93424c676467eed880d1ce7f0418f264.ppt
- Количество слайдов: 31
 Rushing Through the Implementation Pipeline: Hypertension Self-Management Hayden B. Bosworth, Ph. D. George L. Jackson, Ph. D. , MHA Ben J. Powers, MD, MHS Center for Health Services Research in Primary Care Durham VA Medical Center VA Quality Enhancement Research Initiative (QUERI) 2008 Annual Meeting
Rushing Through the Implementation Pipeline: Hypertension Self-Management Hayden B. Bosworth, Ph. D. George L. Jackson, Ph. D. , MHA Ben J. Powers, MD, MHS Center for Health Services Research in Primary Care Durham VA Medical Center VA Quality Enhancement Research Initiative (QUERI) 2008 Annual Meeting
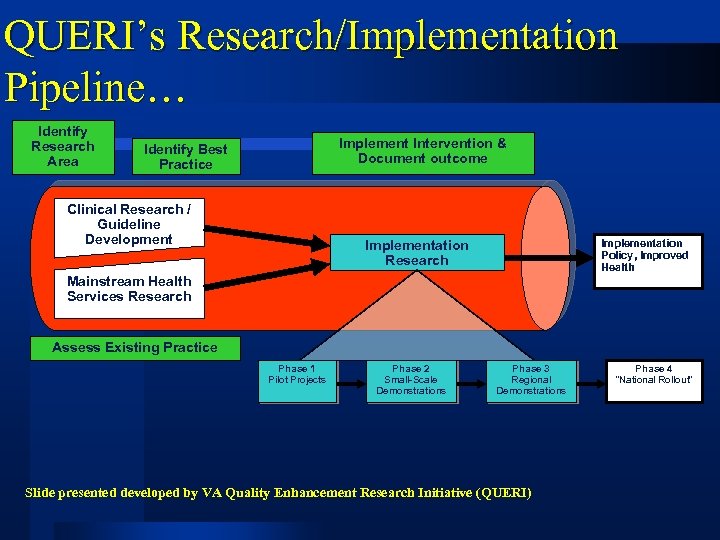 QUERI’s Research/Implementation Pipeline… Identify Research Area Implement Intervention & Document outcome Identify Best Practice Clinical Research / Guideline Development Implementation Research Implementation Policy, Improved Health Mainstream Health Services Research Assess Existing Practice Phase 1 Pilot Projects Phase 2 Small-Scale Demonstrations Phase 3 Regional Demonstrations Slide presented developed by VA Quality Enhancement Research Initiative (QUERI) Phase 4 “National Rollout”
QUERI’s Research/Implementation Pipeline… Identify Research Area Implement Intervention & Document outcome Identify Best Practice Clinical Research / Guideline Development Implementation Research Implementation Policy, Improved Health Mainstream Health Services Research Assess Existing Practice Phase 1 Pilot Projects Phase 2 Small-Scale Demonstrations Phase 3 Regional Demonstrations Slide presented developed by VA Quality Enhancement Research Initiative (QUERI) Phase 4 “National Rollout”
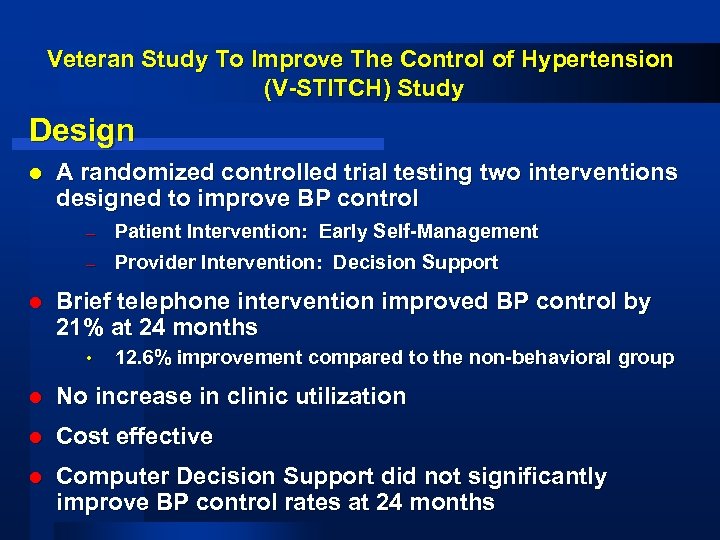 Veteran Study To Improve The Control of Hypertension (V-STITCH) Study Design l A randomized controlled trial testing two interventions designed to improve BP control – – l Patient Intervention: Early Self-Management Provider Intervention: Decision Support Brief telephone intervention improved BP control by 21% at 24 months • 12. 6% improvement compared to the non-behavioral group l No increase in clinic utilization l Cost effective l Computer Decision Support did not significantly improve BP control rates at 24 months
Veteran Study To Improve The Control of Hypertension (V-STITCH) Study Design l A randomized controlled trial testing two interventions designed to improve BP control – – l Patient Intervention: Early Self-Management Provider Intervention: Decision Support Brief telephone intervention improved BP control by 21% at 24 months • 12. 6% improvement compared to the non-behavioral group l No increase in clinic utilization l Cost effective l Computer Decision Support did not significantly improve BP control rates at 24 months
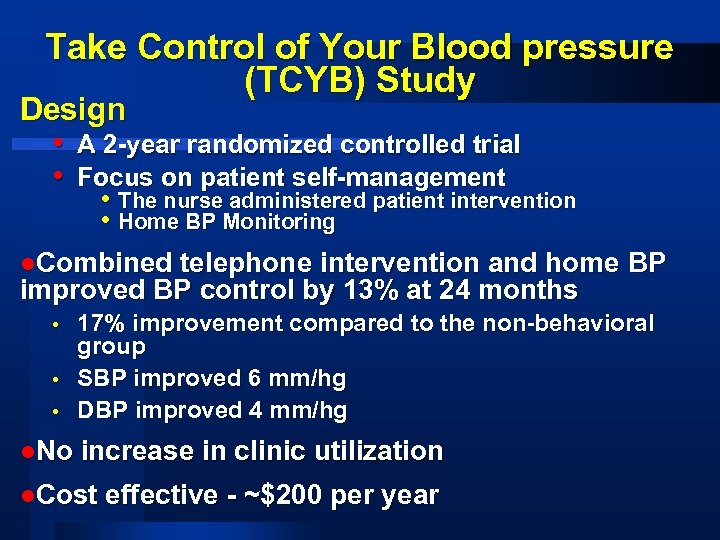 Take Control of Your Blood pressure (TCYB) Study Design • A 2 -year randomized controlled trial • Focus on patient self-management • The nurse administered patient intervention • Home BP Monitoring l. Combined telephone intervention and home BP improved BP control by 13% at 24 months • • • l. No 17% improvement compared to the non-behavioral group SBP improved 6 mm/hg DBP improved 4 mm/hg increase in clinic utilization l. Cost effective - ~$200 per year
Take Control of Your Blood pressure (TCYB) Study Design • A 2 -year randomized controlled trial • Focus on patient self-management • The nurse administered patient intervention • Home BP Monitoring l. Combined telephone intervention and home BP improved BP control by 13% at 24 months • • • l. No 17% improvement compared to the non-behavioral group SBP improved 6 mm/hg DBP improved 4 mm/hg increase in clinic utilization l. Cost effective - ~$200 per year
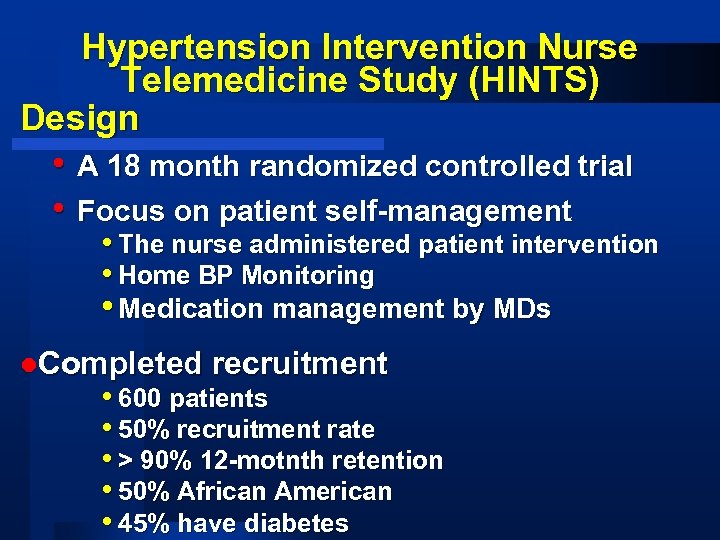 Hypertension Intervention Nurse Telemedicine Study (HINTS) Design • A 18 month randomized controlled trial • Focus on patient self-management • The nurse administered patient intervention • Home BP Monitoring • Medication management by MDs l. Completed recruitment • 600 patients • 50% recruitment rate • > 90% 12 -motnth retention • 50% African American • 45% have diabetes
Hypertension Intervention Nurse Telemedicine Study (HINTS) Design • A 18 month randomized controlled trial • Focus on patient self-management • The nurse administered patient intervention • Home BP Monitoring • Medication management by MDs l. Completed recruitment • 600 patients • 50% recruitment rate • > 90% 12 -motnth retention • 50% African American • 45% have diabetes
 Telephone Intervention l Behavioral interventions to enhance hypertension control l Intervention implemented in nontraditional setting - outside of the clinic, easily administered via the telephone l Delivered by nurses or other clinicians l Tailoring the intervention to patients’ needs - this ensures a more cost efficient method of implementing the intervention l Multiple hypertension-related behaviors addressed l Software allows the integration of patient, medical records, and provider information l Emphasis on cultural issues related to hypertension
Telephone Intervention l Behavioral interventions to enhance hypertension control l Intervention implemented in nontraditional setting - outside of the clinic, easily administered via the telephone l Delivered by nurses or other clinicians l Tailoring the intervention to patients’ needs - this ensures a more cost efficient method of implementing the intervention l Multiple hypertension-related behaviors addressed l Software allows the integration of patient, medical records, and provider information l Emphasis on cultural issues related to hypertension
 HTN IMPROVE: Quality Improvement Project Hypertension Telemedicine Nurse Implementation Project for Veterans
HTN IMPROVE: Quality Improvement Project Hypertension Telemedicine Nurse Implementation Project for Veterans
 In the Pipeline – Summary of HTN-IMPROVE l The study is addressing four specific aims: – 1) Assess the implementation of an evidencebased behavioral intervention to improve BP levels. – 2) Evaluate the clinical impact of the intervention to promote and improve BP levels as it is implemented. – 3) Assess the organizational factors associated with the sustainability of the intervention to improve BP levels. – 4) Assess the cost of the intervention to improve BP levels as it is implemented by VA facilities.
In the Pipeline – Summary of HTN-IMPROVE l The study is addressing four specific aims: – 1) Assess the implementation of an evidencebased behavioral intervention to improve BP levels. – 2) Evaluate the clinical impact of the intervention to promote and improve BP levels as it is implemented. – 3) Assess the organizational factors associated with the sustainability of the intervention to improve BP levels. – 4) Assess the cost of the intervention to improve BP levels as it is implemented by VA facilities.
 In the Pipeline – Summary of HTNIMPROVE l Methods: l 12 geographically diverse VA sites within two Veteran Integrated Service Networks (VISNs) – 6 sites implementing the behavioral telephone intervention – 6 control sites. l l The unit of analysis is patients with an annual inadequate BP control. Phase I – Conducting a needs assessment and evaluating barriers and facilitators for implementing the proposed behavioral intervention at each of the 6 intervention sites. l Phase II – Examining the impact of the interventions by comparing 12 -month pre/post changes in BP control obtained from medical records for individual patients who receive the intervention compared to a individuals from the 6 control sites. l Phase III – Examine the sustainability of the intervention and examine what organizational factors facilitate or hinder the sustained implementation of the study. l Phase IV. – Examine the implementation costs of disseminating the telephone based behavioral interventions.
In the Pipeline – Summary of HTNIMPROVE l Methods: l 12 geographically diverse VA sites within two Veteran Integrated Service Networks (VISNs) – 6 sites implementing the behavioral telephone intervention – 6 control sites. l l The unit of analysis is patients with an annual inadequate BP control. Phase I – Conducting a needs assessment and evaluating barriers and facilitators for implementing the proposed behavioral intervention at each of the 6 intervention sites. l Phase II – Examining the impact of the interventions by comparing 12 -month pre/post changes in BP control obtained from medical records for individual patients who receive the intervention compared to a individuals from the 6 control sites. l Phase III – Examine the sustainability of the intervention and examine what organizational factors facilitate or hinder the sustained implementation of the study. l Phase IV. – Examine the implementation costs of disseminating the telephone based behavioral interventions.
 Intervention Overview l l l l 6 intervention and 6 control facilities. 5 FTE interventionist (e. g. , nurse) 500 patients per facility (250 enrolled every 6 months) Use centralized software on Durham server Call patient every 4 weeks Calls last approximately 5 -10 minutes Interventionist may do several modules each call
Intervention Overview l l l l 6 intervention and 6 control facilities. 5 FTE interventionist (e. g. , nurse) 500 patients per facility (250 enrolled every 6 months) Use centralized software on Durham server Call patient every 4 weeks Calls last approximately 5 -10 minutes Interventionist may do several modules each call
 Eligibility and Referral l Criterion 1 – Blood Pressure: Mean of outpatient BP measurements in the last 365 days. Systolic BP > 140 mm. Hg or Diastolic BP > 90 mm. Hg l Criterion 2 – Assigned Primary Care Provider at the VA The patient must have an assigned primary care provider at the VA l Criterion 3 – Previous Visits to VA Must have had 3 or more visits in the past 730 days at the facility to a primary care clinic. l Criterion 4 – Hypertension ICD-9 CM Diagnoses
Eligibility and Referral l Criterion 1 – Blood Pressure: Mean of outpatient BP measurements in the last 365 days. Systolic BP > 140 mm. Hg or Diastolic BP > 90 mm. Hg l Criterion 2 – Assigned Primary Care Provider at the VA The patient must have an assigned primary care provider at the VA l Criterion 3 – Previous Visits to VA Must have had 3 or more visits in the past 730 days at the facility to a primary care clinic. l Criterion 4 – Hypertension ICD-9 CM Diagnoses
 Eligibility & Referral Primary Method: PDP/CPRS Referral Step 1: Nurse-administered self-management support added as option to hypertension reminder Step 2: Templated consult Step 3: Feedback loop from interventionist to physician (initial note indicating participation co-signed by PCP)
Eligibility & Referral Primary Method: PDP/CPRS Referral Step 1: Nurse-administered self-management support added as option to hypertension reminder Step 2: Templated consult Step 3: Feedback loop from interventionist to physician (initial note indicating participation co-signed by PCP)
 Implementation Staffing Implementation & Core Team: l Site champion(s) l Nurse interventionist(s) l Site administrators l Site IT
Implementation Staffing Implementation & Core Team: l Site champion(s) l Nurse interventionist(s) l Site administrators l Site IT
 Timeline l l l l August 2008 – Confirm facility participation September 2008 – January 2009 – Implementation preparation (surveys, interviews) – Training – Site visit to your facility – Monthly calls to learn from each other January 2009 – Test system with hypothetical patients February 2009 – Fully implement intervention as part of study February 2009 -Frebruay 2010 – implement intervention recruitment – Monthly calls to learn from each other – Support from Durham February 2010 -February 2011 – Patient follow-up completed February 2011 -February 2012 – Secondary data follow-up
Timeline l l l l August 2008 – Confirm facility participation September 2008 – January 2009 – Implementation preparation (surveys, interviews) – Training – Site visit to your facility – Monthly calls to learn from each other January 2009 – Test system with hypothetical patients February 2009 – Fully implement intervention as part of study February 2009 -Frebruay 2010 – implement intervention recruitment – Monthly calls to learn from each other – Support from Durham February 2010 -February 2011 – Patient follow-up completed February 2011 -February 2012 – Secondary data follow-up
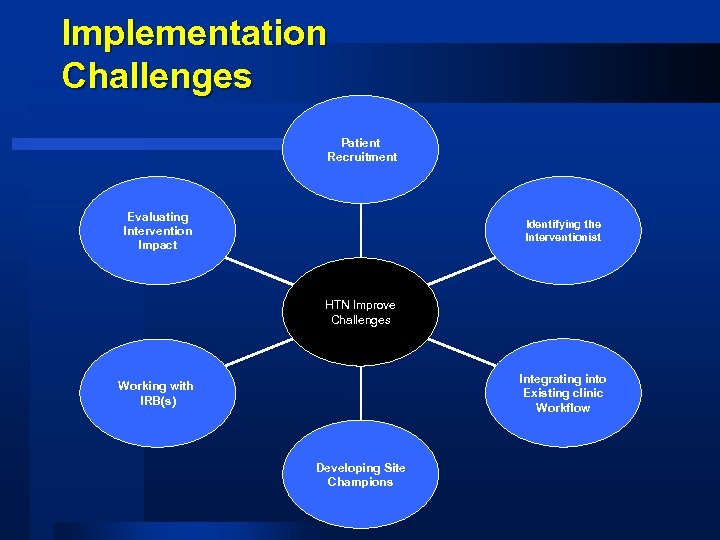 Implementation Challenges Patient Recruitment Evaluating Intervention Impact Identifying the Interventionist HTN Improve Challenges Integrating into Existing clinic Workflow Working with IRB(s) Developing Site Champions
Implementation Challenges Patient Recruitment Evaluating Intervention Impact Identifying the Interventionist HTN Improve Challenges Integrating into Existing clinic Workflow Working with IRB(s) Developing Site Champions
 Developing Site Champions l Clinical Trial – Investigators also part of ambulatory care staff – Local project coordinator keeps things moving l Implementation – Need for administrative, PCP, and nursing champions – Regular teleconference contact with Durham team Key Questions: -How do you identify enthusiastic champions at willing facilities? -Do the site champions have the necessary resources and facility backing?
Developing Site Champions l Clinical Trial – Investigators also part of ambulatory care staff – Local project coordinator keeps things moving l Implementation – Need for administrative, PCP, and nursing champions – Regular teleconference contact with Durham team Key Questions: -How do you identify enthusiastic champions at willing facilities? -Do the site champions have the necessary resources and facility backing?
 Patient Recruitment l Clinical Trial – Identified and recruited through central data pull. l Implementation – Pts referred from providers? OR – Identified and recruited centrally (i. e. central data pull)? Key Questions: -Which recruitment procedure works best with existing clinic workflow? - Which would be most acceptable and sustainable for clinics?
Patient Recruitment l Clinical Trial – Identified and recruited through central data pull. l Implementation – Pts referred from providers? OR – Identified and recruited centrally (i. e. central data pull)? Key Questions: -Which recruitment procedure works best with existing clinic workflow? - Which would be most acceptable and sustainable for clinics?
 Identifying the Interventionist l Clinical Trial – 1 FTE Research Nurse l Implementation – 0. 5 FTE Clinic nurse • 1 person= 0. 5 FTE OR • 5 people =0. 5 FTE? Key Questions: - How did the clinics prefer to allocate their nursing resources? - Can we still maintain the fidelity of the intervention with different models?
Identifying the Interventionist l Clinical Trial – 1 FTE Research Nurse l Implementation – 0. 5 FTE Clinic nurse • 1 person= 0. 5 FTE OR • 5 people =0. 5 FTE? Key Questions: - How did the clinics prefer to allocate their nursing resources? - Can we still maintain the fidelity of the intervention with different models?
 Integrating into Workflow l Clinical Trial – Intervention operates independently of usual care. – Call schedule negotiated between study nurse and patient l Implementation – Scheduled nurse telephone appointments OR – Nurse adds to workflow when possible Key Questions: -Can we fit this into usual clinic operating hours? - How do we document nurse workload credit for time spent on intervention?
Integrating into Workflow l Clinical Trial – Intervention operates independently of usual care. – Call schedule negotiated between study nurse and patient l Implementation – Scheduled nurse telephone appointments OR – Nurse adds to workflow when possible Key Questions: -Can we fit this into usual clinic operating hours? - How do we document nurse workload credit for time spent on intervention?
 Working with IRB(s) l Clinical Trial l – IRB approval Implementation – Addressing multiple interpretations – Is it research at Durham, but QI elsewhere? Key Questions: -What constitutes quality improvement? -Collaborating with people not accustomed to working with IRBs.
Working with IRB(s) l Clinical Trial l – IRB approval Implementation – Addressing multiple interpretations – Is it research at Durham, but QI elsewhere? Key Questions: -What constitutes quality improvement? -Collaborating with people not accustomed to working with IRBs.
 Evaluating intervention Impact l Clinical Trial – Clearly defined control groups – Intent to treat analysis starts at randomization l Implementation – Must define control groups • Same-site controls • Different-site controls – Intent to treat not as clear Key Questions: -Who are the most appropriate control patients/sites? -What causes a patient to become part of the analysis?
Evaluating intervention Impact l Clinical Trial – Clearly defined control groups – Intent to treat analysis starts at randomization l Implementation – Must define control groups • Same-site controls • Different-site controls – Intent to treat not as clear Key Questions: -Who are the most appropriate control patients/sites? -What causes a patient to become part of the analysis?
 Summary Intervention tested in 3 separate trials with > 2500 subjects – takes along time l Moving into the realm of implementation l New challenges l – – l Identifying partners Integrating into regular work of clinic Obtaining resources Measuring success Expanding beyond hypertension to other CVD
Summary Intervention tested in 3 separate trials with > 2500 subjects – takes along time l Moving into the realm of implementation l New challenges l – – l Identifying partners Integrating into regular work of clinic Obtaining resources Measuring success Expanding beyond hypertension to other CVD
 Acknowledgements • VA Health Services Research Investigator Initiated Award, 2001 -06 • NHLBI Grant R 01 HL 070713 (2003 -2009) • Pfizer Health Communication Initiative Award (20042006) • Established Investigator Award, American Heart Association (2006 -2011) Danny Almirall Mike Newell Pam Gentry Bryan Weiner Teresa Damush Daniel Lee Eugene Oddone Amy Kaufman
Acknowledgements • VA Health Services Research Investigator Initiated Award, 2001 -06 • NHLBI Grant R 01 HL 070713 (2003 -2009) • Pfizer Health Communication Initiative Award (20042006) • Established Investigator Award, American Heart Association (2006 -2011) Danny Almirall Mike Newell Pam Gentry Bryan Weiner Teresa Damush Daniel Lee Eugene Oddone Amy Kaufman
 Contact Information • Hayden Bosworth – hayden. bosworth@duke. edu • George L. Jackson – george. l. jackson@duke. edu • Ben Powers – power 017@mc. duke. edu
Contact Information • Hayden Bosworth – hayden. bosworth@duke. edu • George L. Jackson – george. l. jackson@duke. edu • Ben Powers – power 017@mc. duke. edu
 Relevant Publications 1. Bosworth HB, Olsen MK, Mc. Cant F, et al. Hypertension Intervention Nurse Telemedicine Study (HINTS). Am Heart J 2007; 153(6): 918 -24. 2. Bosworth HB, Olsen MK, Goldstein MK, et al. The veterans' study to improve the control of hypertension (V-STITCH): design and methodology. Contemp Clin Trials 2005; 26: 155 -68. 3. Chan AS, Coleman RW, Martins SB, et al. Evaluating provider adherence in a trial of a guideline-based decision support system for hypertension. Medinfo 2004; 11(Pt 1): 125 -9. 4. Goldstein MK, Coleman RW, Tu SW, et al. Translating research into practice: organizational issues in implementing automated decision support for hypertension in three medical centers. J Am Med Inform Assoc 2004; 11(5): 368 -76. 5. Goldstein MK, Hoffman BB, Coleman RW, et al. Implementing clinical practice guidelines while taking account of changing evidence. Proc AMIA Symp 2000: 300 -4. 6. Goldstein MK, Hoffman BB, Coleman RW, et al. Patient safety in guideline-based decision support for hypertension management: ATHENA DSS. Proc AMIA Symp 2001: 214 -8. 7. Lin ND, Martins SB, Chan AS, et al. Identifying barriers to hypertension guideline adherence using clinician feedback at the point of care. AMIA Annu Symp Proc 2006: 494 -8. 8. Bosworth HB, Oddone EZ. Telemedicine and Hypertension. J Clin Outcomes Management 2004; 11(8): 517 -522. 9. Bosworth HB, Oddone EZ, Weinberger M. Patient treatment adherence: Concepts interventions, and measurement. Mahwah, NJ: Lawrence Erlbaum Associates, 2006. 10. Bosworth HB, Dudley T, Olsen MK, et al. Racial differences in blood pressure control: potential explanatory factors. Am J Med 2006; 119(1): 70. 11. Bosworth HB, Oddone EZ. A model of psychosocial and cultural antecedents of blood pressure control. Journal of the National Medical Association 2002; 94: 236 -248. 12. Bosworth HB, Olsen MK, Gentry P, et al. Nurse administered telephone intervention for blood pressure control. Patient Educ Couns 2005; 57(1): 5 -14. 13. Bosworth HB, Olsen MK, Oddone EZ. Improving blood pressure control by tailored feedback to patients and clinicians. Am Heart J 2005; 149(5): 795 -803.
Relevant Publications 1. Bosworth HB, Olsen MK, Mc. Cant F, et al. Hypertension Intervention Nurse Telemedicine Study (HINTS). Am Heart J 2007; 153(6): 918 -24. 2. Bosworth HB, Olsen MK, Goldstein MK, et al. The veterans' study to improve the control of hypertension (V-STITCH): design and methodology. Contemp Clin Trials 2005; 26: 155 -68. 3. Chan AS, Coleman RW, Martins SB, et al. Evaluating provider adherence in a trial of a guideline-based decision support system for hypertension. Medinfo 2004; 11(Pt 1): 125 -9. 4. Goldstein MK, Coleman RW, Tu SW, et al. Translating research into practice: organizational issues in implementing automated decision support for hypertension in three medical centers. J Am Med Inform Assoc 2004; 11(5): 368 -76. 5. Goldstein MK, Hoffman BB, Coleman RW, et al. Implementing clinical practice guidelines while taking account of changing evidence. Proc AMIA Symp 2000: 300 -4. 6. Goldstein MK, Hoffman BB, Coleman RW, et al. Patient safety in guideline-based decision support for hypertension management: ATHENA DSS. Proc AMIA Symp 2001: 214 -8. 7. Lin ND, Martins SB, Chan AS, et al. Identifying barriers to hypertension guideline adherence using clinician feedback at the point of care. AMIA Annu Symp Proc 2006: 494 -8. 8. Bosworth HB, Oddone EZ. Telemedicine and Hypertension. J Clin Outcomes Management 2004; 11(8): 517 -522. 9. Bosworth HB, Oddone EZ, Weinberger M. Patient treatment adherence: Concepts interventions, and measurement. Mahwah, NJ: Lawrence Erlbaum Associates, 2006. 10. Bosworth HB, Dudley T, Olsen MK, et al. Racial differences in blood pressure control: potential explanatory factors. Am J Med 2006; 119(1): 70. 11. Bosworth HB, Oddone EZ. A model of psychosocial and cultural antecedents of blood pressure control. Journal of the National Medical Association 2002; 94: 236 -248. 12. Bosworth HB, Olsen MK, Gentry P, et al. Nurse administered telephone intervention for blood pressure control. Patient Educ Couns 2005; 57(1): 5 -14. 13. Bosworth HB, Olsen MK, Oddone EZ. Improving blood pressure control by tailored feedback to patients and clinicians. Am Heart J 2005; 149(5): 795 -803.
 Single disease vs. multimorbidity self-mgmt? l Two key questions 1. ) Is there a “spillover” effect from disease-focused self-mgmt onto other conditions? 2. ) Is it possible to address multiple conditions simultaneously in a selfmanagement intervention?
Single disease vs. multimorbidity self-mgmt? l Two key questions 1. ) Is there a “spillover” effect from disease-focused self-mgmt onto other conditions? 2. ) Is it possible to address multiple conditions simultaneously in a selfmanagement intervention?
 Self-management spillover l VSTITCH – Hb. A 1 c among patients with diabetes: • 0. 46% reduction in Hb. A 1 c over two years compared to usual care (95% CI, 0. 04% to 0. 89%; p=0. 03). – LDL cholesterol: • 0. 9 mg/dl between group difference (95% CI, -7. 3 mg/dl to 5. 6 mg/dl; p=0. 79). Powers et al. SGIM annual meeting 2008.
Self-management spillover l VSTITCH – Hb. A 1 c among patients with diabetes: • 0. 46% reduction in Hb. A 1 c over two years compared to usual care (95% CI, 0. 04% to 0. 89%; p=0. 03). – LDL cholesterol: • 0. 9 mg/dl between group difference (95% CI, -7. 3 mg/dl to 5. 6 mg/dl; p=0. 79). Powers et al. SGIM annual meeting 2008.
 Comprehensive selfmanagement l Cholesterol, Hypertension, and Glucose Education (CHANGE) study – RWJ Disparities Research for Change l Supporting Post-MI Risk Modification Intervention via Telemedicine Evaluation (SPRITE) – AHA Pharmaceutical Roundtable Outcome Research
Comprehensive selfmanagement l Cholesterol, Hypertension, and Glucose Education (CHANGE) study – RWJ Disparities Research for Change l Supporting Post-MI Risk Modification Intervention via Telemedicine Evaluation (SPRITE) – AHA Pharmaceutical Roundtable Outcome Research
 Eligibility & Referral Secondary Method: Physician referral from general clinic Step 1: Physician refers patient to interventionist Step 2: Feedback loop from interventionist to physician
Eligibility & Referral Secondary Method: Physician referral from general clinic Step 1: Physician refers patient to interventionist Step 2: Feedback loop from interventionist to physician
 Eligibility & Referral Tertiary Method: Interventionist referral Step 1: Patient pull list reviewed for eligible participant Step 2: Nurse contacts patients based on eligibility criteria Step 3: Patients with most recent outpatient BP measurements contacted first Step 4: PCP gets note and can opt out of patient contact within 72 hours
Eligibility & Referral Tertiary Method: Interventionist referral Step 1: Patient pull list reviewed for eligible participant Step 2: Nurse contacts patients based on eligibility criteria Step 3: Patients with most recent outpatient BP measurements contacted first Step 4: PCP gets note and can opt out of patient contact within 72 hours
 Evaluating Successful Implementation l Clinical Trial – Quantitative results patient level l Implementation – Qualitative and quantitative results both organization and patient Key Questions: -How do you develop a research team with needed expertise? -What frameworks will be used for doing the evaluation?
Evaluating Successful Implementation l Clinical Trial – Quantitative results patient level l Implementation – Qualitative and quantitative results both organization and patient Key Questions: -How do you develop a research team with needed expertise? -What frameworks will be used for doing the evaluation?


