Role of Ca ions in mechanisms of intercellular signalling.pptx
- Количество слайдов: 45
 Role of Ca 2+ ions in mechanisms of cell signaling Done by: Maulenova R Moldakozhayev A. Naizabayeva D.
Role of Ca 2+ ions in mechanisms of cell signaling Done by: Maulenova R Moldakozhayev A. Naizabayeva D.
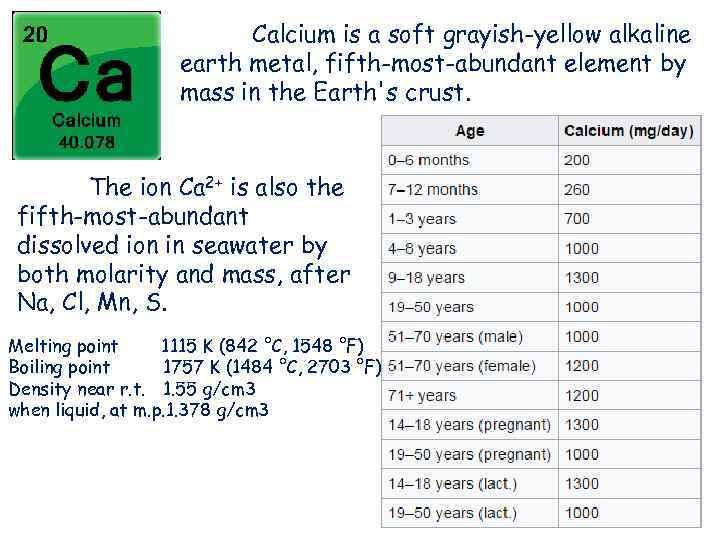 Calcium is a soft grayish-yellow alkaline earth metal, fifth-most-abundant element by mass in the Earth's crust. The ion Ca 2+ is also the fifth-most-abundant dissolved ion in seawater by both molarity and mass, after Na, Cl, Mn, S. Melting point 1115 K (842 °C, 1548 °F) Boiling point 1757 K (1484 °C, 2703 °F) Density near r. t. 1. 55 g/cm 3 when liquid, at m. p. 1. 378 g/cm 3
Calcium is a soft grayish-yellow alkaline earth metal, fifth-most-abundant element by mass in the Earth's crust. The ion Ca 2+ is also the fifth-most-abundant dissolved ion in seawater by both molarity and mass, after Na, Cl, Mn, S. Melting point 1115 K (842 °C, 1548 °F) Boiling point 1757 K (1484 °C, 2703 °F) Density near r. t. 1. 55 g/cm 3 when liquid, at m. p. 1. 378 g/cm 3
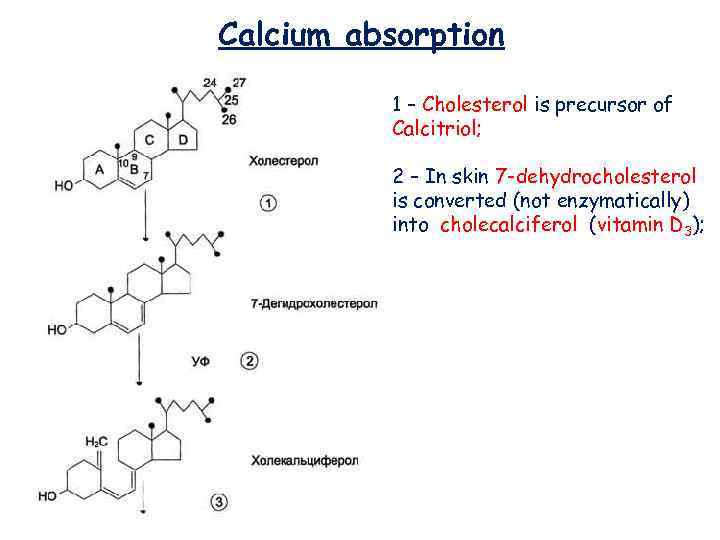 Calcium absorption 1 – Cholesterol is precursor of Calcitriol; 2 – In skin 7 -dehydrocholesterol is converted (not enzymatically) into cholecalciferol (vitamin D 3);
Calcium absorption 1 – Cholesterol is precursor of Calcitriol; 2 – In skin 7 -dehydrocholesterol is converted (not enzymatically) into cholecalciferol (vitamin D 3);
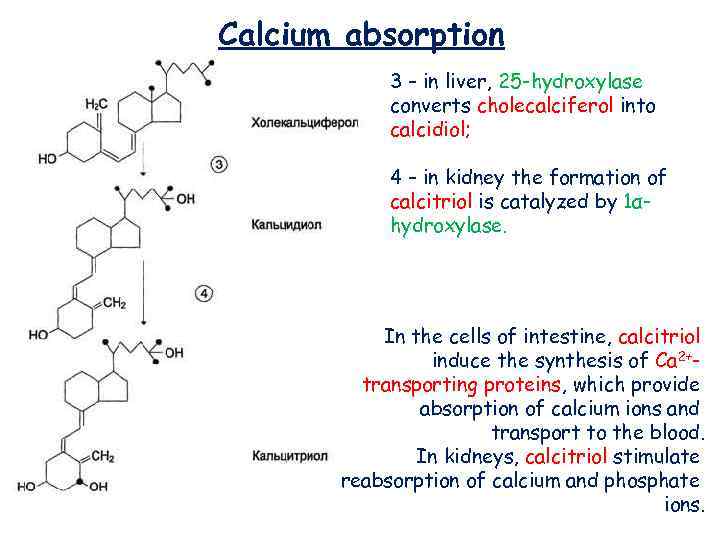 Calcium absorption 3 – in liver, 25 -hydroxylase converts cholecalciferol into calcidiol; 4 – in kidney the formation of calcitriol is catalyzed by 1αhydroxylase. In the cells of intestine, calcitriol induce the synthesis of Са 2+transporting proteins, which provide absorption of calcium ions and transport to the blood. In kidneys, calcitriol stimulate reabsorption of calcium and phosphate ions.
Calcium absorption 3 – in liver, 25 -hydroxylase converts cholecalciferol into calcidiol; 4 – in kidney the formation of calcitriol is catalyzed by 1αhydroxylase. In the cells of intestine, calcitriol induce the synthesis of Са 2+transporting proteins, which provide absorption of calcium ions and transport to the blood. In kidneys, calcitriol stimulate reabsorption of calcium and phosphate ions.
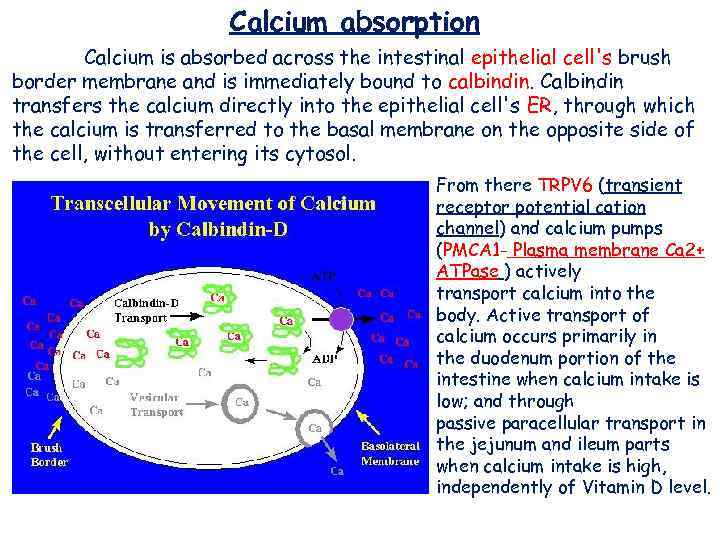 Calcium absorption Calcium is absorbed across the intestinal epithelial cell's brush border membrane and is immediately bound to calbindin. Calbindin transfers the calcium directly into the epithelial cell's ER, through which the calcium is transferred to the basal membrane on the opposite side of the cell, without entering its cytosol. From there TRPV 6 (transient receptor potential cation channel) and calcium pumps (PMCA 1 - Plasma membrane Ca 2+ ATPase ) actively transport calcium into the body. Active transport of calcium occurs primarily in the duodenum portion of the intestine when calcium intake is low; and through passive paracellular transport in the jejunum and ileum parts when calcium intake is high, independently of Vitamin D level.
Calcium absorption Calcium is absorbed across the intestinal epithelial cell's brush border membrane and is immediately bound to calbindin. Calbindin transfers the calcium directly into the epithelial cell's ER, through which the calcium is transferred to the basal membrane on the opposite side of the cell, without entering its cytosol. From there TRPV 6 (transient receptor potential cation channel) and calcium pumps (PMCA 1 - Plasma membrane Ca 2+ ATPase ) actively transport calcium into the body. Active transport of calcium occurs primarily in the duodenum portion of the intestine when calcium intake is low; and through passive paracellular transport in the jejunum and ileum parts when calcium intake is high, independently of Vitamin D level.
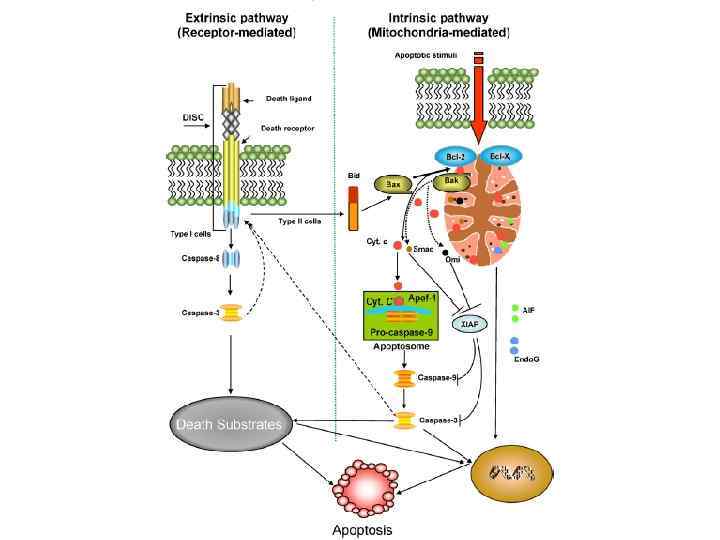
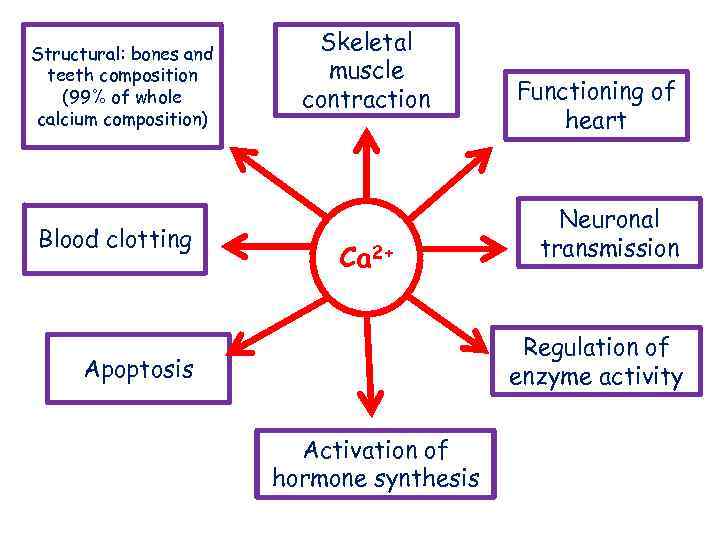 Structural: bones and teeth composition (99% of whole calcium composition) Blood clotting Skeletal muscle contraction Ca 2+ Functioning of heart Neuronal transmission Regulation of enzyme activity Apoptosis Activation of hormone synthesis
Structural: bones and teeth composition (99% of whole calcium composition) Blood clotting Skeletal muscle contraction Ca 2+ Functioning of heart Neuronal transmission Regulation of enzyme activity Apoptosis Activation of hormone synthesis
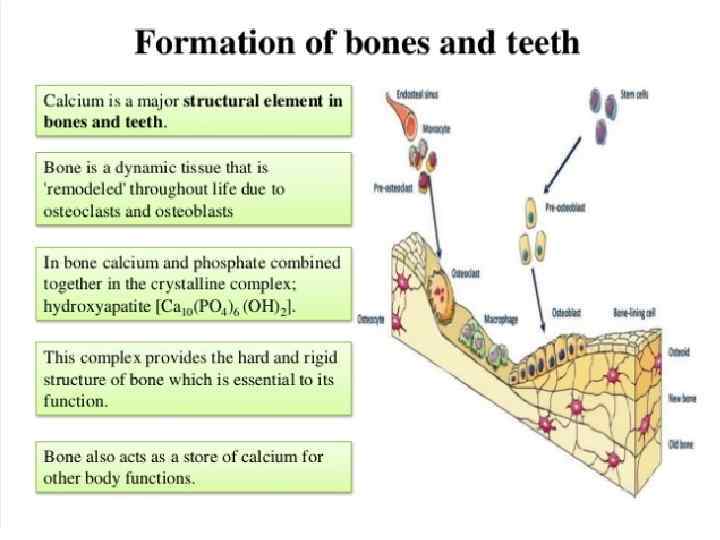
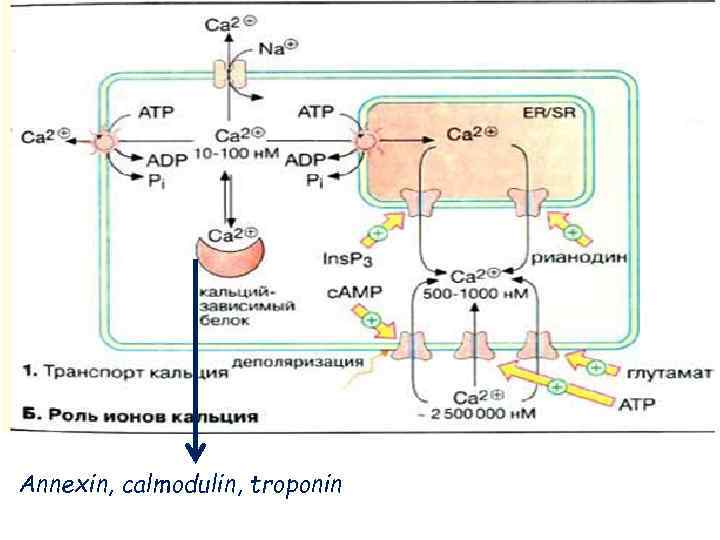 Annexin, calmodulin, troponin
Annexin, calmodulin, troponin
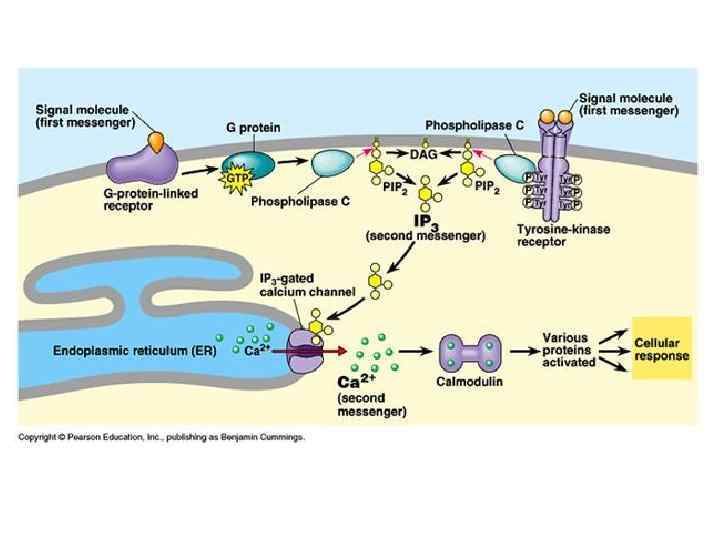
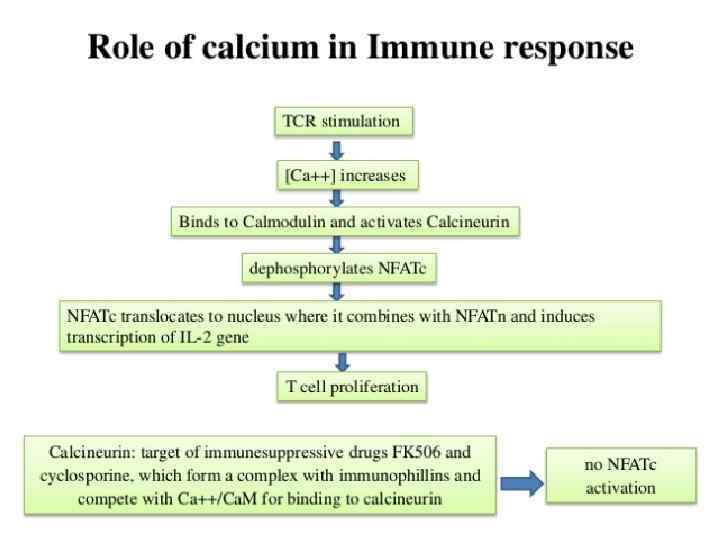
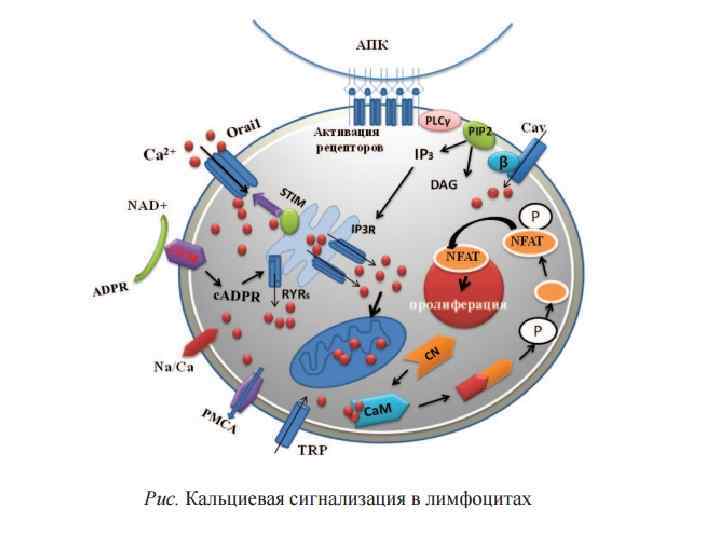
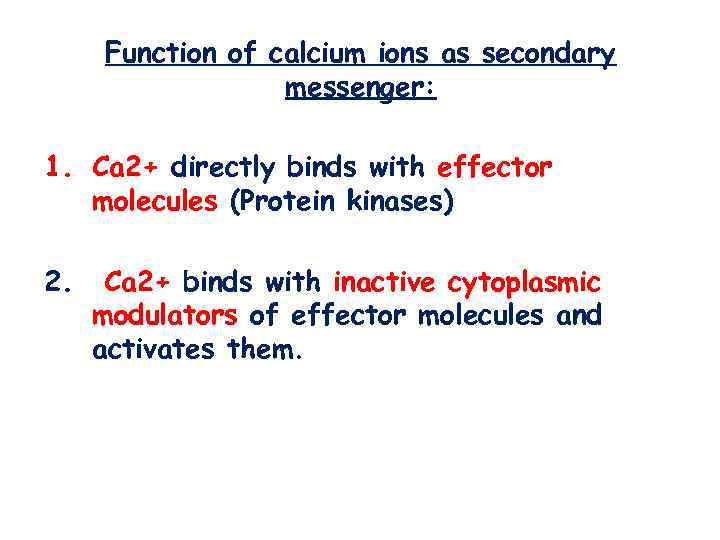 Function of calcium ions as secondary messenger: 1. Са 2+ directly binds with effector molecules (Protein kinases) 2. Са 2+ binds with inactive cytoplasmic modulators of effector molecules and activates them.
Function of calcium ions as secondary messenger: 1. Са 2+ directly binds with effector molecules (Protein kinases) 2. Са 2+ binds with inactive cytoplasmic modulators of effector molecules and activates them.
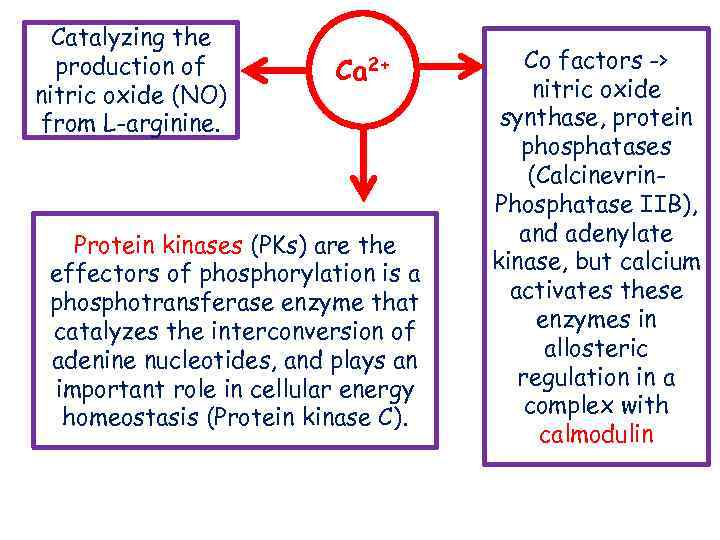 Catalyzing the production of nitric oxide (NO) from L-arginine. Ca 2+ Protein kinases (PKs) are the effectors of phosphorylation is a phosphotransferase enzyme that catalyzes the interconversion of adenine nucleotides, and plays an important role in cellular energy homeostasis (Protein kinase C). Co factors -> nitric oxide synthase, protein phosphatases (Calcinevrin. Phosphatase IIB), and adenylate kinase, but calcium activates these enzymes in allosteric regulation in a complex with calmodulin
Catalyzing the production of nitric oxide (NO) from L-arginine. Ca 2+ Protein kinases (PKs) are the effectors of phosphorylation is a phosphotransferase enzyme that catalyzes the interconversion of adenine nucleotides, and plays an important role in cellular energy homeostasis (Protein kinase C). Co factors -> nitric oxide synthase, protein phosphatases (Calcinevrin. Phosphatase IIB), and adenylate kinase, but calcium activates these enzymes in allosteric regulation in a complex with calmodulin
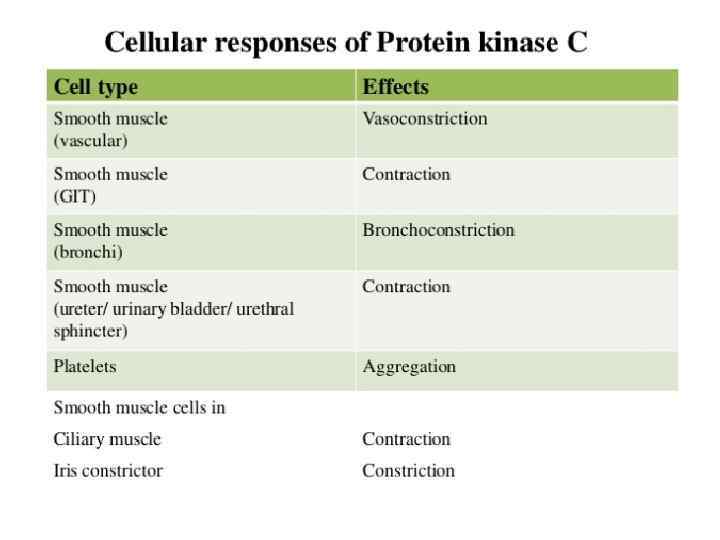
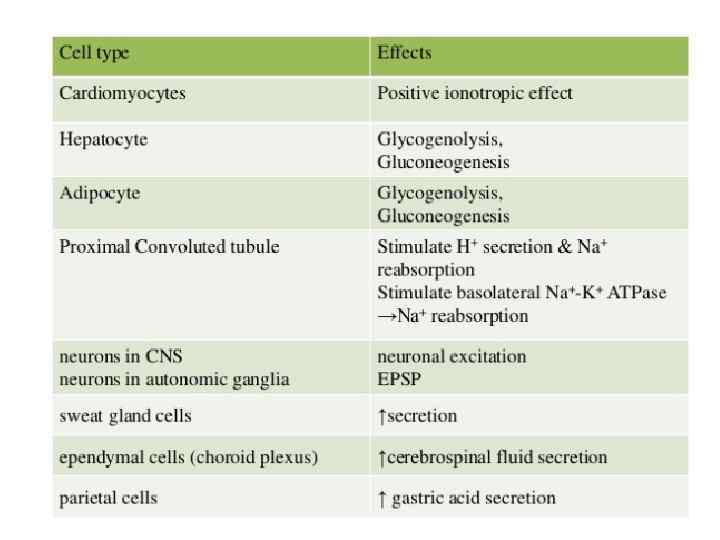
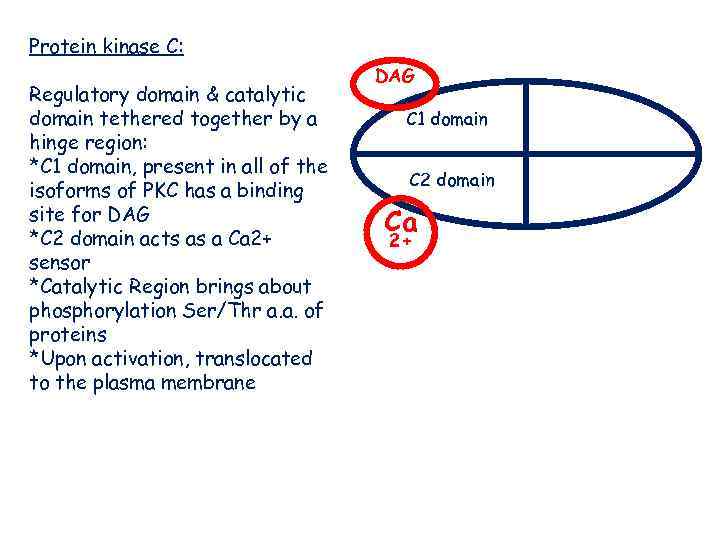 Protein kinase C: Regulatory domain & catalytic domain tethered together by a hinge region: *C 1 domain, present in all of the isoforms of PKC has a binding site for DAG *C 2 domain acts as a Ca 2+ sensor *Catalytic Region brings about phosphorylation Ser/Thr a. a. of proteins *Upon activation, translocated to the plasma membrane DAG C 1 domain C 2 domain Ca 2+
Protein kinase C: Regulatory domain & catalytic domain tethered together by a hinge region: *C 1 domain, present in all of the isoforms of PKC has a binding site for DAG *C 2 domain acts as a Ca 2+ sensor *Catalytic Region brings about phosphorylation Ser/Thr a. a. of proteins *Upon activation, translocated to the plasma membrane DAG C 1 domain C 2 domain Ca 2+
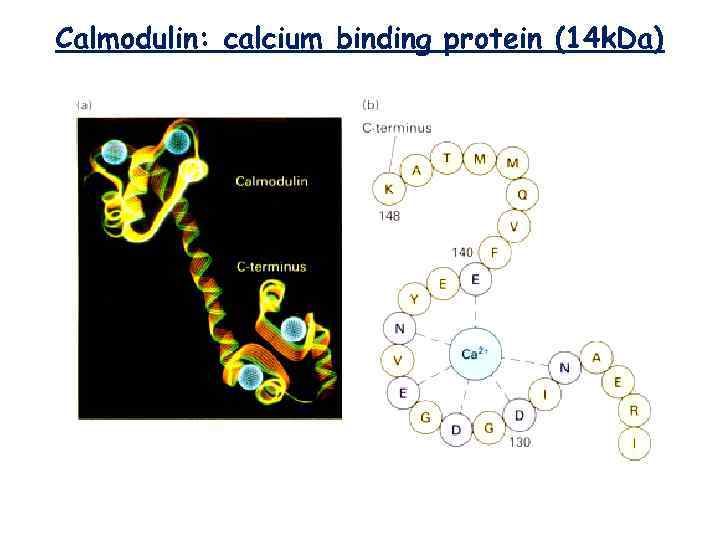 Calmodulin: calcium binding protein (14 k. Da)
Calmodulin: calcium binding protein (14 k. Da)
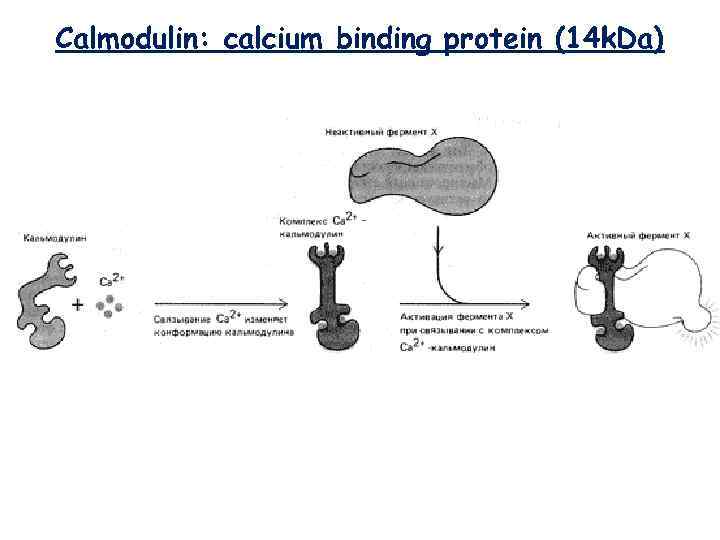 Calmodulin: calcium binding protein (14 k. Da)
Calmodulin: calcium binding protein (14 k. Da)
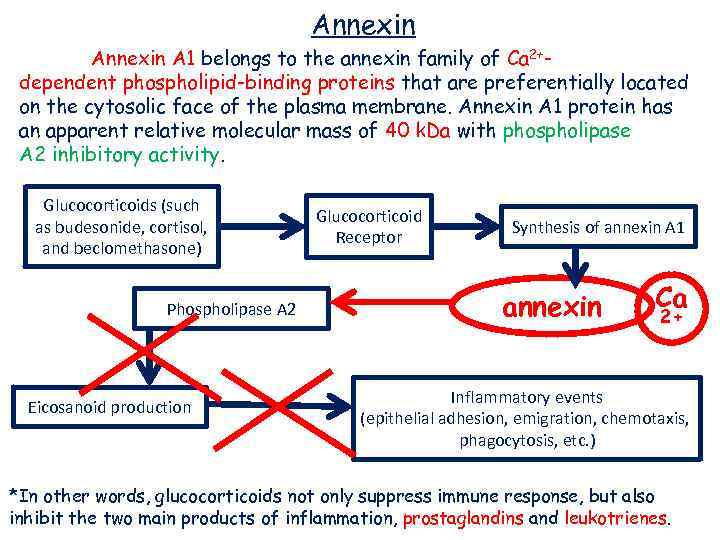 Annexin A 1 belongs to the annexin family of Ca 2+dependent phospholipid-binding proteins that are preferentially located on the cytosolic face of the plasma membrane. Annexin A 1 protein has an apparent relative molecular mass of 40 k. Da with phospholipase A 2 inhibitory activity. Glucocorticoids (such as budesonide, cortisol, and beclomethasone) Phospholipase A 2 Eicosanoid production Glucocorticoid Receptor Synthesis of annexin A 1 annexin Ca 2+ Inflammatory events (epithelial adhesion, emigration, chemotaxis, phagocytosis, etc. ) *In other words, glucocorticoids not only suppress immune response, but also inhibit the two main products of inflammation, prostaglandins and leukotrienes.
Annexin A 1 belongs to the annexin family of Ca 2+dependent phospholipid-binding proteins that are preferentially located on the cytosolic face of the plasma membrane. Annexin A 1 protein has an apparent relative molecular mass of 40 k. Da with phospholipase A 2 inhibitory activity. Glucocorticoids (such as budesonide, cortisol, and beclomethasone) Phospholipase A 2 Eicosanoid production Glucocorticoid Receptor Synthesis of annexin A 1 annexin Ca 2+ Inflammatory events (epithelial adhesion, emigration, chemotaxis, phagocytosis, etc. ) *In other words, glucocorticoids not only suppress immune response, but also inhibit the two main products of inflammation, prostaglandins and leukotrienes.
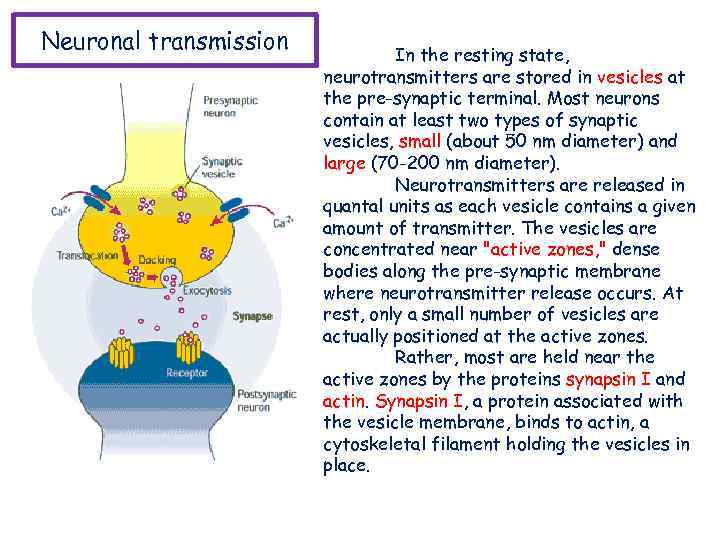 Neuronal transmission In the resting state, neurotransmitters are stored in vesicles at the pre-synaptic terminal. Most neurons contain at least two types of synaptic vesicles, small (about 50 nm diameter) and large (70 -200 nm diameter). Neurotransmitters are released in quantal units as each vesicle contains a given amount of transmitter. The vesicles are concentrated near "active zones, " dense bodies along the pre-synaptic membrane where neurotransmitter release occurs. At rest, only a small number of vesicles are actually positioned at the active zones. Rather, most are held near the active zones by the proteins synapsin I and actin. Synapsin I, a protein associated with the vesicle membrane, binds to actin, a cytoskeletal filament holding the vesicles in place.
Neuronal transmission In the resting state, neurotransmitters are stored in vesicles at the pre-synaptic terminal. Most neurons contain at least two types of synaptic vesicles, small (about 50 nm diameter) and large (70 -200 nm diameter). Neurotransmitters are released in quantal units as each vesicle contains a given amount of transmitter. The vesicles are concentrated near "active zones, " dense bodies along the pre-synaptic membrane where neurotransmitter release occurs. At rest, only a small number of vesicles are actually positioned at the active zones. Rather, most are held near the active zones by the proteins synapsin I and actin. Synapsin I, a protein associated with the vesicle membrane, binds to actin, a cytoskeletal filament holding the vesicles in place.
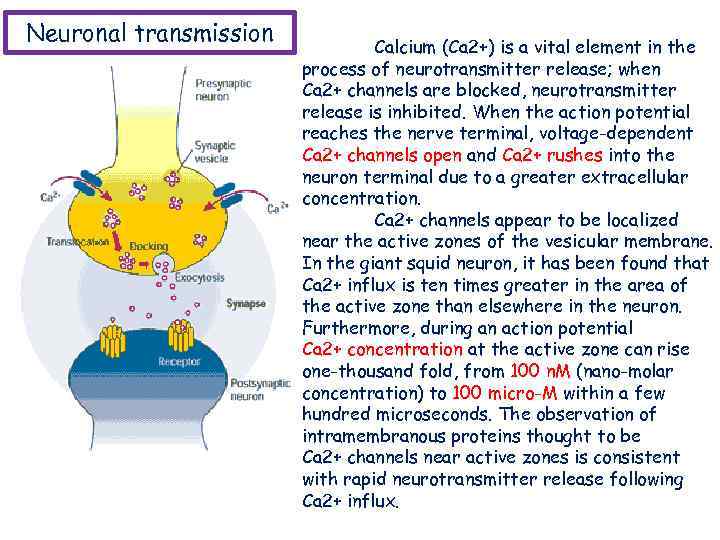 Neuronal transmission Calcium (Ca 2+) is a vital element in the process of neurotransmitter release; when Ca 2+ channels are blocked, neurotransmitter release is inhibited. When the action potential reaches the nerve terminal, voltage-dependent Ca 2+ channels open and Ca 2+ rushes into the neuron terminal due to a greater extracellular concentration. Ca 2+ channels appear to be localized near the active zones of the vesicular membrane. In the giant squid neuron, it has been found that Ca 2+ influx is ten times greater in the area of the active zone than elsewhere in the neuron. Furthermore, during an action potential Ca 2+ concentration at the active zone can rise one-thousand fold, from 100 n. M (nano-molar concentration) to 100 micro-M within a few hundred microseconds. The observation of intramembranous proteins thought to be Ca 2+ channels near active zones is consistent with rapid neurotransmitter release following Ca 2+ influx.
Neuronal transmission Calcium (Ca 2+) is a vital element in the process of neurotransmitter release; when Ca 2+ channels are blocked, neurotransmitter release is inhibited. When the action potential reaches the nerve terminal, voltage-dependent Ca 2+ channels open and Ca 2+ rushes into the neuron terminal due to a greater extracellular concentration. Ca 2+ channels appear to be localized near the active zones of the vesicular membrane. In the giant squid neuron, it has been found that Ca 2+ influx is ten times greater in the area of the active zone than elsewhere in the neuron. Furthermore, during an action potential Ca 2+ concentration at the active zone can rise one-thousand fold, from 100 n. M (nano-molar concentration) to 100 micro-M within a few hundred microseconds. The observation of intramembranous proteins thought to be Ca 2+ channels near active zones is consistent with rapid neurotransmitter release following Ca 2+ influx.
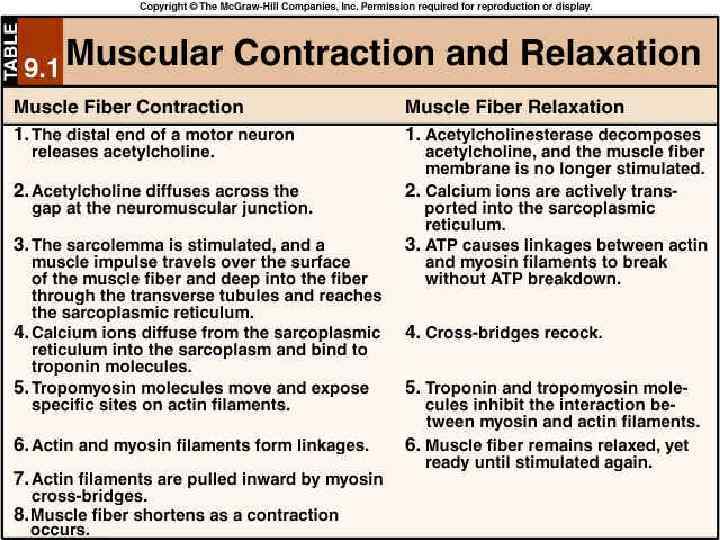
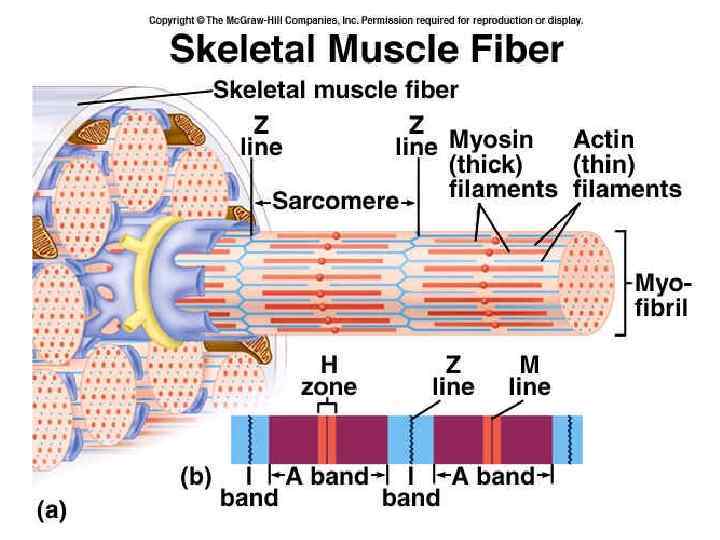
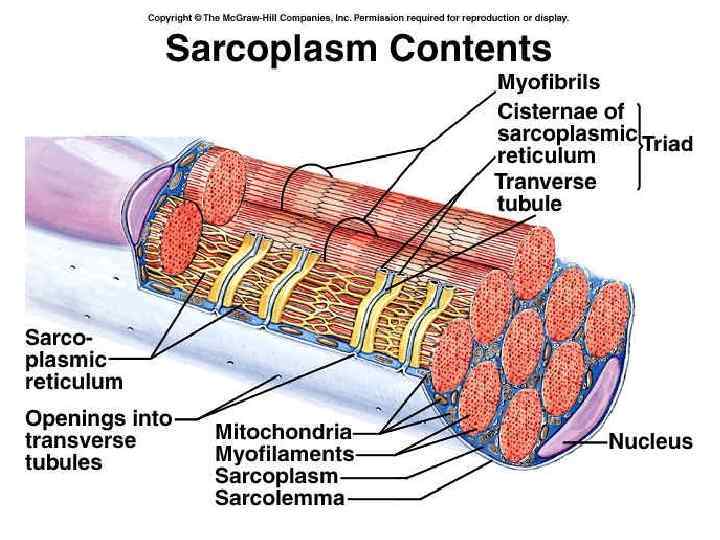
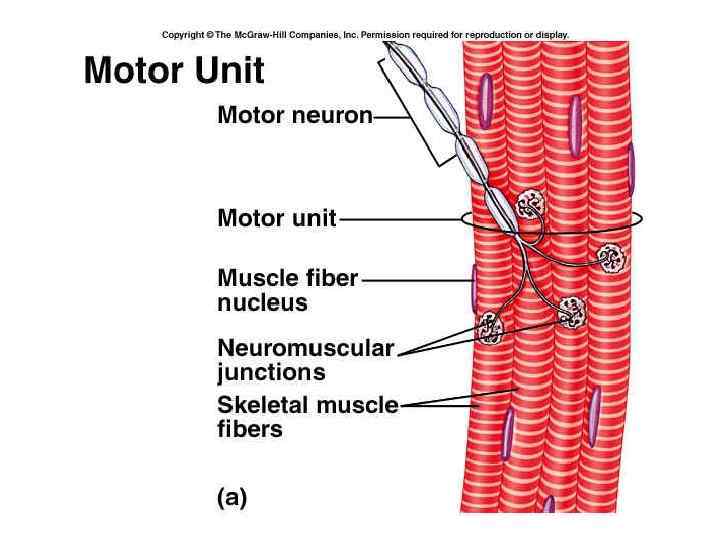
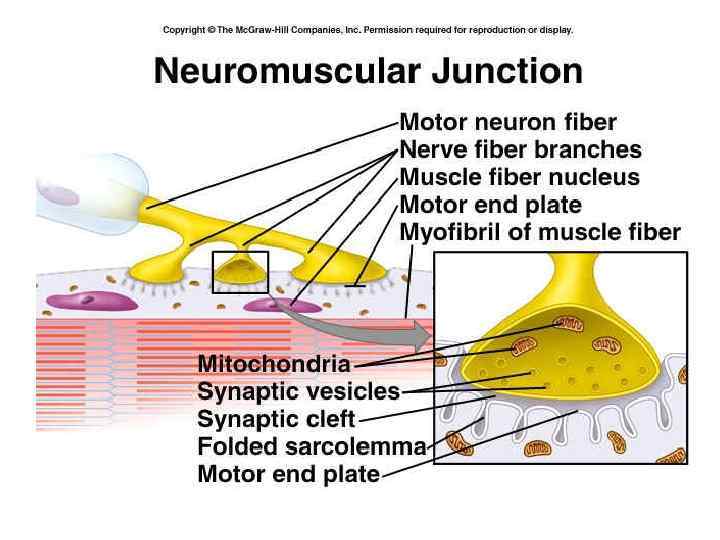
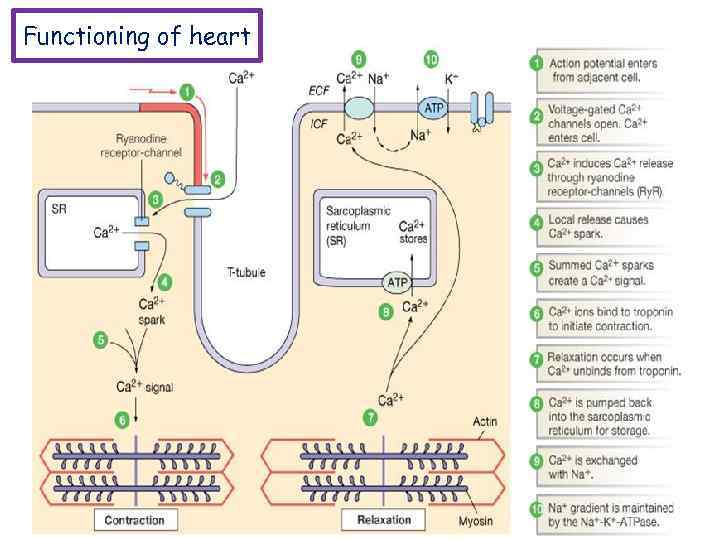
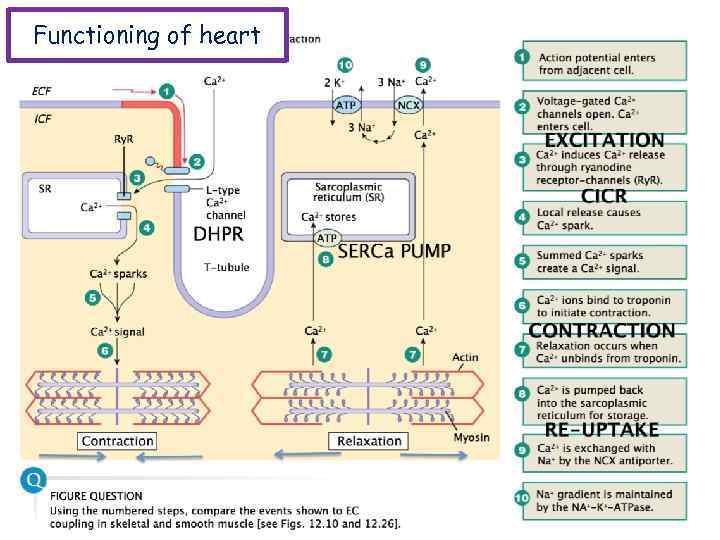
 Functioning of heart The importance of calcium-dependent signaling in the heart has been appreciated for decades. For example, it is well accepted that intracellular calcium release from the sarcoplasmic reticulum (SR) is required for cardiac muscle contraction. Indeed, with each heart beat the calcium concentration in the cytosol of cardiac myocytes is elevated approximately 10 -fold from a resting level of ∼ 100 n. M to ∼ 1 μM. Presumably, a defect in signaling that prevents effective elevation of cytosolic calcium would impair contractility as the contraction of heart muscle is directly determined by the level of calcium elevation during systole. Similarly, a defect in the removal of calcium from the cytosol during diastole would impair cardiac relaxation, which is critically important in that it allows the heart chambers to refill with blood in preparation for the next beat.
Functioning of heart The importance of calcium-dependent signaling in the heart has been appreciated for decades. For example, it is well accepted that intracellular calcium release from the sarcoplasmic reticulum (SR) is required for cardiac muscle contraction. Indeed, with each heart beat the calcium concentration in the cytosol of cardiac myocytes is elevated approximately 10 -fold from a resting level of ∼ 100 n. M to ∼ 1 μM. Presumably, a defect in signaling that prevents effective elevation of cytosolic calcium would impair contractility as the contraction of heart muscle is directly determined by the level of calcium elevation during systole. Similarly, a defect in the removal of calcium from the cytosol during diastole would impair cardiac relaxation, which is critically important in that it allows the heart chambers to refill with blood in preparation for the next beat.
 Functioning of heart Cardiac muscle fibers contract via excitationcontraction coupling, using a mechanism unique to cardiac muscle called calcium-induced calcium release. CICR is A process whereby calcium can trigger release of further calcium from the muscle sarcoplasmic reticulum.
Functioning of heart Cardiac muscle fibers contract via excitationcontraction coupling, using a mechanism unique to cardiac muscle called calcium-induced calcium release. CICR is A process whereby calcium can trigger release of further calcium from the muscle sarcoplasmic reticulum.
 Functioning of heart During stimulation of the muscle cell, the motor neuron releases the neurotransmitter acetylcholine, which then binds to a post-synaptic nicotinic acetylcholine receptor. The inward flow of calcium from the L-type calcium channels activates ryanodine receptors to release calcium ions from the sarcoplasmic reticulum.
Functioning of heart During stimulation of the muscle cell, the motor neuron releases the neurotransmitter acetylcholine, which then binds to a post-synaptic nicotinic acetylcholine receptor. The inward flow of calcium from the L-type calcium channels activates ryanodine receptors to release calcium ions from the sarcoplasmic reticulum.
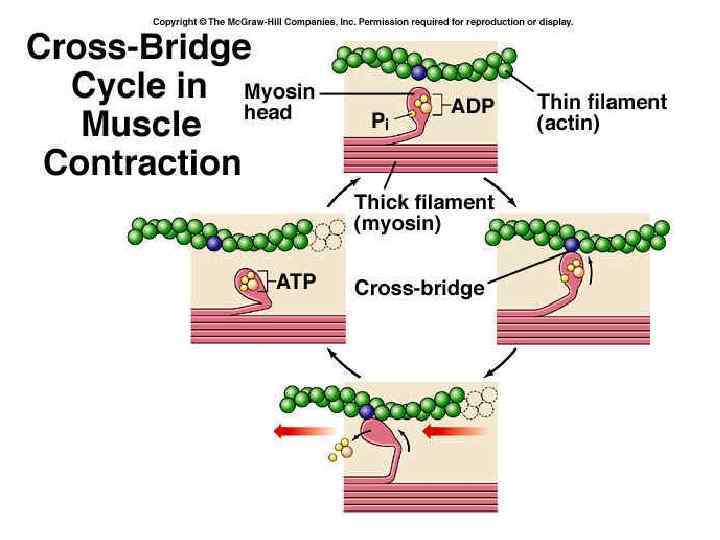
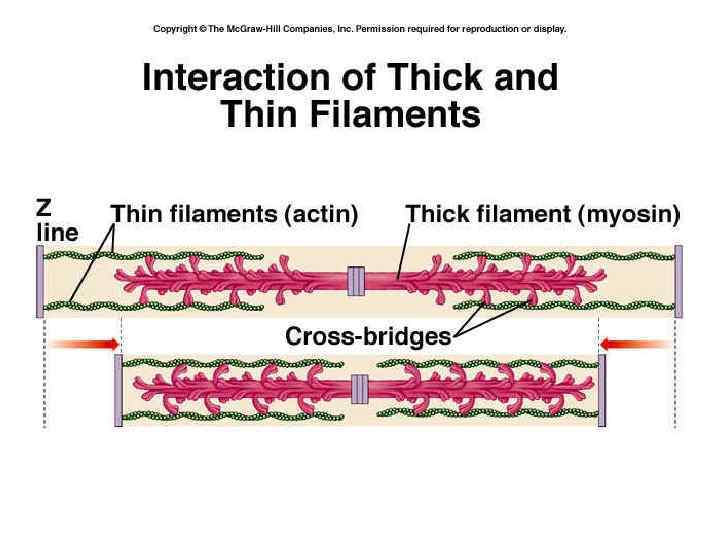
 Functioning of heart Excitation-contraction coupling describes the process of converting an electrical stimulus (action potential) into a mechanical response (muscle contraction). Calcium-induced calcium release involves the conduction of calcium ions into the cardiomyocyte, triggering further release of ions into the cytoplasm. Calcium prolongs the duration of muscle cell depolarization before repolarization occurs. Contraction in cardiac muscle occurs due to the binding of the myosin head to adenosine triphosphate (ATP), which then pulls the actin filaments to the center of the sarcomere, the mechanical force of contraction. In the sliding filament model, myosin filaments slide along actin filaments to shorten or lengthen the muscle fiber for contraction and relaxation
Functioning of heart Excitation-contraction coupling describes the process of converting an electrical stimulus (action potential) into a mechanical response (muscle contraction). Calcium-induced calcium release involves the conduction of calcium ions into the cardiomyocyte, triggering further release of ions into the cytoplasm. Calcium prolongs the duration of muscle cell depolarization before repolarization occurs. Contraction in cardiac muscle occurs due to the binding of the myosin head to adenosine triphosphate (ATP), which then pulls the actin filaments to the center of the sarcomere, the mechanical force of contraction. In the sliding filament model, myosin filaments slide along actin filaments to shorten or lengthen the muscle fiber for contraction and relaxation
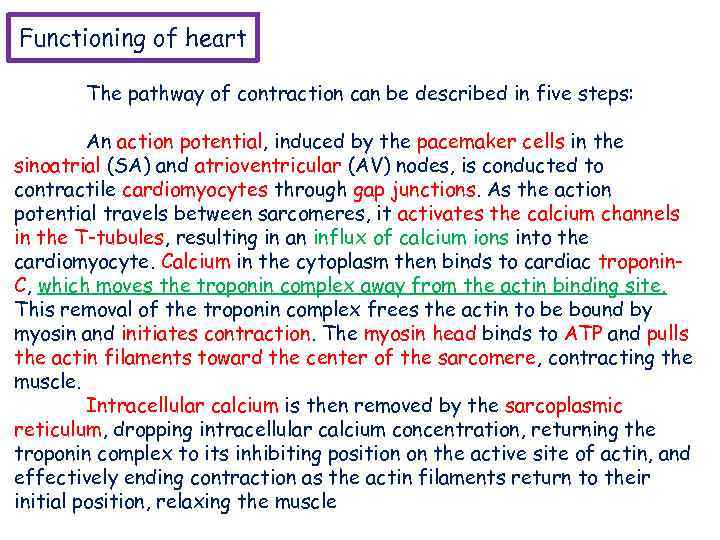 Functioning of heart The pathway of contraction can be described in five steps: An action potential, induced by the pacemaker cells in the sinoatrial (SA) and atrioventricular (AV) nodes, is conducted to contractile cardiomyocytes through gap junctions. As the action potential travels between sarcomeres, it activates the calcium channels in the T-tubules, resulting in an influx of calcium ions into the cardiomyocyte. Calcium in the cytoplasm then binds to cardiac troponin. C, which moves the troponin complex away from the actin binding site. This removal of the troponin complex frees the actin to be bound by myosin and initiates contraction. The myosin head binds to ATP and pulls the actin filaments toward the center of the sarcomere, contracting the muscle. Intracellular calcium is then removed by the sarcoplasmic reticulum, dropping intracellular calcium concentration, returning the troponin complex to its inhibiting position on the active site of actin, and effectively ending contraction as the actin filaments return to their initial position, relaxing the muscle
Functioning of heart The pathway of contraction can be described in five steps: An action potential, induced by the pacemaker cells in the sinoatrial (SA) and atrioventricular (AV) nodes, is conducted to contractile cardiomyocytes through gap junctions. As the action potential travels between sarcomeres, it activates the calcium channels in the T-tubules, resulting in an influx of calcium ions into the cardiomyocyte. Calcium in the cytoplasm then binds to cardiac troponin. C, which moves the troponin complex away from the actin binding site. This removal of the troponin complex frees the actin to be bound by myosin and initiates contraction. The myosin head binds to ATP and pulls the actin filaments toward the center of the sarcomere, contracting the muscle. Intracellular calcium is then removed by the sarcoplasmic reticulum, dropping intracellular calcium concentration, returning the troponin complex to its inhibiting position on the active site of actin, and effectively ending contraction as the actin filaments return to their initial position, relaxing the muscle
 Functioning of heart
Functioning of heart
 Functioning of heart
Functioning of heart
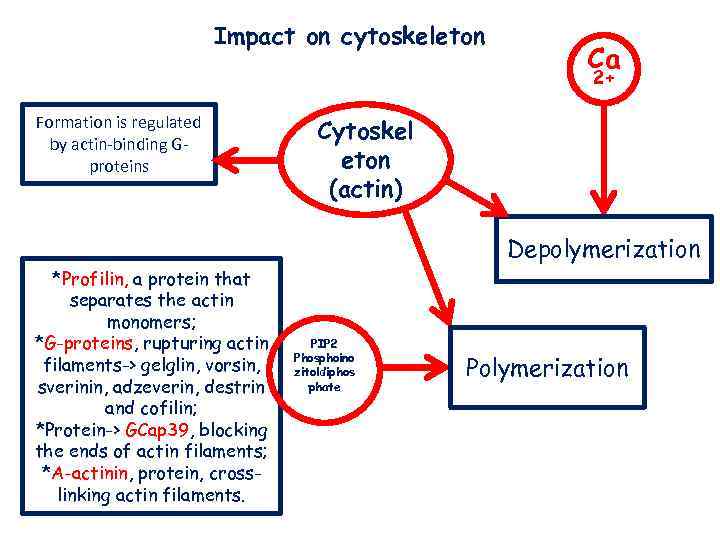 Impact on cytoskeleton Ca 2+ Formation is regulated by actin-binding Gproteins Cytoskel eton (actin) Depolymerization *Profilin, a protein that separates the actin monomers; *G-proteins, rupturing actin filaments-> gelglin, vorsin, sverinin, adzeverin, destrin and cofilin; *Protein-> GCap 39, blocking the ends of actin filaments; *A-actinin, protein, crosslinking actin filaments. PIP 2 Phosphoino zitoldiphos phate Polymerization
Impact on cytoskeleton Ca 2+ Formation is regulated by actin-binding Gproteins Cytoskel eton (actin) Depolymerization *Profilin, a protein that separates the actin monomers; *G-proteins, rupturing actin filaments-> gelglin, vorsin, sverinin, adzeverin, destrin and cofilin; *Protein-> GCap 39, blocking the ends of actin filaments; *A-actinin, protein, crosslinking actin filaments. PIP 2 Phosphoino zitoldiphos phate Polymerization
 Regulation of Ca 2+ ions in blood The main regulators of the exchange of Ca 2 + in the blood are parathyroid hormone, calcitriol and calcitonin. Parathyroid hormone (PTH) is a single-chain polypeptide consisting of 84 amino acid residues (about 9. 5 k. D), whose action is aimed at increasing the concentration of calcium ions and reducing the concentration of phosphates in the blood plasma.
Regulation of Ca 2+ ions in blood The main regulators of the exchange of Ca 2 + in the blood are parathyroid hormone, calcitriol and calcitonin. Parathyroid hormone (PTH) is a single-chain polypeptide consisting of 84 amino acid residues (about 9. 5 k. D), whose action is aimed at increasing the concentration of calcium ions and reducing the concentration of phosphates in the blood plasma.
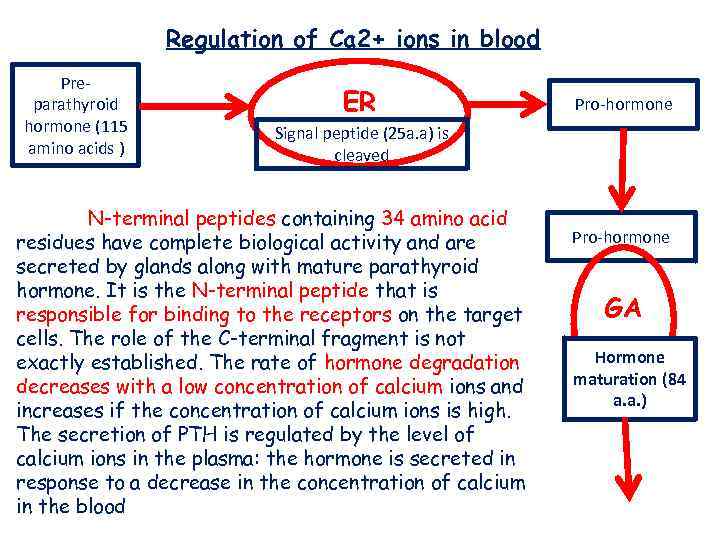 Regulation of Ca 2+ ions in blood Preparathyroid hormone (115 amino acids ) ER Pro-hormone Signal peptide (25 a. a) is cleaved N-terminal peptides containing 34 amino acid residues have complete biological activity and are secreted by glands along with mature parathyroid hormone. It is the N-terminal peptide that is responsible for binding to the receptors on the target cells. The role of the C-terminal fragment is not exactly established. The rate of hormone degradation decreases with a low concentration of calcium ions and increases if the concentration of calcium ions is high. The secretion of PTH is regulated by the level of calcium ions in the plasma: the hormone is secreted in response to a decrease in the concentration of calcium in the blood Pro-hormone GA Hormone maturation (84 a. a. )
Regulation of Ca 2+ ions in blood Preparathyroid hormone (115 amino acids ) ER Pro-hormone Signal peptide (25 a. a) is cleaved N-terminal peptides containing 34 amino acid residues have complete biological activity and are secreted by glands along with mature parathyroid hormone. It is the N-terminal peptide that is responsible for binding to the receptors on the target cells. The role of the C-terminal fragment is not exactly established. The rate of hormone degradation decreases with a low concentration of calcium ions and increases if the concentration of calcium ions is high. The secretion of PTH is regulated by the level of calcium ions in the plasma: the hormone is secreted in response to a decrease in the concentration of calcium in the blood Pro-hormone GA Hormone maturation (84 a. a. )
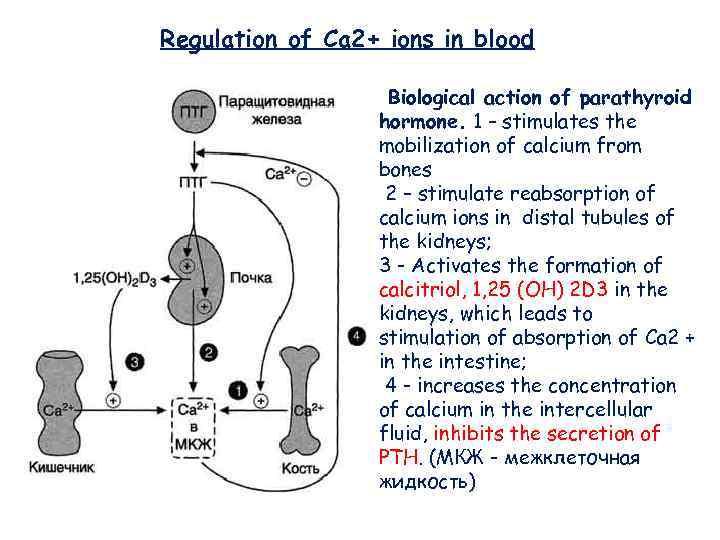 Regulation of Ca 2+ ions in blood Biological action of parathyroid hormone. 1 – stimulates the mobilization of calcium from bones 2 – stimulate reabsorption of calcium ions in distal tubules of the kidneys; 3 - Activates the formation of calcitriol, 1, 25 (OH) 2 D 3 in the kidneys, which leads to stimulation of absorption of Ca 2 + in the intestine; 4 - increases the concentration of calcium in the intercellular fluid, inhibits the secretion of PTH. (МКЖ - межклеточная жидкость)
Regulation of Ca 2+ ions in blood Biological action of parathyroid hormone. 1 – stimulates the mobilization of calcium from bones 2 – stimulate reabsorption of calcium ions in distal tubules of the kidneys; 3 - Activates the formation of calcitriol, 1, 25 (OH) 2 D 3 in the kidneys, which leads to stimulation of absorption of Ca 2 + in the intestine; 4 - increases the concentration of calcium in the intercellular fluid, inhibits the secretion of PTH. (МКЖ - межклеточная жидкость)
 Regulation of Ca 2+ ions in blood Calcitonin is a polypeptide consisting of 32 amino acid residues with one disulfide bond. The hormone is secreted by parafollicular Kcells of the thyroid gland or C-cells of parathyroid glands in the form of a high-molecular precursor protein. Secretion of calcitonin increases with increasing concentration of Ca 2 + and decreases with decreasing Ca 2 + concentration in the blood. Calcitonin is a parathyroid hormone antagonist. It inhibits: 1) the release of Ca 2 + from the bone, reducing the activity of osteoclasts. 2) inhibits tubular reabsorption calcium ions, stimulating their excretion by kidneys with urea. *The rate of secretion of calcitonin in women strongly depends on the level of estrogens. With a lack of estrogens, the secretion of calcitonin decreases. This causes an acceleration of the mobilization of calcium from bone tissue, which leads to the development of osteoporosis.
Regulation of Ca 2+ ions in blood Calcitonin is a polypeptide consisting of 32 amino acid residues with one disulfide bond. The hormone is secreted by parafollicular Kcells of the thyroid gland or C-cells of parathyroid glands in the form of a high-molecular precursor protein. Secretion of calcitonin increases with increasing concentration of Ca 2 + and decreases with decreasing Ca 2 + concentration in the blood. Calcitonin is a parathyroid hormone antagonist. It inhibits: 1) the release of Ca 2 + from the bone, reducing the activity of osteoclasts. 2) inhibits tubular reabsorption calcium ions, stimulating their excretion by kidneys with urea. *The rate of secretion of calcitonin in women strongly depends on the level of estrogens. With a lack of estrogens, the secretion of calcitonin decreases. This causes an acceleration of the mobilization of calcium from bone tissue, which leads to the development of osteoporosis.
 Thanks for attention!!!
Thanks for attention!!!


