26f92395cc4e480644b7fee6966a67df.ppt
- Количество слайдов: 23
 Safety of smoking cessation medications Last updated March 2011
Safety of smoking cessation medications Last updated March 2011
 Safety section Chair Neal Benowitz Carolyn Dresler Steve S Hecht John Hughes Anne M Joseph Jacques Le Houezec Cheryl Oncken Maxine Stitzer Last updated March 2011 University of California San Francisco, USA Arkansas Department of Health, USA University of Minnesota, USA University of Vermont, USA Minneapolis VA Medical Center, USA Freelance consultant (France), and University of Nottingham, UK University of Conneticut, USA Johns Hopkins University, USA
Safety section Chair Neal Benowitz Carolyn Dresler Steve S Hecht John Hughes Anne M Joseph Jacques Le Houezec Cheryl Oncken Maxine Stitzer Last updated March 2011 University of California San Francisco, USA Arkansas Department of Health, USA University of Minnesota, USA University of Vermont, USA Minneapolis VA Medical Center, USA Freelance consultant (France), and University of Nottingham, UK University of Conneticut, USA Johns Hopkins University, USA
 Nicotine vs. tobacco smoke • The main adverse effect of nicotine from tobacco is addiction, which sustains tobacco use 1 -2 • Other toxicants in tobacco smoke, not nicotine, are responsible for majority of adverse health effects 3, 4 – > 4000 different chemicals – tar, carbon monoxide, irritant and oxidant gases – > 80 carcinogens (11 are group 1 carcinogens)5 • Nicotine dependence leads to continued exposure to toxicants in tobacco smoke 1 USDHHS. The Health Consequences of Smoking: Nicotine Addition. 1988. 2 Royal College of Physicians. London: 2008. 3 Benowitrz. In Nicotine Safety and Toxicity; pp 185– 195 NY: OUP. 4 Hoffman and Hoffman. J Toxicol Environ Health 1997; 50: 307– 64. 5 Smith et al. Food Chem Toxicol 2003; 41: 807– 817. Last updated March 2011
Nicotine vs. tobacco smoke • The main adverse effect of nicotine from tobacco is addiction, which sustains tobacco use 1 -2 • Other toxicants in tobacco smoke, not nicotine, are responsible for majority of adverse health effects 3, 4 – > 4000 different chemicals – tar, carbon monoxide, irritant and oxidant gases – > 80 carcinogens (11 are group 1 carcinogens)5 • Nicotine dependence leads to continued exposure to toxicants in tobacco smoke 1 USDHHS. The Health Consequences of Smoking: Nicotine Addition. 1988. 2 Royal College of Physicians. London: 2008. 3 Benowitrz. In Nicotine Safety and Toxicity; pp 185– 195 NY: OUP. 4 Hoffman and Hoffman. J Toxicol Environ Health 1997; 50: 307– 64. 5 Smith et al. Food Chem Toxicol 2003; 41: 807– 817. Last updated March 2011
 Nicotine and cancer • Nicotine per se is not a substantial cause of cancer 1 -2 • Any cancer-related risks from short-term NRT are insignificant compared to the risks of smoking • Other tobacco smoke constituents are believed to be responsible for cancers 3, 4 1 US Department of Health and Human Services, Bethesda, MD, 2001. 2 Surgeon General's report 2010 3 Hoffman and Hecht. IN Handbook of Experimental Pharmacolgy, pp 63– 102; 1990 Heidelberg: Springer-Verlag 4 Hecht. J Natl Cancer Inst. 1999; 91: 1194– 1210. Last updated March 2011
Nicotine and cancer • Nicotine per se is not a substantial cause of cancer 1 -2 • Any cancer-related risks from short-term NRT are insignificant compared to the risks of smoking • Other tobacco smoke constituents are believed to be responsible for cancers 3, 4 1 US Department of Health and Human Services, Bethesda, MD, 2001. 2 Surgeon General's report 2010 3 Hoffman and Hecht. IN Handbook of Experimental Pharmacolgy, pp 63– 102; 1990 Heidelberg: Springer-Verlag 4 Hecht. J Natl Cancer Inst. 1999; 91: 1194– 1210. Last updated March 2011
 Nicotine and cancer • Studies carried out in rodents demonstrate that under normal conditions nicotine is not carcinogenic 1 • Carcinogenic, nicotine-derived nitrosamines may be formed in the body under certain conditions 2– 4 – levels would be low and represent minimal risk during short term NRT – further research required to establish any risk associated with long term use of NRT • Nicotine can inhibit apoptosis (cell death) and enhance angiogenesis in cell and experimental animal test systems 5 -9 • This could in theory promote the spread of cancers. However this risk has not been documented in people 1 US Department of Health and Human Services, Bethesda, MD, 2001. 2 Benowitz et al. J Pharmacol Exp Ther 1994; 268: 296– 303. 3 Hecht et al. Proc Natl Acad Sci USA. 2000; 97: 12493 -12497. 4 Carmella et al. Carcinogenesis 1997; 18: 101– 106. Last updated March 2011 5 Porubin et al. J Agric Food Chem, 2007; 55: 7199 -7204. 6 Stepanov et al. Cancer Res. 2009; 69: 8236 -8240. 7 Cooke. Life Sci, 2007; 80: 2347 -2351. 8 West et al. J Clin Invest, 2003; 111: 81 -90. 9 Dasgupta et al. Proc Natl Acad Sci USA, 2006; 103: 6332 -6337.
Nicotine and cancer • Studies carried out in rodents demonstrate that under normal conditions nicotine is not carcinogenic 1 • Carcinogenic, nicotine-derived nitrosamines may be formed in the body under certain conditions 2– 4 – levels would be low and represent minimal risk during short term NRT – further research required to establish any risk associated with long term use of NRT • Nicotine can inhibit apoptosis (cell death) and enhance angiogenesis in cell and experimental animal test systems 5 -9 • This could in theory promote the spread of cancers. However this risk has not been documented in people 1 US Department of Health and Human Services, Bethesda, MD, 2001. 2 Benowitz et al. J Pharmacol Exp Ther 1994; 268: 296– 303. 3 Hecht et al. Proc Natl Acad Sci USA. 2000; 97: 12493 -12497. 4 Carmella et al. Carcinogenesis 1997; 18: 101– 106. Last updated March 2011 5 Porubin et al. J Agric Food Chem, 2007; 55: 7199 -7204. 6 Stepanov et al. Cancer Res. 2009; 69: 8236 -8240. 7 Cooke. Life Sci, 2007; 80: 2347 -2351. 8 West et al. J Clin Invest, 2003; 111: 81 -90. 9 Dasgupta et al. Proc Natl Acad Sci USA, 2006; 103: 6332 -6337.
 Smoking and cardiovascular disease • Smoking increases risk of CVD • Smoking-related factors contributing to CV risk: – – increased thrombogenesis carbon monoxide oxidative damage hyperlipidaemia • Smoking cessation significantly reduces risk within 1– 3 years 1 -2 • Smoking cessation using NRT has a favourable effect on these contributing factors 3 1 Rosenberg et al. N Engl J Med. 1990; 322: 213– 217. 2 Rosenberg et al. N Engl J Med. 1985; 313: 1511– 1514. 3 Ludviksdottir et al. J Intern Med. 1999; 246: 61– 66. Last updated March 2011
Smoking and cardiovascular disease • Smoking increases risk of CVD • Smoking-related factors contributing to CV risk: – – increased thrombogenesis carbon monoxide oxidative damage hyperlipidaemia • Smoking cessation significantly reduces risk within 1– 3 years 1 -2 • Smoking cessation using NRT has a favourable effect on these contributing factors 3 1 Rosenberg et al. N Engl J Med. 1990; 322: 213– 217. 2 Rosenberg et al. N Engl J Med. 1985; 313: 1511– 1514. 3 Ludviksdottir et al. J Intern Med. 1999; 246: 61– 66. Last updated March 2011
 NRT and cardiovascular disease • NRT can be used safely by majority of people with cardiovascular disease, even with concomitant smoking 1– 3 • Meta-analyses show no difference in rate of acute MI between NRT patch and placebo 4 -6 – N. B. majority of studies excluded patients with CVD at baseline • The benefits of NRT outweigh the risks, even in smokers with cardiovascular disease 7– 9 1 Joseph et al. N Engl J Med. 1996; 335: 1792– 1798. 2 Tzivoni et al. Cardiovasc Drugs Ther. 1998; 12: 239– 244. 3 Working Group. Arch Intern Med. 1994; 154: 989– 995. 4 Greenland et al. Drug Saf. 1998; 18: 297– 308. Last updated March 2011 5 Mills et al. Tob Induc Dis. 2010; 8: 8. 6 Hays & Ebbert. Drugs. 2010; 70: 2357 -2372. 7 Benowitz. Prog Cardiovasc Dis. 2003; 46: 91 -111. 8 Joseph & Fu. Prog Cardiovasc Dis. 2003; 45: 429 -441. 9 Hubbard et al. Tob Control. 2005; 14: 416– 21.
NRT and cardiovascular disease • NRT can be used safely by majority of people with cardiovascular disease, even with concomitant smoking 1– 3 • Meta-analyses show no difference in rate of acute MI between NRT patch and placebo 4 -6 – N. B. majority of studies excluded patients with CVD at baseline • The benefits of NRT outweigh the risks, even in smokers with cardiovascular disease 7– 9 1 Joseph et al. N Engl J Med. 1996; 335: 1792– 1798. 2 Tzivoni et al. Cardiovasc Drugs Ther. 1998; 12: 239– 244. 3 Working Group. Arch Intern Med. 1994; 154: 989– 995. 4 Greenland et al. Drug Saf. 1998; 18: 297– 308. Last updated March 2011 5 Mills et al. Tob Induc Dis. 2010; 8: 8. 6 Hays & Ebbert. Drugs. 2010; 70: 2357 -2372. 7 Benowitz. Prog Cardiovasc Dis. 2003; 46: 91 -111. 8 Joseph & Fu. Prog Cardiovasc Dis. 2003; 45: 429 -441. 9 Hubbard et al. Tob Control. 2005; 14: 416– 21.
 Smoking, NRT and pregnancy • Maternal smoking is associated with poor pregnancy and childhood outcomes 1– 5 • Many toxicants in tobacco smoke could be responsible • Nicotine is a potential foetal teratogen • Nicotine may contribute to obstetrical complications in pregnant women and to sudden infant death syndrome 6, 7 • Benefits of NRT outweigh the risks of smoking during pregnancy 8 – reduce or eliminate the exposure of fetus to other toxicants in tobacco smoke – reduce overall dose and duration of exposure to nicotine 1 USDHHS. Surgeon General's Report. 2001. 2 Steyn et al. Paediatr Perinat Epidemiol. 2006; 20: 90 -99. 3 Fried et al. Neurotoxicol Teratol. 1998; 20: 293 -306. 4 Jacobsen et al. Neuropsychopharmacology. 2007; 32: 2453 -2464. Last updated March 2011 5 Romano et al. Pediatrics, 2006; 117: 2101 -2110. 6 Slotkin et al. . Brain Res Bull. 1995; 38: 69 -75. 7 Slotkin et al. Neuropsychopharmacology. 2006; 31: 2462 -2475. 8 Benowitz & Dempsey. Nicotine Tob Res. 2004; 6 Suppl 2: S 189 -202.
Smoking, NRT and pregnancy • Maternal smoking is associated with poor pregnancy and childhood outcomes 1– 5 • Many toxicants in tobacco smoke could be responsible • Nicotine is a potential foetal teratogen • Nicotine may contribute to obstetrical complications in pregnant women and to sudden infant death syndrome 6, 7 • Benefits of NRT outweigh the risks of smoking during pregnancy 8 – reduce or eliminate the exposure of fetus to other toxicants in tobacco smoke – reduce overall dose and duration of exposure to nicotine 1 USDHHS. Surgeon General's Report. 2001. 2 Steyn et al. Paediatr Perinat Epidemiol. 2006; 20: 90 -99. 3 Fried et al. Neurotoxicol Teratol. 1998; 20: 293 -306. 4 Jacobsen et al. Neuropsychopharmacology. 2007; 32: 2453 -2464. Last updated March 2011 5 Romano et al. Pediatrics, 2006; 117: 2101 -2110. 6 Slotkin et al. . Brain Res Bull. 1995; 38: 69 -75. 7 Slotkin et al. Neuropsychopharmacology. 2006; 31: 2462 -2475. 8 Benowitz & Dempsey. Nicotine Tob Res. 2004; 6 Suppl 2: S 189 -202.
 Nicotine replacement therapy • NRT delivers nicotine without the toxicants from tobacco 1 • NRT helps combat the symptoms of withdrawal 2 • Nicotine dose from NRT is lower and administered more gradually than with smoking and this reduces the addictive potential 1, 3 1 Benowitz & Gourlay. J Am Coll Cardiol. 1997; 29: 1422 -1431. 2 Silagy et al. Cochrane Database Syst Rev. 2004; (3): CD 000146. 3 Le Houezec. Int J Tuberc Lung Dis. 2003; 7: 811 -819. Last updated March 2011
Nicotine replacement therapy • NRT delivers nicotine without the toxicants from tobacco 1 • NRT helps combat the symptoms of withdrawal 2 • Nicotine dose from NRT is lower and administered more gradually than with smoking and this reduces the addictive potential 1, 3 1 Benowitz & Gourlay. J Am Coll Cardiol. 1997; 29: 1422 -1431. 2 Silagy et al. Cochrane Database Syst Rev. 2004; (3): CD 000146. 3 Le Houezec. Int J Tuberc Lung Dis. 2003; 7: 811 -819. Last updated March 2011
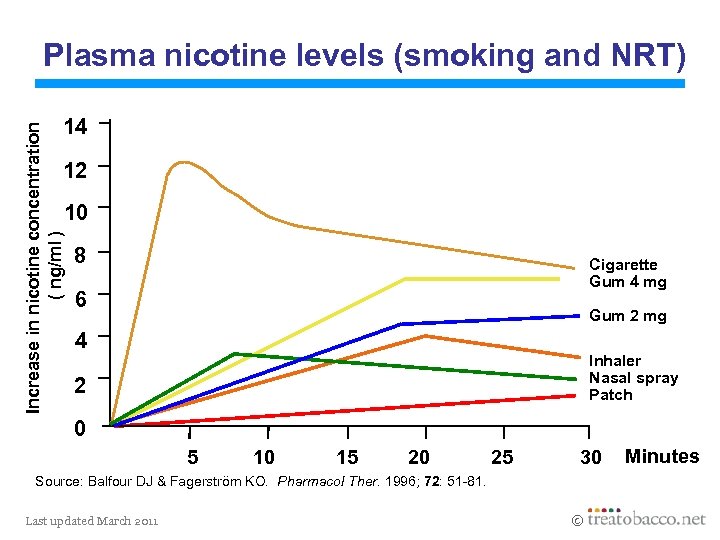 Plasma nicotine levels (smoking and NRT) Increase in nicotine concentration ( ng/ml ) 14 12 10 8 Cigarette Gum 4 mg 6 Gum 2 mg 4 Inhaler Nasal spray Patch 2 0 5 10 15 20 25 30 Source: Balfour DJ & Fagerström KO. Pharmacol Ther. 1996; 72: 51 -81. Last updated March 2011 Minutes
Plasma nicotine levels (smoking and NRT) Increase in nicotine concentration ( ng/ml ) 14 12 10 8 Cigarette Gum 4 mg 6 Gum 2 mg 4 Inhaler Nasal spray Patch 2 0 5 10 15 20 25 30 Source: Balfour DJ & Fagerström KO. Pharmacol Ther. 1996; 72: 51 -81. Last updated March 2011 Minutes
 Abuse liability of NRT • Prevalence of abuse and dependence with current NRT products is nil (patch) or very low (<10% gum, nasal spray, inhaler)1 • Likely to be greatest with products that deliver nicotine rapidly, but less than that of cigarettes 2– 4 • Even if dependence develops, there is likely to be overall health benefit if individual no longer smokes 1 West et al. Psychopharmacology. 2000; 149: 198 -202. 2 Stitzer & de Wit. In: Benowitz, NL. Nicotine Safety and Toxicity. pp. 1998 119 -131 New York, Oxford University Press. 3 Henningfield & Keenan. J Consult Clin Psychol. 1993; 61: 743 -750. 4 Hughes. In Nicotine Safety and Toxicity, 1998; pp. 147 -157. New York: OUP Last updated March 2011
Abuse liability of NRT • Prevalence of abuse and dependence with current NRT products is nil (patch) or very low (<10% gum, nasal spray, inhaler)1 • Likely to be greatest with products that deliver nicotine rapidly, but less than that of cigarettes 2– 4 • Even if dependence develops, there is likely to be overall health benefit if individual no longer smokes 1 West et al. Psychopharmacology. 2000; 149: 198 -202. 2 Stitzer & de Wit. In: Benowitz, NL. Nicotine Safety and Toxicity. pp. 1998 119 -131 New York, Oxford University Press. 3 Henningfield & Keenan. J Consult Clin Psychol. 1993; 61: 743 -750. 4 Hughes. In Nicotine Safety and Toxicity, 1998; pp. 147 -157. New York: OUP Last updated March 2011
 Safety of Bupropion • Bupropion is generally well tolerated by smokers • The benefits of buproprion to aid smoking cessation outweigh the risks of buproprion in most smokers 1– 3 • Clinical trials of bupropion in smokers with cardiovascular disease found no significant adverse cardiovascular side effects 4 -5 • Data on safety in pregnancy are inconclusive • Bupropion appears not to have significant abuse liability 6 – 9 1 Hurt et al. N Engl J Med. 1997; 337: 1195 -1202. 2 Jorenby et al. N Engl J Med. 1999; 340: 685 -691. 3 Cox et al. J Gen Intern Med. 2004; 19: 828 -834. 4 Joseph & Fu. Prog Cardiovasc Dis. 2003; 45: 429 -441. 5 Tonstad et al. Eur Heart J. 2003; 24: 946 -955. Last updated March 2011 6 Peck et al. Br J Clin Pharmacol. 1979; 7: 469 -78. 7 Miller & Griffith. Psychopharmacology. 1983; 80: 199 -205. 8 Margolin et al. Drug Alcohol Depend. 1995; 40: 125 -31. 9 Rush et al. Exp and Clin Psychopharmacol. 1998; 6: 32 -44.
Safety of Bupropion • Bupropion is generally well tolerated by smokers • The benefits of buproprion to aid smoking cessation outweigh the risks of buproprion in most smokers 1– 3 • Clinical trials of bupropion in smokers with cardiovascular disease found no significant adverse cardiovascular side effects 4 -5 • Data on safety in pregnancy are inconclusive • Bupropion appears not to have significant abuse liability 6 – 9 1 Hurt et al. N Engl J Med. 1997; 337: 1195 -1202. 2 Jorenby et al. N Engl J Med. 1999; 340: 685 -691. 3 Cox et al. J Gen Intern Med. 2004; 19: 828 -834. 4 Joseph & Fu. Prog Cardiovasc Dis. 2003; 45: 429 -441. 5 Tonstad et al. Eur Heart J. 2003; 24: 946 -955. Last updated March 2011 6 Peck et al. Br J Clin Pharmacol. 1979; 7: 469 -78. 7 Miller & Griffith. Psychopharmacology. 1983; 80: 199 -205. 8 Margolin et al. Drug Alcohol Depend. 1995; 40: 125 -31. 9 Rush et al. Exp and Clin Psychopharmacol. 1998; 6: 32 -44.
 Safety of Varenicline • Varenicline is generally well tolerated by smokers 1 -3 • The most common side effect is nausea (30 -40%), sometimes with vomiting 4 -6 • Varenicline is licensed for all smokers 18, except those with severe renal impairment, pregnant or breastfeeding smokers • Warnings have been issued after a number of reports of psychiatric disturbances, including suicide in smokers taking varenicline, but not observed in studies 7 -8 1 Williams et al. Curr Med Res Opin. 2007; 23: 793 -801. 2 Cahill et al. Cochrane Database Syst Rev. 2011; (2): CD 006103. 3 Stapleton et al. Addiction. 2008; 103: 146 -54. 4 Gonzales et al. JAMA. 2006; 296: 47 -55. Last updated March 2011 5 Jorenby et al. JAMA. 2006; 296: 56 -63. Erratum in: JAMA, 2006; 296: 1355. 6 Tonstad et al. JAMA. 2006; 296: 64 -71. 7 Gunnell et al. BMJ. 2009; 339: b 3805. 8 Stapleton et al. Addiction. 2008; 103: 146 -154.
Safety of Varenicline • Varenicline is generally well tolerated by smokers 1 -3 • The most common side effect is nausea (30 -40%), sometimes with vomiting 4 -6 • Varenicline is licensed for all smokers 18, except those with severe renal impairment, pregnant or breastfeeding smokers • Warnings have been issued after a number of reports of psychiatric disturbances, including suicide in smokers taking varenicline, but not observed in studies 7 -8 1 Williams et al. Curr Med Res Opin. 2007; 23: 793 -801. 2 Cahill et al. Cochrane Database Syst Rev. 2011; (2): CD 006103. 3 Stapleton et al. Addiction. 2008; 103: 146 -54. 4 Gonzales et al. JAMA. 2006; 296: 47 -55. Last updated March 2011 5 Jorenby et al. JAMA. 2006; 296: 56 -63. Erratum in: JAMA, 2006; 296: 1355. 6 Tonstad et al. JAMA. 2006; 296: 64 -71. 7 Gunnell et al. BMJ. 2009; 339: b 3805. 8 Stapleton et al. Addiction. 2008; 103: 146 -154.
 Safety of other medications • Nortriptyline, moclobemide and clonidine have been found in smoking cessation trials in healthy smokers to be safe in doses approved for the treatment of depression/hypertension 1 -5 • Not approved by regulatory authorities for such use • All of these drugs are associated with severe toxicities in overdose 1 Hughes et al. Cochrane Database Syst Rev. 2007; (1): CD 000031. 2 Hughes et al. Nicotine Tob Res. 2005; 7: 491 -499. 3 Berlin et al. Clin Pharmacol Ther. 1995; 58: 444 -452. 4 Gourlay et al. Cochrane Database Syst Rev. 2004; (3): CD 000058. 5 Fiore. Respir Care. 2000; 45: 1200 -1262. Last updated March 2011
Safety of other medications • Nortriptyline, moclobemide and clonidine have been found in smoking cessation trials in healthy smokers to be safe in doses approved for the treatment of depression/hypertension 1 -5 • Not approved by regulatory authorities for such use • All of these drugs are associated with severe toxicities in overdose 1 Hughes et al. Cochrane Database Syst Rev. 2007; (1): CD 000031. 2 Hughes et al. Nicotine Tob Res. 2005; 7: 491 -499. 3 Berlin et al. Clin Pharmacol Ther. 1995; 58: 444 -452. 4 Gourlay et al. Cochrane Database Syst Rev. 2004; (3): CD 000058. 5 Fiore. Respir Care. 2000; 45: 1200 -1262. Last updated March 2011
 Concomitant smoking • Concomitant use of NRT, bupropion or varenicline and smoking is well tolerated 1– 7 • Number of cigarettes smoked is likely to be less than at baseline • This might result in reduced health hazards • Such benefit has yet to be scientifically demonstrated • NRT-assisted reduction does increase later quitting 8 -12 1 Benowitz et al. J Pharmacol Exp Ther. 1998; 287: 958 -962 2 Bjornson-Benson et al. Addict Behav. 1993; 18: 491 -502. 3 Hurt et al. N Engl J Med. 1997; 337: 1195 -202. 4 Jorenby et al. N Engl J Med. 1999; 340: 685 -91. 5 Zevin et al. Clin Pharmacol Ther. 1998; 64: 87 -96. 6 Gonzales et al. JAMA. 2006; 296: 47 -55. Last updated March 2011 7 Jorenby et al. JAMA. 2006; 296: 56 -63. 8 Bolliger et al. BMJ. 2000; 321: 329 -333. 9 Wennike et al. Addiction. 2003; 98: 1395 -1402. 10 Batra et al. Clin Pharmacol Ther. 2005; 78: 689 -696. 11 Rennard et al. Nicotine Tob Res. 2006; 8: 555 -564. 12 Kralikova et al. BMC Public Health. 2009; 9: 433.
Concomitant smoking • Concomitant use of NRT, bupropion or varenicline and smoking is well tolerated 1– 7 • Number of cigarettes smoked is likely to be less than at baseline • This might result in reduced health hazards • Such benefit has yet to be scientifically demonstrated • NRT-assisted reduction does increase later quitting 8 -12 1 Benowitz et al. J Pharmacol Exp Ther. 1998; 287: 958 -962 2 Bjornson-Benson et al. Addict Behav. 1993; 18: 491 -502. 3 Hurt et al. N Engl J Med. 1997; 337: 1195 -202. 4 Jorenby et al. N Engl J Med. 1999; 340: 685 -91. 5 Zevin et al. Clin Pharmacol Ther. 1998; 64: 87 -96. 6 Gonzales et al. JAMA. 2006; 296: 47 -55. Last updated March 2011 7 Jorenby et al. JAMA. 2006; 296: 56 -63. 8 Bolliger et al. BMJ. 2000; 321: 329 -333. 9 Wennike et al. Addiction. 2003; 98: 1395 -1402. 10 Batra et al. Clin Pharmacol Ther. 2005; 78: 689 -696. 11 Rennard et al. Nicotine Tob Res. 2006; 8: 555 -564. 12 Kralikova et al. BMC Public Health. 2009; 9: 433.
 ‘Reduced risk’ cigarettes • Includes low tar and ‘light’ cigarettes, and novel products that deliver nicotine with minimal tobacco combustion • Low tar cigarettes have not be shown to substantially reduce health hazards of smoking but do provide sufficient nicotine to sustain addiction 1 -6 • Some novel products may deliver fewer or lower levels of toxicants but some deliver more carbon monoxide. Not shown to reduce risk of smoking or aid cessation 7 -9 • Smoking cessation medications are most likely safer than any ‘reduced risk’ cigarette 1 NCI Monograph 13. 2001. 2 NCI Monograph 7. 1996. 3 Hecht et al. Cancer Epidemiol Biomarkers Prev. 2005; 14: 693 -698. Erratum in: Cancer Epidemiol Biomarkers Prev. 2006; 15: 1568. 4 Benowitz et al. Cancer Epidemiol Biomarkers Prev. 2005; 14: 1376 -1383. Last updated March 2011 5 Bernert et al. Nicotine Tob Res. 2005; 7: 729 -738. 6 Blackford et al. Cancer Epidemiol Biomarkers Prev. 2006; 15: 1799 -1804. 7 Fagerström et al. Tob Control. 2000; 9: 327 -333. 8 Pauly et al. Cancer Epidemiol Biomarkers Prev. 1998; 7: 967 -979. 9 Buchhalter & Eissenberg. Nicotine Tob Res. 2000; 2: 39 -43.
‘Reduced risk’ cigarettes • Includes low tar and ‘light’ cigarettes, and novel products that deliver nicotine with minimal tobacco combustion • Low tar cigarettes have not be shown to substantially reduce health hazards of smoking but do provide sufficient nicotine to sustain addiction 1 -6 • Some novel products may deliver fewer or lower levels of toxicants but some deliver more carbon monoxide. Not shown to reduce risk of smoking or aid cessation 7 -9 • Smoking cessation medications are most likely safer than any ‘reduced risk’ cigarette 1 NCI Monograph 13. 2001. 2 NCI Monograph 7. 1996. 3 Hecht et al. Cancer Epidemiol Biomarkers Prev. 2005; 14: 693 -698. Erratum in: Cancer Epidemiol Biomarkers Prev. 2006; 15: 1568. 4 Benowitz et al. Cancer Epidemiol Biomarkers Prev. 2005; 14: 1376 -1383. Last updated March 2011 5 Bernert et al. Nicotine Tob Res. 2005; 7: 729 -738. 6 Blackford et al. Cancer Epidemiol Biomarkers Prev. 2006; 15: 1799 -1804. 7 Fagerström et al. Tob Control. 2000; 9: 327 -333. 8 Pauly et al. Cancer Epidemiol Biomarkers Prev. 1998; 7: 967 -979. 9 Buchhalter & Eissenberg. Nicotine Tob Res. 2000; 2: 39 -43.
 Electronic Nicotine Delivery Devices • Electronic Nicotine Delivery Devices are basically oropharyngeal nicotine delivery devices • Systemic dose of nicotine obtained with ENDD is relatively small, but amount of nicotine in the cartridge is substantial (up to 16 -18 mg)1 • Small amounts of contaminants (e. g. TSNA) found in some products which raise health concerns 2 • some case reports, surveys and small studies seem to indicate that it may help smokers to quit smoking 3 -6 • Standardisation of products (regulatory oversight) and clinical and non-clinical studies are needed 2 1 Bullen et al. Tob Control. 2010; 19: 98 -103. 2 Etter et al. Tob Control. 2011; 20: 243 -248. 3 Etter. BMC Public Health. 2010; 10: 231. 19: 1945 -1953. Last updated March 2011 4 Siegel et al. Am J Prev Med. 2011; 40: 472 -475. 5 Trtchounian et al. Nicotine Tob Res. 2010; 12: 905 -912. 6 Vansickel et al. Cancer Epidemiol Biomarkers Prev. 2010;
Electronic Nicotine Delivery Devices • Electronic Nicotine Delivery Devices are basically oropharyngeal nicotine delivery devices • Systemic dose of nicotine obtained with ENDD is relatively small, but amount of nicotine in the cartridge is substantial (up to 16 -18 mg)1 • Small amounts of contaminants (e. g. TSNA) found in some products which raise health concerns 2 • some case reports, surveys and small studies seem to indicate that it may help smokers to quit smoking 3 -6 • Standardisation of products (regulatory oversight) and clinical and non-clinical studies are needed 2 1 Bullen et al. Tob Control. 2010; 19: 98 -103. 2 Etter et al. Tob Control. 2011; 20: 243 -248. 3 Etter. BMC Public Health. 2010; 10: 231. 19: 1945 -1953. Last updated March 2011 4 Siegel et al. Am J Prev Med. 2011; 40: 472 -475. 5 Trtchounian et al. Nicotine Tob Res. 2010; 12: 905 -912. 6 Vansickel et al. Cancer Epidemiol Biomarkers Prev. 2010;
 Smokeless tobacco • Snuff or chewing tobacco has been suggested as a potential aid to harm reduction or smoking cessation 1 • However, such products known to cause oral cancer 2– 3 • Country variation in composition may mean toxicity of smokeless tobacco products varies 4– 7 • Smokeless tobacco is addictive and not recommended for smoking cessation • Safety and efficacy of NRT, bupropion, and varenicline are better demonstrated 1 Radical strategies for prevention and harm reduction in nicotine addiction. Royal College of Physicians; 2008. 2 International Agency for Research on Cancer. IARC Monographs Vol. 89. Smokeless Tobacco and Some Tobacco-specific N-Nitrosamines. 2007. 3 US Surgeon General. Public Health Service, Bethesda, MD, NIH Publ. No. 86 -2874, 1986 4 Hecht. Chem Res Toxicol. 1998; 11: 559 -603. 5 Hoffmann et al. J Natl Cancer Inst. 1995; 87: 1832 -1869. 6 Osterdahl. International Agency for Research on Cancer Scientific Publications 105, 235 -237, 1991. 7 Stepanov et al. Nicotine Tob Res. 2008; 10: 1773 -1782. Last updated March 2011
Smokeless tobacco • Snuff or chewing tobacco has been suggested as a potential aid to harm reduction or smoking cessation 1 • However, such products known to cause oral cancer 2– 3 • Country variation in composition may mean toxicity of smokeless tobacco products varies 4– 7 • Smokeless tobacco is addictive and not recommended for smoking cessation • Safety and efficacy of NRT, bupropion, and varenicline are better demonstrated 1 Radical strategies for prevention and harm reduction in nicotine addiction. Royal College of Physicians; 2008. 2 International Agency for Research on Cancer. IARC Monographs Vol. 89. Smokeless Tobacco and Some Tobacco-specific N-Nitrosamines. 2007. 3 US Surgeon General. Public Health Service, Bethesda, MD, NIH Publ. No. 86 -2874, 1986 4 Hecht. Chem Res Toxicol. 1998; 11: 559 -603. 5 Hoffmann et al. J Natl Cancer Inst. 1995; 87: 1832 -1869. 6 Osterdahl. International Agency for Research on Cancer Scientific Publications 105, 235 -237, 1991. 7 Stepanov et al. Nicotine Tob Res. 2008; 10: 1773 -1782. Last updated March 2011
 Long-term pharmacotherapy • Goal of long-term pharmacotherapy is to replace smoking 1 -3 • Long-term use of NRT, bupropion or varenicline is likely to be much safer than smoking but efficacy has not yet been demonstrated 4 -7 • Experience with long term buproprion use for depression suggests it is well tolerated 6 • Evidence of a persistent health risk, even with prolonged reduction of cigarette consumption 8 1 Anderson & Hughes. Addiction. 2000; 95 Suppl 1: S 9 -11. 2 IOM report. Clearing the Smoke: Assessing the Science Base for Tobacco Harm Reduction. Washington DC, 2001. 3 Radical strategies for prevention and harm reduction in nicotine addiction. Royal College of Physicians; 2008. 4 Stead & Lancaster. Cochrane Database Syst Rev. 2007; (3): CD 005231. 5 Murray et al. Chest. 1996; 109: 438 -445. 6 Cox et al. J Gen Intern Med. 2004; 19: 828 -834. 7 Tonstad et al. JAMA. 2006; 296: 64 -71. 8 Godtfredsen et al. J Epidemiol Community Health. 2003; 57: 412 -416. Last updated March 2011
Long-term pharmacotherapy • Goal of long-term pharmacotherapy is to replace smoking 1 -3 • Long-term use of NRT, bupropion or varenicline is likely to be much safer than smoking but efficacy has not yet been demonstrated 4 -7 • Experience with long term buproprion use for depression suggests it is well tolerated 6 • Evidence of a persistent health risk, even with prolonged reduction of cigarette consumption 8 1 Anderson & Hughes. Addiction. 2000; 95 Suppl 1: S 9 -11. 2 IOM report. Clearing the Smoke: Assessing the Science Base for Tobacco Harm Reduction. Washington DC, 2001. 3 Radical strategies for prevention and harm reduction in nicotine addiction. Royal College of Physicians; 2008. 4 Stead & Lancaster. Cochrane Database Syst Rev. 2007; (3): CD 005231. 5 Murray et al. Chest. 1996; 109: 438 -445. 6 Cox et al. J Gen Intern Med. 2004; 19: 828 -834. 7 Tonstad et al. JAMA. 2006; 296: 64 -71. 8 Godtfredsen et al. J Epidemiol Community Health. 2003; 57: 412 -416. Last updated March 2011
 Safety in adolescents • Many adolescents are addicted to nicotine 1 • Little reason to believe that any risk associated with NRT, bupropion or varenicline is greater in adolescents who smoke than in adults who smoke • Efficacy of NRT and bupropion in adolescents is unclear 2 -10 • Efficacy of varenicline in adolescents has not been studied 1 USDHHS. A Report of the Surgeon General. 1994. 2 Colby & Gwaltney. JAMA. 2007; 298: 2182 -2184. 3 Hanson et al. Nicotine Tob Res. 2003; 5: 515 -526. 4 Hanson et al. Drug Alcohol Depend. 2008; 95: 164 -168. 5 Killen et al. J Consult Clin Psychol. 2004; 72: 729 -735. Last updated March 2011 6 Monuteaux et al. J Clin Psychiatry. 2007; 68: 1094 -1101. 7 Moolchan et al. Pediatrics. 2005; 115: e 407 -e 414. 8 Muramoto et al. Arch Pediatr Adolesc Med. 2007; 161: 1068 -1074. 9 Roddy et al. Tob Control. 2006; 15: 373 -376. 10 Upadhyaya et al. J Am Acad Child Adolesc Psychiatry. 2004; 43: 199 -205.
Safety in adolescents • Many adolescents are addicted to nicotine 1 • Little reason to believe that any risk associated with NRT, bupropion or varenicline is greater in adolescents who smoke than in adults who smoke • Efficacy of NRT and bupropion in adolescents is unclear 2 -10 • Efficacy of varenicline in adolescents has not been studied 1 USDHHS. A Report of the Surgeon General. 1994. 2 Colby & Gwaltney. JAMA. 2007; 298: 2182 -2184. 3 Hanson et al. Nicotine Tob Res. 2003; 5: 515 -526. 4 Hanson et al. Drug Alcohol Depend. 2008; 95: 164 -168. 5 Killen et al. J Consult Clin Psychol. 2004; 72: 729 -735. Last updated March 2011 6 Monuteaux et al. J Clin Psychiatry. 2007; 68: 1094 -1101. 7 Moolchan et al. Pediatrics. 2005; 115: e 407 -e 414. 8 Muramoto et al. Arch Pediatr Adolesc Med. 2007; 161: 1068 -1074. 9 Roddy et al. Tob Control. 2006; 15: 373 -376. 10 Upadhyaya et al. J Am Acad Child Adolesc Psychiatry. 2004; 43: 199 -205.
 Recommendations • All smokers, even those with cardiovascular disease, should be offered nicotine replacement therapy, bupropion or varenicline to aid cessation. • Pregnant smokers should be encouraged to attempt cessation using educational and behavioral interventions before using pharmacological approaches. If such quit attempts are unsuccessful, the risks and benefits of pharmacotherapy should be considered and explained to the woman. The decision to use pharmacotherapy should be made on an individualized basis after discussion between the woman and her physician. Last updated March 2011
Recommendations • All smokers, even those with cardiovascular disease, should be offered nicotine replacement therapy, bupropion or varenicline to aid cessation. • Pregnant smokers should be encouraged to attempt cessation using educational and behavioral interventions before using pharmacological approaches. If such quit attempts are unsuccessful, the risks and benefits of pharmacotherapy should be considered and explained to the woman. The decision to use pharmacotherapy should be made on an individualized basis after discussion between the woman and her physician. Last updated March 2011
 Areas for further research • Safety of nicotine and other medications to aid smoking cessation in pregnant smokers, including impact on the health and development of newborns of mothers who received medication during pregnancy. • Preclincal and clinical studies on the role of nicotine vs other toxicants in the onset of SIDS. • Safety of bupropion in high risk groups (pregnancy, alcohol abuse, history of stroke or other brain injury). • Safety of varenicline in high risk groups (psychiatric disease, cardiovascular disease, alcohol and drug abuse, pregnancy) • Safety of nicotine and other medications to aid smoking cessation in adolescents. • Beneficial and harmful health effects of novel nicotine delivery devices, including their potential for use to initiate smoking (in adolescents), to increase overall nicotine intake via concurrent use with cigarettes, and potential adverse effects on smoking cessation (i. e. switching instead of quitting). Last updated March 2011
Areas for further research • Safety of nicotine and other medications to aid smoking cessation in pregnant smokers, including impact on the health and development of newborns of mothers who received medication during pregnancy. • Preclincal and clinical studies on the role of nicotine vs other toxicants in the onset of SIDS. • Safety of bupropion in high risk groups (pregnancy, alcohol abuse, history of stroke or other brain injury). • Safety of varenicline in high risk groups (psychiatric disease, cardiovascular disease, alcohol and drug abuse, pregnancy) • Safety of nicotine and other medications to aid smoking cessation in adolescents. • Beneficial and harmful health effects of novel nicotine delivery devices, including their potential for use to initiate smoking (in adolescents), to increase overall nicotine intake via concurrent use with cigarettes, and potential adverse effects on smoking cessation (i. e. switching instead of quitting). Last updated March 2011
 Areas for further research • Development of biomarkers of exposure and surrogate markers of health effects of tobacco and related products. • Safety of long term nicotine replacement or other medication as would be used to reduce cigarette smoking or maintain tobacco abstinence. • Safety and efficacy of oral tobacco products (e. g. snus) for smoking cessation, for harm reduction or for withdrawal relief during temporary abstinence. • Does use of novel tobacco products or oral tobacco products for non -cessation reasons undermine motivation to quit? • More rigorous studies of the association of varenicline with psychiatric disorders, including prospective studies. • Better epidemiological studies of the association of smoking reduction and reduced risk of smoking. Last updated March 2011
Areas for further research • Development of biomarkers of exposure and surrogate markers of health effects of tobacco and related products. • Safety of long term nicotine replacement or other medication as would be used to reduce cigarette smoking or maintain tobacco abstinence. • Safety and efficacy of oral tobacco products (e. g. snus) for smoking cessation, for harm reduction or for withdrawal relief during temporary abstinence. • Does use of novel tobacco products or oral tobacco products for non -cessation reasons undermine motivation to quit? • More rigorous studies of the association of varenicline with psychiatric disorders, including prospective studies. • Better epidemiological studies of the association of smoking reduction and reduced risk of smoking. Last updated March 2011


