e2bc3424f5fe82809aebc722cfa3183c.ppt
- Количество слайдов: 56
 Ru-Catalyzed C-H Activation Wang cheng ming 2013. 2. 25
Ru-Catalyzed C-H Activation Wang cheng ming 2013. 2. 25
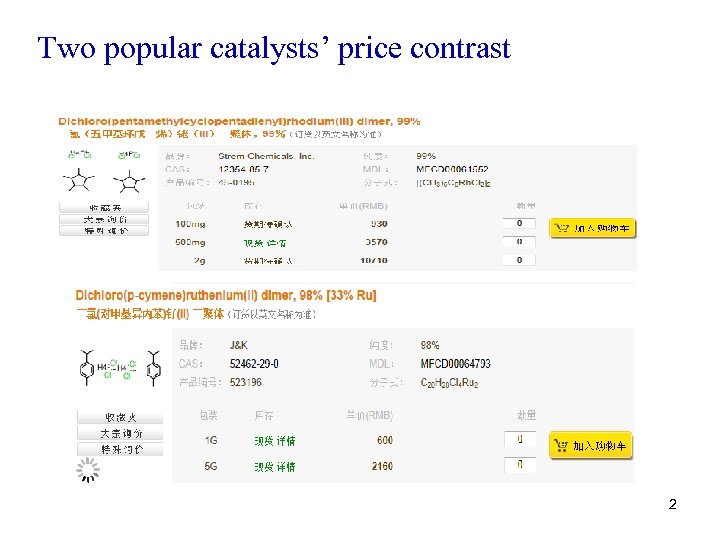 Two popular catalysts’ price contrast 2
Two popular catalysts’ price contrast 2
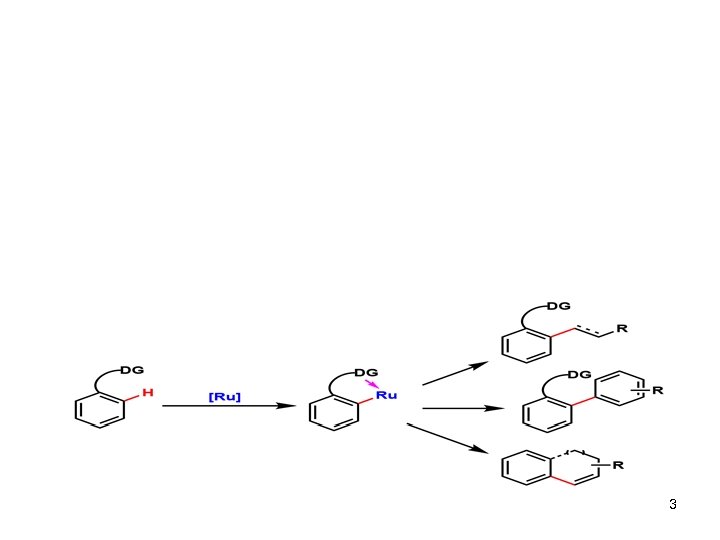 3
3
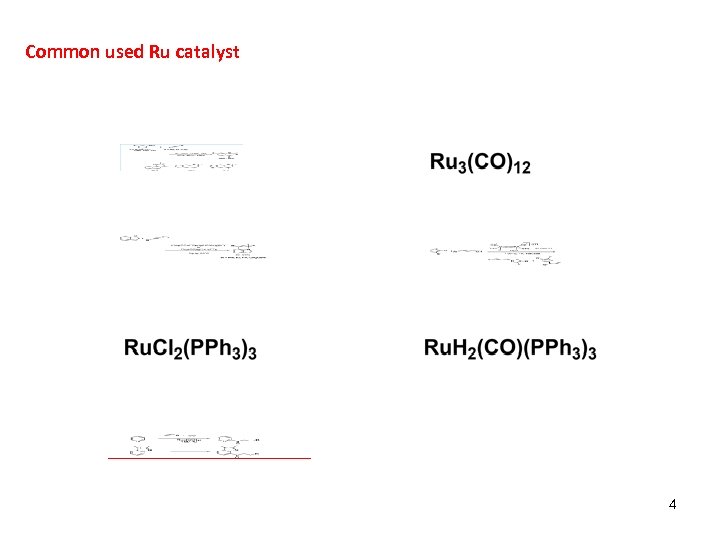 Common used Ru catalyst 4
Common used Ru catalyst 4
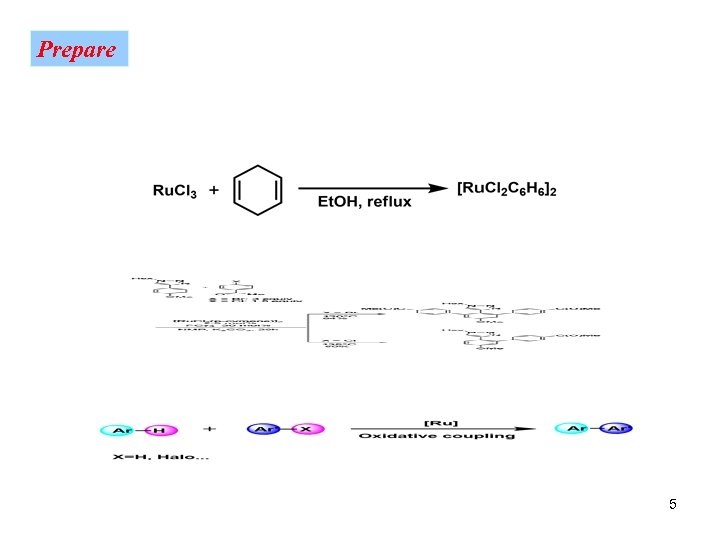 Prepare 5
Prepare 5
 Ru-catalyzed C-H activation u. Cross oxidative coupling u. Directing C-H activation u. Summary 6
Ru-catalyzed C-H activation u. Cross oxidative coupling u. Directing C-H activation u. Summary 6
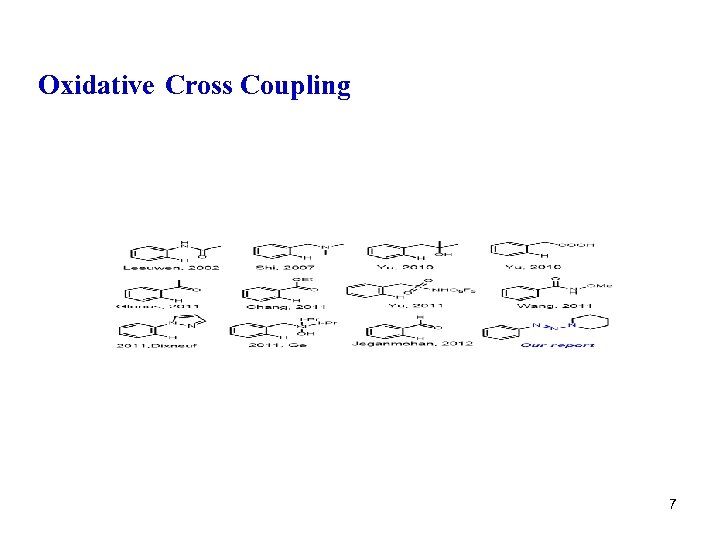 Oxidative Cross Coupling 7
Oxidative Cross Coupling 7
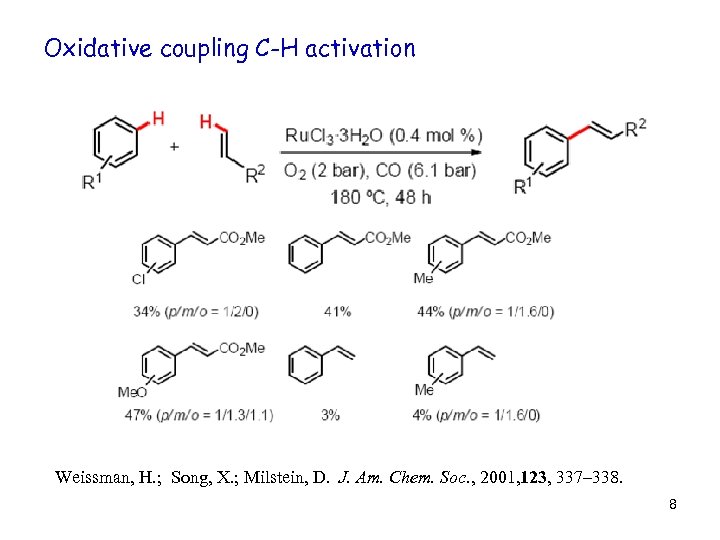 Oxidative coupling C-H activation Weissman, H. ; Song, X. ; Milstein, D. J. Am. Chem. Soc. , 2001, 123, 337– 338. 8
Oxidative coupling C-H activation Weissman, H. ; Song, X. ; Milstein, D. J. Am. Chem. Soc. , 2001, 123, 337– 338. 8
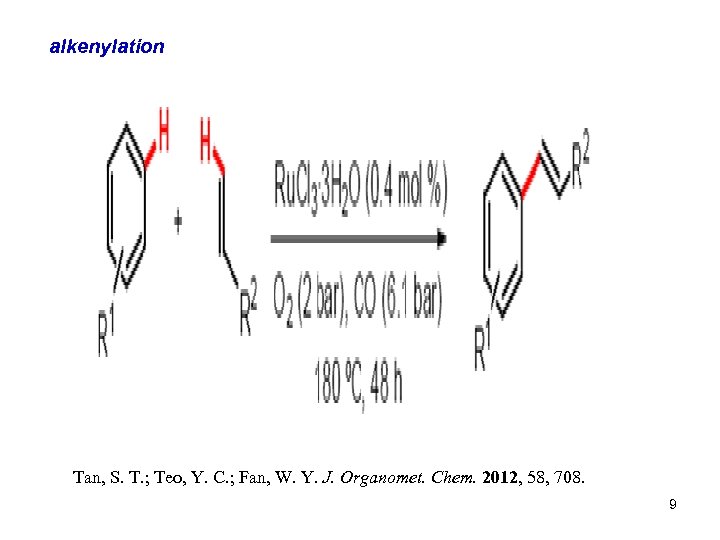 alkenylation Tan, S. T. ; Teo, Y. C. ; Fan, W. Y. J. Organomet. Chem. 2012, 58, 708. 9
alkenylation Tan, S. T. ; Teo, Y. C. ; Fan, W. Y. J. Organomet. Chem. 2012, 58, 708. 9
 alkylation Onodera, G. ; Imajima, H. ; Yamanashi, M. ; Nishibayashi, Y. ; Hidai, M. ; Uemura, S. Organometallics 2004, 23, 5841. 10
alkylation Onodera, G. ; Imajima, H. ; Yamanashi, M. ; Nishibayashi, Y. ; Hidai, M. ; Uemura, S. Organometallics 2004, 23, 5841. 10
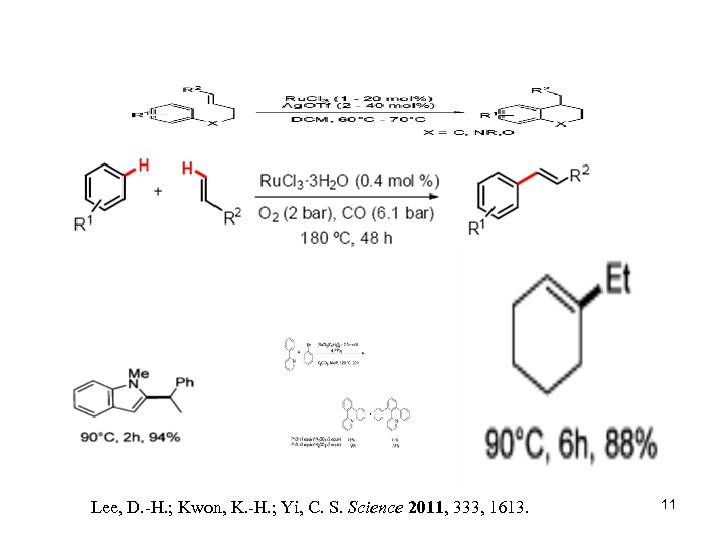 Lee, D. -H. ; Kwon, K. -H. ; Yi, C. S. Science 2011, 333, 1613. 11
Lee, D. -H. ; Kwon, K. -H. ; Yi, C. S. Science 2011, 333, 1613. 11
 acylation 12
acylation 12
 cyclization Yi, C. S. ; Yun, S. Y. ; Guzei, I. A. J. Am. Chem. Soc. 2005, 127, 5782. 13
cyclization Yi, C. S. ; Yun, S. Y. ; Guzei, I. A. J. Am. Chem. Soc. 2005, 127, 5782. 13
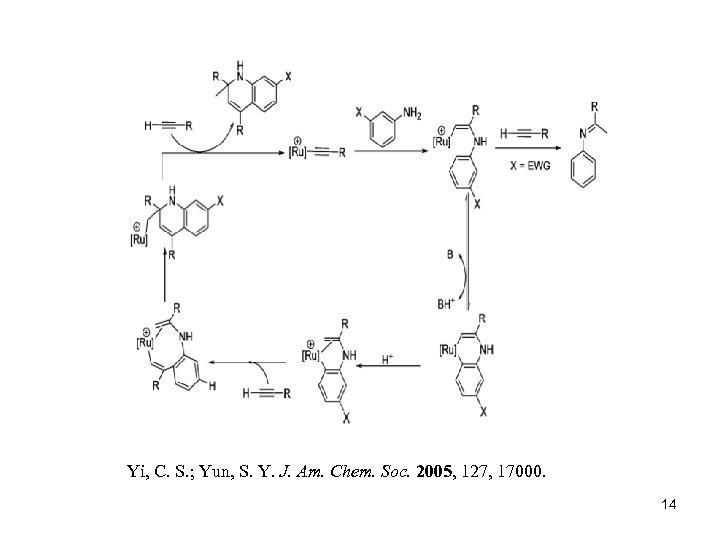 Yi, C. S. ; Yun, S. Y. J. Am. Chem. Soc. 2005, 127, 17000. 14
Yi, C. S. ; Yun, S. Y. J. Am. Chem. Soc. 2005, 127, 17000. 14
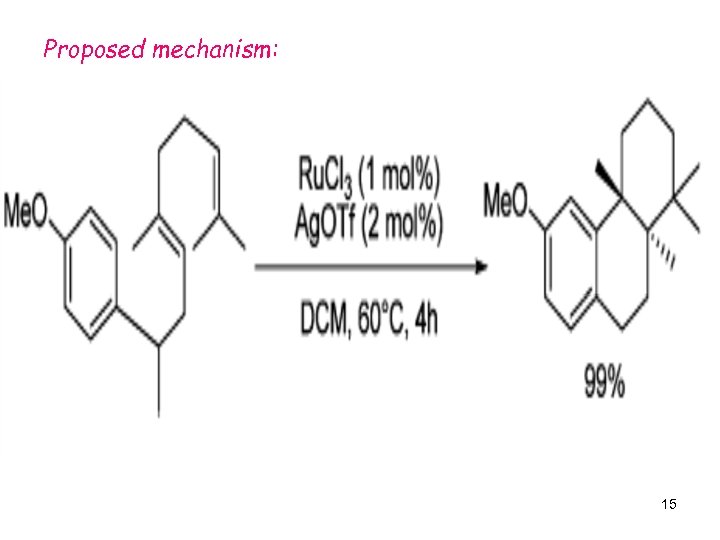 Proposed mechanism: 15
Proposed mechanism: 15
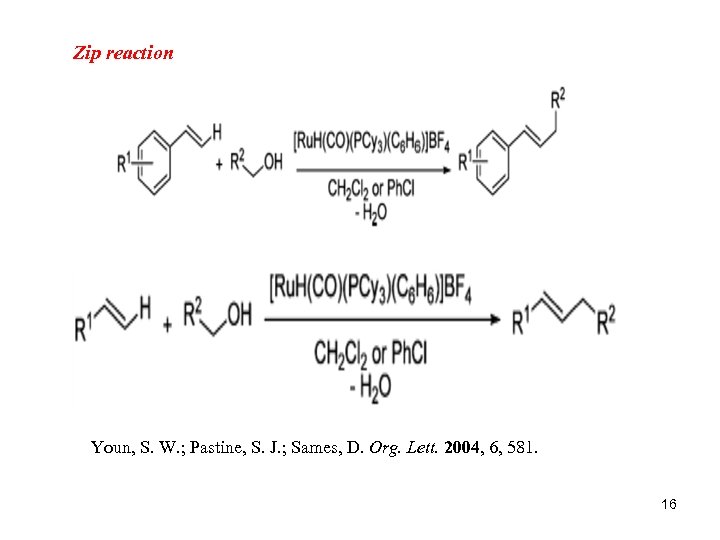 Zip reaction Youn, S. W. ; Pastine, S. J. ; Sames, D. Org. Lett. 2004, 6, 581. 16
Zip reaction Youn, S. W. ; Pastine, S. J. ; Sames, D. Org. Lett. 2004, 6, 581. 16
 Directing C-H activation 17
Directing C-H activation 17
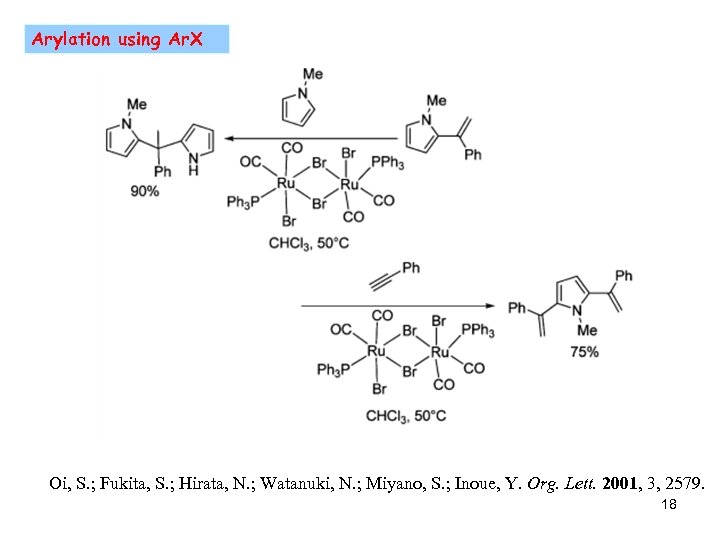 Arylation using Ar. X Oi, S. ; Fukita, S. ; Hirata, N. ; Watanuki, N. ; Miyano, S. ; Inoue, Y. Org. Lett. 2001, 3, 2579. 18
Arylation using Ar. X Oi, S. ; Fukita, S. ; Hirata, N. ; Watanuki, N. ; Miyano, S. ; Inoue, Y. Org. Lett. 2001, 3, 2579. 18
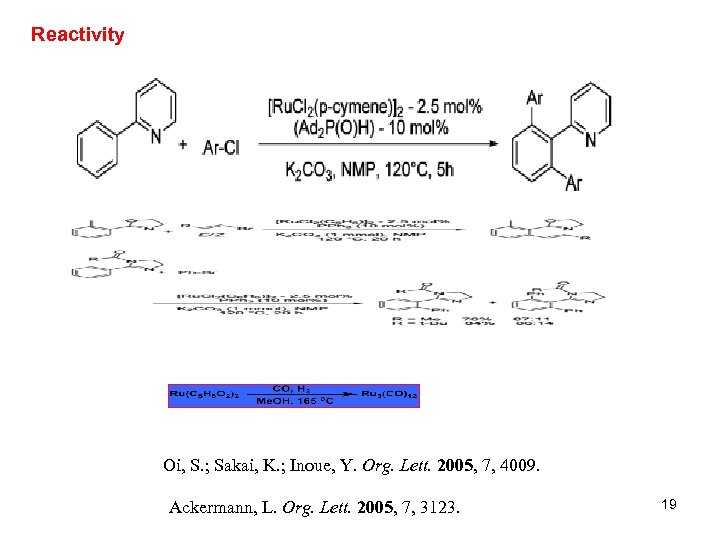 Reactivity Oi, S. ; Sakai, K. ; Inoue, Y. Org. Lett. 2005, 7, 4009. Ackermann, L. Org. Lett. 2005, 7, 3123. 19
Reactivity Oi, S. ; Sakai, K. ; Inoue, Y. Org. Lett. 2005, 7, 4009. Ackermann, L. Org. Lett. 2005, 7, 3123. 19
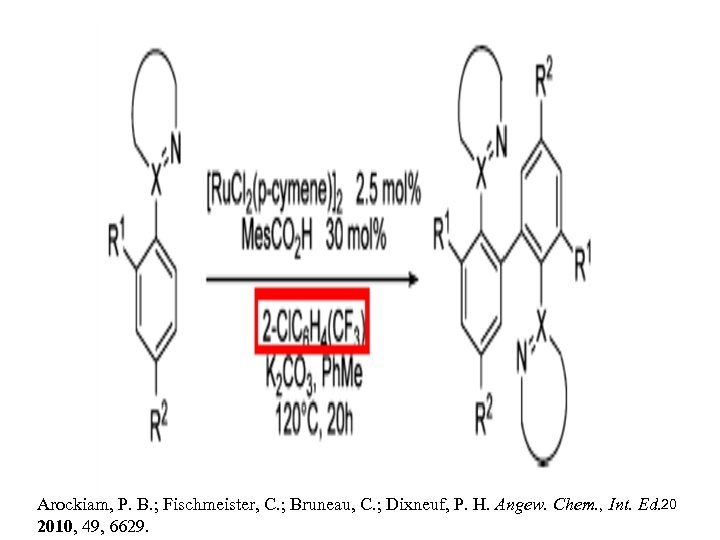 Arockiam, P. B. ; Fischmeister, C. ; Bruneau, C. ; Dixneuf, P. H. Angew. Chem. , Int. Ed. 20 2010, 49, 6629.
Arockiam, P. B. ; Fischmeister, C. ; Bruneau, C. ; Dixneuf, P. H. Angew. Chem. , Int. Ed. 20 2010, 49, 6629.
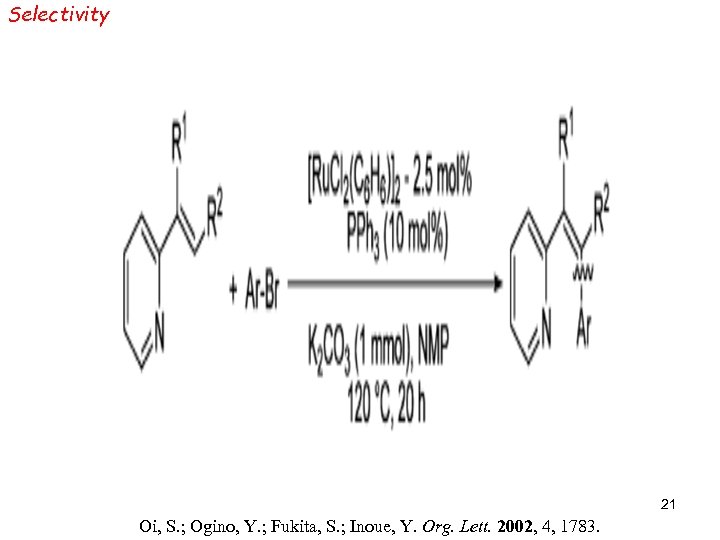 Selectivity 21 Oi, S. ; Ogino, Y. ; Fukita, S. ; Inoue, Y. Org. Lett. 2002, 4, 1783.
Selectivity 21 Oi, S. ; Ogino, Y. ; Fukita, S. ; Inoue, Y. Org. Lett. 2002, 4, 1783.
 Oi, S. ; Aizawa, E. ; Ogino, Y. ; Inoue, Y. J. Org. Chem. 2005, 70, 3113. 22
Oi, S. ; Aizawa, E. ; Ogino, Y. ; Inoue, Y. J. Org. Chem. 2005, 70, 3113. 22
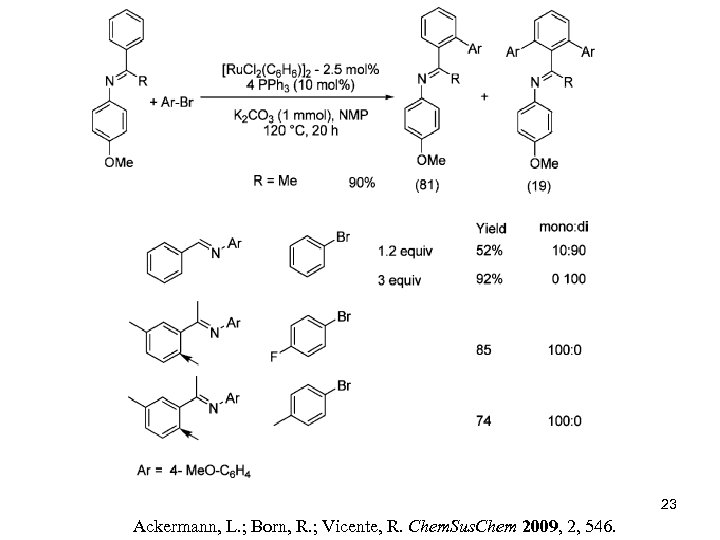 23 Ackermann, L. ; Born, R. ; Vicente, R. Chem. Sus. Chem 2009, 2, 546.
23 Ackermann, L. ; Born, R. ; Vicente, R. Chem. Sus. Chem 2009, 2, 546.
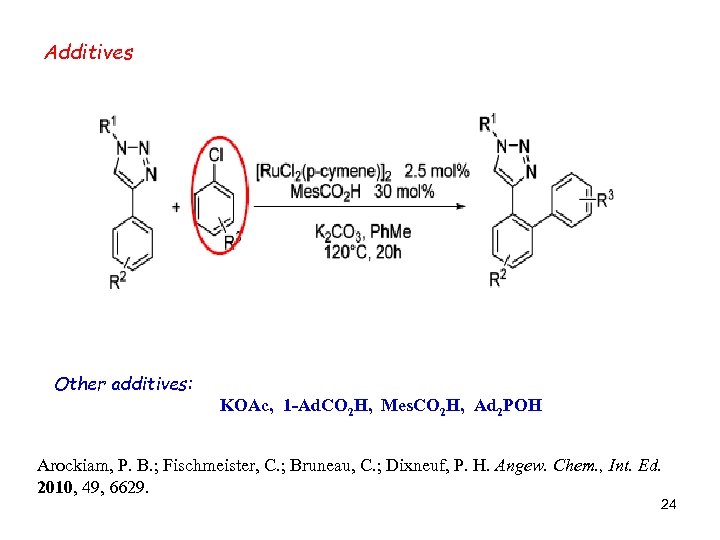 Additives Other additives: KOAc, 1 -Ad. CO 2 H, Mes. CO 2 H, Ad 2 POH Arockiam, P. B. ; Fischmeister, C. ; Bruneau, C. ; Dixneuf, P. H. Angew. Chem. , Int. Ed. 2010, 49, 6629. 24
Additives Other additives: KOAc, 1 -Ad. CO 2 H, Mes. CO 2 H, Ad 2 POH Arockiam, P. B. ; Fischmeister, C. ; Bruneau, C. ; Dixneuf, P. H. Angew. Chem. , Int. Ed. 2010, 49, 6629. 24
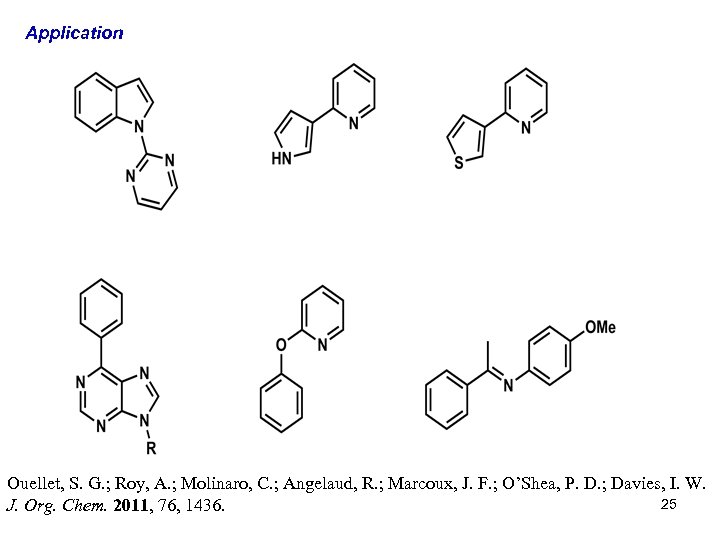 Application Ouellet, S. G. ; Roy, A. ; Molinaro, C. ; Angelaud, R. ; Marcoux, J. F. ; O’Shea, P. D. ; Davies, I. W. 25 J. Org. Chem. 2011, 76, 1436.
Application Ouellet, S. G. ; Roy, A. ; Molinaro, C. ; Angelaud, R. ; Marcoux, J. F. ; O’Shea, P. D. ; Davies, I. W. 25 J. Org. Chem. 2011, 76, 1436.
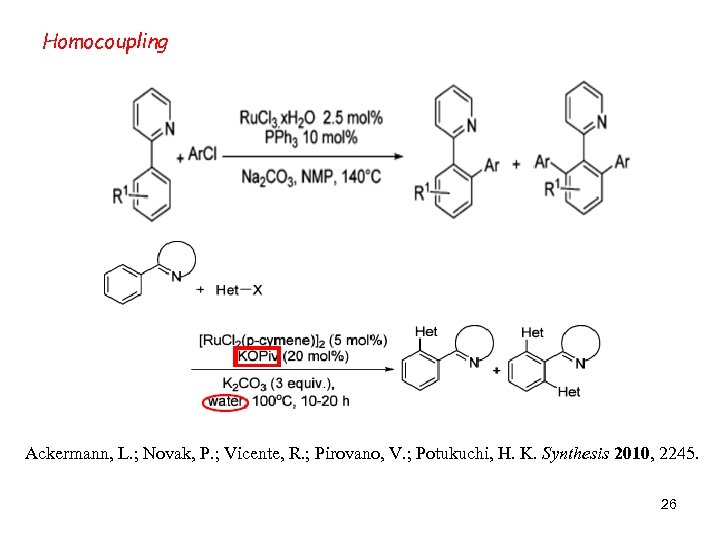 Homocoupling Ackermann, L. ; Novak, P. ; Vicente, R. ; Pirovano, V. ; Potukuchi, H. K. Synthesis 2010, 2245. 26
Homocoupling Ackermann, L. ; Novak, P. ; Vicente, R. ; Pirovano, V. ; Potukuchi, H. K. Synthesis 2010, 2245. 26
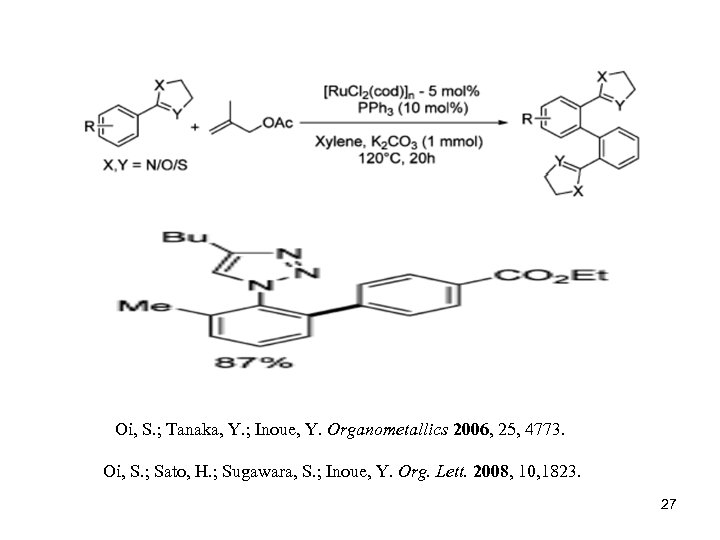 Oi, S. ; Tanaka, Y. ; Inoue, Y. Organometallics 2006, 25, 4773. Oi, S. ; Sato, H. ; Sugawara, S. ; Inoue, Y. Org. Lett. 2008, 10, 1823. 27
Oi, S. ; Tanaka, Y. ; Inoue, Y. Organometallics 2006, 25, 4773. Oi, S. ; Sato, H. ; Sugawara, S. ; Inoue, Y. Org. Lett. 2008, 10, 1823. 27
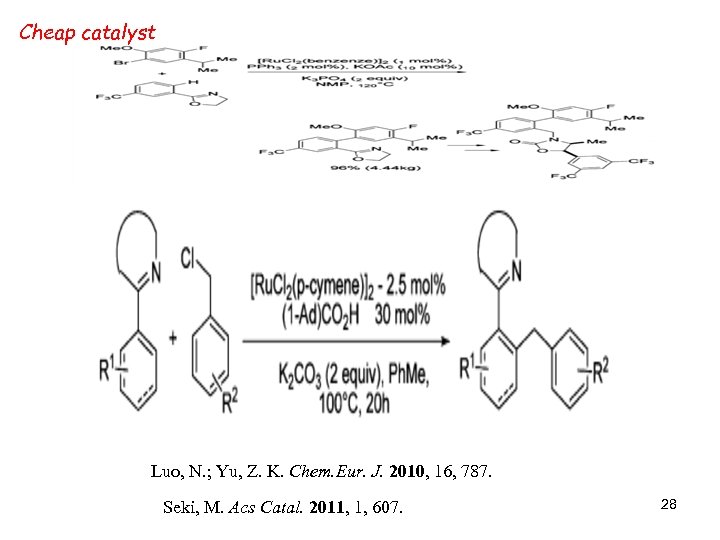 Cheap catalyst Luo, N. ; Yu, Z. K. Chem. Eur. J. 2010, 16, 787. Seki, M. Acs Catal. 2011, 1, 607. 28
Cheap catalyst Luo, N. ; Yu, Z. K. Chem. Eur. J. 2010, 16, 787. Seki, M. Acs Catal. 2011, 1, 607. 28
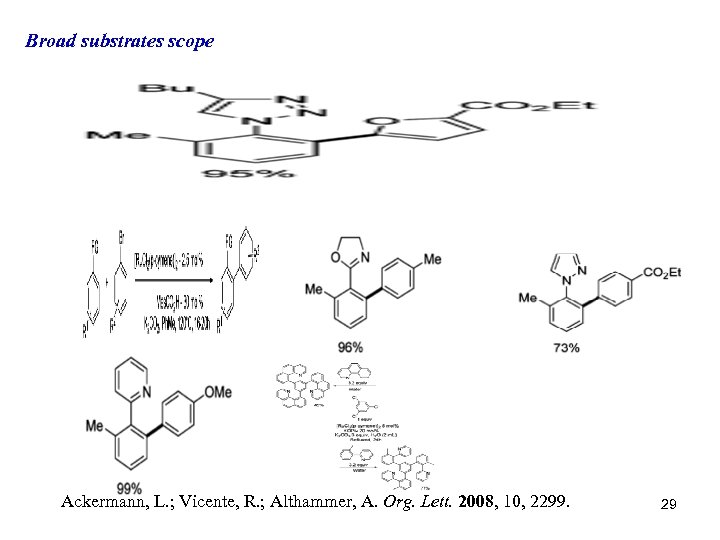 Broad substrates scope Ackermann, L. ; Vicente, R. ; Althammer, A. Org. Lett. 2008, 10, 2299. 29
Broad substrates scope Ackermann, L. ; Vicente, R. ; Althammer, A. Org. Lett. 2008, 10, 2299. 29
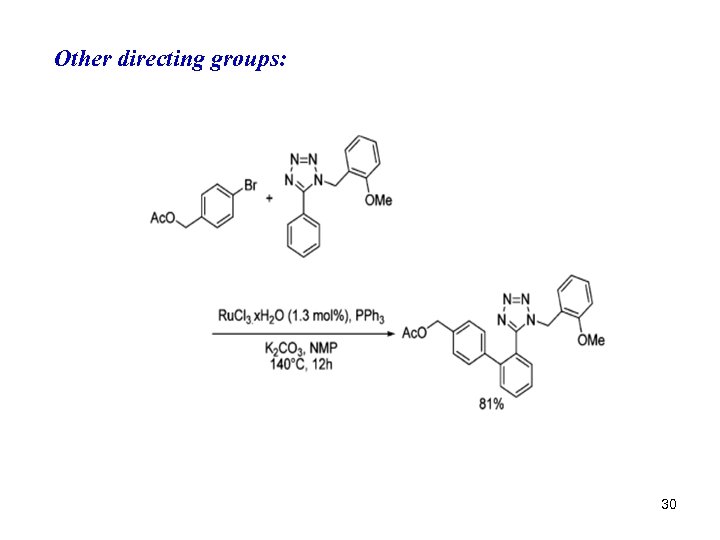 Other directing groups: 30
Other directing groups: 30
 Alkylation using sp 3 RX 31 Ackermann, L. ; Novak, P. ; Vicente, R. ; Hofmann, N. Angew. Chem. , Int. Ed. 2009, 48, 6045.
Alkylation using sp 3 RX 31 Ackermann, L. ; Novak, P. ; Vicente, R. ; Hofmann, N. Angew. Chem. , Int. Ed. 2009, 48, 6045.
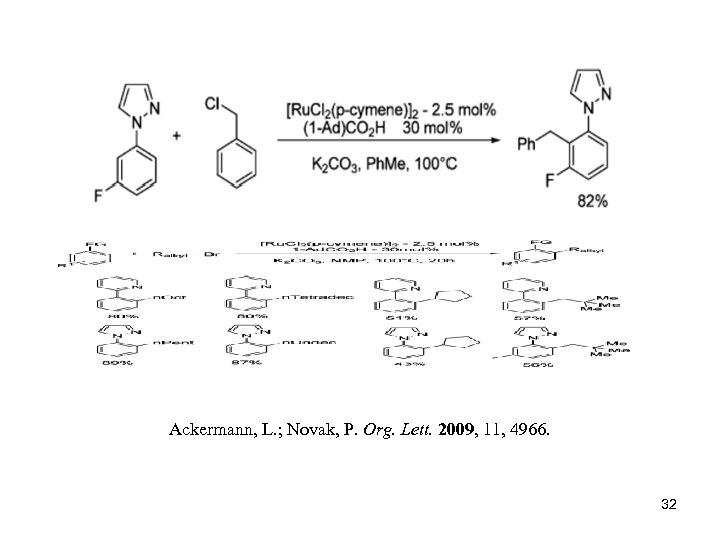 Ackermann, L. ; Novak, P. Org. Lett. 2009, 11, 4966. 32
Ackermann, L. ; Novak, P. Org. Lett. 2009, 11, 4966. 32
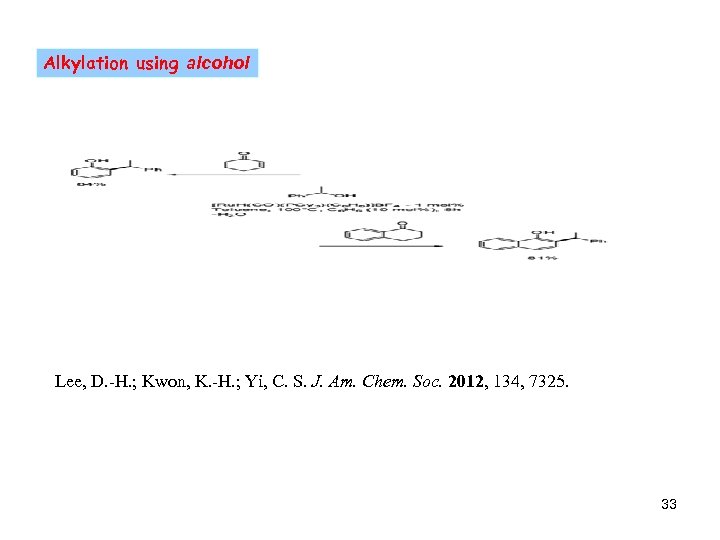 Alkylation using alcohol Lee, D. -H. ; Kwon, K. -H. ; Yi, C. S. J. Am. Chem. Soc. 2012, 134, 7325. 33
Alkylation using alcohol Lee, D. -H. ; Kwon, K. -H. ; Yi, C. S. J. Am. Chem. Soc. 2012, 134, 7325. 33
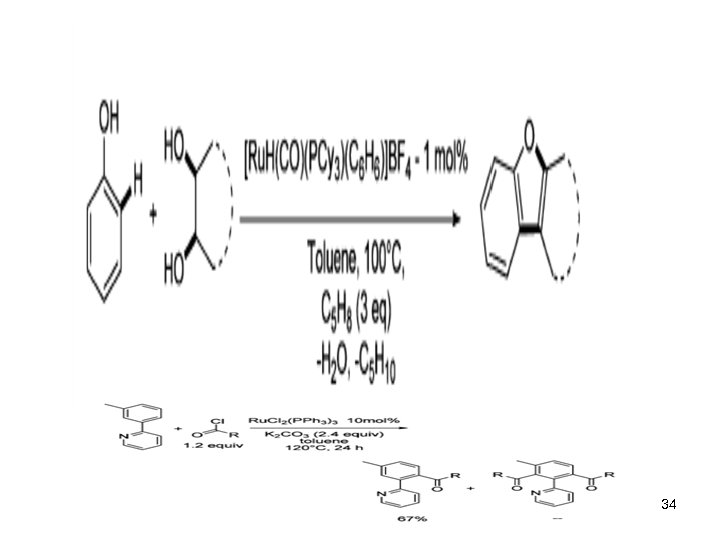 34
34
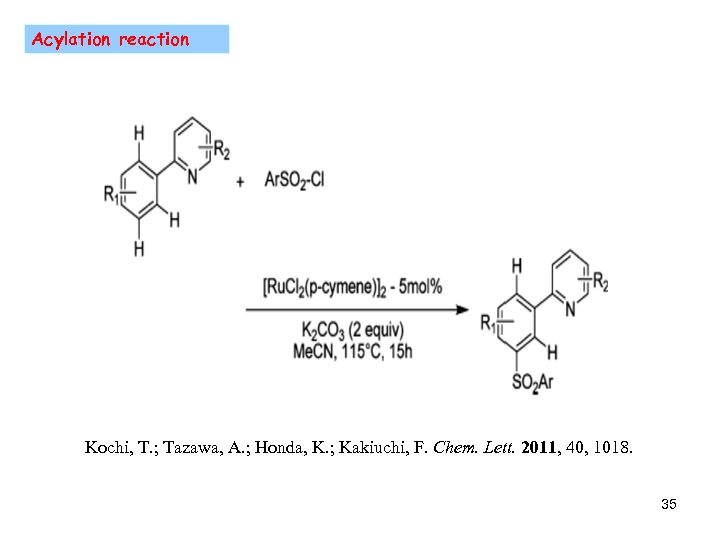 Acylation reaction Kochi, T. ; Tazawa, A. ; Honda, K. ; Kakiuchi, F. Chem. Lett. 2011, 40, 1018. 35
Acylation reaction Kochi, T. ; Tazawa, A. ; Honda, K. ; Kakiuchi, F. Chem. Lett. 2011, 40, 1018. 35
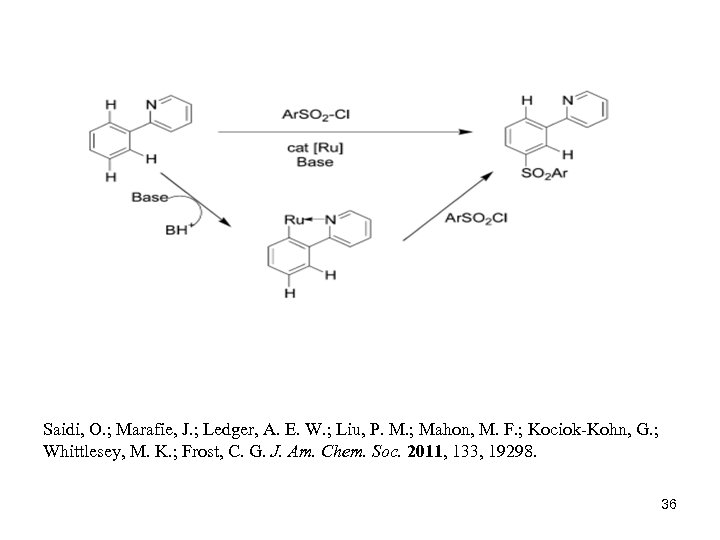 Saidi, O. ; Marafie, J. ; Ledger, A. E. W. ; Liu, P. M. ; Mahon, M. F. ; Kociok-Kohn, G. ; Whittlesey, M. K. ; Frost, C. G. J. Am. Chem. Soc. 2011, 133, 19298. 36
Saidi, O. ; Marafie, J. ; Ledger, A. E. W. ; Liu, P. M. ; Mahon, M. F. ; Kociok-Kohn, G. ; Whittlesey, M. K. ; Frost, C. G. J. Am. Chem. Soc. 2011, 133, 19298. 36
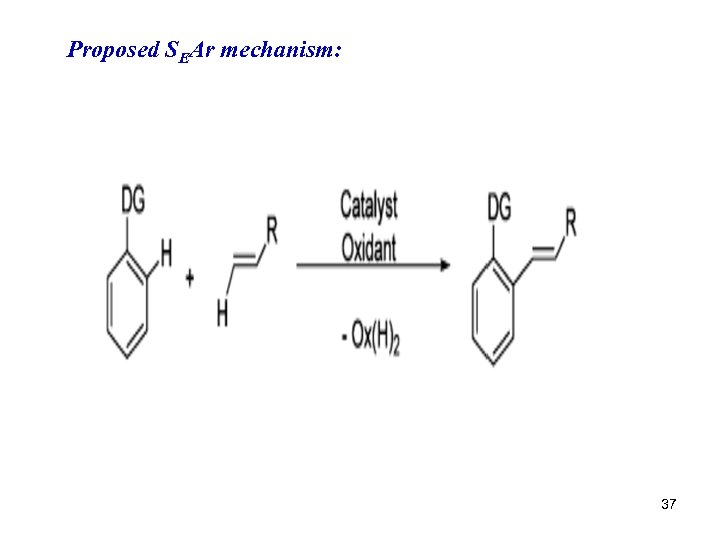 Proposed SEAr mechanism: 37
Proposed SEAr mechanism: 37
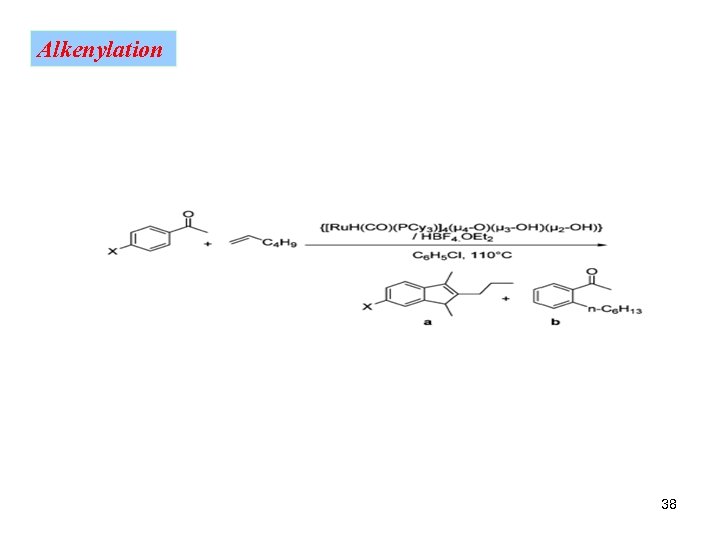 Alkenylation 38
Alkenylation 38
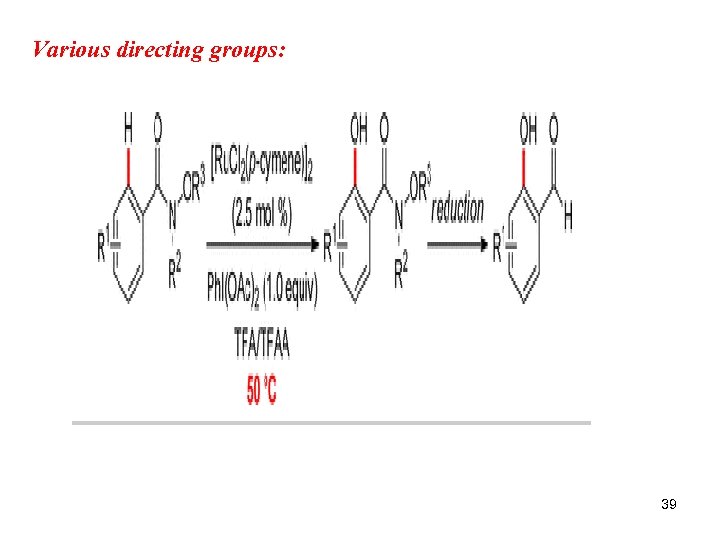 Various directing groups: 39
Various directing groups: 39
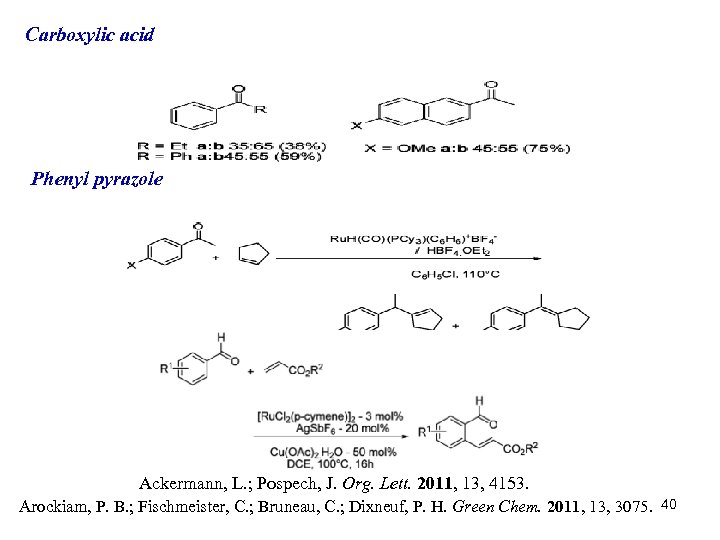 Carboxylic acid Phenyl pyrazole Ackermann, L. ; Pospech, J. Org. Lett. 2011, 13, 4153. Arockiam, P. B. ; Fischmeister, C. ; Bruneau, C. ; Dixneuf, P. H. Green Chem. 2011, 13, 3075. 40
Carboxylic acid Phenyl pyrazole Ackermann, L. ; Pospech, J. Org. Lett. 2011, 13, 4153. Arockiam, P. B. ; Fischmeister, C. ; Bruneau, C. ; Dixneuf, P. H. Green Chem. 2011, 13, 3075. 40
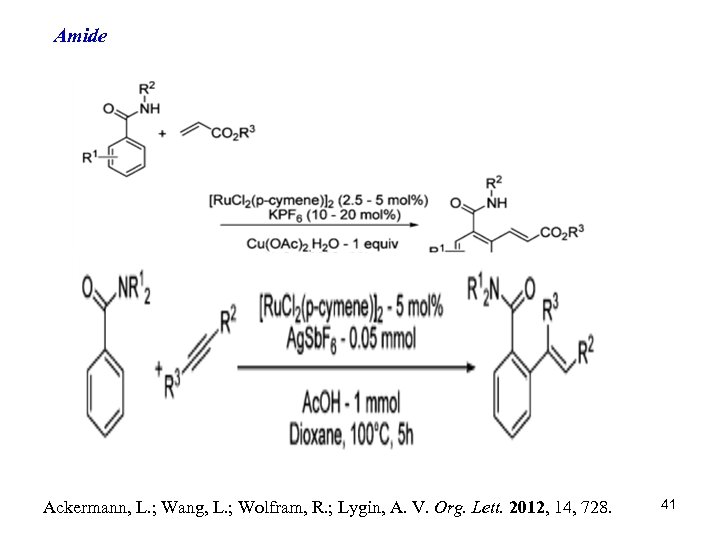 Amide Ackermann, L. ; Wang, L. ; Wolfram, R. ; Lygin, A. V. Org. Lett. 2012, 14, 728. 41
Amide Ackermann, L. ; Wang, L. ; Wolfram, R. ; Lygin, A. V. Org. Lett. 2012, 14, 728. 41
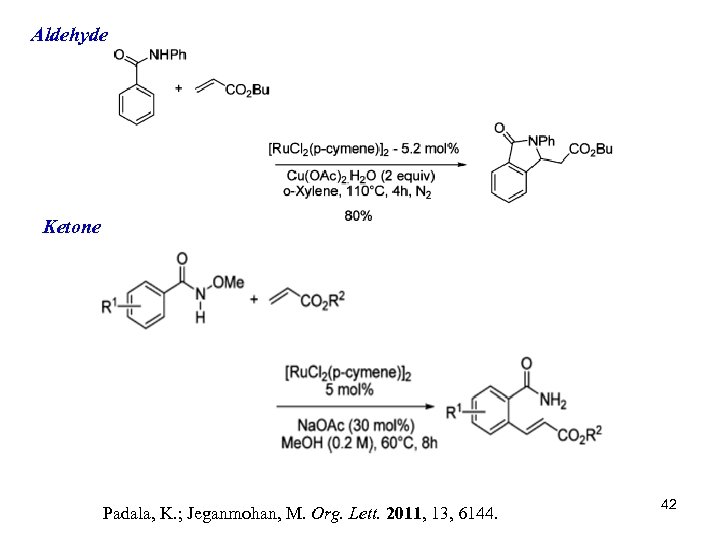 Aldehyde Ketone Padala, K. ; Jeganmohan, M. Org. Lett. 2011, 13, 6144. 42
Aldehyde Ketone Padala, K. ; Jeganmohan, M. Org. Lett. 2011, 13, 6144. 42
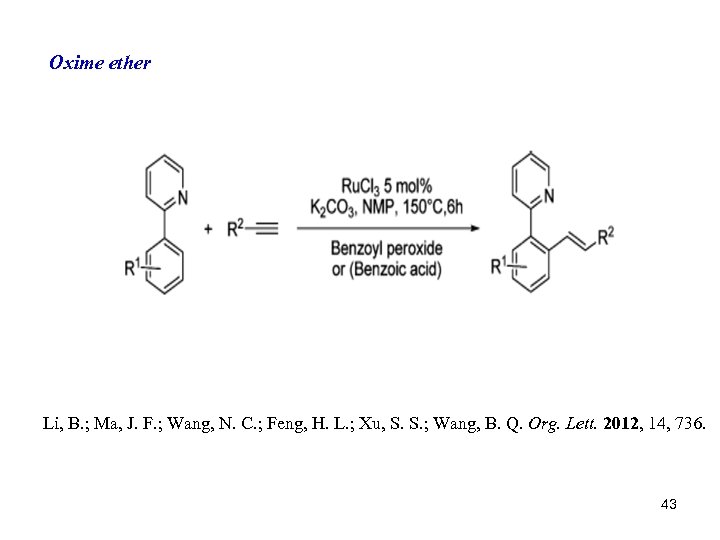 Oxime ether Li, B. ; Ma, J. F. ; Wang, N. C. ; Feng, H. L. ; Xu, S. S. ; Wang, B. Q. Org. Lett. 2012, 14, 736. 43
Oxime ether Li, B. ; Ma, J. F. ; Wang, N. C. ; Feng, H. L. ; Xu, S. S. ; Wang, B. Q. Org. Lett. 2012, 14, 736. 43
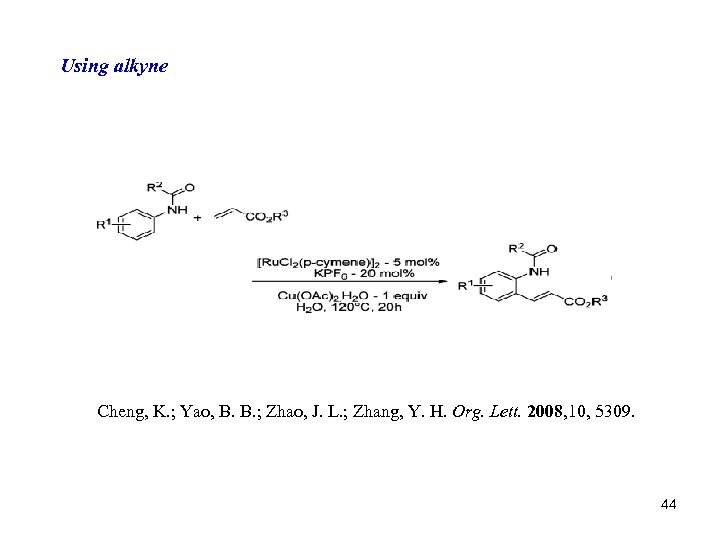 Using alkyne Cheng, K. ; Yao, B. B. ; Zhao, J. L. ; Zhang, Y. H. Org. Lett. 2008, 10, 5309. 44
Using alkyne Cheng, K. ; Yao, B. B. ; Zhao, J. L. ; Zhang, Y. H. Org. Lett. 2008, 10, 5309. 44
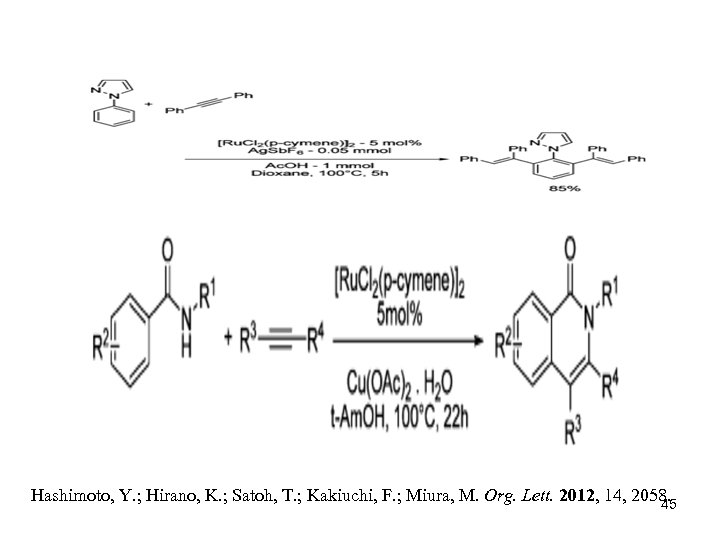 Hashimoto, Y. ; Hirano, K. ; Satoh, T. ; Kakiuchi, F. ; Miura, M. Org. Lett. 2012, 14, 2058. 45
Hashimoto, Y. ; Hirano, K. ; Satoh, T. ; Kakiuchi, F. ; Miura, M. Org. Lett. 2012, 14, 2058. 45
 Cyclization 46
Cyclization 46
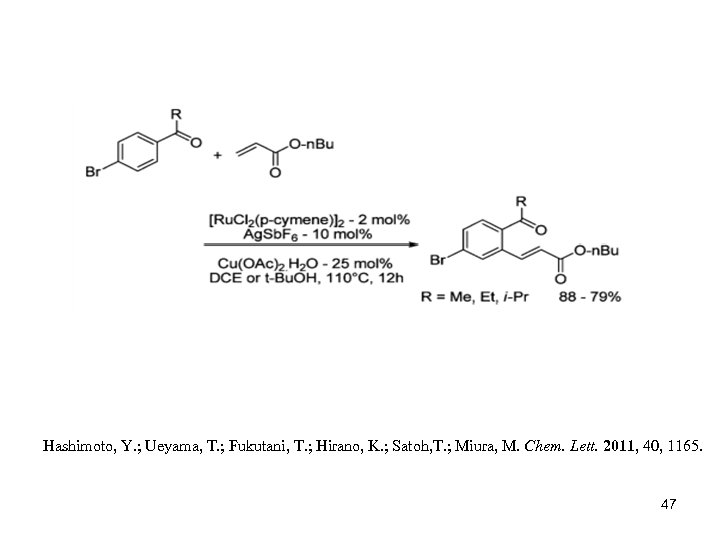 Hashimoto, Y. ; Ueyama, T. ; Fukutani, T. ; Hirano, K. ; Satoh, T. ; Miura, M. Chem. Lett. 2011, 40, 1165. 47
Hashimoto, Y. ; Ueyama, T. ; Fukutani, T. ; Hirano, K. ; Satoh, T. ; Miura, M. Chem. Lett. 2011, 40, 1165. 47
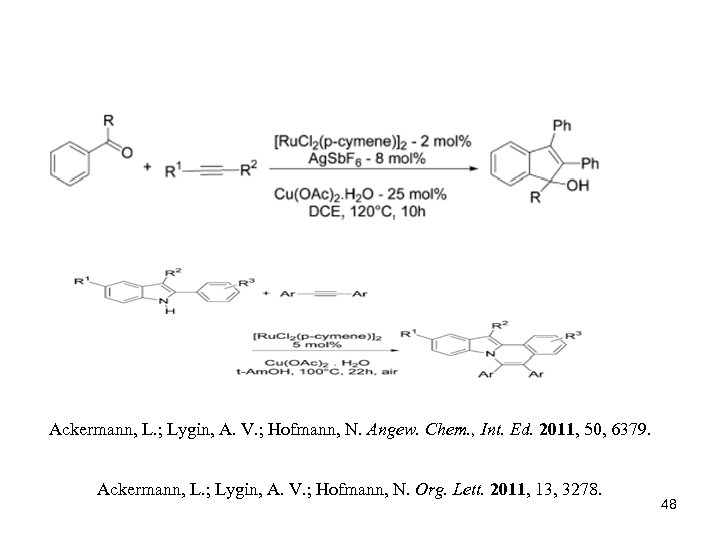 Ackermann, L. ; Lygin, A. V. ; Hofmann, N. Angew. Chem. , Int. Ed. 2011, 50, 6379. Ackermann, L. ; Lygin, A. V. ; Hofmann, N. Org. Lett. 2011, 13, 3278. 48
Ackermann, L. ; Lygin, A. V. ; Hofmann, N. Angew. Chem. , Int. Ed. 2011, 50, 6379. Ackermann, L. ; Lygin, A. V. ; Hofmann, N. Org. Lett. 2011, 13, 3278. 48
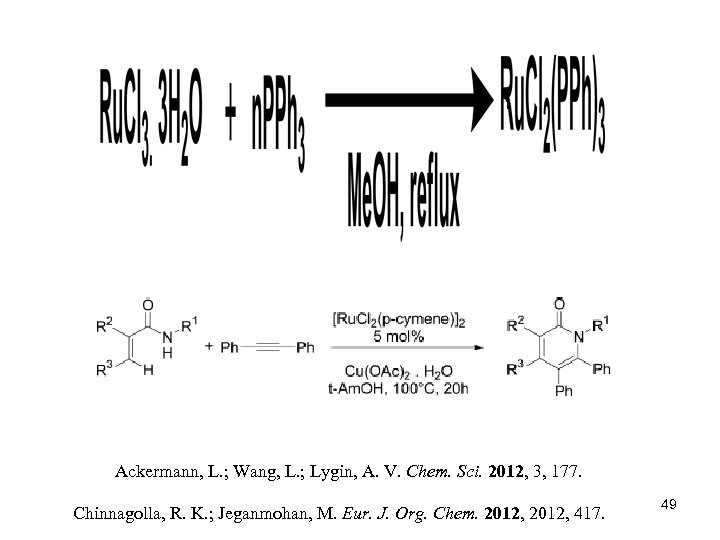 Ackermann, L. ; Wang, L. ; Lygin, A. V. Chem. Sci. 2012, 3, 177. Chinnagolla, R. K. ; Jeganmohan, M. Eur. J. Org. Chem. 2012, 417. 49
Ackermann, L. ; Wang, L. ; Lygin, A. V. Chem. Sci. 2012, 3, 177. Chinnagolla, R. K. ; Jeganmohan, M. Eur. J. Org. Chem. 2012, 417. 49
 selectivity 50 Kakiuchi, F. ; Sato, T. ; Tsujimoto, T. ; Yamauchi, M. ; Chatani, N. ; Murai, S. Chem. Lett. 1998, 27, 1053.
selectivity 50 Kakiuchi, F. ; Sato, T. ; Tsujimoto, T. ; Yamauchi, M. ; Chatani, N. ; Murai, S. Chem. Lett. 1998, 27, 1053.
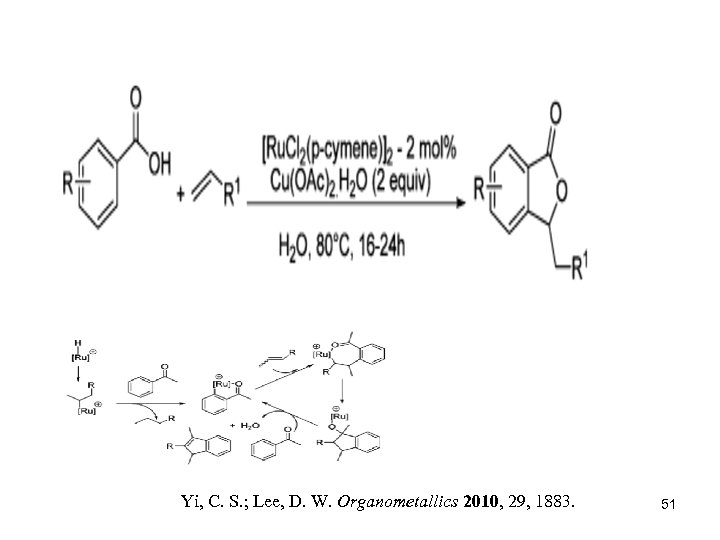 Yi, C. S. ; Lee, D. W. Organometallics 2010, 29, 1883. 51
Yi, C. S. ; Lee, D. W. Organometallics 2010, 29, 1883. 51
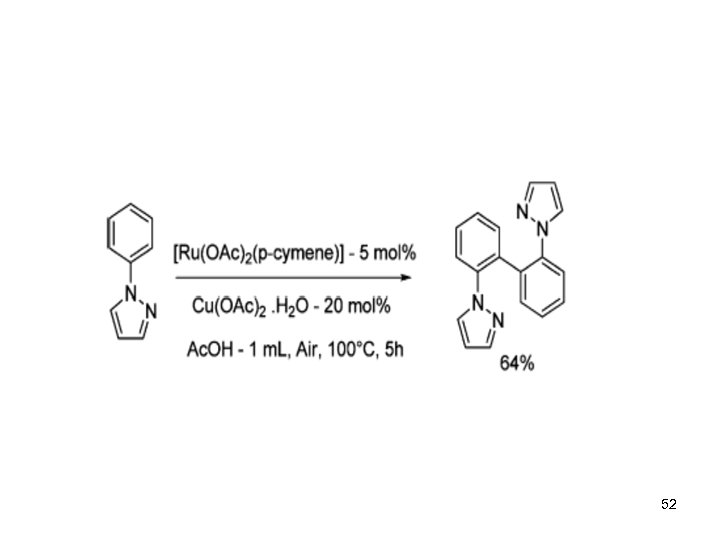 52
52
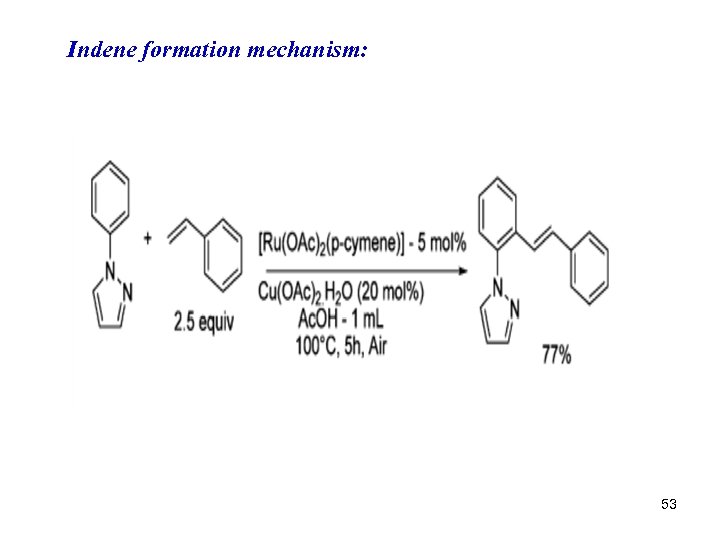 Indene formation mechanism: 53
Indene formation mechanism: 53
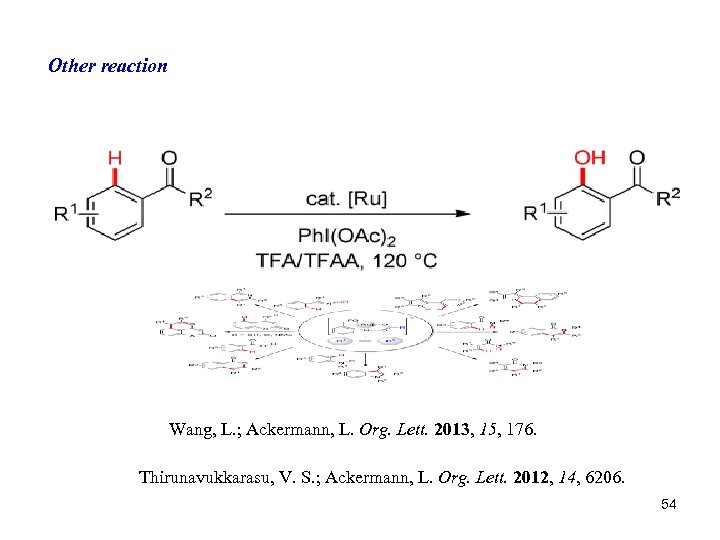 Other reaction Wang, L. ; Ackermann, L. Org. Lett. 2013, 15, 176. Thirunavukkarasu, V. S. ; Ackermann, L. Org. Lett. 2012, 14, 6206. 54
Other reaction Wang, L. ; Ackermann, L. Org. Lett. 2013, 15, 176. Thirunavukkarasu, V. S. ; Ackermann, L. Org. Lett. 2012, 14, 6206. 54
 Future may focus on: u. React at other C-H site other than ortho. u. Catalytic sp 3 C-H activation. u. Aerobic oxidations. u. Other new reaction. 55
Future may focus on: u. React at other C-H site other than ortho. u. Catalytic sp 3 C-H activation. u. Aerobic oxidations. u. Other new reaction. 55
 Further reviews Rh: Satoh, T. ; Miura, M. Chem. Eur. J. 2010, 16, 11212. Ru: Kozhushkov, S. I. ; Ackermann, L. Chem. Sci. , 2012, 3, 886. Ackermann, L. Acc. Chem. Res. , 2013, ASAP, DOI: 10. 1021/ar 3002798. Lutz Ackermann http: //www. ackermann. chemie. uni-goettingen. de/ 56
Further reviews Rh: Satoh, T. ; Miura, M. Chem. Eur. J. 2010, 16, 11212. Ru: Kozhushkov, S. I. ; Ackermann, L. Chem. Sci. , 2012, 3, 886. Ackermann, L. Acc. Chem. Res. , 2013, ASAP, DOI: 10. 1021/ar 3002798. Lutz Ackermann http: //www. ackermann. chemie. uni-goettingen. de/ 56


