ad6e6bf6f1b138ac8caa777f6ffc4d51.ppt
- Количество слайдов: 56
 Sampling of Nutrients in the Environment a QA/QC perspective Dan Wruck Supervisor, Nutrients & Environmental Waters Queensland Health Scientific Services E-mail: dan_wruck@health. qld. gov. au Manilla, Philippines, October 2006
Sampling of Nutrients in the Environment a QA/QC perspective Dan Wruck Supervisor, Nutrients & Environmental Waters Queensland Health Scientific Services E-mail: dan_wruck@health. qld. gov. au Manilla, Philippines, October 2006
 Brisbane Sydney Melbourne
Brisbane Sydney Melbourne

 re ? he W South of the Great Divide
re ? he W South of the Great Divide
 Nutrients Lab üLeading Australian Lab - analysing approx 20, 000 samples annually üAccredited to ISO/IEC 17025 üSamples sourced from: – pristine freshwater – water storages – estuaries – seawater – effluent monitoring – compliance monitoring
Nutrients Lab üLeading Australian Lab - analysing approx 20, 000 samples annually üAccredited to ISO/IEC 17025 üSamples sourced from: – pristine freshwater – water storages – estuaries – seawater – effluent monitoring – compliance monitoring
 Nutrients Lab üAccredited Proficiency Testing Provider üAccredited to ILAC Guide 13: 2000 – based on ISO guide 43 -1 and relevant elements of ISO/IEC 17025 üManages and Coordinates National Low Level Nutrient Collaborative Trials (NLLNCT) – More than 60 Australian & overseas labs participate – trials evaluate & provides feedback through Summary Reports, Workshops
Nutrients Lab üAccredited Proficiency Testing Provider üAccredited to ILAC Guide 13: 2000 – based on ISO guide 43 -1 and relevant elements of ISO/IEC 17025 üManages and Coordinates National Low Level Nutrient Collaborative Trials (NLLNCT) – More than 60 Australian & overseas labs participate – trials evaluate & provides feedback through Summary Reports, Workshops
 Nutrients Lab üCertified Reference Material Producer üAccredited to ILAC Guide 12: 2000 – based on ISO guide 34: 1996 (quality system guidelines for the production of reference materials) üCertified Reference Materials in natural freshwaters and seawaters üSuitable for: – method development – quality control samples
Nutrients Lab üCertified Reference Material Producer üAccredited to ILAC Guide 12: 2000 – based on ISO guide 34: 1996 (quality system guidelines for the production of reference materials) üCertified Reference Materials in natural freshwaters and seawaters üSuitable for: – method development – quality control samples
 p. H Conductivity Turbidity (NTU) (mg/L ) 140 875 600 (µS/cm at 25 o. C) 7. 4 Hello… any crocs around? ? ? TSS
p. H Conductivity Turbidity (NTU) (mg/L ) 140 875 600 (µS/cm at 25 o. C) 7. 4 Hello… any crocs around? ? ? TSS
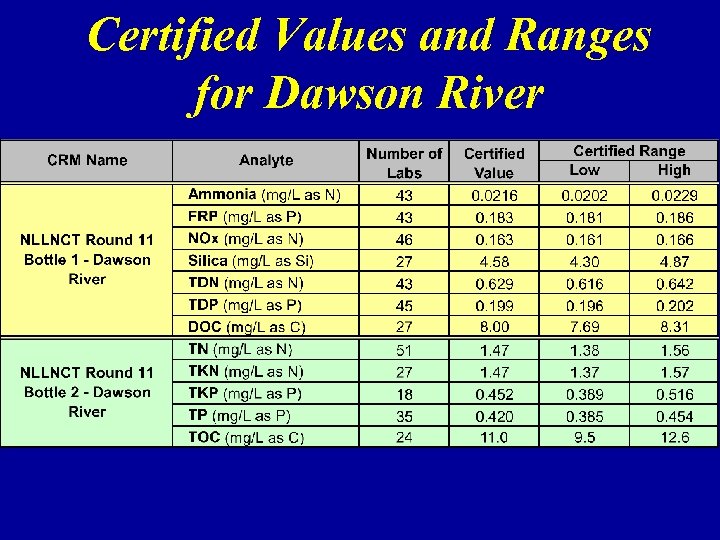 Certified Values and Ranges for Dawson River
Certified Values and Ranges for Dawson River
 NLLNCT Round 9 Pristine Freshwater Site üSomerset Dam üPotable water source for Brisbane population. üBloody good fishing spot Conductivity Turbidity ( m. S/cm ) ( NTU ) Total Suspended Solids ( mg/L ) 5 6 300
NLLNCT Round 9 Pristine Freshwater Site üSomerset Dam üPotable water source for Brisbane population. üBloody good fishing spot Conductivity Turbidity ( m. S/cm ) ( NTU ) Total Suspended Solids ( mg/L ) 5 6 300
 NLLNCT Round 9 Pristine Seawater üPumicestone Passage üPristine estuary üCan be good fishing
NLLNCT Round 9 Pristine Seawater üPumicestone Passage üPristine estuary üCan be good fishing
 Impacted seawater üPrawn Farm Conductivity Turbidity ( m. S/cm ) ( NTU ) 40, 000 28 Total Suspended Solids ( mg/L ) 150
Impacted seawater üPrawn Farm Conductivity Turbidity ( m. S/cm ) ( NTU ) 40, 000 28 Total Suspended Solids ( mg/L ) 150
 QHSS Nutrient’s Laboratory Gary with 5 channel FIA automated analyser Tuyet preparing samples for Kjeldahl analysis
QHSS Nutrient’s Laboratory Gary with 5 channel FIA automated analyser Tuyet preparing samples for Kjeldahl analysis
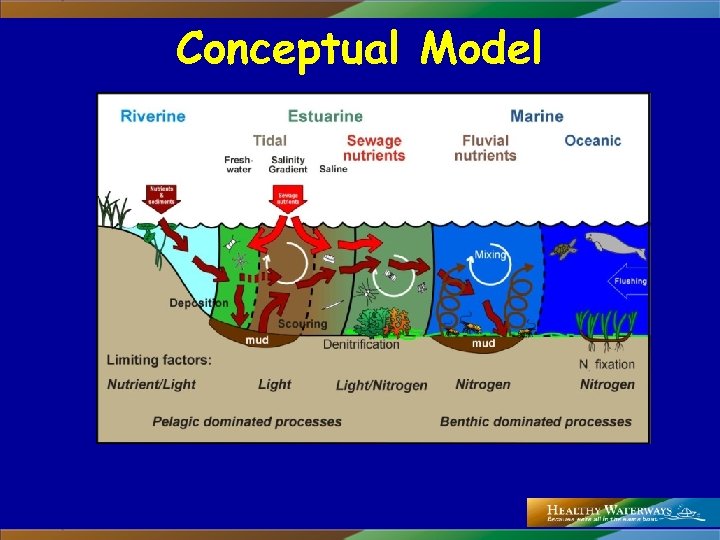 Conceptual Model
Conceptual Model
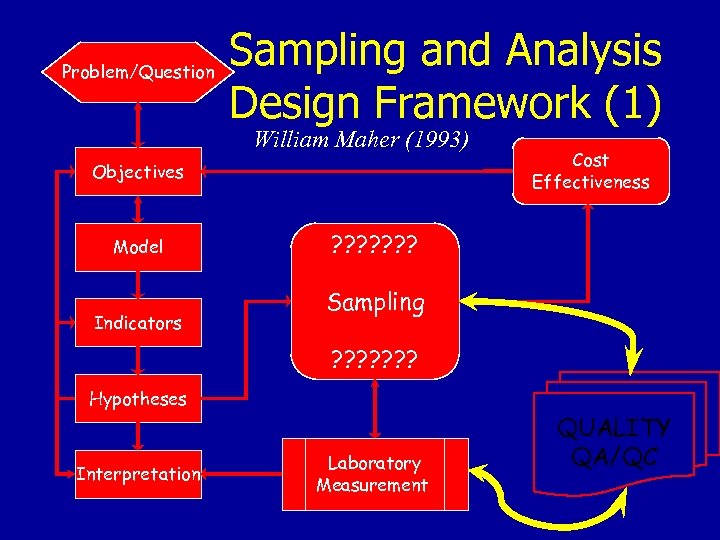 Problem/Question Sampling and Analysis Design Framework (1) William Maher (1993) Objectives Model Indicators Cost Effectiveness ? ? ? ? Sampling ? ? ? ? Hypotheses Interpretation Laboratory Measurement QUALITY QA/QC
Problem/Question Sampling and Analysis Design Framework (1) William Maher (1993) Objectives Model Indicators Cost Effectiveness ? ? ? ? Sampling ? ? ? ? Hypotheses Interpretation Laboratory Measurement QUALITY QA/QC
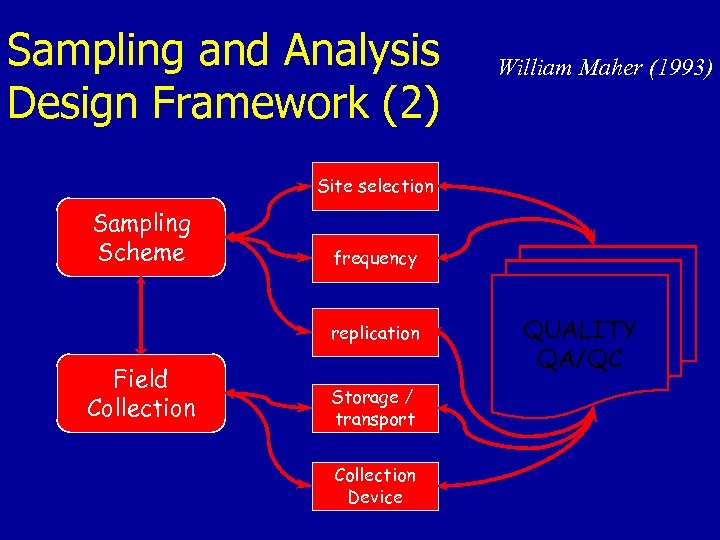 Sampling and Analysis Design Framework (2) William Maher (1993) Site selection Sampling Scheme frequency replication Field Collection Storage / transport Collection Device QUALITY QA/QC
Sampling and Analysis Design Framework (2) William Maher (1993) Site selection Sampling Scheme frequency replication Field Collection Storage / transport Collection Device QUALITY QA/QC
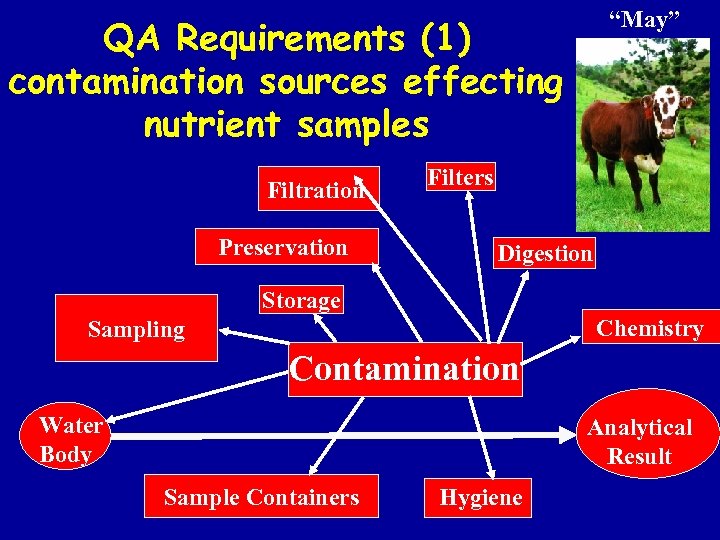 “May” QA Requirements (1) contamination sources effecting nutrient samples Filtration Preservation Filters Digestion Storage Chemistry Sampling Contamination Water Body Analytical Result Sample Containers Hygiene
“May” QA Requirements (1) contamination sources effecting nutrient samples Filtration Preservation Filters Digestion Storage Chemistry Sampling Contamination Water Body Analytical Result Sample Containers Hygiene
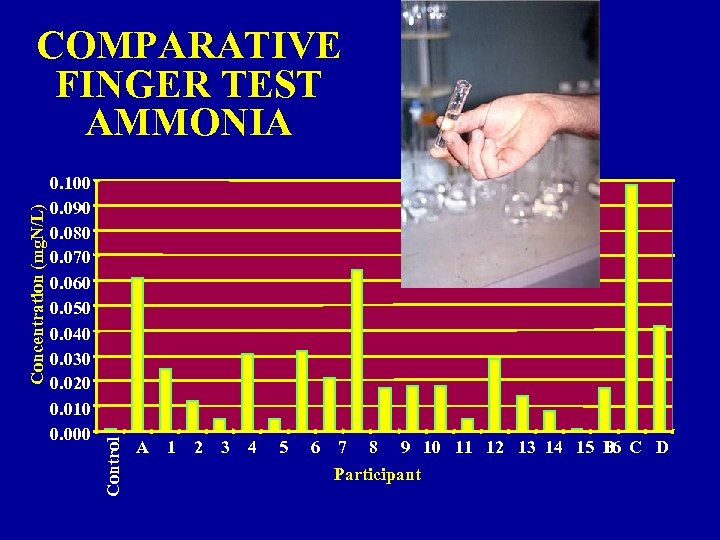 0. 100 0. 090 0. 080 0. 070 0. 060 0. 050 0. 040 0. 030 0. 020 0. 010 0. 000 Control Concentration (mg. N/L) COMPARATIVE FINGER TEST AMMONIA A 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 B C D 16 Participant
0. 100 0. 090 0. 080 0. 070 0. 060 0. 050 0. 040 0. 030 0. 020 0. 010 0. 000 Control Concentration (mg. N/L) COMPARATIVE FINGER TEST AMMONIA A 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 B C D 16 Participant
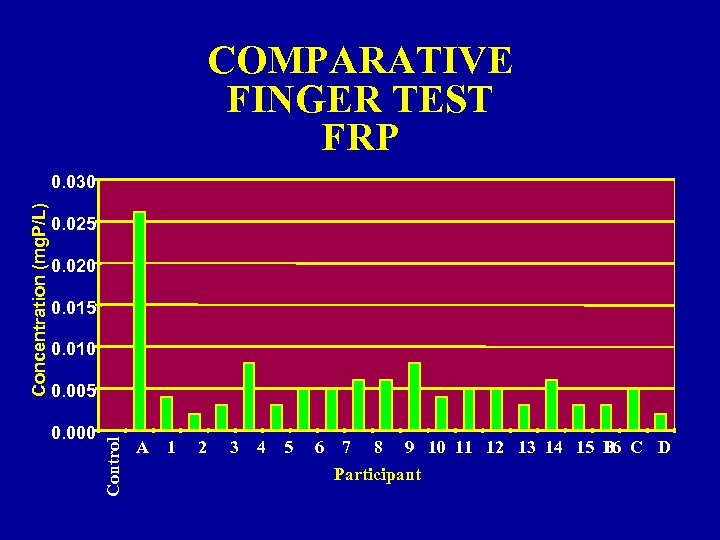 COMPARATIVE FINGER TEST FRP 0. 025 0. 020 0. 015 0. 010 0. 005 0. 000 Control Concentration (mg. P/L) 0. 030 A 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 B C D 16 Participant
COMPARATIVE FINGER TEST FRP 0. 025 0. 020 0. 015 0. 010 0. 005 0. 000 Control Concentration (mg. P/L) 0. 030 A 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 B C D 16 Participant
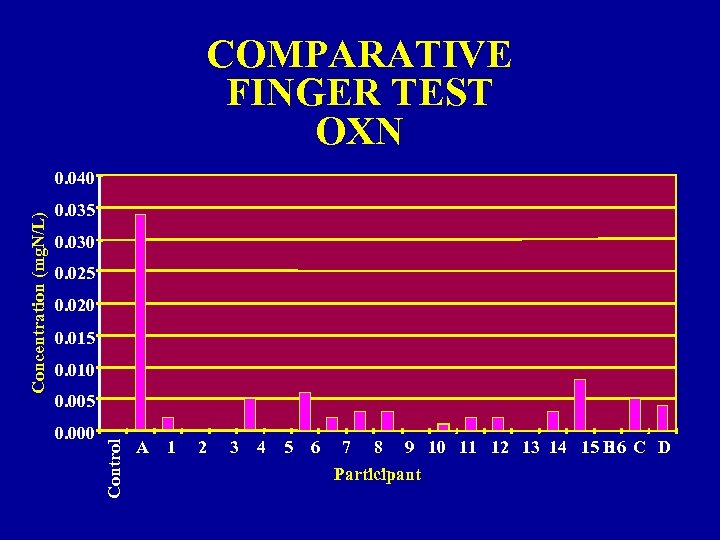 COMPARATIVE FINGER TEST OXN 0. 035 0. 030 0. 025 0. 020 0. 015 0. 010 0. 005 0. 000 Control Concentration (mg. N/L) 0. 040 A 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 B C D 16 Participant
COMPARATIVE FINGER TEST OXN 0. 035 0. 030 0. 025 0. 020 0. 015 0. 010 0. 005 0. 000 Control Concentration (mg. N/L) 0. 040 A 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 B C D 16 Participant
 QA Requirements How to effectively monitor QA for sampling Some Examples üAudit sample bottles before use üfield blanks - take through whole sampling process ütransport blank - background check ücontrol sites üreplicate samples
QA Requirements How to effectively monitor QA for sampling Some Examples üAudit sample bottles before use üfield blanks - take through whole sampling process ütransport blank - background check ücontrol sites üreplicate samples
 WHY DO WE COLLECT SAMPLES? In-situ Techniques or Field Instruments üNot Robust – site access & location – equipment fouling üQuality assurance – difficult to incorporate üInadequate detection Limits üMatrix correction
WHY DO WE COLLECT SAMPLES? In-situ Techniques or Field Instruments üNot Robust – site access & location – equipment fouling üQuality assurance – difficult to incorporate üInadequate detection Limits üMatrix correction
 Monitoring Techniques üKey Processes – Water quality – Phytoplankton bioassays – Sediment nutrient fluxes üHuman Impacts Sewage plume mapping N signatures of mangroves üCritical Habitat – Seagrass distribution and depth range
Monitoring Techniques üKey Processes – Water quality – Phytoplankton bioassays – Sediment nutrient fluxes üHuman Impacts Sewage plume mapping N signatures of mangroves üCritical Habitat – Seagrass distribution and depth range
 PRESERVATION & STORAGE NO universal procedure will satisfy all requirements WHY üAll water bodies contain particulate matter – the nature of the particulate matter determines how a sample behaves üAny procedure WILL require validation
PRESERVATION & STORAGE NO universal procedure will satisfy all requirements WHY üAll water bodies contain particulate matter – the nature of the particulate matter determines how a sample behaves üAny procedure WILL require validation
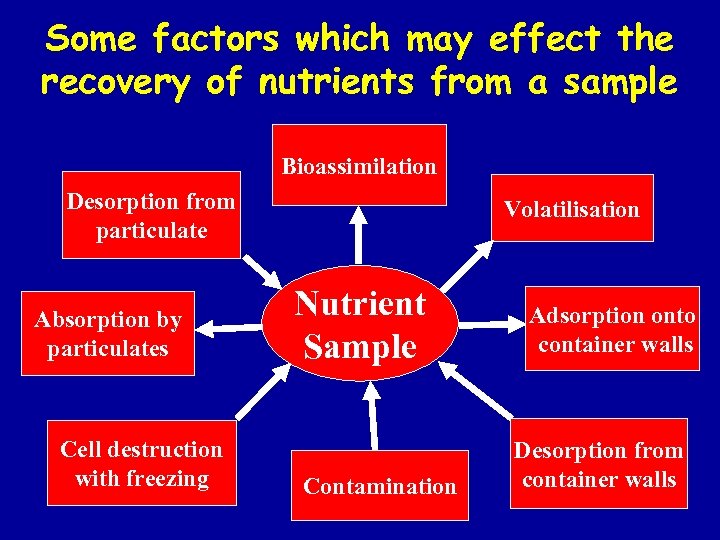 Some factors which may effect the recovery of nutrients from a sample Bioassimilation Desorption from particulate Absorption by particulates Cell destruction with freezing Volatilisation Nutrient Sample Contamination Adsorption onto container walls Desorption from container walls
Some factors which may effect the recovery of nutrients from a sample Bioassimilation Desorption from particulate Absorption by particulates Cell destruction with freezing Volatilisation Nutrient Sample Contamination Adsorption onto container walls Desorption from container walls
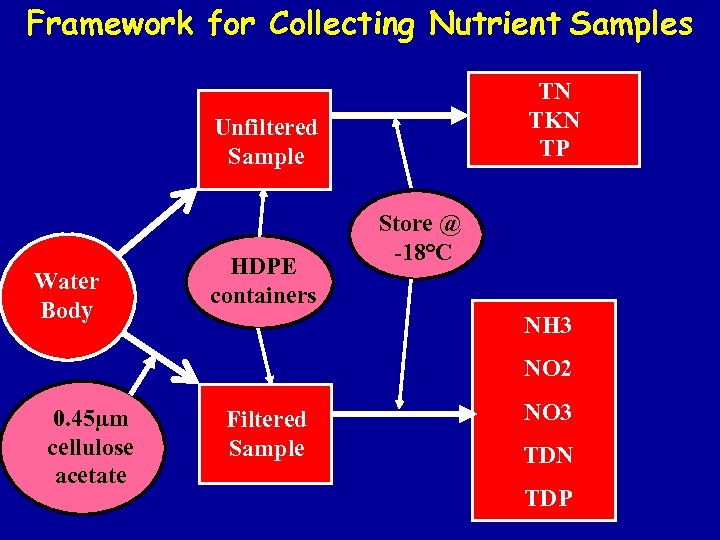 Framework for Collecting Nutrient Samples TN TKN TP Unfiltered Sample Water Body HDPE containers Store @ -18°C NH 3 NO 2 0. 45µm cellulose acetate Filtered Sample NO 3 TDN TDP
Framework for Collecting Nutrient Samples TN TKN TP Unfiltered Sample Water Body HDPE containers Store @ -18°C NH 3 NO 2 0. 45µm cellulose acetate Filtered Sample NO 3 TDN TDP
 Sample Storage
Sample Storage
 Some Common Sampling Devices & Situations
Some Common Sampling Devices & Situations
 “GRAB” - Stream • Sample into the flow • Face neck of bottle down • Invert bottle at depth of 150 mm
“GRAB” - Stream • Sample into the flow • Face neck of bottle down • Invert bottle at depth of 150 mm
 “GRAB” - Pond Do not disturb bottom sediments Beaker with telescopic pole for “inspector gadget” situations
“GRAB” - Pond Do not disturb bottom sediments Beaker with telescopic pole for “inspector gadget” situations
 “GRAB” - Sample away from boat slick
“GRAB” - Sample away from boat slick
 Automatic Samplers Install in secure environment Sampling MUST take account of preservation requirements
Automatic Samplers Install in secure environment Sampling MUST take account of preservation requirements
 Multi-parameter Probe • p. H • conductivity • DO • salinity • temperature
Multi-parameter Probe • p. H • conductivity • DO • salinity • temperature
 Van Veen Depth Sampling Van Dorn
Van Veen Depth Sampling Van Dorn
 Vacum Filtration Metals Chlorophyll
Vacum Filtration Metals Chlorophyll
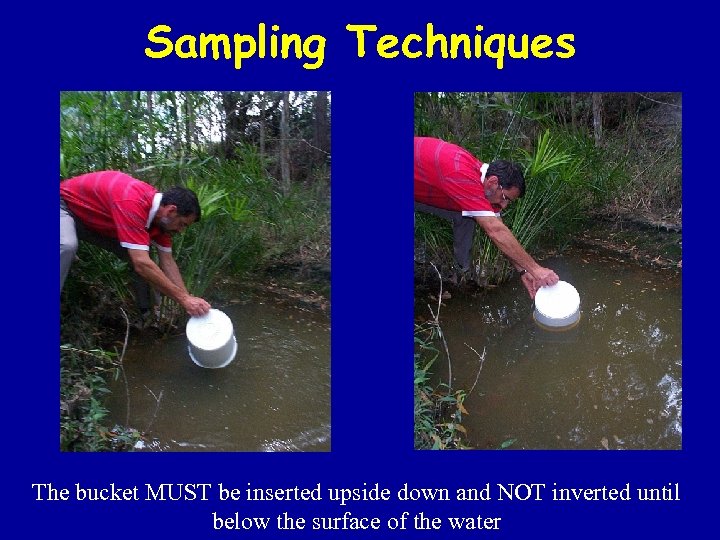 Sampling Techniques The bucket MUST be inserted upside down and NOT inverted until below the surface of the water
Sampling Techniques The bucket MUST be inserted upside down and NOT inverted until below the surface of the water
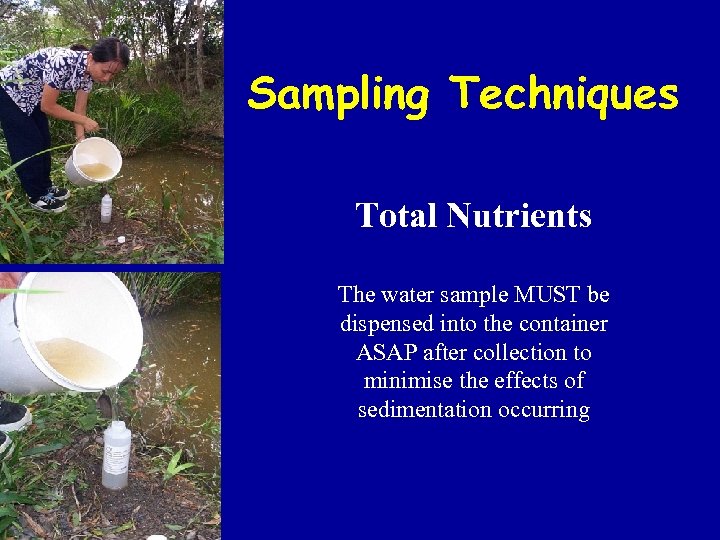 Sampling Techniques Total Nutrients The water sample MUST be dispensed into the container ASAP after collection to minimise the effects of sedimentation occurring
Sampling Techniques Total Nutrients The water sample MUST be dispensed into the container ASAP after collection to minimise the effects of sedimentation occurring
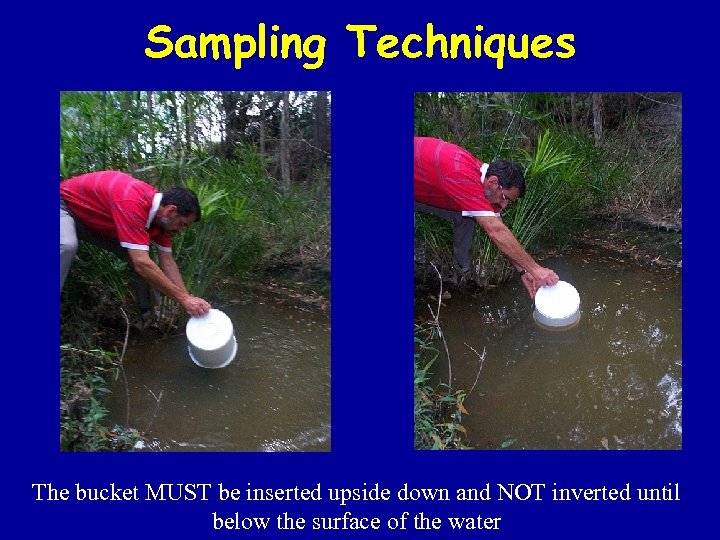 Sampling Techniques The bucket MUST be inserted upside down and NOT inverted until below the surface of the water
Sampling Techniques The bucket MUST be inserted upside down and NOT inverted until below the surface of the water
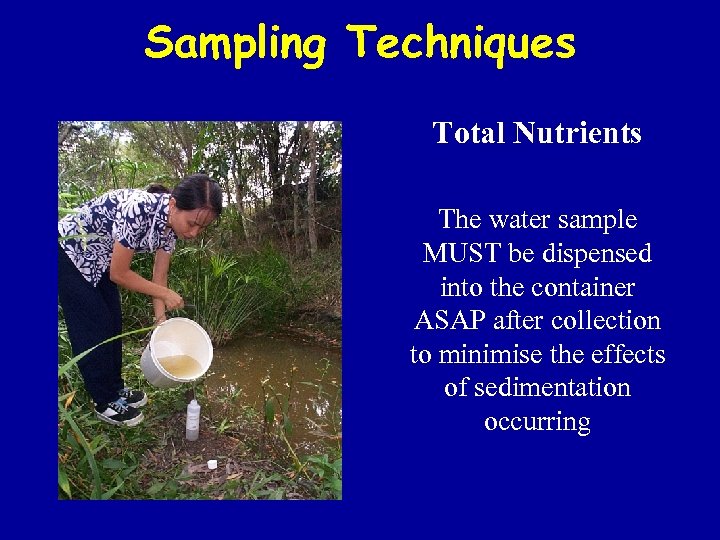 Sampling Techniques Total Nutrients The water sample MUST be dispensed into the container ASAP after collection to minimise the effects of sedimentation occurring
Sampling Techniques Total Nutrients The water sample MUST be dispensed into the container ASAP after collection to minimise the effects of sedimentation occurring
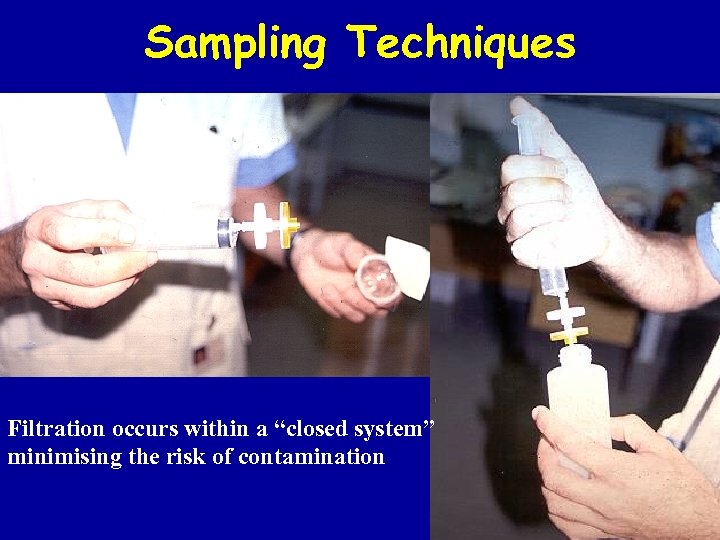 Sampling Techniques Filtration occurs within a “closed system” minimising the risk of contamination
Sampling Techniques Filtration occurs within a “closed system” minimising the risk of contamination
 ? ? ?
? ? ?
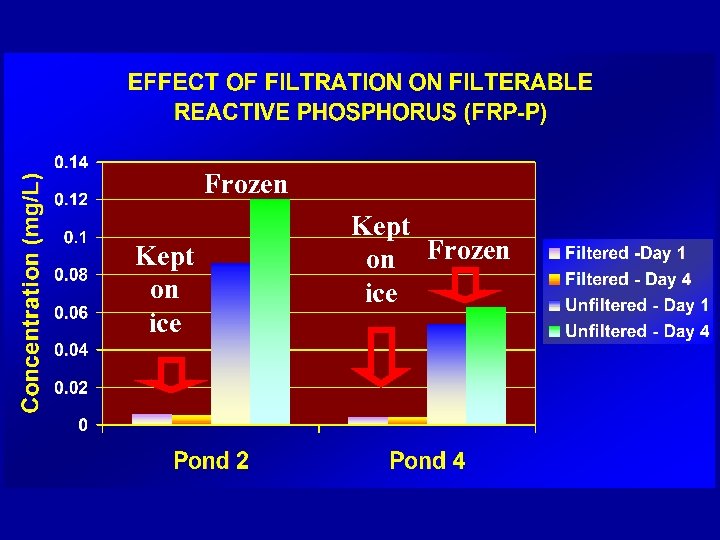 Frozen Kept on ice Kept on Frozen ice
Frozen Kept on ice Kept on Frozen ice
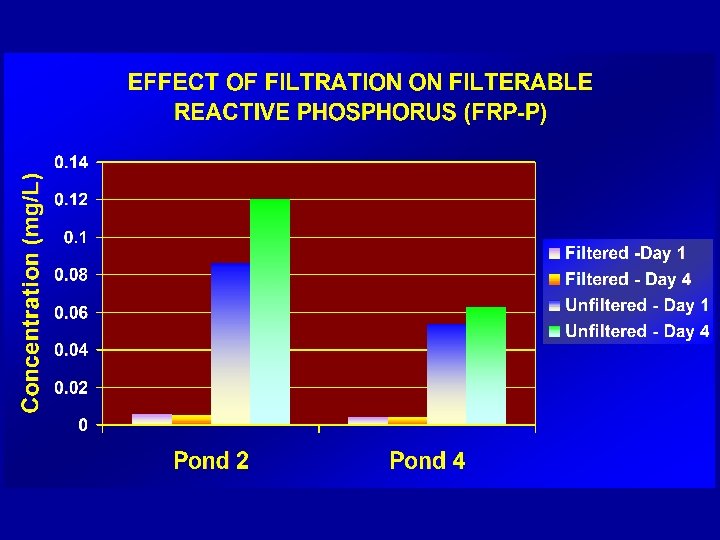
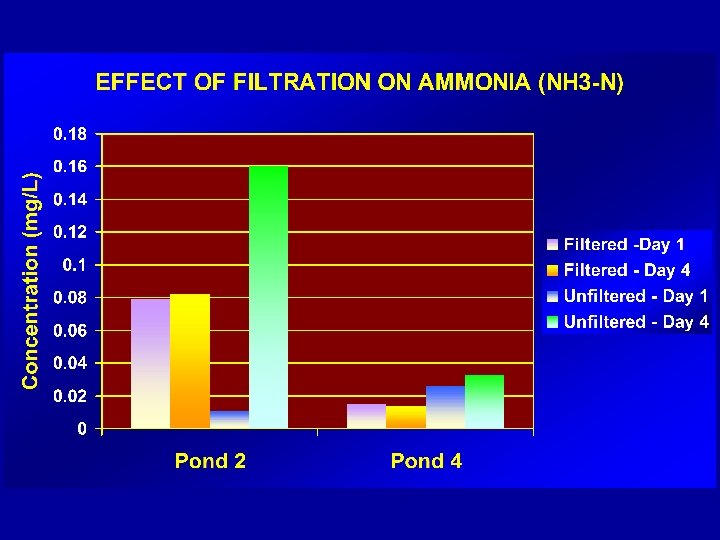
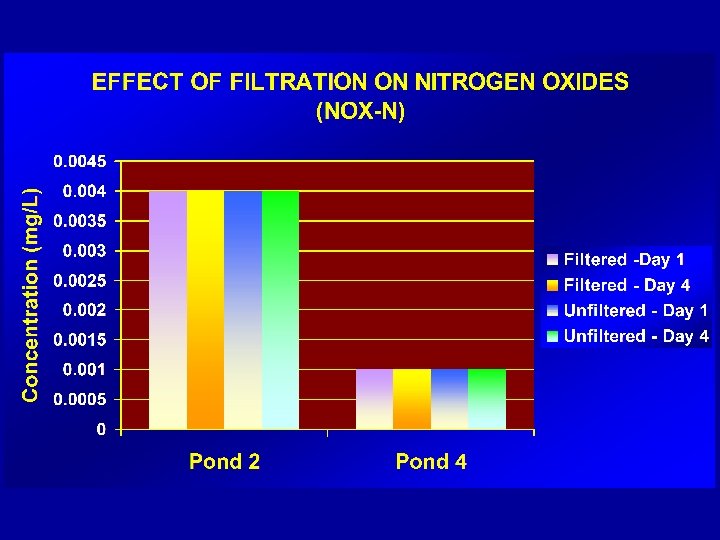
 PRESSURE - PROCEDURE üROBUST üPRACTICAL üSMALL VOLUMES/BOTTLES üMINIMAL CONTAMINATION – (procedure is performed in a “closed environment”) üREPRODUCIBLE üHIGH ACCEPTANCE AMONG FIELD OFFICERS üMEETS REQUIREMENTS OF AS/NZS 5667 -1998 & ISO 5667. 3: 2003)
PRESSURE - PROCEDURE üROBUST üPRACTICAL üSMALL VOLUMES/BOTTLES üMINIMAL CONTAMINATION – (procedure is performed in a “closed environment”) üREPRODUCIBLE üHIGH ACCEPTANCE AMONG FIELD OFFICERS üMEETS REQUIREMENTS OF AS/NZS 5667 -1998 & ISO 5667. 3: 2003)
 ? ? ? • Impossible to model • Impact assessments - meaningless • Potential high costs • incorrect decisions on treatment processes • legal implications • Professional reputation
? ? ? • Impossible to model • Impact assessments - meaningless • Potential high costs • incorrect decisions on treatment processes • legal implications • Professional reputation
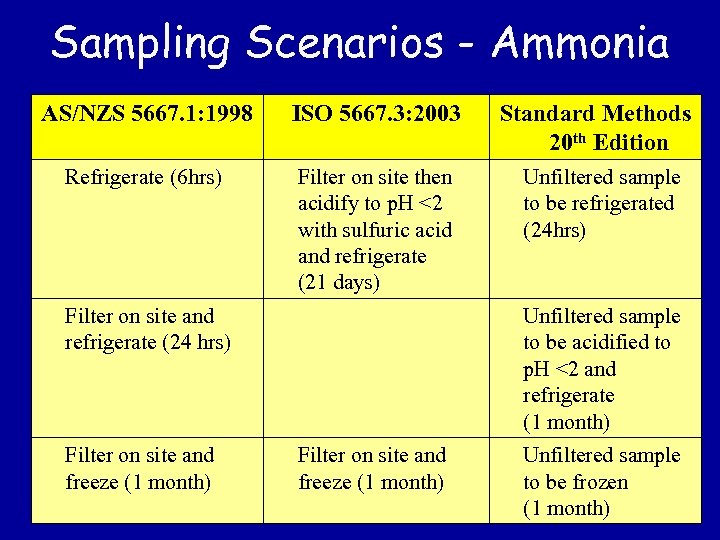 Sampling Scenarios - Ammonia AS/NZS 5667. 1: 1998 ISO 5667. 3: 2003 Refrigerate (6 hrs) Filter on site then acidify to p. H <2 with sulfuric acid and refrigerate (21 days) Filter on site and refrigerate (24 hrs) Filter on site and freeze (1 month) Standard Methods 20 th Edition Unfiltered sample to be refrigerated (24 hrs) Unfiltered sample to be acidified to p. H <2 and refrigerate (1 month) Filter on site and freeze (1 month) Unfiltered sample to be frozen (1 month)
Sampling Scenarios - Ammonia AS/NZS 5667. 1: 1998 ISO 5667. 3: 2003 Refrigerate (6 hrs) Filter on site then acidify to p. H <2 with sulfuric acid and refrigerate (21 days) Filter on site and refrigerate (24 hrs) Filter on site and freeze (1 month) Standard Methods 20 th Edition Unfiltered sample to be refrigerated (24 hrs) Unfiltered sample to be acidified to p. H <2 and refrigerate (1 month) Filter on site and freeze (1 month) Unfiltered sample to be frozen (1 month)
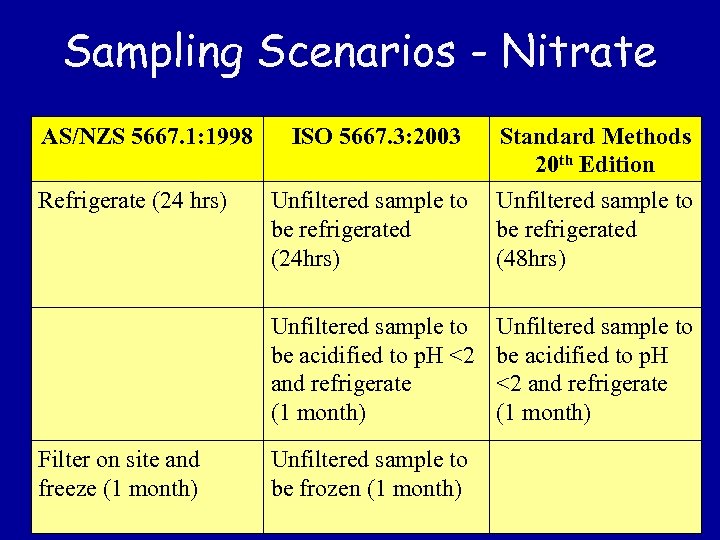 Sampling Scenarios - Nitrate AS/NZS 5667. 1: 1998 Filter on site and freeze (1 month) Unfiltered sample to be refrigerated (24 hrs) Standard Methods 20 th Edition Unfiltered sample to be refrigerated (48 hrs) Unfiltered sample to be acidified to p. H <2 and refrigerate (1 month) Refrigerate (24 hrs) ISO 5667. 3: 2003 Unfiltered sample to be acidified to p. H <2 and refrigerate (1 month) Unfiltered sample to be frozen (1 month)
Sampling Scenarios - Nitrate AS/NZS 5667. 1: 1998 Filter on site and freeze (1 month) Unfiltered sample to be refrigerated (24 hrs) Standard Methods 20 th Edition Unfiltered sample to be refrigerated (48 hrs) Unfiltered sample to be acidified to p. H <2 and refrigerate (1 month) Refrigerate (24 hrs) ISO 5667. 3: 2003 Unfiltered sample to be acidified to p. H <2 and refrigerate (1 month) Unfiltered sample to be frozen (1 month)
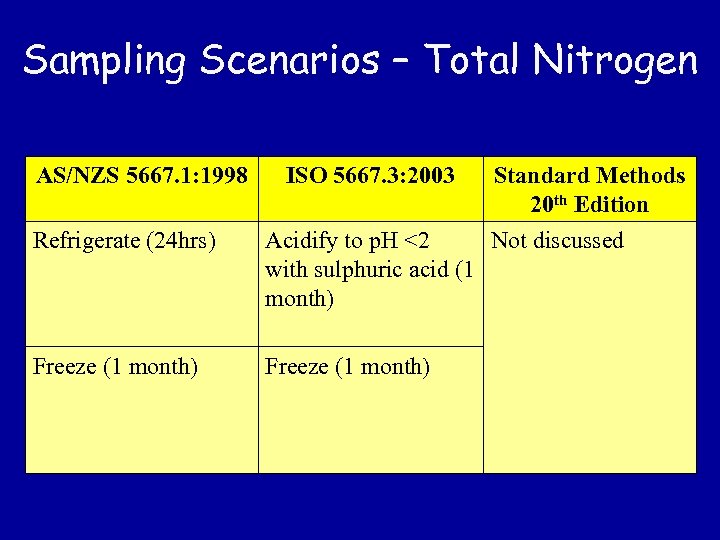 Sampling Scenarios – Total Nitrogen AS/NZS 5667. 1: 1998 ISO 5667. 3: 2003 Standard Methods 20 th Edition Refrigerate (24 hrs) Acidify to p. H <2 Not discussed with sulphuric acid (1 month) Freeze (1 month)
Sampling Scenarios – Total Nitrogen AS/NZS 5667. 1: 1998 ISO 5667. 3: 2003 Standard Methods 20 th Edition Refrigerate (24 hrs) Acidify to p. H <2 Not discussed with sulphuric acid (1 month) Freeze (1 month)
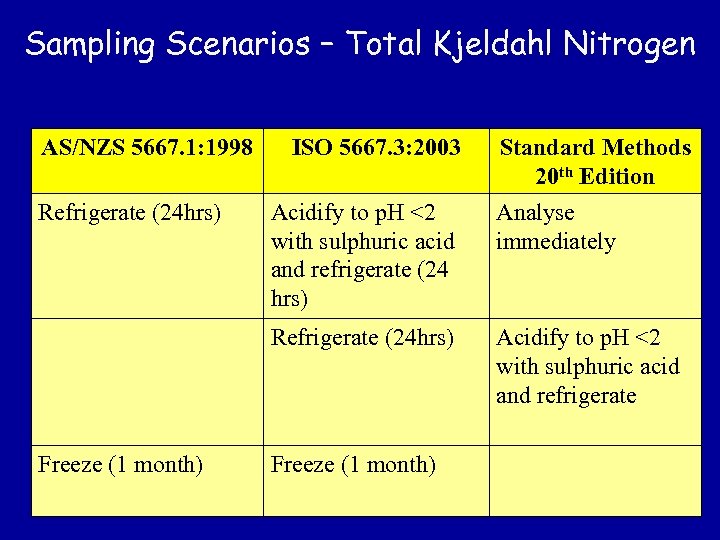 Sampling Scenarios – Total Kjeldahl Nitrogen AS/NZS 5667. 1: 1998 Refrigerate (24 hrs) ISO 5667. 3: 2003 Standard Methods 20 th Edition Analyse immediately Refrigerate (24 hrs) Freeze (1 month) Acidify to p. H <2 with sulphuric acid and refrigerate (24 hrs) Acidify to p. H <2 with sulphuric acid and refrigerate Freeze (1 month)
Sampling Scenarios – Total Kjeldahl Nitrogen AS/NZS 5667. 1: 1998 Refrigerate (24 hrs) ISO 5667. 3: 2003 Standard Methods 20 th Edition Analyse immediately Refrigerate (24 hrs) Freeze (1 month) Acidify to p. H <2 with sulphuric acid and refrigerate (24 hrs) Acidify to p. H <2 with sulphuric acid and refrigerate Freeze (1 month)
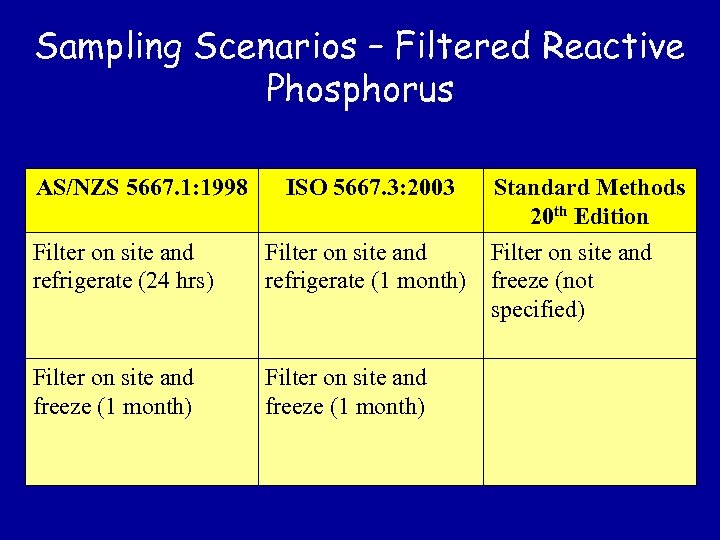 Sampling Scenarios – Filtered Reactive Phosphorus AS/NZS 5667. 1: 1998 ISO 5667. 3: 2003 Filter on site and refrigerate (24 hrs) Filter on site and refrigerate (1 month) Filter on site and freeze (1 month) Standard Methods 20 th Edition Filter on site and freeze (not specified)
Sampling Scenarios – Filtered Reactive Phosphorus AS/NZS 5667. 1: 1998 ISO 5667. 3: 2003 Filter on site and refrigerate (24 hrs) Filter on site and refrigerate (1 month) Filter on site and freeze (1 month) Standard Methods 20 th Edition Filter on site and freeze (not specified)
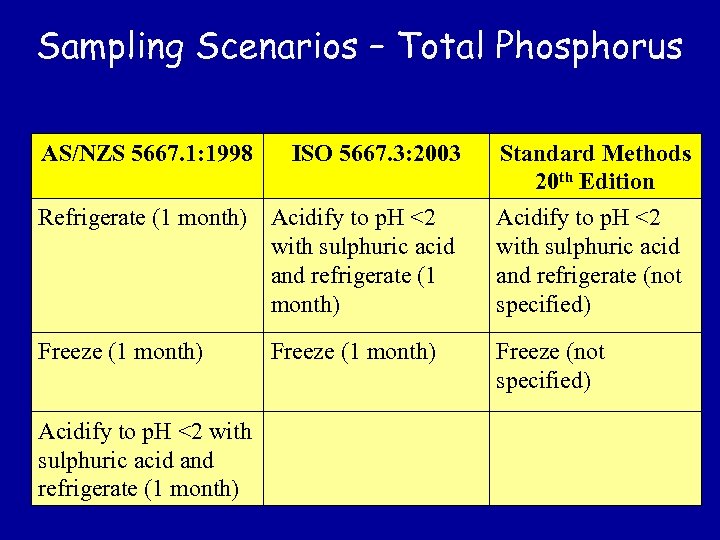 Sampling Scenarios – Total Phosphorus AS/NZS 5667. 1: 1998 ISO 5667. 3: 2003 Standard Methods 20 th Edition Refrigerate (1 month) Acidify to p. H <2 with sulphuric acid and refrigerate (not specified) Freeze (1 month) Freeze (not specified) Acidify to p. H <2 with sulphuric acid and refrigerate (1 month) Freeze (1 month)
Sampling Scenarios – Total Phosphorus AS/NZS 5667. 1: 1998 ISO 5667. 3: 2003 Standard Methods 20 th Edition Refrigerate (1 month) Acidify to p. H <2 with sulphuric acid and refrigerate (not specified) Freeze (1 month) Freeze (not specified) Acidify to p. H <2 with sulphuric acid and refrigerate (1 month) Freeze (1 month)
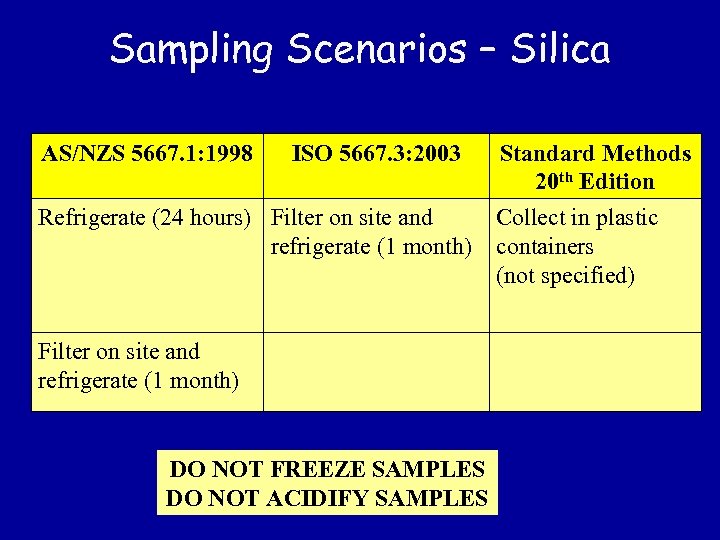 Sampling Scenarios – Silica AS/NZS 5667. 1: 1998 ISO 5667. 3: 2003 Refrigerate (24 hours) Filter on site and refrigerate (1 month) DO NOT FREEZE SAMPLES DO NOT ACIDIFY SAMPLES Standard Methods 20 th Edition Collect in plastic containers (not specified)
Sampling Scenarios – Silica AS/NZS 5667. 1: 1998 ISO 5667. 3: 2003 Refrigerate (24 hours) Filter on site and refrigerate (1 month) DO NOT FREEZE SAMPLES DO NOT ACIDIFY SAMPLES Standard Methods 20 th Edition Collect in plastic containers (not specified)
 Farmer Danny
Farmer Danny



