3686336f158e7302f5dd7fbee8553a9d.ppt
- Количество слайдов: 21

Safety of the factor Xa inhibitor, apixaban, in combination with antiplatelet therapy after acute coronary syndromes: Results from the APPRAISE-1 dose guiding trial John H. Alexander, MD, MHSc Duke Clinical Research Institute, Durham, NC, USA Lars Wallentin, MD, Ph. D Uppsala Clinical Research Center, Uppsala, Sweden On behalf of the APPRAISE Investigators

Disclosures APPRAISE-1 was supported by Bristol-Myers Squibb John Alexander n Research Support: Bristol-Myers Squibb, Medicure, Medtronic Japan, Millennium, NIH, Regado Biosciences, Schering Plough n Consulting: Adolor, Daiichi Sankyo, Medicure, NIH, Novartis Lars Wallentin n Research Support: Astra-Zeneca, Bristol-Myers Squibb, Boehringer -Ingelheim, Lilly, Glaxo. Smith. Klein, Schering-Plough This presentation discusses the unapproved use of apixaban in patients with acute coronary syndromes
Background n Patients with acute coronary syndromes continue to have recurrent ischemic events despite contemporary evidence based care including revascularization and potent antiplatelet therapy. n Oral anticoagulation (warfarin and the direct thrombin inhibitor, ximelagatran) is superior to aspirin following acute coronary syndromes, however warfarin is rarely used because of its narrow therapeutic window and requirement for monitoring. n No placebo controlled trial of oral anticoagulation has been performed in patients taking dual antiplatelet therapy. n Novel anticoagulants offer an opportunity to reduce recurrent ischemic events beyond dual antiplatelet therapy but also pose a risk of bleeding.
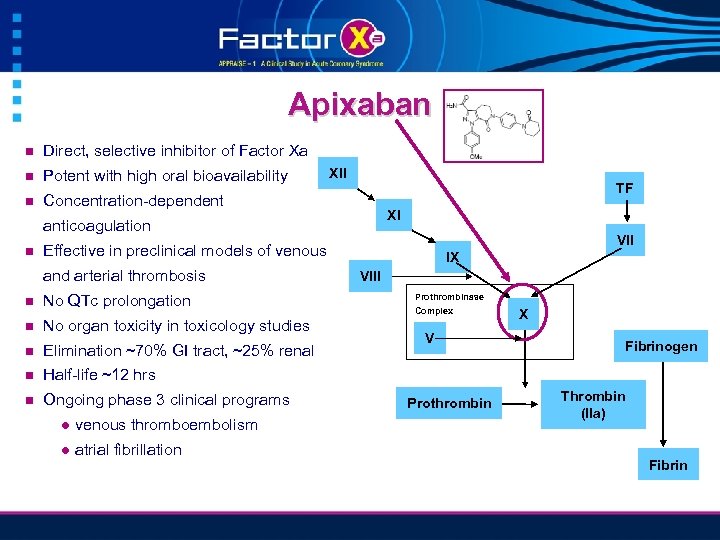
Apixaban n Direct, selective inhibitor of Factor Xa n Potent with high oral bioavailability n Concentration-dependent XII TF XI anticoagulation n VII Effective in preclinical models of venous and arterial thrombosis n No QTc prolongation n No organ toxicity in toxicology studies n Elimination ~70% GI tract, ~25% renal n Ongoing phase 3 clinical programs VIII Half-life ~12 hrs n IX l venous thromboembolism l atrial fibrillation Prothrombinase Complex V Prothrombin X Fibrinogen Thrombin (IIa) Fibrin

APPRAISE Objectives n To evaluate the effect on bleeding of 4 doses of apixaban vs. placebo given over 26 weeks in patients with a recent acute coronary syndrome receiving current evidence based care. n To determine the optimal dose of apixaban for further investigation.
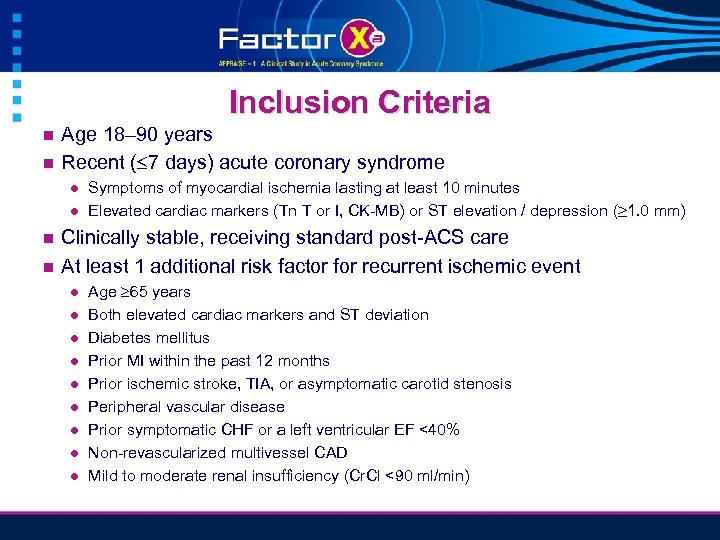
Inclusion Criteria n n Age 18– 90 years Recent ( 7 days) acute coronary syndrome l l n n Symptoms of myocardial ischemia lasting at least 10 minutes Elevated cardiac markers (Tn T or I, CK-MB) or ST elevation / depression (≥ 1. 0 mm) Clinically stable, receiving standard post-ACS care At least 1 additional risk factor for recurrent ischemic event l l l l l Age 65 years Both elevated cardiac markers and ST deviation Diabetes mellitus Prior MI within the past 12 months Prior ischemic stroke, TIA, or asymptomatic carotid stenosis Peripheral vascular disease Prior symptomatic CHF or a left ventricular EF <40% Non-revascularized multivessel CAD Mild to moderate renal insufficiency (Cr. Cl <90 ml/min)

Exclusion Criteria n Planned catheterization, PCI, CABG or other invasive procedure n Persistent severe HTN (SBP ≥ 180, DBP ≥ 110 mm. Hg) n Severe renal dysfunction (Cr. Cl <30 ml/min) n Active bleeding or at high risk for bleeding n Acute pericarditis or pericardial effusion n Recent stroke ( 3 months) n Severe heart failure (NYHA Class IV) n Platelet count 100, 000/mm 3, hemoglobin 10 g/dl n Need for ongoing parenteral or oral anticoagulant n Chronic NSAID or high-dose (>325 mg/day) aspirin
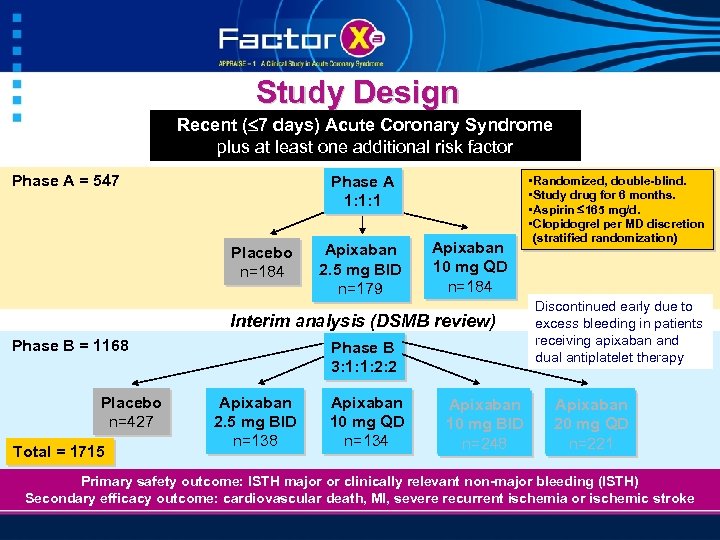
Study Design Recent ( 7 days) Acute Coronary Syndrome plus at least one additional risk factor Phase A = 547 Phase A 1: 1: 1 Placebo n=184 Apixaban 2. 5 mg BID n=179 Apixaban 10 mg QD n=184 Interim analysis (DSMB review) Phase B = 1168 Placebo n=427 Total = 1715 Phase B 3: 1: 1: 2: 2 Apixaban 2. 5 mg BID n=138 Apixaban 10 mg QD n=134 Apixaban 10 mg BID n=248 • Randomized, double-blind. • Study drug for 6 months. • Aspirin 165 mg/d. • Clopidogrel per MD discretion (stratified randomization) Discontinued early due to excess bleeding in patients receiving apixaban and dual antiplatelet therapy Apixaban 20 mg QD n=221 Primary safety outcome: ISTH major or clinically relevant non-major bleeding (ISTH) Secondary efficacy outcome: cardiovascular death, MI, severe recurrent ischemia or ischemic stroke
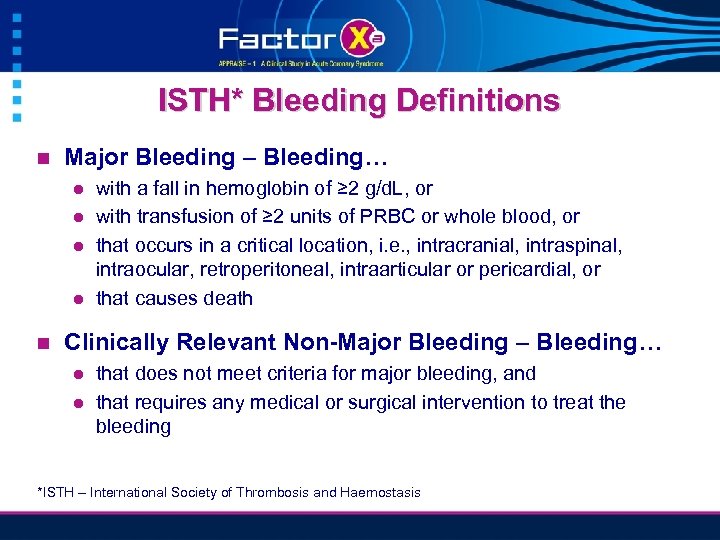
ISTH* Bleeding Definitions n Major Bleeding – Bleeding… l l n with a fall in hemoglobin of ≥ 2 g/d. L, or with transfusion of ≥ 2 units of PRBC or whole blood, or that occurs in a critical location, i. e. , intracranial, intraspinal, intraocular, retroperitoneal, intraarticular or pericardial, or that causes death Clinically Relevant Non-Major Bleeding – Bleeding… l l that does not meet criteria for major bleeding, and that requires any medical or surgical intervention to treat the bleeding *ISTH – International Society of Thrombosis and Haemostasis

APPRAISE Enrollment Europe (plus) 1305 n n n Austria 14 Belgium 62 Denmark 54 France 56 Germany 95 Israel 163 Italy 2 Poland 166 Russia 442 Spain 99 Sweden 111 United Kingdom North America 410 n n Canada 219 United States 191 41
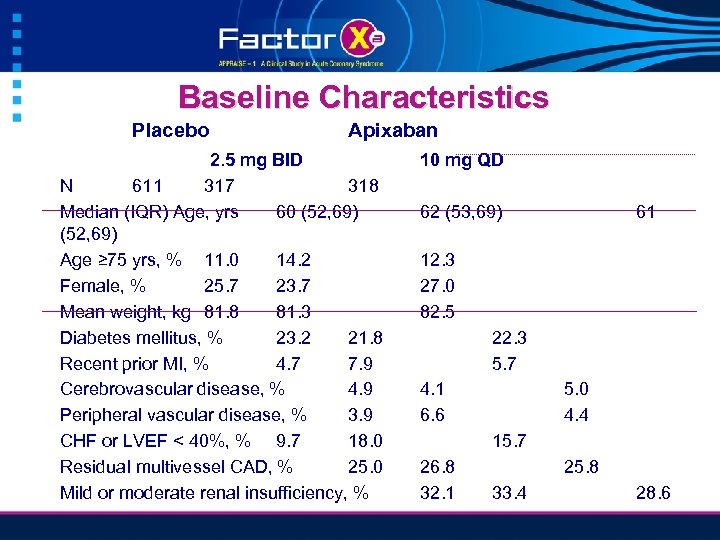
Baseline Characteristics Placebo Apixaban 2. 5 mg BID N 611 317 318 Median (IQR) Age, yrs 60 (52, 69) Age ≥ 75 yrs, % 11. 0 14. 2 Female, % 25. 7 23. 7 Mean weight, kg 81. 8 81. 3 Diabetes mellitus, % 23. 2 21. 8 Recent prior MI, % 4. 7 7. 9 Cerebrovascular disease, % 4. 9 Peripheral vascular disease, % 3. 9 CHF or LVEF < 40%, % 9. 7 18. 0 Residual multivessel CAD, % 25. 0 Mild or moderate renal insufficiency, % 10 mg QD 62 (53, 69) 61 12. 3 27. 0 82. 5 22. 3 5. 7 4. 1 6. 6 5. 0 4. 4 15. 7 26. 8 32. 1 25. 8 33. 4 28. 6
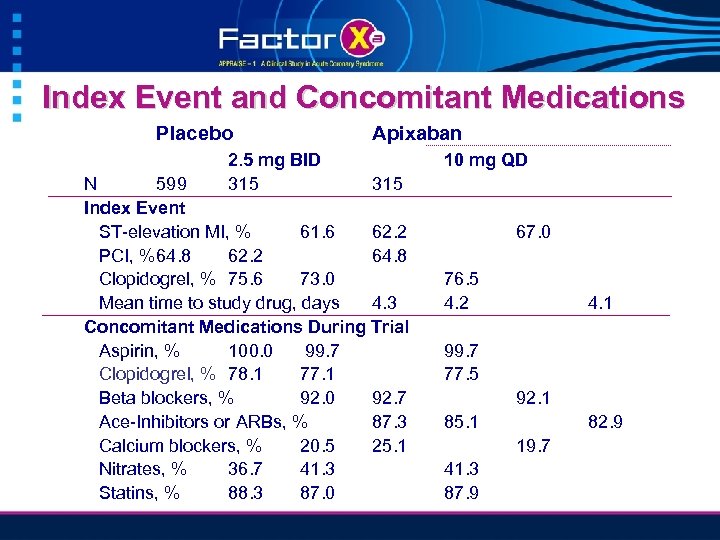
Index Event and Concomitant Medications Placebo 2. 5 mg BID 315 Apixaban N 599 315 Index Event ST-elevation MI, % 61. 6 62. 2 PCI, %64. 8 62. 2 64. 8 Clopidogrel, % 75. 6 73. 0 Mean time to study drug, days 4. 3 Concomitant Medications During Trial Aspirin, % 100. 0 99. 7 Clopidogrel, % 78. 1 77. 1 Beta blockers, % 92. 0 92. 7 Ace-Inhibitors or ARBs, % 87. 3 Calcium blockers, % 20. 5 25. 1 Nitrates, % 36. 7 41. 3 Statins, % 88. 3 87. 0 10 mg QD 67. 0 76. 5 4. 2 4. 1 99. 7 77. 5 92. 1 85. 1 82. 9 19. 7 41. 3 87. 9
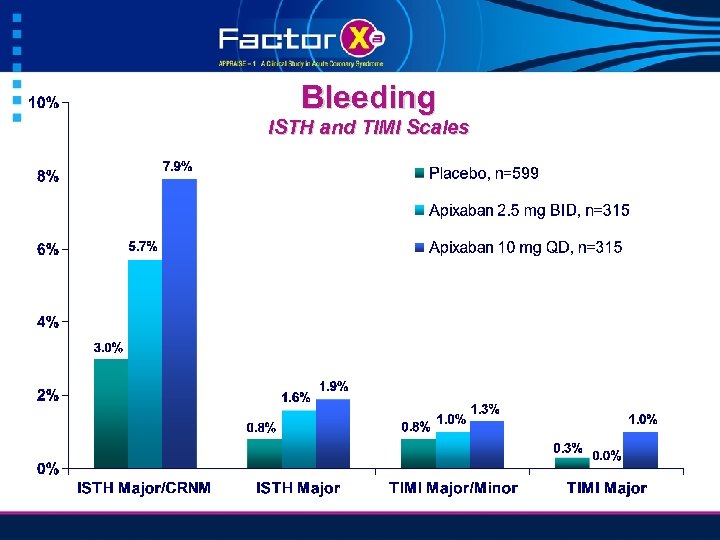
Bleeding ISTH and TIMI Scales
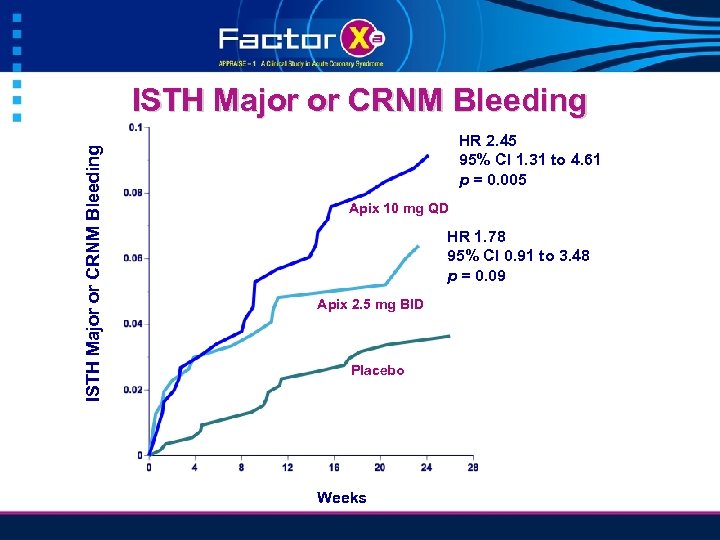
ISTH Major or CRNM Bleeding HR 2. 45 95% CI 1. 31 to 4. 61 p = 0. 005 Apix 10 mg QD HR 1. 78 95% CI 0. 91 to 3. 48 p = 0. 09 Apix 2. 5 mg BID Placebo Weeks
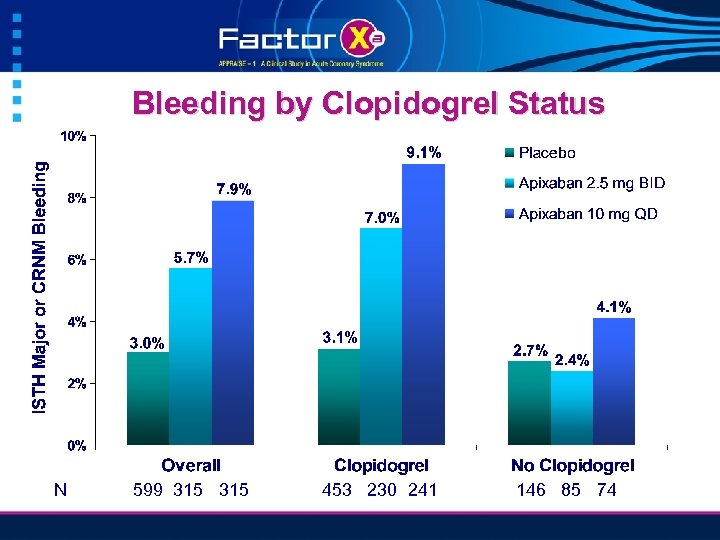
Bleeding by Clopidogrel Status N 599 315 453 230 241 146 85 74
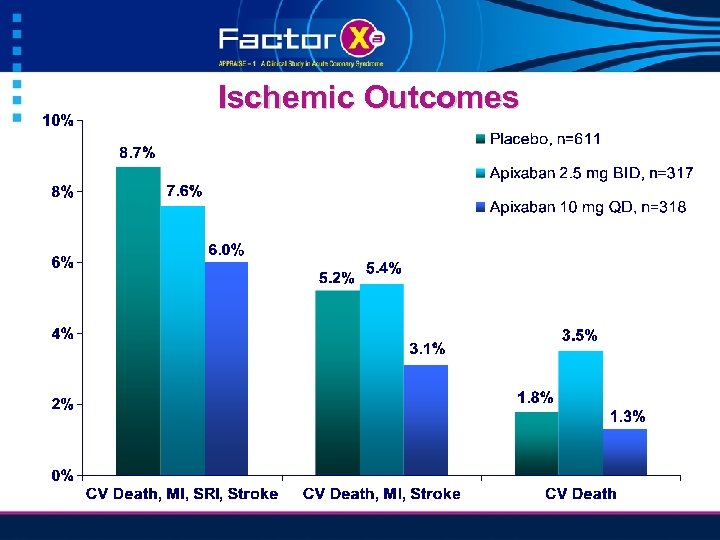
Ischemic Outcomes
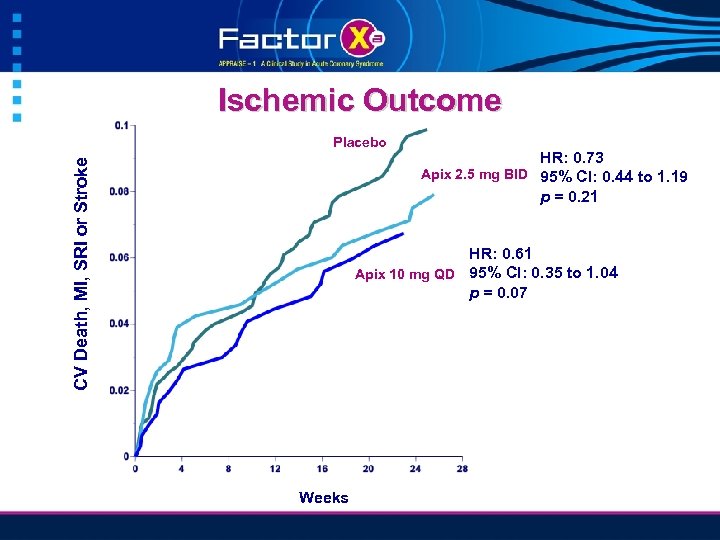
Ischemic Outcome CV Death, MI, SRI or Stroke Placebo HR: 0. 73 Apix 2. 5 mg BID 95% CI: 0. 44 to 1. 19 p = 0. 21 HR: 0. 61 Apix 10 mg QD 95% CI: 0. 35 to 1. 04 p = 0. 07 Weeks
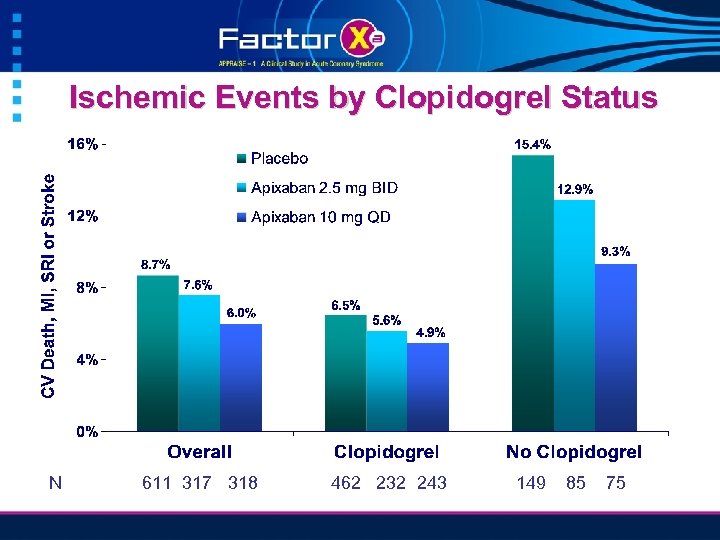
Ischemic Events by Clopidogrel Status N 611 317 318 462 232 243 149 85 75
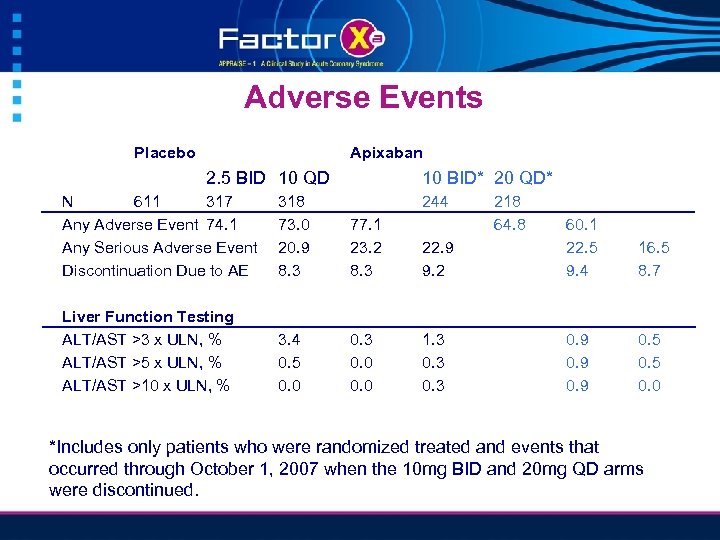
Adverse Events Placebo Apixaban 2. 5 BID 10 QD 10 BID* 20 QD* N 611 317 Any Adverse Event 74. 1 Any Serious Adverse Event Discontinuation Due to AE 318 73. 0 20. 9 8. 3 244 77. 1 23. 2 8. 3 Liver Function Testing ALT/AST >3 x ULN, % ALT/AST >5 x ULN, % ALT/AST >10 x ULN, % 3. 4 0. 5 0. 0 0. 3 0. 0 218 64. 8 22. 9 9. 2 60. 1 22. 5 9. 4 16. 5 8. 7 1. 3 0. 9 0. 5 0. 0 *Includes only patients who were randomized treated and events that occurred through October 1, 2007 when the 10 mg BID and 20 mg QD arms were discontinued.

Conclusions n This is the first experience using anticoagulation with a direct factor Xa inhibitor for secondary prevention in patients with an acute coronary syndrome treated with dual antiplatelet therapy. n We found that the addition of apixaban to contemporary antiplatelet therapy for 6 months following an acute coronary syndrome results in a dose dependent increase in bleeding and a promising trend toward a reduction in clinically important ischemic events. n The relative increase in bleeding and reduction in ischemic events appears similar among patients taking single (aspirin) or dual (aspirin plus clopidogrel) antiplatelet therapy. n Apixaban, at a total daily dose of between 5 and 10 mg, appears promising in patients with recent acute coronary syndromes receiving either aspirin or dual antiplatelet therapy and deserves further clinical investigation.

Study Organization n APPRAISE Investigators and Research Coordinators n Steering Committee (National Coordinators): Lars Wallentin and Robert Harrington (co-chairs), John Alexander (PI, USA), Richard Becker, Deepak Bhatt, Frank Cools (Belgium), Filippo Crea (Italy), Harald Darius (Germany), Mikael Dellborg (Sweden), Keith Fox (UK), Shaun Goodman (Canada), Kurt Huber (Austria), Steen Husted (Denmark), Basil Lewis (Israel), Jose Lopez. Sendon (Spain), Puneet Mohan (BMS), Giles Montalescot (France), Mikhail Ruda (Russia), Witold Ruzyllo (Poland), Freek Verheugt. n Data Monitoring Committee: Maarten Simoons (chair), Eric Boersma, James De. Lemos, Fred Spencer n DCRI (CEC, Stats): Kenneth Mahaffey, Meredith Smith, Laura Melton, Robert Clare n CRO (PPD): Clark Weaver, Keven Griffith n Sponsor (BMS): Rajnish Saini, Leigh Townes, Heather Knowles, Helen He
3686336f158e7302f5dd7fbee8553a9d.ppt