable prognosis • Embryonal Variants: – Embryonal, now includes botryoid; favorable to very favorable (WHO Classification: Fletcher et al. , IARC Press, Lyon 2013)
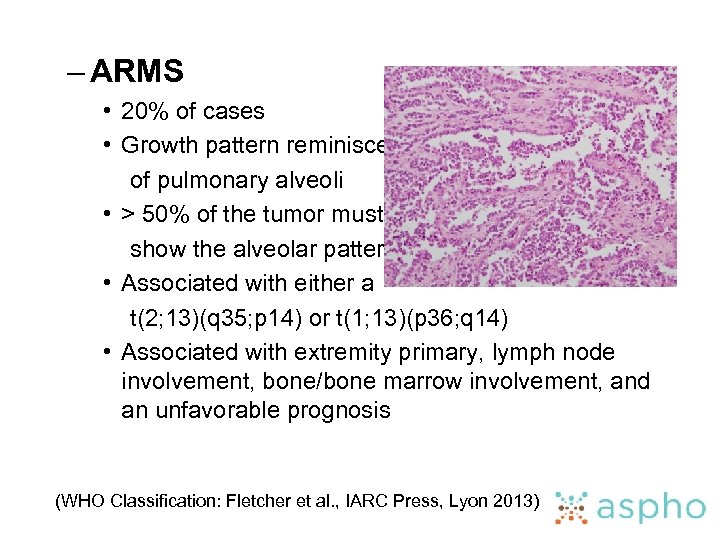 – ARMS • 20% of cases • Growth pattern reminiscent of pulmonary alveoli • > 50% of the tumor must show the alveolar pattern • Associated with either a t(2; 13)(q 35; p 14) or t(1; 13)(p 36; q 14) • Associated with extremity primary, lymph node involvement, bone/bone marrow involvement, and an unfavorable prognosis (WHO Classification: Fletcher et al. , IARC Press, Lyon 2013)
– ARMS • 20% of cases • Growth pattern reminiscent of pulmonary alveoli • > 50% of the tumor must show the alveolar pattern • Associated with either a t(2; 13)(q 35; p 14) or t(1; 13)(p 36; q 14) • Associated with extremity primary, lymph node involvement, bone/bone marrow involvement, and an unfavorable prognosis (WHO Classification: Fletcher et al. , IARC Press, Lyon 2013)
 RMS Clinical Presentation • Recognize the clinical presentation of RMS affecting the head/neck, orbit, nasopharynx, trunk, bladder, prostate, vagina, extremities – Mass, +/- pain, +/- disturbance in function Primary site distribution (IRS-II) Patterns of spread Site Frequency Lymphatic: 40% of paratesticular Head/Neck 34% and 20% of extremity tumors Orbit (8%) Non-Parameningeal (8%) CNS extension: 50% of Parameningeal (18%) parameningeal (cranial nerve GU 23% palsies, erosion of cranial bone, Paratestis/Vag/Uterus (12%) direct intracranial growth) Bladder/Prostate (11%) Hematogenous: 10 -20% at Extremity 17% diagnosis (lung, bone Other 25% marrow, liver) (Maurer et al. , Cancer 1993; 71: 1904)
RMS Clinical Presentation • Recognize the clinical presentation of RMS affecting the head/neck, orbit, nasopharynx, trunk, bladder, prostate, vagina, extremities – Mass, +/- pain, +/- disturbance in function Primary site distribution (IRS-II) Patterns of spread Site Frequency Lymphatic: 40% of paratesticular Head/Neck 34% and 20% of extremity tumors Orbit (8%) Non-Parameningeal (8%) CNS extension: 50% of Parameningeal (18%) parameningeal (cranial nerve GU 23% palsies, erosion of cranial bone, Paratestis/Vag/Uterus (12%) direct intracranial growth) Bladder/Prostate (11%) Hematogenous: 10 -20% at Extremity 17% diagnosis (lung, bone Other 25% marrow, liver) (Maurer et al. , Cancer 1993; 71: 1904)
 RMS Diagnosis and Staging • Be able to appropriately utilize imaging and laboratory modalities to determine the extent and metastatic spread of RMS – Staging workup • • MRI or CT of primary site CT of chest/CXR CT of liver and retroperitoneum Bone scan Bone marrow aspirate/biopsy (bilateral) PET scan (under investigation) Special situations – MRI or CT of head for paramengineal tumors – CSF cytology for parameningeal/paraspinal tumors – Lymph node assessment for paratesticular/extremity (Weiss et al. J Clin Oncol 2013; 31: 3226)
RMS Diagnosis and Staging • Be able to appropriately utilize imaging and laboratory modalities to determine the extent and metastatic spread of RMS – Staging workup • • MRI or CT of primary site CT of chest/CXR CT of liver and retroperitoneum Bone scan Bone marrow aspirate/biopsy (bilateral) PET scan (under investigation) Special situations – MRI or CT of head for paramengineal tumors – CSF cytology for parameningeal/paraspinal tumors – Lymph node assessment for paratesticular/extremity (Weiss et al. J Clin Oncol 2013; 31: 3226)
 – IRS staging system (clinical system) Stg 1 Sites Orbit, non-para. No, N 1, or Nx meningeal head and neck, nonbladder/prostate GU, biliary tract T T 1 or T 2 Size N a or b 2 or Nx Parameningeal, T 1 or T 2 a 3 Parameningeal, bladder/prostate, T 1 or T 2 a N 1 N 0, N 1, or N 0 bladder/prostate, extremity, other Nx b extremity, other 4 or N 1 All with distant T 1 or T 2 a or b metastases Definitions: T=tumor invasiveness; T 1=confined to anatomic site of origin; N 0
– IRS staging system (clinical system) Stg 1 Sites Orbit, non-para. No, N 1, or Nx meningeal head and neck, nonbladder/prostate GU, biliary tract T T 1 or T 2 Size N a or b 2 or Nx Parameningeal, T 1 or T 2 a 3 Parameningeal, bladder/prostate, T 1 or T 2 a N 1 N 0, N 1, or N 0 bladder/prostate, extremity, other Nx b extremity, other 4 or N 1 All with distant T 1 or T 2 a or b metastases Definitions: T=tumor invasiveness; T 1=confined to anatomic site of origin; N 0
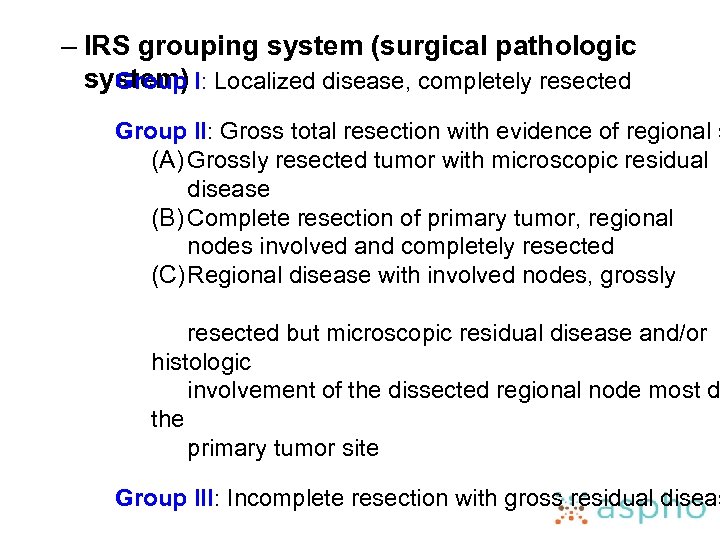 – IRS grouping system (surgical pathologic system) I: Localized disease, completely resected Group II: Gross total resection with evidence of regional s (A) Grossly resected tumor with microscopic residual disease (B) Complete resection of primary tumor, regional nodes involved and completely resected (C) Regional disease with involved nodes, grossly resected but microscopic residual disease and/or histologic involvement of the dissected regional node most d the primary tumor site Group III: Incomplete resection with gross residual diseas
– IRS grouping system (surgical pathologic system) I: Localized disease, completely resected Group II: Gross total resection with evidence of regional s (A) Grossly resected tumor with microscopic residual disease (B) Complete resection of primary tumor, regional nodes involved and completely resected (C) Regional disease with involved nodes, grossly resected but microscopic residual disease and/or histologic involvement of the dissected regional node most d the primary tumor site Group III: Incomplete resection with gross residual diseas
 RMS Treatment • Know the principles of management of RMS – Treatment philosophy • Optimal treatment requires a coordinated multidisciplinary management plan: SURGERY, RADIOTHERAPY, CHEMOTHERAPY – Treatment assigned based on risk-
RMS Treatment • Know the principles of management of RMS – Treatment philosophy • Optimal treatment requires a coordinated multidisciplinary management plan: SURGERY, RADIOTHERAPY, CHEMOTHERAPY – Treatment assigned based on risk-
 RMS Treatment - Surgery • Know the role of surgery in the treatment of RMS – Form of local therapy – Excision of the primary tumor upfront whenever possible without causing major functional or cosmetic deficits – Primary re-excision considered for residual tumor – Sites requiring surgical assessment of lymph nodes • Paratesticular (ISRLND if ≥ 10 years)
RMS Treatment - Surgery • Know the role of surgery in the treatment of RMS – Form of local therapy – Excision of the primary tumor upfront whenever possible without causing major functional or cosmetic deficits – Primary re-excision considered for residual tumor – Sites requiring surgical assessment of lymph nodes • Paratesticular (ISRLND if ≥ 10 years)
 RMS Treatment – Radiation Therapy • Know the role of irradiation in the treatment of RMS – Form of local therapy • Local/regional relapse rates (IRS-IV): local (51%), regional (17%), and distant (32%) (Crist et al. , J Clin Oncol 2001; 12: 3091) – Patients with group I ERMS do not receive RT – RT usually begins during weeks 3 -15 of therapy • Exception: Early RT for paramengineal with ICE – Treatment volume determined by pretreatment (presurgical) tumor size – Doses of 3600 -5040 c. Gy used; dose depends on group,
RMS Treatment – Radiation Therapy • Know the role of irradiation in the treatment of RMS – Form of local therapy • Local/regional relapse rates (IRS-IV): local (51%), regional (17%), and distant (32%) (Crist et al. , J Clin Oncol 2001; 12: 3091) – Patients with group I ERMS do not receive RT – RT usually begins during weeks 3 -15 of therapy • Exception: Early RT for paramengineal with ICE – Treatment volume determined by pretreatment (presurgical) tumor size – Doses of 3600 -5040 c. Gy used; dose depends on group,
 • RMS Treatment Chemotherapy Know the role of chemo in the treatment of RMS – Local and systemic tumor control – Standard (intermediate-risk) • Vincristine, Dactinomycin, and Cyclophosphamide (VAC) x approximately 45 weeks • Alternatively, VAC alternating with Vincristine/Irinotecan – Low-risk (low stage, low group, ERMS) • VA, VA + lower CPM dose, or shorter duration therapy – High-risk (metastatic) • Additional active agents and interval compression
• RMS Treatment Chemotherapy Know the role of chemo in the treatment of RMS – Local and systemic tumor control – Standard (intermediate-risk) • Vincristine, Dactinomycin, and Cyclophosphamide (VAC) x approximately 45 weeks • Alternatively, VAC alternating with Vincristine/Irinotecan – Low-risk (low stage, low group, ERMS) • VA, VA + lower CPM dose, or shorter duration therapy – High-risk (metastatic) • Additional active agents and interval compression
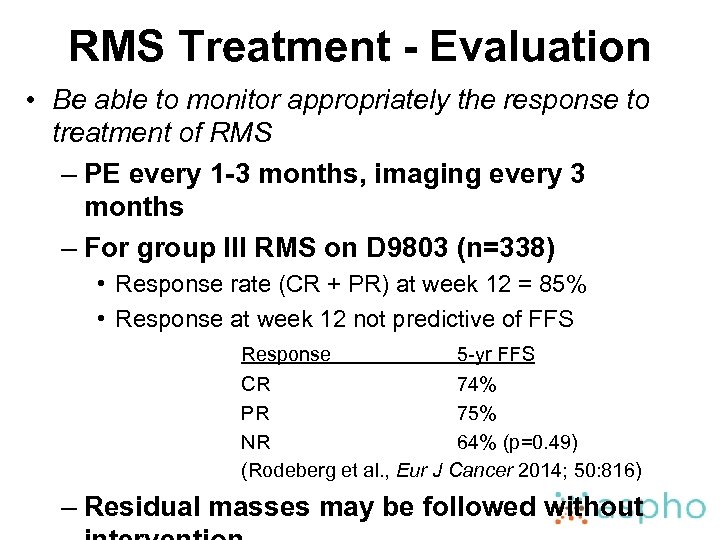 RMS Treatment - Evaluation • Be able to monitor appropriately the response to treatment of RMS – PE every 1 -3 months, imaging every 3 months – For group III RMS on D 9803 (n=338) • Response rate (CR + PR) at week 12 = 85% • Response at week 12 not predictive of FFS Response 5 -yr FFS CR 74% PR 75% NR 64% (p=0. 49) (Rodeberg et al. , Eur J Cancer 2014; 50: 816) – Residual masses may be followed without
RMS Treatment - Evaluation • Be able to monitor appropriately the response to treatment of RMS – PE every 1 -3 months, imaging every 3 months – For group III RMS on D 9803 (n=338) • Response rate (CR + PR) at week 12 = 85% • Response at week 12 not predictive of FFS Response 5 -yr FFS CR 74% PR 75% NR 64% (p=0. 49) (Rodeberg et al. , Eur J Cancer 2014; 50: 816) – Residual masses may be followed without
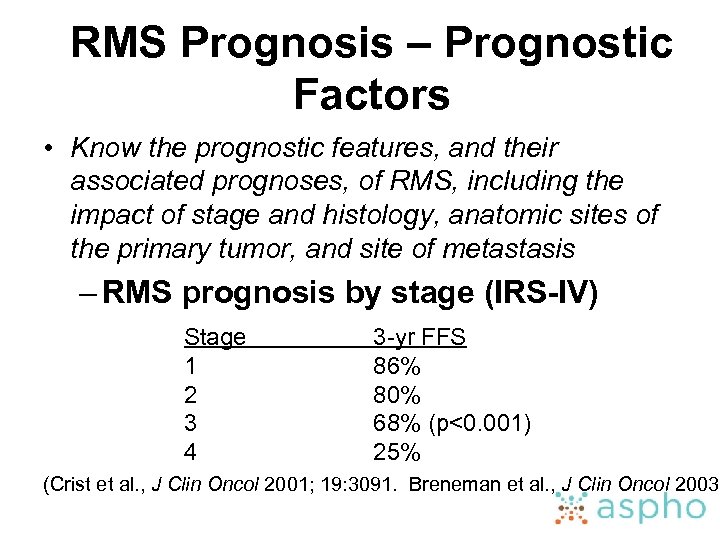 RMS Prognosis – Prognostic Factors • Know the prognostic features, and their associated prognoses, of RMS, including the impact of stage and histology, anatomic sites of the primary tumor, and site of metastasis – RMS prognosis by stage (IRS-IV) Stage 1 2 3 4 3 -yr FFS 86% 80% 68% (p<0. 001) 25% (Crist et al. , J Clin Oncol 2001; 19: 3091. Breneman et al. , J Clin Oncol 2003;
RMS Prognosis – Prognostic Factors • Know the prognostic features, and their associated prognoses, of RMS, including the impact of stage and histology, anatomic sites of the primary tumor, and site of metastasis – RMS prognosis by stage (IRS-IV) Stage 1 2 3 4 3 -yr FFS 86% 80% 68% (p<0. 001) 25% (Crist et al. , J Clin Oncol 2001; 19: 3091. Breneman et al. , J Clin Oncol 2003;
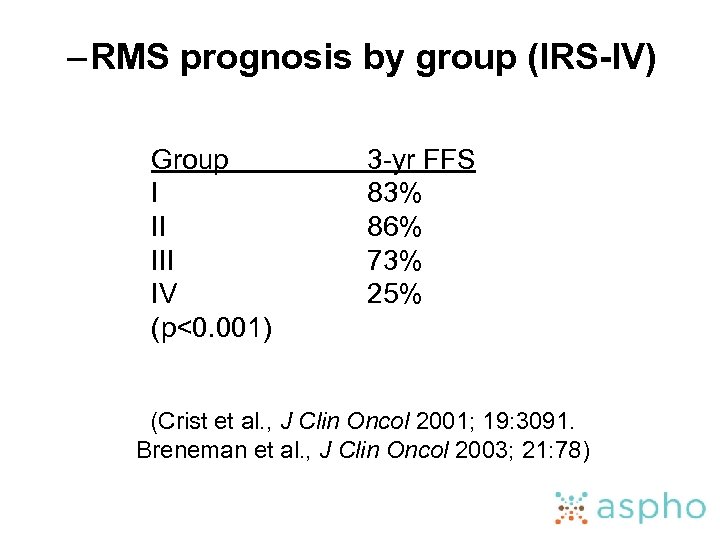 – RMS prognosis by group (IRS-IV) Group I II IV (p<0. 001) 3 -yr FFS 83% 86% 73% 25% (Crist et al. , J Clin Oncol 2001; 19: 3091. Breneman et al. , J Clin Oncol 2003; 21: 78)
– RMS prognosis by group (IRS-IV) Group I II IV (p<0. 001) 3 -yr FFS 83% 86% 73% 25% (Crist et al. , J Clin Oncol 2001; 19: 3091. Breneman et al. , J Clin Oncol 2003; 21: 78)
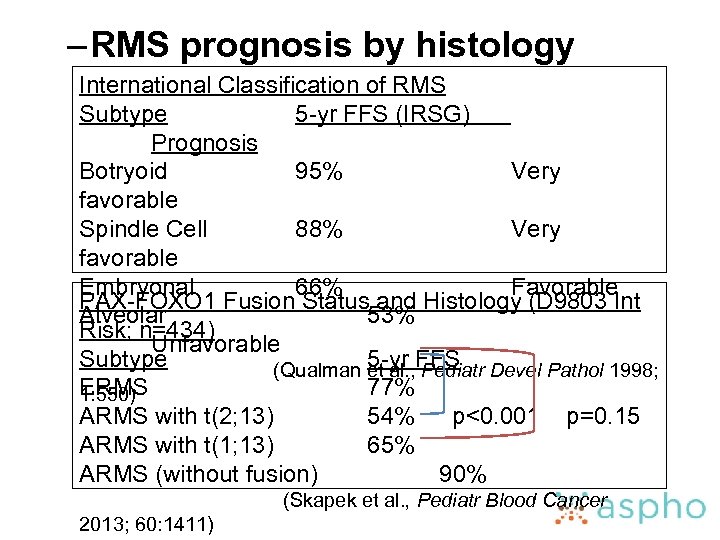 – RMS prognosis by histology International Classification of RMS Subtype 5 -yr FFS (IRSG) Prognosis Botryoid 95% Very favorable Spindle Cell 88% Very favorable Embryonal 66% Favorable PAX-FOXO 1 Fusion Status and Histology (D 9803 Int Alveolar 53% Risk; n=434) Unfavorable Subtype 5 -yr FFS (Qualman et al. , Pediatr Devel Pathol 1998; ERMS 77% 1: 550) ARMS with t(2; 13) 54% p<0. 001 p=0. 15 ARMS with t(1; 13) 65% ARMS (without fusion) 90% (Skapek et al. , Pediatr Blood Cancer 2013; 60: 1411)
– RMS prognosis by histology International Classification of RMS Subtype 5 -yr FFS (IRSG) Prognosis Botryoid 95% Very favorable Spindle Cell 88% Very favorable Embryonal 66% Favorable PAX-FOXO 1 Fusion Status and Histology (D 9803 Int Alveolar 53% Risk; n=434) Unfavorable Subtype 5 -yr FFS (Qualman et al. , Pediatr Devel Pathol 1998; ERMS 77% 1: 550) ARMS with t(2; 13) 54% p<0. 001 p=0. 15 ARMS with t(1; 13) 65% ARMS (without fusion) 90% (Skapek et al. , Pediatr Blood Cancer 2013; 60: 1411)
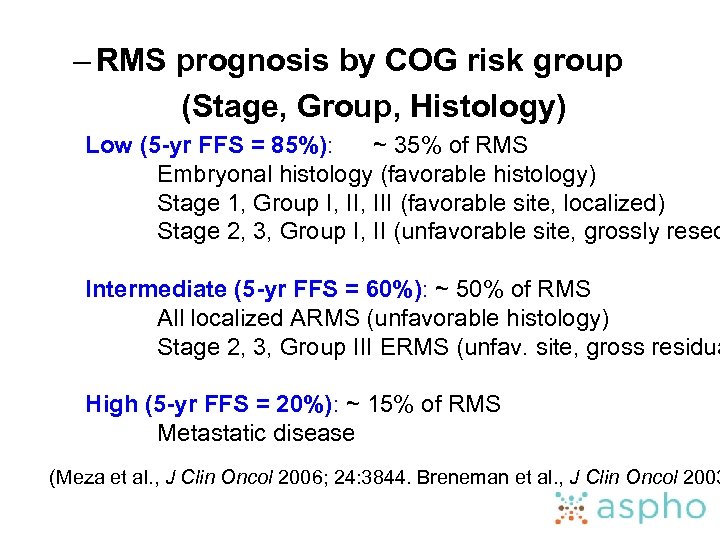 – RMS prognosis by COG risk group (Stage, Group, Histology) Low (5 -yr FFS = 85%): ~ 35% of RMS Embryonal histology (favorable histology) Stage 1, Group I, III (favorable site, localized) Stage 2, 3, Group I, II (unfavorable site, grossly resec Intermediate (5 -yr FFS = 60%): ~ 50% of RMS All localized ARMS (unfavorable histology) Stage 2, 3, Group III ERMS (unfav. site, gross residua High (5 -yr FFS = 20%): ~ 15% of RMS Metastatic disease (Meza et al. , J Clin Oncol 2006; 24: 3844. Breneman et al. , J Clin Oncol 2003
– RMS prognosis by COG risk group (Stage, Group, Histology) Low (5 -yr FFS = 85%): ~ 35% of RMS Embryonal histology (favorable histology) Stage 1, Group I, III (favorable site, localized) Stage 2, 3, Group I, II (unfavorable site, grossly resec Intermediate (5 -yr FFS = 60%): ~ 50% of RMS All localized ARMS (unfavorable histology) Stage 2, 3, Group III ERMS (unfav. site, gross residua High (5 -yr FFS = 20%): ~ 15% of RMS Metastatic disease (Meza et al. , J Clin Oncol 2006; 24: 3844. Breneman et al. , J Clin Oncol 2003
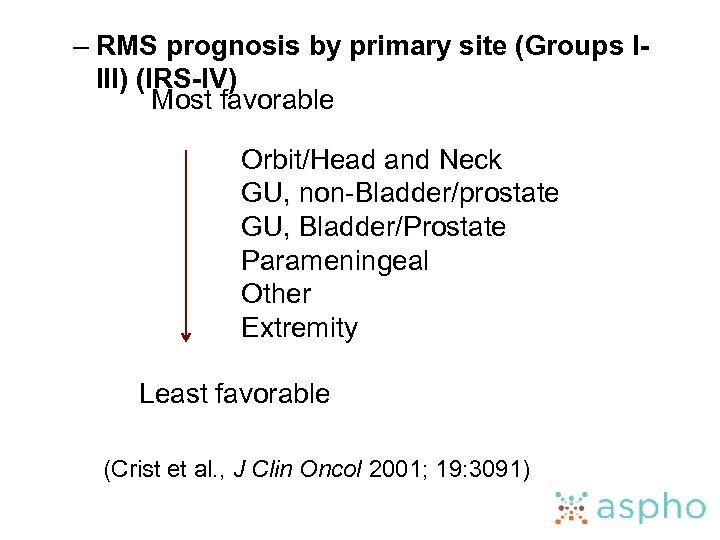 – RMS prognosis by primary site (Groups IIII) (IRS-IV) Most favorable Orbit/Head and Neck GU, non-Bladder/prostate GU, Bladder/Prostate Parameningeal Other Extremity Least favorable (Crist et al. , J Clin Oncol 2001; 19: 3091)
– RMS prognosis by primary site (Groups IIII) (IRS-IV) Most favorable Orbit/Head and Neck GU, non-Bladder/prostate GU, Bladder/Prostate Parameningeal Other Extremity Least favorable (Crist et al. , J Clin Oncol 2001; 19: 3091)
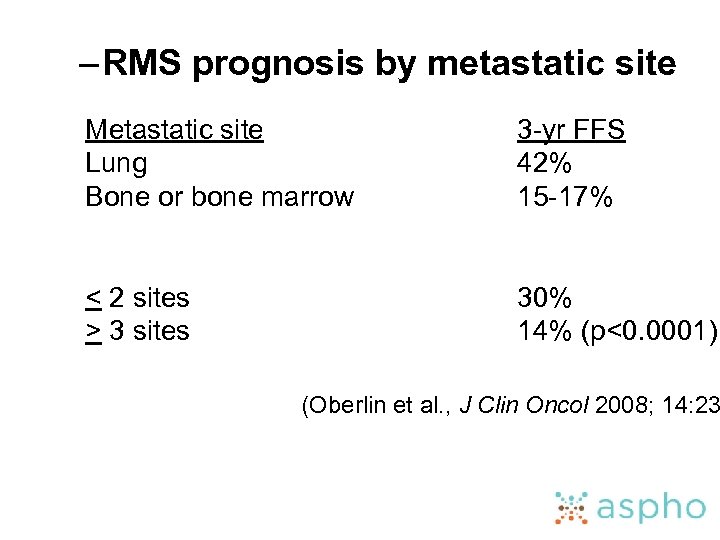 – RMS prognosis by metastatic site Metastatic site Lung Bone or bone marrow 3 -yr FFS 42% 15 -17% < 2 sites > 3 sites 30% 14% (p<0. 0001) (Oberlin et al. , J Clin Oncol 2008; 14: 23
– RMS prognosis by metastatic site Metastatic site Lung Bone or bone marrow 3 -yr FFS 42% 15 -17% < 2 sites > 3 sites 30% 14% (p<0. 0001) (Oberlin et al. , J Clin Oncol 2008; 14: 23
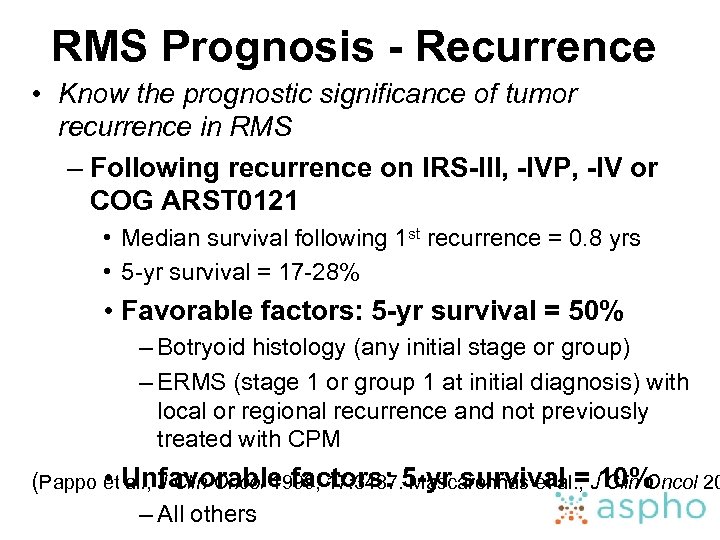 RMS Prognosis - Recurrence • Know the prognostic significance of tumor recurrence in RMS – Following recurrence on IRS-III, -IVP, -IV or COG ARST 0121 • Median survival following 1 st recurrence = 0. 8 yrs • 5 -yr survival = 17 -28% • Favorable factors: 5 -yr survival = 50% – Botryoid histology (any initial stage or group) – ERMS (stage 1 or group 1 at initial diagnosis) with local or regional recurrence and not previously treated with CPM • al. , J Clin Oncol 1999; 17: 3487. Mascarenhas et al. , Clin Oncol 20 (Pappo et Unfavorable factors: 5 -yr survival =J 10% – All others
RMS Prognosis - Recurrence • Know the prognostic significance of tumor recurrence in RMS – Following recurrence on IRS-III, -IVP, -IV or COG ARST 0121 • Median survival following 1 st recurrence = 0. 8 yrs • 5 -yr survival = 17 -28% • Favorable factors: 5 -yr survival = 50% – Botryoid histology (any initial stage or group) – ERMS (stage 1 or group 1 at initial diagnosis) with local or regional recurrence and not previously treated with CPM • al. , J Clin Oncol 1999; 17: 3487. Mascarenhas et al. , Clin Oncol 20 (Pappo et Unfavorable factors: 5 -yr survival =J 10% – All others
 RMS Complications/Late Effects • Know the complications and late effects of RMS and its therapy – Acute toxicity • Hematologic toxicity: > 85% of patients per VAC course • Infectious complications: 20% of patients per VAC course • Sinusoidal obstruction syndrome (VOD): associated with dactinomycin and cyclophosphamide; rate 4 -5% on VA or VAC (Arndt et al. , J Clin Oncol 2004; 22: 1894) – Long term effects • Infertility: associated with higher cumulative doses of alkylating agents (Kenney et al. , Cancer 2001; 91: 613, Green et al. , Lancet Oncol 2014; 15: 1215)
RMS Complications/Late Effects • Know the complications and late effects of RMS and its therapy – Acute toxicity • Hematologic toxicity: > 85% of patients per VAC course • Infectious complications: 20% of patients per VAC course • Sinusoidal obstruction syndrome (VOD): associated with dactinomycin and cyclophosphamide; rate 4 -5% on VA or VAC (Arndt et al. , J Clin Oncol 2004; 22: 1894) – Long term effects • Infertility: associated with higher cumulative doses of alkylating agents (Kenney et al. , Cancer 2001; 91: 613, Green et al. , Lancet Oncol 2014; 15: 1215)
 NRSTS – Epidemiology: 40% of soft tissue sarcomas < 5 yrs of age, but 77% of soft tissue sarcomas among 15 – 19 yr olds – Clinical presentation/Staging: may arise in any part of the body, most commonly the extremities and trunk; metastatic spread to lungs, bone, lymph nodes – Prognosis: depends on tumor type, stage (localized vs metastatic), age, group (extent of resection), tumor size (≤ 5 cm vs > 5 cm), and grade (mitotic rate, necrosis, nuclear atypia) – Treatment: Surgery, RT for residual, +/- Chemotherapy • Group I; observe unless grade 3 and > 5 cm, then consider RT and/or chemotherapy (Dox + Ifos) • Group II; RT alone unless grade 3 and/or > 5 cm, then consider chemotherapy (Dox + Ifos) • Group III-IV or grade 3 and > 5 cm any group; chemotherapy (Dox + Ifos), RT, delayed surgery • Role of tyrosine kinase inhibitors (pazopanib) under investigation (Spunt et al. , J Clin Oncol 2002; 20: 3225. Spunt et al. Proc Annu Meet Am So Oncol 2014; 32: 10008)
NRSTS – Epidemiology: 40% of soft tissue sarcomas < 5 yrs of age, but 77% of soft tissue sarcomas among 15 – 19 yr olds – Clinical presentation/Staging: may arise in any part of the body, most commonly the extremities and trunk; metastatic spread to lungs, bone, lymph nodes – Prognosis: depends on tumor type, stage (localized vs metastatic), age, group (extent of resection), tumor size (≤ 5 cm vs > 5 cm), and grade (mitotic rate, necrosis, nuclear atypia) – Treatment: Surgery, RT for residual, +/- Chemotherapy • Group I; observe unless grade 3 and > 5 cm, then consider RT and/or chemotherapy (Dox + Ifos) • Group II; RT alone unless grade 3 and/or > 5 cm, then consider chemotherapy (Dox + Ifos) • Group III-IV or grade 3 and > 5 cm any group; chemotherapy (Dox + Ifos), RT, delayed surgery • Role of tyrosine kinase inhibitors (pazopanib) under investigation (Spunt et al. , J Clin Oncol 2002; 20: 3225. Spunt et al. Proc Annu Meet Am So Oncol 2014; 32: 10008)
 NRSTS Select Types Chemotherapy Responsive Synovial sarcoma Most common type; associated with t(X; 18)(p 11; q 11) = SYTSSX fusion; 56% response rate to chemo (Pappo et al. , J Clin Oncol 2005; 23: 4031) Undifferentiated sarcoma One of the more common NRSTS; No identifiable line of differentiation when analyzed by available technology Infantile fibrosarcoma Infantile form (< 2 yrs) associated with t(12; 15)(p 13; q 25) = ETV 6 -NTRK 3 fusion; most common STS in children < 1 yr; VA or VAC if not resected Poorly Responsive to Chemotherapy MPNST associated with Leiomyosarcoma HIV One of the more common NRSTS; 20 -50% NF 1; 5 -16% of NF 1 patients develop Associated with immunosuppression and
NRSTS Select Types Chemotherapy Responsive Synovial sarcoma Most common type; associated with t(X; 18)(p 11; q 11) = SYTSSX fusion; 56% response rate to chemo (Pappo et al. , J Clin Oncol 2005; 23: 4031) Undifferentiated sarcoma One of the more common NRSTS; No identifiable line of differentiation when analyzed by available technology Infantile fibrosarcoma Infantile form (< 2 yrs) associated with t(12; 15)(p 13; q 25) = ETV 6 -NTRK 3 fusion; most common STS in children < 1 yr; VA or VAC if not resected Poorly Responsive to Chemotherapy MPNST associated with Leiomyosarcoma HIV One of the more common NRSTS; 20 -50% NF 1; 5 -16% of NF 1 patients develop Associated with immunosuppression and
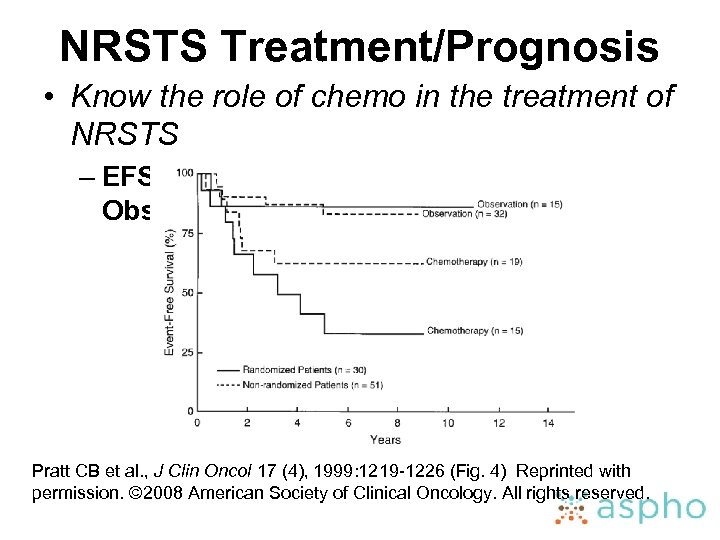 NRSTS Treatment/Prognosis • Know the role of chemo in the treatment of NRSTS – EFS for group I/II NRSTS: Chemo vs Observation Pratt CB et al. , J Clin Oncol 17 (4), 1999: 1219 -1226 (Fig. 4) Reprinted with permission. © 2008 American Society of Clinical Oncology. All rights reserved.
NRSTS Treatment/Prognosis • Know the role of chemo in the treatment of NRSTS – EFS for group I/II NRSTS: Chemo vs Observation Pratt CB et al. , J Clin Oncol 17 (4), 1999: 1219 -1226 (Fig. 4) Reprinted with permission. © 2008 American Society of Clinical Oncology. All rights reserved.
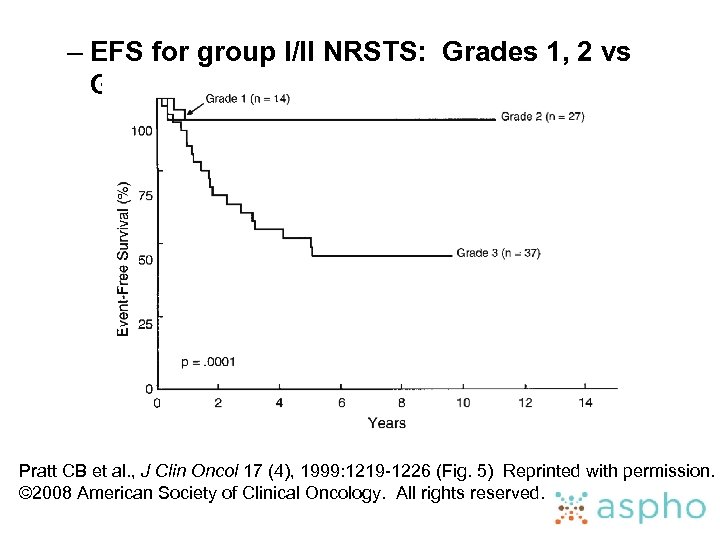 – EFS for group I/II NRSTS: Grades 1, 2 vs Grade 3 Pratt CB et al. , J Clin Oncol 17 (4), 1999: 1219 -1226 (Fig. 5) Reprinted with permission. © 2008 American Society of Clinical Oncology. All rights reserved.
– EFS for group I/II NRSTS: Grades 1, 2 vs Grade 3 Pratt CB et al. , J Clin Oncol 17 (4), 1999: 1219 -1226 (Fig. 5) Reprinted with permission. © 2008 American Society of Clinical Oncology. All rights reserved.
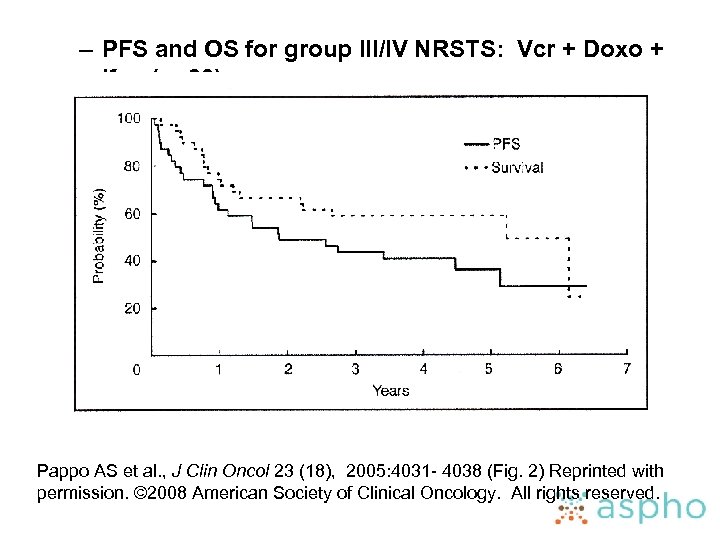 – PFS and OS for group III/IV NRSTS: Vcr + Doxo + Ifos (n=39) Pappo AS et al. , J Clin Oncol 23 (18), 2005: 4031 - 4038 (Fig. 2) Reprinted with permission. © 2008 American Society of Clinical Oncology. All rights reserved.
– PFS and OS for group III/IV NRSTS: Vcr + Doxo + Ifos (n=39) Pappo AS et al. , J Clin Oncol 23 (18), 2005: 4031 - 4038 (Fig. 2) Reprinted with permission. © 2008 American Society of Clinical Oncology. All rights reserved.
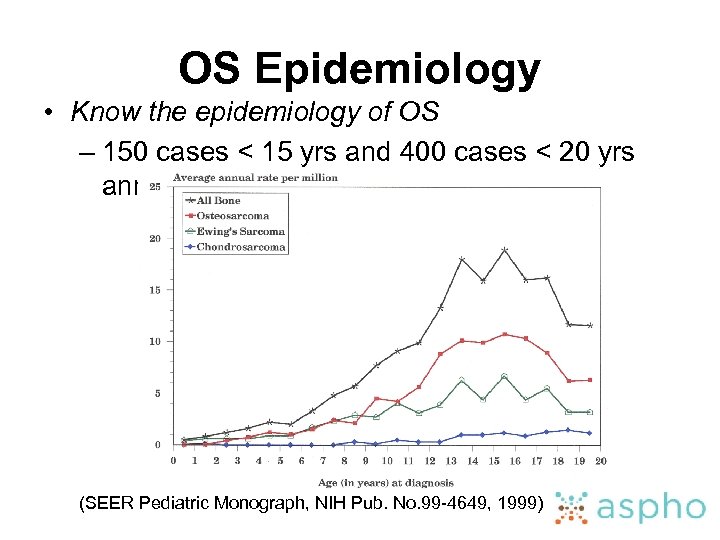 OS Epidemiology • Know the epidemiology of OS – 150 cases < 15 yrs and 400 cases < 20 yrs annually (SEER Pediatric Monograph, NIH Pub. No. 99 -4649, 1999)
OS Epidemiology • Know the epidemiology of OS – 150 cases < 15 yrs and 400 cases < 20 yrs annually (SEER Pediatric Monograph, NIH Pub. No. 99 -4649, 1999)
 OS Predisposing Factors • Know that OS can occur as a late effect of RT and that patients with hereditary retinoblastoma are at increased risk of OS – Ionizing radiation • Cause of 3% of OS • Median time of 9 – 11 yrs following exposure • Dose response (RT dose usually 45 -60 Gy) (Newton et al. , Cancer 2001; 67: 193) – Hereditary Rb (germline Rb mutations) • Cumulative incidence of bone sarcoma 7 -8% at 20 yrs and 9 -10% at 30 yrs • OS develops within and outside of radiation fields (Kleinerman et al. , J Clin Oncol 2012;
OS Predisposing Factors • Know that OS can occur as a late effect of RT and that patients with hereditary retinoblastoma are at increased risk of OS – Ionizing radiation • Cause of 3% of OS • Median time of 9 – 11 yrs following exposure • Dose response (RT dose usually 45 -60 Gy) (Newton et al. , Cancer 2001; 67: 193) – Hereditary Rb (germline Rb mutations) • Cumulative incidence of bone sarcoma 7 -8% at 20 yrs and 9 -10% at 30 yrs • OS develops within and outside of radiation fields (Kleinerman et al. , J Clin Oncol 2012;
 OS Genetics • Know the cytogenetic and molecular genetic abnormalities associated with OS – Cytogenetic abnormalities • Majority have karyotypic abnormalities • Genetic heterogeneity/chromosomal instability, including chromothripsis (shattering of small groups of chromosomes and random re-assembly) • Supernumerary ring chromosome (12 q 13 -15) characteristic of parosteal OS – Molecular genetic abnormalities • Tumor suppressor gene mutations – Rb: germline or somatic (35% of OS) – p 53: germline (Li-Fraumeni Syndrome) or somatic • RECQL 4 helicase mutations (WHO Classification of Tumors of Soft Tissues and Bone: developet al. , IARC Press, L – Rothmund-Thomson Syndrome; 32% Fletcher OS
OS Genetics • Know the cytogenetic and molecular genetic abnormalities associated with OS – Cytogenetic abnormalities • Majority have karyotypic abnormalities • Genetic heterogeneity/chromosomal instability, including chromothripsis (shattering of small groups of chromosomes and random re-assembly) • Supernumerary ring chromosome (12 q 13 -15) characteristic of parosteal OS – Molecular genetic abnormalities • Tumor suppressor gene mutations – Rb: germline or somatic (35% of OS) – p 53: germline (Li-Fraumeni Syndrome) or somatic • RECQL 4 helicase mutations (WHO Classification of Tumors of Soft Tissues and Bone: developet al. , IARC Press, L – Rothmund-Thomson Syndrome; 32% Fletcher OS
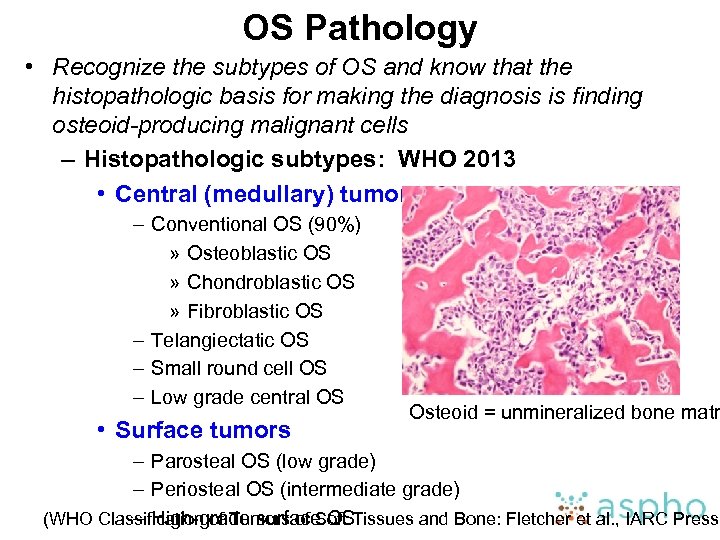 OS Pathology • Recognize the subtypes of OS and know that the histopathologic basis for making the diagnosis is finding osteoid-producing malignant cells – Histopathologic subtypes: WHO 2013 • Central (medullary) tumors – Conventional OS (90%) » Osteoblastic OS » Chondroblastic OS » Fibroblastic OS – Telangiectatic OS – Small round cell OS – Low grade central OS • Surface tumors Osteoid = unmineralized bone matr – Parosteal OS (low grade) – Periosteal OS (intermediate grade) (WHO Classification of Tumors of Soft Tissues and Bone: Fletcher et al. , IARC Press, – High-grade surface OS
OS Pathology • Recognize the subtypes of OS and know that the histopathologic basis for making the diagnosis is finding osteoid-producing malignant cells – Histopathologic subtypes: WHO 2013 • Central (medullary) tumors – Conventional OS (90%) » Osteoblastic OS » Chondroblastic OS » Fibroblastic OS – Telangiectatic OS – Small round cell OS – Low grade central OS • Surface tumors Osteoid = unmineralized bone matr – Parosteal OS (low grade) – Periosteal OS (intermediate grade) (WHO Classification of Tumors of Soft Tissues and Bone: Fletcher et al. , IARC Press, – High-grade surface OS
 OS Clinical Presentation • Recognize the most common skeletal locations for OS and know the common metastatic sites Patterns of spread Clinical presentation Pain, +/- mass at the primary site, typically involves metaphyses of long bones Most common primary sites: distal femur (55%) > proximal tibia (27%) > proximal humerus (11%) > other sites (Meyers et al. , J Clin Oncol 2005; 23: 2004) Skip lesions: Occur in up to 20% of cases several cm from primary Hematogenous: 1520% at diagnosis (lung [68 -93%] > bone [15 -29%]) (Goorin et al. , J Clin Oncol 2002; 20: 426. Meyers et al. , J Clin Oncol 1993; 11: 449)
OS Clinical Presentation • Recognize the most common skeletal locations for OS and know the common metastatic sites Patterns of spread Clinical presentation Pain, +/- mass at the primary site, typically involves metaphyses of long bones Most common primary sites: distal femur (55%) > proximal tibia (27%) > proximal humerus (11%) > other sites (Meyers et al. , J Clin Oncol 2005; 23: 2004) Skip lesions: Occur in up to 20% of cases several cm from primary Hematogenous: 1520% at diagnosis (lung [68 -93%] > bone [15 -29%]) (Goorin et al. , J Clin Oncol 2002; 20: 426. Meyers et al. , J Clin Oncol 1993; 11: 449)
 OS Diagnosis and Staging • Utilize appropriate imaging modalities to determine the extent and metastatic spread of OS – Staging workup • • Plain films of involved bone MRI of involved bone CT of chest/CXR Bone scan/PET scan (under investigation) – Stage assignment • Localized vs Metastatic • Resectable vs Unresectable
OS Diagnosis and Staging • Utilize appropriate imaging modalities to determine the extent and metastatic spread of OS – Staging workup • • Plain films of involved bone MRI of involved bone CT of chest/CXR Bone scan/PET scan (under investigation) – Stage assignment • Localized vs Metastatic • Resectable vs Unresectable
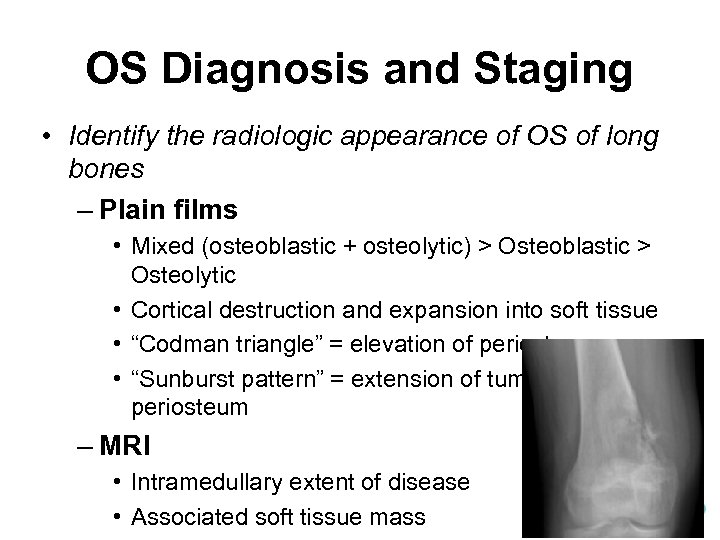 OS Diagnosis and Staging • Identify the radiologic appearance of OS of long bones – Plain films • Mixed (osteoblastic + osteolytic) > Osteoblastic > Osteolytic • Cortical destruction and expansion into soft tissue • “Codman triangle” = elevation of periosteum • “Sunburst pattern” = extension of tumor through the periosteum – MRI • Intramedullary extent of disease • Associated soft tissue mass
OS Diagnosis and Staging • Identify the radiologic appearance of OS of long bones – Plain films • Mixed (osteoblastic + osteolytic) > Osteoblastic > Osteolytic • Cortical destruction and expansion into soft tissue • “Codman triangle” = elevation of periosteum • “Sunburst pattern” = extension of tumor through the periosteum – MRI • Intramedullary extent of disease • Associated soft tissue mass
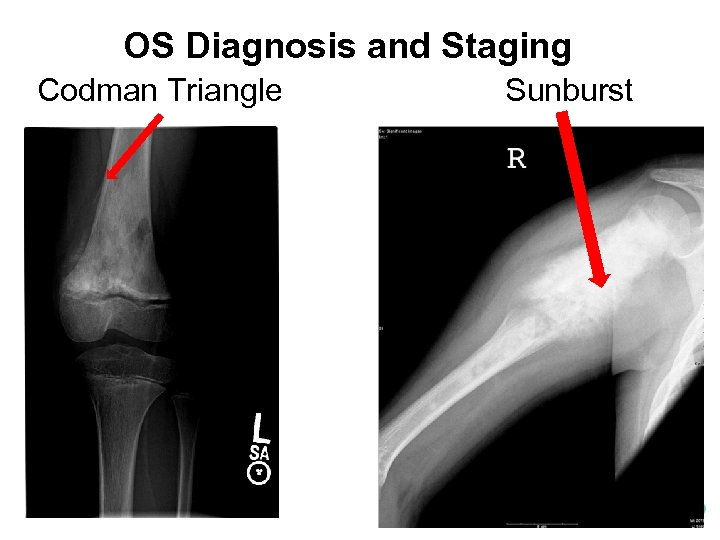 OS Diagnosis and Staging Codman Triangle Sunburst
OS Diagnosis and Staging Codman Triangle Sunburst
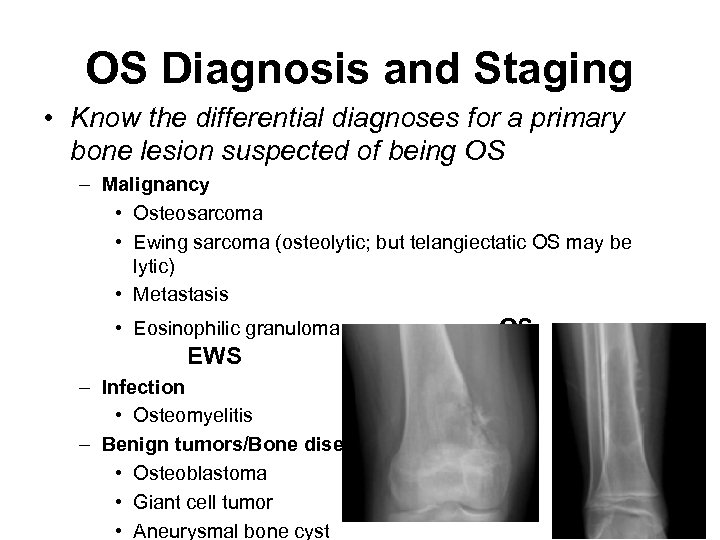 OS Diagnosis and Staging • Know the differential diagnoses for a primary bone lesion suspected of being OS – Malignancy • Osteosarcoma • Ewing sarcoma (osteolytic; but telangiectatic OS may be lytic) • Metastasis • Eosinophilic granuloma OS EWS – Infection • Osteomyelitis – Benign tumors/Bone diseases (less likely) • Osteoblastoma • Giant cell tumor • Aneurysmal bone cyst
OS Diagnosis and Staging • Know the differential diagnoses for a primary bone lesion suspected of being OS – Malignancy • Osteosarcoma • Ewing sarcoma (osteolytic; but telangiectatic OS may be lytic) • Metastasis • Eosinophilic granuloma OS EWS – Infection • Osteomyelitis – Benign tumors/Bone diseases (less likely) • Osteoblastoma • Giant cell tumor • Aneurysmal bone cyst
 OS Treatment - Surgery • Know the role of surgery and the available surgical options in the treatment of primary and metastatic OS – Role of surgery • Removal of all gross tumor with margins en bloc and biopsy site through normal tissue planes is required • Metastatic sites must be resected – Surgical options • Amputation or limb salvage procedure (metallic endoprosthesis, allograft, vascularized or non-vascularized autograft) • Type of procedure depends on tumor location, size, extra-medullary extent, presence of metastases, age, skeletal development, and life-style preference – Timing • Following neoadjuvant chemotherapy (week 10)
OS Treatment - Surgery • Know the role of surgery and the available surgical options in the treatment of primary and metastatic OS – Role of surgery • Removal of all gross tumor with margins en bloc and biopsy site through normal tissue planes is required • Metastatic sites must be resected – Surgical options • Amputation or limb salvage procedure (metallic endoprosthesis, allograft, vascularized or non-vascularized autograft) • Type of procedure depends on tumor location, size, extra-medullary extent, presence of metastases, age, skeletal development, and life-style preference – Timing • Following neoadjuvant chemotherapy (week 10)
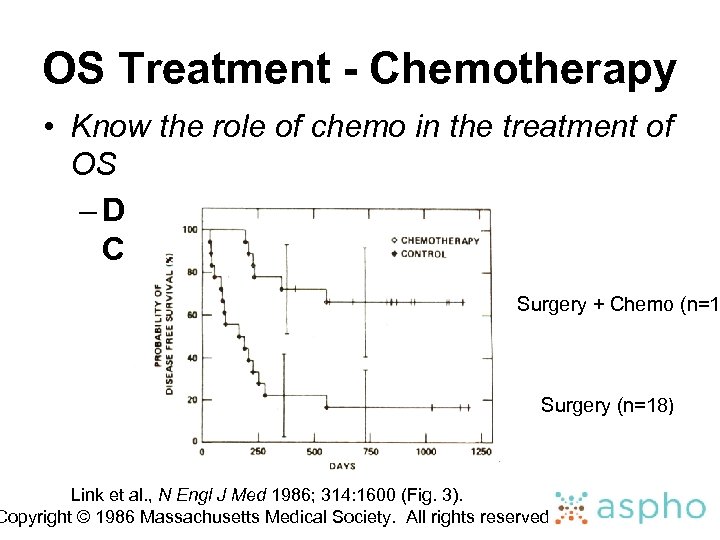 OS Treatment - Chemotherapy • Know the role of chemo in the treatment of OS – DFS: Surgery vs Surgery + Chemotherapy Surgery + Chemo (n=1 Surgery (n=18) Link et al. , N Engl J Med 1986; 314: 1600 (Fig. 3). Copyright © 1986 Massachusetts Medical Society. All rights reserved
OS Treatment - Chemotherapy • Know the role of chemo in the treatment of OS – DFS: Surgery vs Surgery + Chemotherapy Surgery + Chemo (n=1 Surgery (n=18) Link et al. , N Engl J Med 1986; 314: 1600 (Fig. 3). Copyright © 1986 Massachusetts Medical Society. All rights reserved
 OS Treatment - Chemotherapy • Know the role of neoadjuvant and post-operative chemo in the treatment of OS and the principles of therapy for different stages of OS – Treatment schedule • Chemo administered pre-surgically (neoadjuvant) and post-surgically • Neoadjuvant therapy allows starting therapy more rapidly, may facilitate limb salvage, and provides histologic response – Agents • Primarily systemic tumor control • Localized/resectable disease: HD-MTX, Dox + Cisplat (MAP)
OS Treatment - Chemotherapy • Know the role of neoadjuvant and post-operative chemo in the treatment of OS and the principles of therapy for different stages of OS – Treatment schedule • Chemo administered pre-surgically (neoadjuvant) and post-surgically • Neoadjuvant therapy allows starting therapy more rapidly, may facilitate limb salvage, and provides histologic response – Agents • Primarily systemic tumor control • Localized/resectable disease: HD-MTX, Dox + Cisplat (MAP)
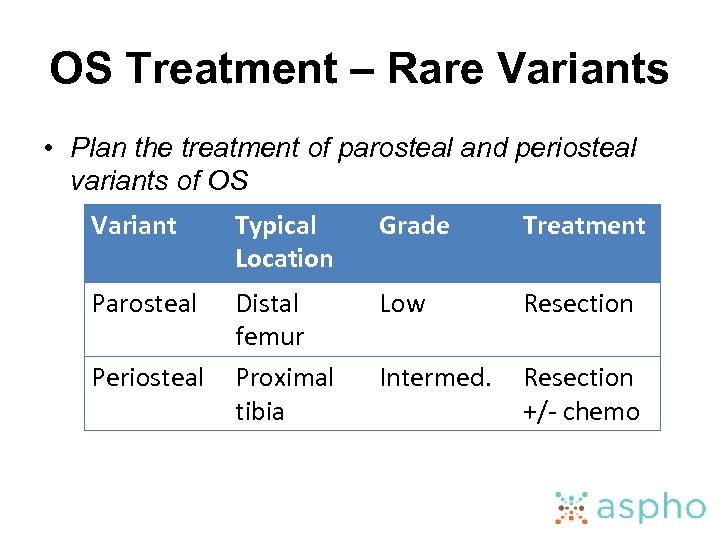 OS Treatment – Rare Variants • Plan the treatment of parosteal and periosteal variants of OS Variant Typical Location Grade Treatment Parosteal Distal femur Proximal tibia Low Resection Intermed. Resection +/- chemo Periosteal
OS Treatment – Rare Variants • Plan the treatment of parosteal and periosteal variants of OS Variant Typical Location Grade Treatment Parosteal Distal femur Proximal tibia Low Resection Intermed. Resection +/- chemo Periosteal
 OS Treatment - Evaluation • Be able to monitor appropriately a patient’s response to treatment of OS – PE every 1 -3 months, imaging every 3 months – Pre-operative clinical response • Decrease pain/mass – Pre-operative radiographic response • Increased ossification • Sclerosis or calcification of border • Reduction in soft tissue mass – Pathologic response • Assessment of necrosis
OS Treatment - Evaluation • Be able to monitor appropriately a patient’s response to treatment of OS – PE every 1 -3 months, imaging every 3 months – Pre-operative clinical response • Decrease pain/mass – Pre-operative radiographic response • Increased ossification • Sclerosis or calcification of border • Reduction in soft tissue mass – Pathologic response • Assessment of necrosis
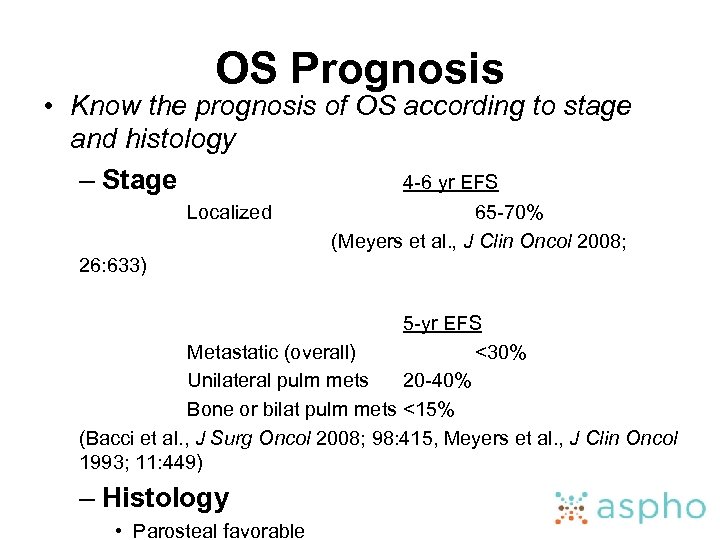 OS Prognosis • Know the prognosis of OS according to stage and histology – Stage 4 -6 yr EFS Localized 65 -70% (Meyers et al. , J Clin Oncol 2008; 26: 633) 5 -yr EFS Metastatic (overall) <30% Unilateral pulm mets 20 -40% Bone or bilat pulm mets <15% (Bacci et al. , J Surg Oncol 2008; 98: 415, Meyers et al. , J Clin Oncol 1993; 11: 449) – Histology • Parosteal favorable
OS Prognosis • Know the prognosis of OS according to stage and histology – Stage 4 -6 yr EFS Localized 65 -70% (Meyers et al. , J Clin Oncol 2008; 26: 633) 5 -yr EFS Metastatic (overall) <30% Unilateral pulm mets 20 -40% Bone or bilat pulm mets <15% (Bacci et al. , J Surg Oncol 2008; 98: 415, Meyers et al. , J Clin Oncol 1993; 11: 449) – Histology • Parosteal favorable
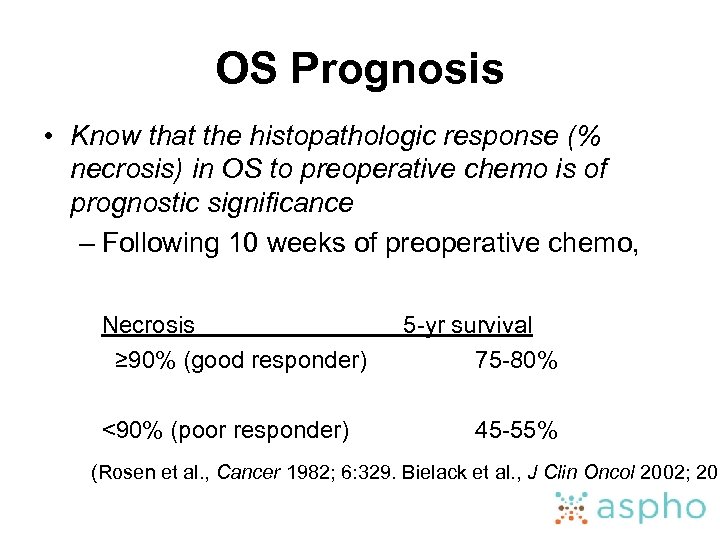 OS Prognosis • Know that the histopathologic response (% necrosis) in OS to preoperative chemo is of prognostic significance – Following 10 weeks of preoperative chemo, Necrosis ≥ 90% (good responder) <90% (poor responder) 5 -yr survival 75 -80% 45 -55% (Rosen et al. , Cancer 1982; 6: 329. Bielack et al. , J Clin Oncol 2002; 20:
OS Prognosis • Know that the histopathologic response (% necrosis) in OS to preoperative chemo is of prognostic significance – Following 10 weeks of preoperative chemo, Necrosis ≥ 90% (good responder) <90% (poor responder) 5 -yr survival 75 -80% 45 -55% (Rosen et al. , Cancer 1982; 6: 329. Bielack et al. , J Clin Oncol 2002; 20:
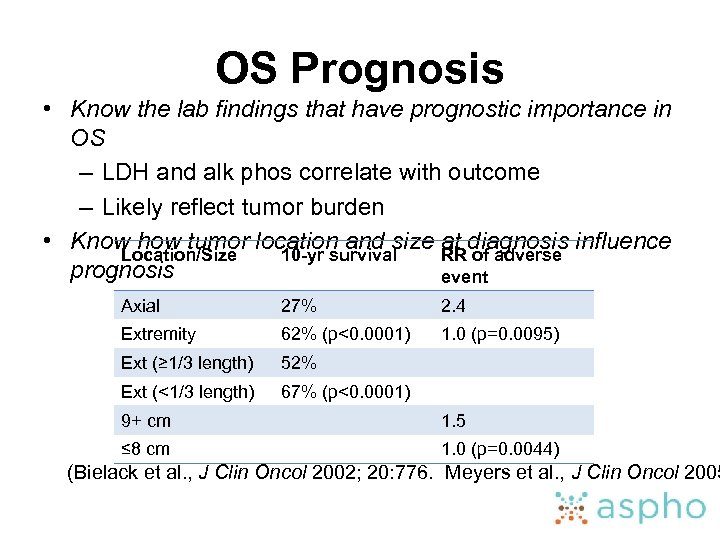 OS Prognosis • Know the lab findings that have prognostic importance in OS – LDH and alk phos correlate with outcome – Likely reflect tumor burden • Know how tumor location and size at diagnosis influence Location/Size 10 -yr survival RR of adverse prognosis event Axial 27% 2. 4 Extremity 62% (p<0. 0001) 1. 0 (p=0. 0095) Ext (≥ 1/3 length) 52% Ext (<1/3 length) 67% (p<0. 0001) 9+ cm 1. 5 ≤ 8 cm 1. 0 (p=0. 0044) (Bielack et al. , J Clin Oncol 2002; 20: 776. Meyers et al. , J Clin Oncol 2005
OS Prognosis • Know the lab findings that have prognostic importance in OS – LDH and alk phos correlate with outcome – Likely reflect tumor burden • Know how tumor location and size at diagnosis influence Location/Size 10 -yr survival RR of adverse prognosis event Axial 27% 2. 4 Extremity 62% (p<0. 0001) 1. 0 (p=0. 0095) Ext (≥ 1/3 length) 52% Ext (<1/3 length) 67% (p<0. 0001) 9+ cm 1. 5 ≤ 8 cm 1. 0 (p=0. 0044) (Bielack et al. , J Clin Oncol 2002; 20: 776. Meyers et al. , J Clin Oncol 2005
 OS Complications/Late Effects • Know the complications and late effects of OS, surgery for OS and chemo for OS – Surgical • Studies continue to look at functional outcomes for patients undergoing limb salvage vs amputation (Nagarajan et al. , J Cancer Surviv 2009; 3: 59) – Chemotherapy • Anthracycline-induced cardiomyopathy; 7. 5% cumulative incidence following approximately 300 mg/m 2; may arise > 15 yrs post therapy (Paulides et al. , Pediatr Blood Cancer 2006; 46: 489) • Cisplatin-induced ototoxicity; 11% of patients (Meyers et al. , J Clin Oncol 2005; 23: 2004)
OS Complications/Late Effects • Know the complications and late effects of OS, surgery for OS and chemo for OS – Surgical • Studies continue to look at functional outcomes for patients undergoing limb salvage vs amputation (Nagarajan et al. , J Cancer Surviv 2009; 3: 59) – Chemotherapy • Anthracycline-induced cardiomyopathy; 7. 5% cumulative incidence following approximately 300 mg/m 2; may arise > 15 yrs post therapy (Paulides et al. , Pediatr Blood Cancer 2006; 46: 489) • Cisplatin-induced ototoxicity; 11% of patients (Meyers et al. , J Clin Oncol 2005; 23: 2004)
 Ewing Family Tumors • • Ewing Sarcoma of Bone Extraosseous Ewing Sarcoma Primitive Neuroectodermal Tumor Askin Tumor (PNET of the chest wall)
Ewing Family Tumors • • Ewing Sarcoma of Bone Extraosseous Ewing Sarcoma Primitive Neuroectodermal Tumor Askin Tumor (PNET of the chest wall)
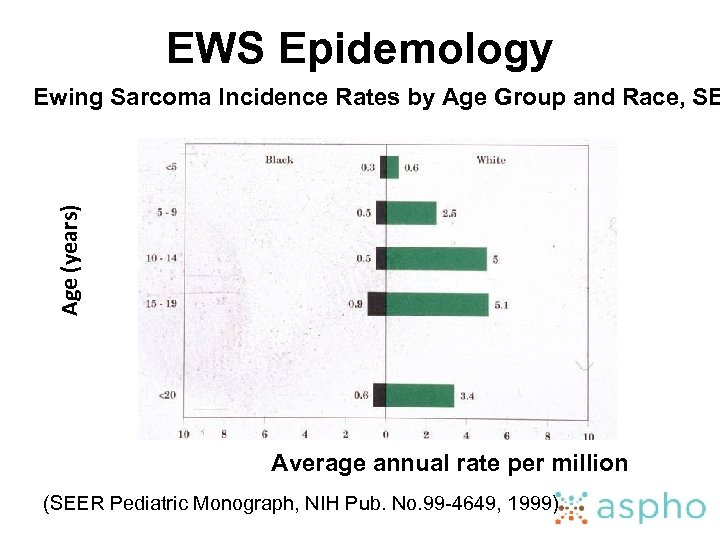 EWS Epidemology Age (years) Ewing Sarcoma Incidence Rates by Age Group and Race, SE Average annual rate per million (SEER Pediatric Monograph, NIH Pub. No. 99 -4649, 1999)
EWS Epidemology Age (years) Ewing Sarcoma Incidence Rates by Age Group and Race, SE Average annual rate per million (SEER Pediatric Monograph, NIH Pub. No. 99 -4649, 1999)
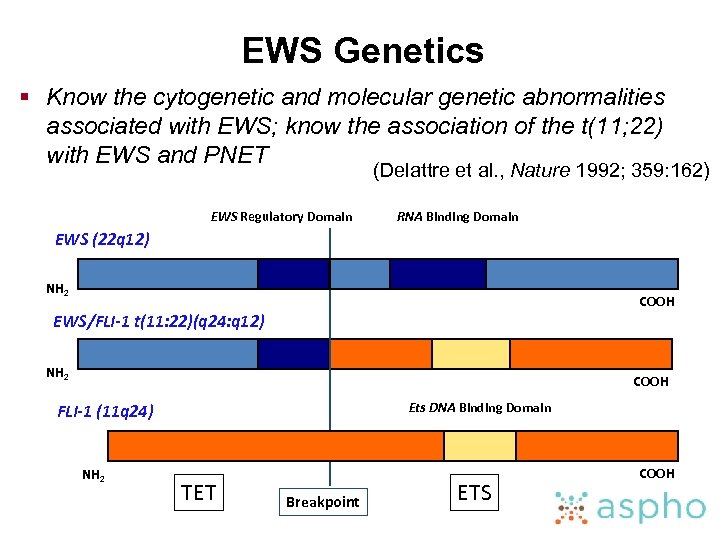 EWS Genetics § Know the cytogenetic and molecular genetic abnormalities associated with EWS; know the association of the t(11; 22) with EWS and PNET (Delattre et al. , Nature 1992; 359: 162) EWS Regulatory Domain RNA Binding Domain EWS (22 q 12) NH 2 COOH EWS/FLI-1 t(11: 22)(q 24: q 12) NH 2 COOH Ets DNA Binding Domain FLI-1 (11 q 24) NH 2 TET Breakpoint ETS COOH
EWS Genetics § Know the cytogenetic and molecular genetic abnormalities associated with EWS; know the association of the t(11; 22) with EWS and PNET (Delattre et al. , Nature 1992; 359: 162) EWS Regulatory Domain RNA Binding Domain EWS (22 q 12) NH 2 COOH EWS/FLI-1 t(11: 22)(q 24: q 12) NH 2 COOH Ets DNA Binding Domain FLI-1 (11 q 24) NH 2 TET Breakpoint ETS COOH
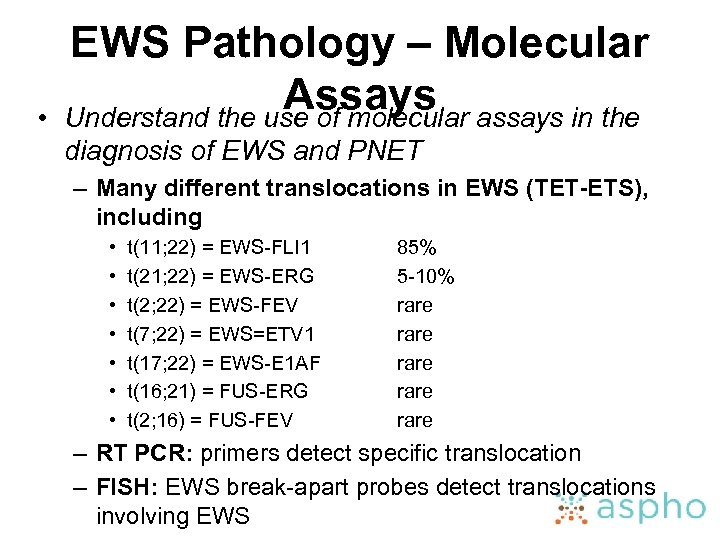 • EWS Pathology – Molecular Assays assays in the Understand the use of molecular diagnosis of EWS and PNET – Many different translocations in EWS (TET-ETS), including • • t(11; 22) = EWS-FLI 1 t(21; 22) = EWS-ERG t(2; 22) = EWS-FEV t(7; 22) = EWS=ETV 1 t(17; 22) = EWS-E 1 AF t(16; 21) = FUS-ERG t(2; 16) = FUS-FEV 85% 5 -10% rare rare – RT PCR: primers detect specific translocation – FISH: EWS break-apart probes detect translocations involving EWS
• EWS Pathology – Molecular Assays assays in the Understand the use of molecular diagnosis of EWS and PNET – Many different translocations in EWS (TET-ETS), including • • t(11; 22) = EWS-FLI 1 t(21; 22) = EWS-ERG t(2; 22) = EWS-FEV t(7; 22) = EWS=ETV 1 t(17; 22) = EWS-E 1 AF t(16; 21) = FUS-ERG t(2; 16) = FUS-FEV 85% 5 -10% rare rare – RT PCR: primers detect specific translocation – FISH: EWS break-apart probes detect translocations involving EWS
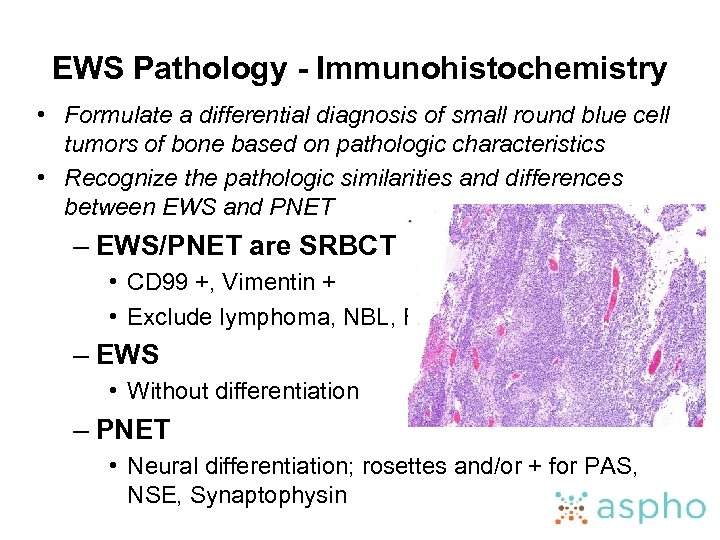 EWS Pathology - Immunohistochemistry • Formulate a differential diagnosis of small round blue cell tumors of bone based on pathologic characteristics • Recognize the pathologic similarities and differences between EWS and PNET – EWS/PNET are SRBCT • CD 99 +, Vimentin + • Exclude lymphoma, NBL, RMS – EWS • Without differentiation – PNET • Neural differentiation; rosettes and/or + for PAS, NSE, Synaptophysin
EWS Pathology - Immunohistochemistry • Formulate a differential diagnosis of small round blue cell tumors of bone based on pathologic characteristics • Recognize the pathologic similarities and differences between EWS and PNET – EWS/PNET are SRBCT • CD 99 +, Vimentin + • Exclude lymphoma, NBL, RMS – EWS • Without differentiation – PNET • Neural differentiation; rosettes and/or + for PAS, NSE, Synaptophysin
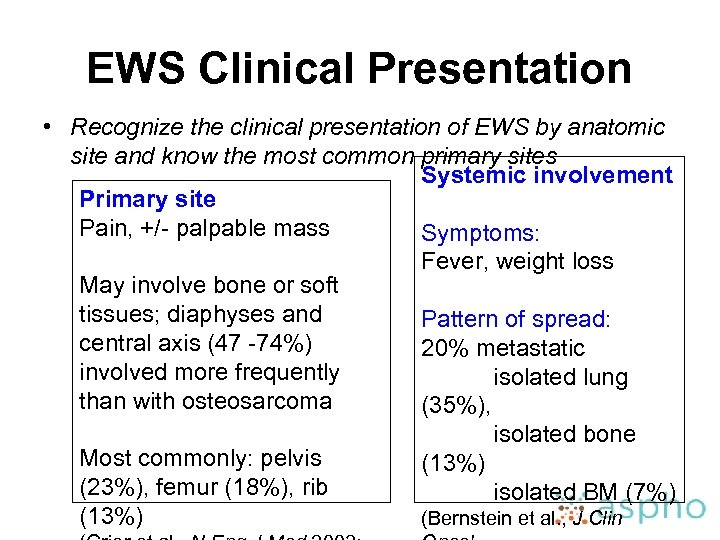 EWS Clinical Presentation • Recognize the clinical presentation of EWS by anatomic site and know the most common primary sites Systemic involvement Primary site Pain, +/- palpable mass Symptoms: Fever, weight loss May involve bone or soft tissues; diaphyses and Pattern of spread: central axis (47 -74%) 20% metastatic involved more frequently isolated lung than with osteosarcoma (35%), isolated bone Most commonly: pelvis (13%) (23%), femur (18%), rib isolated BM (7%) (13%) (Bernstein et al. , J Clin
EWS Clinical Presentation • Recognize the clinical presentation of EWS by anatomic site and know the most common primary sites Systemic involvement Primary site Pain, +/- palpable mass Symptoms: Fever, weight loss May involve bone or soft tissues; diaphyses and Pattern of spread: central axis (47 -74%) 20% metastatic involved more frequently isolated lung than with osteosarcoma (35%), isolated bone Most commonly: pelvis (13%) (23%), femur (18%), rib isolated BM (7%) (13%) (Bernstein et al. , J Clin
 EWS Diagnosis and Staging • Utilize appropriate imaging modalities to determine the extent and metastatic spread of EWS and PNET – Staging workup • Plain films of primary site • MRI or CT of primary site • CT of chest/CXR • Bone scan/PET scan (under investigation) • Bone marrow aspirate/biopsy (bilateral) – Stage assignment • Localized vs Metastatic
EWS Diagnosis and Staging • Utilize appropriate imaging modalities to determine the extent and metastatic spread of EWS and PNET – Staging workup • Plain films of primary site • MRI or CT of primary site • CT of chest/CXR • Bone scan/PET scan (under investigation) • Bone marrow aspirate/biopsy (bilateral) – Stage assignment • Localized vs Metastatic
 EWS Diagnosis and Staging • Recognize the radiologic findings of EWS – Plain films • Permeative lytic lesion with periosteal reaction • Aggressive periosteal reaction may be demonstrated by Codman triangles, “sunburst” pattern, or “onion-skin” pattern • Sclerotic lesions are more rare and may be mistaken for OS; whereas telangiectatic OS may be mistaken for EWS • Differential also include metastasis, eosinophilic granuloma, osteomyelitis
EWS Diagnosis and Staging • Recognize the radiologic findings of EWS – Plain films • Permeative lytic lesion with periosteal reaction • Aggressive periosteal reaction may be demonstrated by Codman triangles, “sunburst” pattern, or “onion-skin” pattern • Sclerotic lesions are more rare and may be mistaken for OS; whereas telangiectatic OS may be mistaken for EWS • Differential also include metastasis, eosinophilic granuloma, osteomyelitis
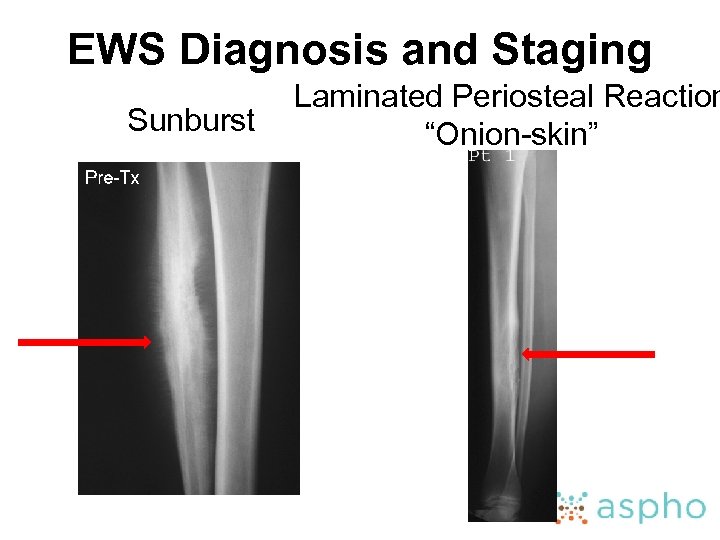 EWS Diagnosis and Staging Sunburst Laminated Periosteal Reaction “Onion-skin”
EWS Diagnosis and Staging Sunburst Laminated Periosteal Reaction “Onion-skin”
 EWS Treatment • Know the principles of treatment for different stages of EWS and PNET – Treatment philosophy • A multidisciplinary approach must provide local control and systemic therapy: SURGERY, RADIOTHERAPY, CHEMO, SCT • Local control measures should not compromise systemic therapy because when treatment fails it is usually due to the development of metastatic disease
EWS Treatment • Know the principles of treatment for different stages of EWS and PNET – Treatment philosophy • A multidisciplinary approach must provide local control and systemic therapy: SURGERY, RADIOTHERAPY, CHEMO, SCT • Local control measures should not compromise systemic therapy because when treatment fails it is usually due to the development of metastatic disease
 • EWS Treatment – Local Control Know the role of surgery and irradiation in the treatment of EWS/PNET – Local control: surgery and/or radiation therapy • No randomized studies comparing surgery and RT • Current trend favors surgery when possible – IESS-1: 21% underwent surgery (Nesbit et al. , J Clin Oncol 1990; 8: 1664) – IESS-2: 42% underwent surgery (Burgert et al. , J Clin Oncol 1990; 8: 1514) – IESS-3: 61% underwent surgery (Grier et al. , N Engl J Med 2003; 348: 692) – AEWS 0031: 79% underwent surgery (Womer et al, J Clin Oncol 2012; 30: 4148) • Combined surgery/RT approach sometimes used • Definitive local control usually done after several weeks of
• EWS Treatment – Local Control Know the role of surgery and irradiation in the treatment of EWS/PNET – Local control: surgery and/or radiation therapy • No randomized studies comparing surgery and RT • Current trend favors surgery when possible – IESS-1: 21% underwent surgery (Nesbit et al. , J Clin Oncol 1990; 8: 1664) – IESS-2: 42% underwent surgery (Burgert et al. , J Clin Oncol 1990; 8: 1514) – IESS-3: 61% underwent surgery (Grier et al. , N Engl J Med 2003; 348: 692) – AEWS 0031: 79% underwent surgery (Womer et al, J Clin Oncol 2012; 30: 4148) • Combined surgery/RT approach sometimes used • Definitive local control usually done after several weeks of
 EWS – Systemic Therapy • Know the role of chemo, preoperative chemo and possible SCT in the treatment of EWS and PNET – Systemic therapy: chemotherapy, (SCT) • All patients require chemotherapy • Standard therapy: Vincristine, Doxorubicin, Cyclophosphamide, Ifosfamide, Etoposide (VDC + IE) • Improved outcomes using interval compression (Womer • Other. Clin Oncol 2012 30; 4148) et al. , J active agents: Topotecan, Irinotecan,
EWS – Systemic Therapy • Know the role of chemo, preoperative chemo and possible SCT in the treatment of EWS and PNET – Systemic therapy: chemotherapy, (SCT) • All patients require chemotherapy • Standard therapy: Vincristine, Doxorubicin, Cyclophosphamide, Ifosfamide, Etoposide (VDC + IE) • Improved outcomes using interval compression (Womer • Other. Clin Oncol 2012 30; 4148) et al. , J active agents: Topotecan, Irinotecan,
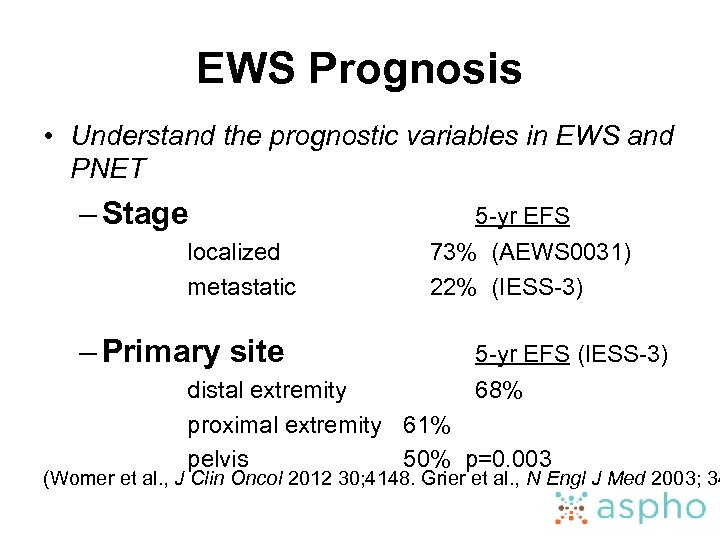 EWS Prognosis • Understand the prognostic variables in EWS and PNET – Stage localized metastatic – Primary site 5 -yr EFS 73% (AEWS 0031) 22% (IESS-3) 5 -yr EFS (IESS-3) distal extremity 68% proximal extremity 61% pelvis 50% p=0. 003 (Womer et al. , J Clin Oncol 2012 30; 4148. Grier et al. , N Engl J Med 2003; 34
EWS Prognosis • Understand the prognostic variables in EWS and PNET – Stage localized metastatic – Primary site 5 -yr EFS 73% (AEWS 0031) 22% (IESS-3) 5 -yr EFS (IESS-3) distal extremity 68% proximal extremity 61% pelvis 50% p=0. 003 (Womer et al. , J Clin Oncol 2012 30; 4148. Grier et al. , N Engl J Med 2003; 34
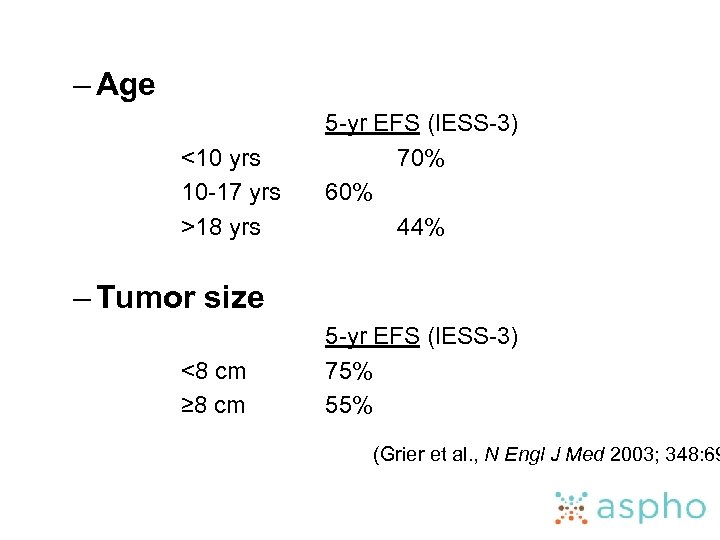 – Age <10 yrs 10 -17 yrs >18 yrs 5 -yr EFS (IESS-3) 70% 60% 44% – Tumor size <8 cm ≥ 8 cm 5 -yr EFS (IESS-3) 75% 55% (Grier et al. , N Engl J Med 2003; 348: 69
– Age <10 yrs 10 -17 yrs >18 yrs 5 -yr EFS (IESS-3) 70% 60% 44% – Tumor size <8 cm ≥ 8 cm 5 -yr EFS (IESS-3) 75% 55% (Grier et al. , N Engl J Med 2003; 348: 69
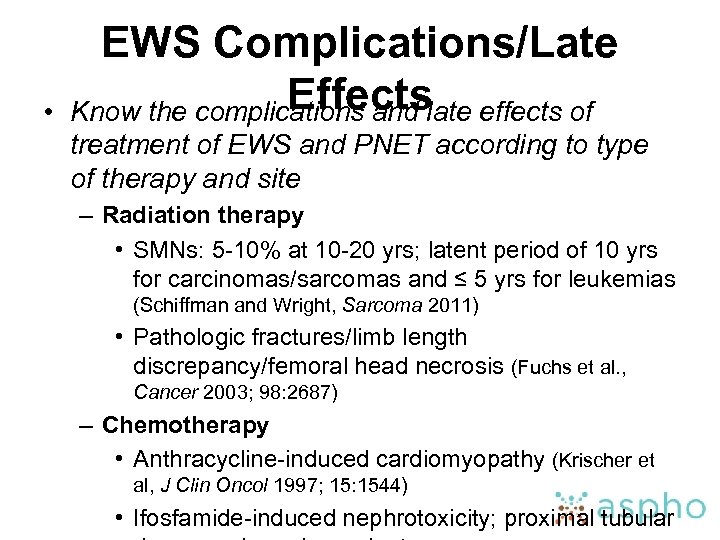 • EWS Complications/Late Effects Know the complications and late effects of treatment of EWS and PNET according to type of therapy and site – Radiation therapy • SMNs: 5 -10% at 10 -20 yrs; latent period of 10 yrs for carcinomas/sarcomas and ≤ 5 yrs for leukemias (Schiffman and Wright, Sarcoma 2011) • Pathologic fractures/limb length discrepancy/femoral head necrosis (Fuchs et al. , Cancer 2003; 98: 2687) – Chemotherapy • Anthracycline-induced cardiomyopathy (Krischer et al, J Clin Oncol 1997; 15: 1544) • Ifosfamide-induced nephrotoxicity; proximal tubular
• EWS Complications/Late Effects Know the complications and late effects of treatment of EWS and PNET according to type of therapy and site – Radiation therapy • SMNs: 5 -10% at 10 -20 yrs; latent period of 10 yrs for carcinomas/sarcomas and ≤ 5 yrs for leukemias (Schiffman and Wright, Sarcoma 2011) • Pathologic fractures/limb length discrepancy/femoral head necrosis (Fuchs et al. , Cancer 2003; 98: 2687) – Chemotherapy • Anthracycline-induced cardiomyopathy (Krischer et al, J Clin Oncol 1997; 15: 1544) • Ifosfamide-induced nephrotoxicity; proximal tubular
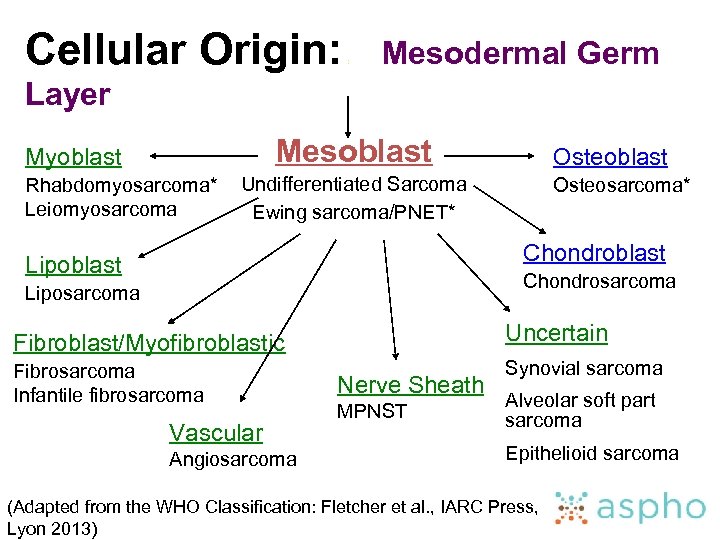 Cellular Origin: : Mesodermal Germ Layer Mesoblast Myoblast Rhabdomyosarcoma* Leiomyosarcoma Osteoblast Undifferentiated Sarcoma Ewing sarcoma/PNET* Osteosarcoma* Chondroblast Lipoblast Chondrosarcoma Liposarcoma Fibroblast/Myofibroblastic Uncertain Fibrosarcoma Infantile fibrosarcoma Synovial sarcoma Vascular Angiosarcoma Nerve Sheath MPNST Alveolar soft part sarcoma Epithelioid sarcoma (Adapted from the WHO Classification: Fletcher et al. , IARC Press, Lyon 2013)
Cellular Origin: : Mesodermal Germ Layer Mesoblast Myoblast Rhabdomyosarcoma* Leiomyosarcoma Osteoblast Undifferentiated Sarcoma Ewing sarcoma/PNET* Osteosarcoma* Chondroblast Lipoblast Chondrosarcoma Liposarcoma Fibroblast/Myofibroblastic Uncertain Fibrosarcoma Infantile fibrosarcoma Synovial sarcoma Vascular Angiosarcoma Nerve Sheath MPNST Alveolar soft part sarcoma Epithelioid sarcoma (Adapted from the WHO Classification: Fletcher et al. , IARC Press, Lyon 2013)


