16ee185a3eb69a181e03bde107839220.ppt
- Количество слайдов: 32

Robotics meets Packaging 2007 Trends and Opportunities in Pharmaceutical Packaging Automation Bill Cairns President BCM Group, LLC © RMP 2007 - 1 - Las Vegas, USA October 12 - 13

Trends – Case Histories – Challenges - Opportunities n n n © RMP 2007 - 2 - n Trends in the Pharmaceutical Industry Market Drivers For Robot Based Solutions Trends Effecting Packaging and Automation Case History – High speed carton loading and case packing Challenges for Automation Opportunities for Robotic Handling of Medical Devices Opportunities for Automation - Late Stage Customization Opportunities For or Automated Container Handling Case Study – Astra Zeneca Solid Dosage End of Line Case Packing Secondary Packaging ABB Integration with Skinetta Case Study Sanofi Aventis Industry Statistics

Trends in the Pharmaceutical Industry n n n n © RMP 2007 - 3 - n n The Pharmaceutical Industry is coming under increasing pressure to reduce its manufacturing costs, increase its flexibility and improve asset utilization. Most industry leading companies are planning for the loss of patent protection that will impact billions in sales revenue and earnings. Numerous Pharmaceutical and Biotech companies have completed mergers and acquisitions over the past two years. Additional activity is rumored. The Industry is consolidating at an unprecedented rate. Big Pharma’s financial and human resources will be gradually redeployed to drug discovery and development until pipelines are reinforced with promising NCEs. Big Pharma will continue to establish alliances with innovative companies for drug discovery and leverage its strengths, sales, distribution and manufacturing infrastructure. The vaccine market is growing rapidly as R&D efforts have experienced success in this area. GSK, Novartis and Merck all have major vaccine manufacturing facility projects in-process. Greater dependence on suppliers and integrators for standardized solutions Changing business models from single large blockbuster products to a multiple smaller-volume product portfolio Increase in compliance packaging (sf or cr) Anti-counterfeiting technologies
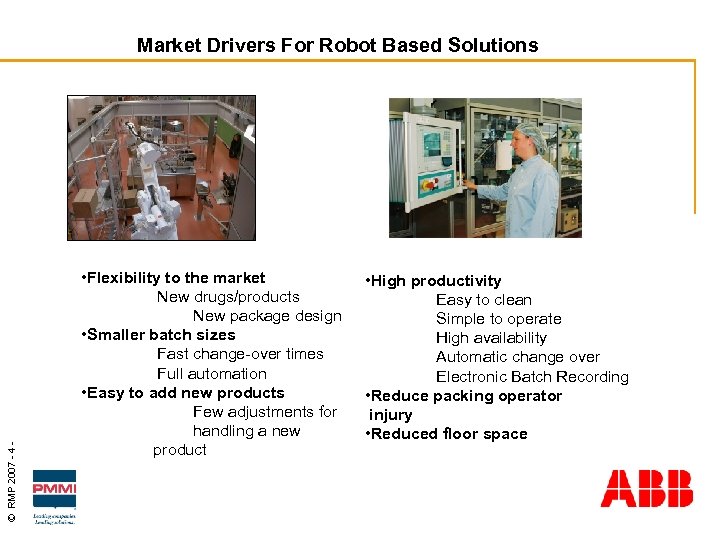
© RMP 2007 - 4 - Market Drivers For Robot Based Solutions • Flexibility to the market New drugs/products New package design • Smaller batch sizes Fast change-over times Full automation • Easy to add new products Few adjustments for handling a new product • High productivity Easy to clean Simple to operate High availability Automatic change over Electronic Batch Recording • Reduce packing operator injury • Reduced floor space
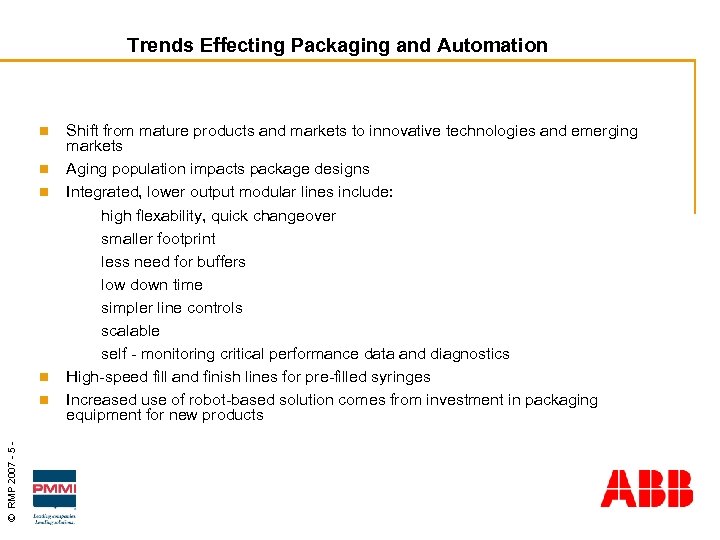
Trends Effecting Packaging and Automation n n © RMP 2007 - 5 - n Shift from mature products and markets to innovative technologies and emerging markets Aging population impacts package designs Integrated, lower output modular lines include: high flexability, quick changeover smaller footprint less need for buffers low down time simpler line controls scalable self - monitoring critical performance data and diagnostics High-speed fill and finish lines for pre-filled syringes Increased use of robot-based solution comes from investment in packaging equipment for new products
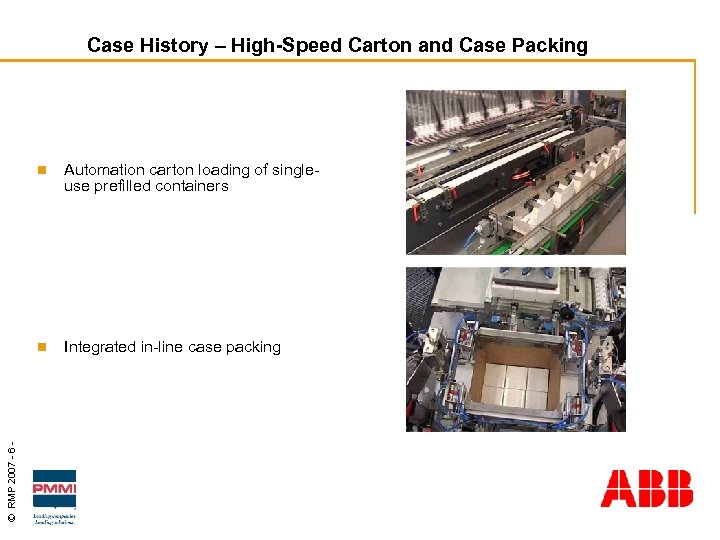
Case History – High-Speed Carton and Case Packing Automation carton loading of singleuse prefilled containers n © RMP 2007 - 6 - n Integrated in-line case packing
Challenges for Automation n n n n © RMP 2007 - n Building the justification of the cost reduction Creating a mind set Finding and training the right personnel Anticipating down time to prevent breakdowns Implementing a managable line optimization Increase an operator’s ability to diagnose packaging line issues and to raise overall equipment effectiveness (OEE) Integration of third party devices (vision, weight, inspection) Complying with GMP standards Guaranteeing traceability through supply chain

Opportunities for Robotic Handling of Medical Devices New devices and delivery systems will require unique equipment and packaging requirements n © RMP 2007 - 8 - n Loading infeed of thermoformer reduces labor , enables different pack patterns
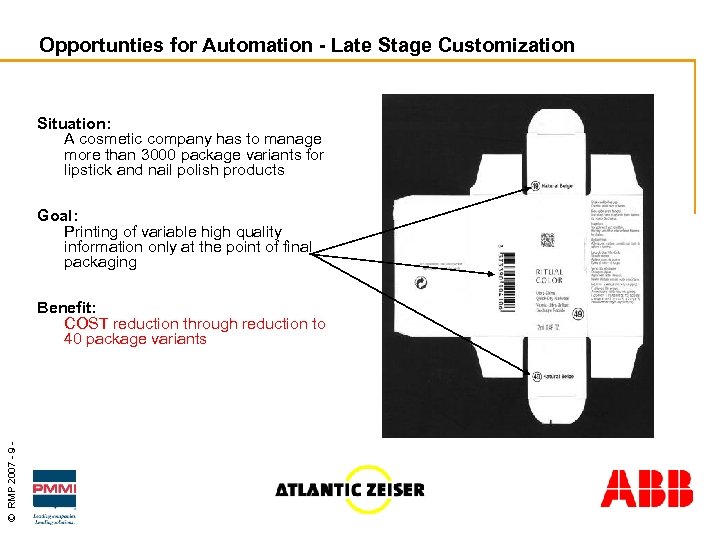
Opportunties for Automation - Late Stage Customization Situation: A cosmetic company has to manage more than 3000 package variants for lipstick and nail polish products Goal: Printing of variable high quality information only at the point of final packaging © RMP 2007 - 9 - Benefit: COST reduction through reduction to 40 package variants
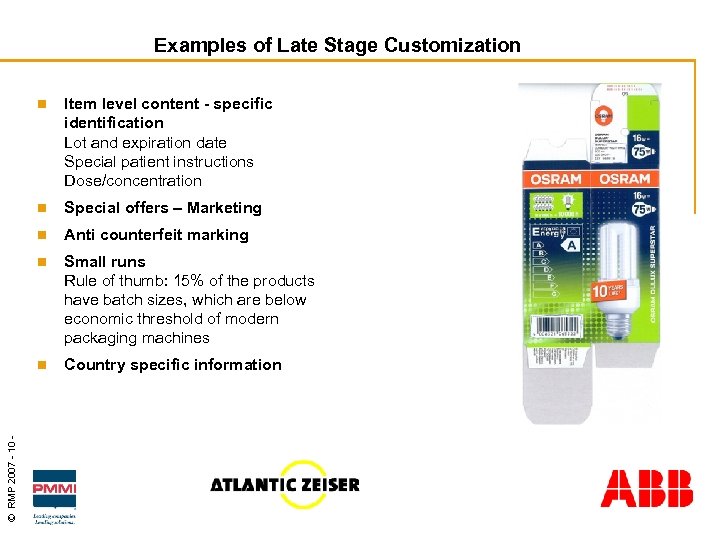
Examples of Late Stage Customization Item level content - specific identification Lot and expiration date Special patient instructions Dose/concentration n Special offers – Marketing n Anti counterfeit marking n Small runs Rule of thumb: 15% of the products have batch sizes, which are below economic threshold of modern packaging machines n © RMP 2007 - 10 - n Country specific information

What are the benefits of Automating Late Stage Customization Reduction in package cost for small order volumes - Reduction of inventory investment - Reduction in inventory storage area - Fast change over - Multiple feeding stations allow format change on the fly - Lower administration cost - Eliminates write-off cost for discontinued inventory - Quick response lowers lead times - Prevents potential lost opportunities to meet market demand © RMP 2007 - 11 - - Mo minimum order quantity penalty
Financial Impact of late Stage Customization 25 million packages a year - Savings by ordering larger volumes: US$0. 01 per package - US$ 268 K savings only in purchasing - US$0. 01 cent/package will be saved by admin, storage and handling cost - Lost opportunity is soft factor, ( hard to calculate ) - © RMP 2007 - 12 - - TOTAL: US$ 446 K per year savings

Opportunities For or Automated Container Handling Automating the application and reading of 2 D code on syringes with Frewitt SA laser marking and reading system n © RMP 2007 - 13 - n Coding and reading on vials and ampules

Automated Marking and Reading for Tracability , Anticounterfeiting © RMP 2007 - 14 -
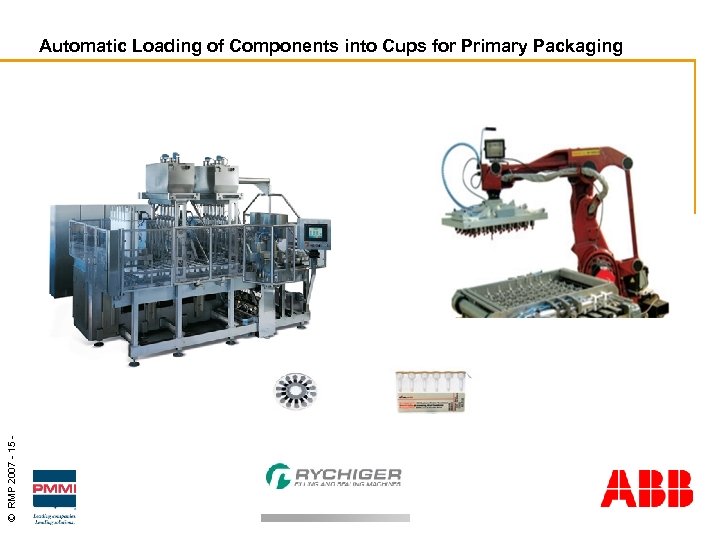
© RMP 2007 - 15 - Automatic Loading of Components into Cups for Primary Packaging

© RMP 2007 - 16 -

Case Study – Astra Zeneca Requirements § Minimize adjustments when changing products § Higher capacity in comparison with manual line § Simple cleaning requirements when changing products § The system must perform with lower downtime than predecessor § Much smaller foot print than conventional case packer § Maximum uptime required © RMP 2007 - 17 - § Minimal operator injury
Case Study – Astra Zeneca Requirements § Case packaging line for tablets § Variation of package type § 4 different sizes of product cartons § 3 different final packing case possibilities § PC based HMI and machine control § Automatic changeover of product/package type § Over 99% availability over the last 4 years © RMP 2007 - 18 - § 17 systems delivered to Astra. Zeneca

© RMP 2007 - 19 - Case Study Astra Zeneca
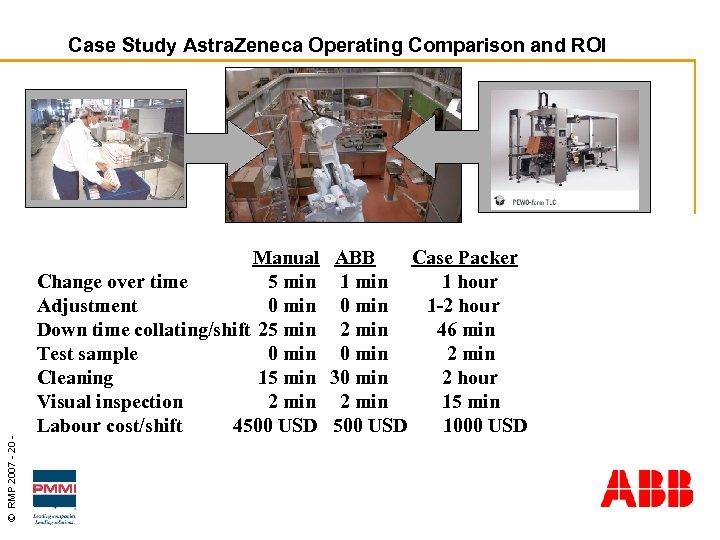
© RMP 2007 - 20 - Case Study Astra. Zeneca Operating Comparison and ROI Manual ABB Case Packer Change over time 5 min 1 hour Adjustment 0 min 1 -2 hour Down time collating/shift 25 min 2 min 46 min Test sample 0 min 2 min Cleaning 15 min 30 min 2 hour Visual inspection 2 min 15 min Labour cost/shift 4500 USD 1000 USD

© RMP 2007 - 21 - Astra. Zeneca’s Automated Solution

Secondary Packaging © RMP 2007 - 22 - n Why use a robot based packaging solution? n Robot is standard product (over 10, 000 per year, 150, 000 total in use) n Already established on the market (food, automotive, consumer, . . ) n High MTBF (Mean Time Between Failure) n High availability 99. 6 % Up time n High accuracy repeatability 0. 1 mm n High Flexibility n Easy to reuse in new lines n Lower maintenance n Less risk of operator injury
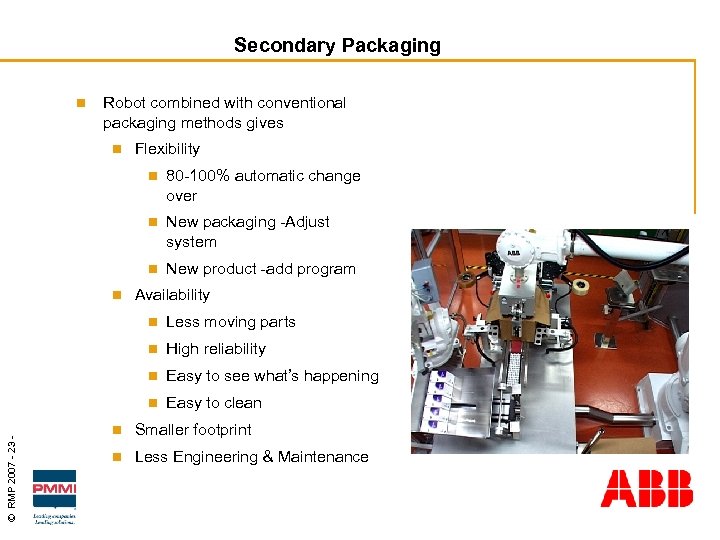
Secondary Packaging n Robot combined with conventional packaging methods gives n Flexibility n n New packaging -Adjust system n n 80 -100% automatic change over New product -add program Availability Less moving parts n High reliability n Easy to see what’s happening n © RMP 2007 - 23 - n Easy to clean n Smaller footprint n Less Engineering & Maintenance
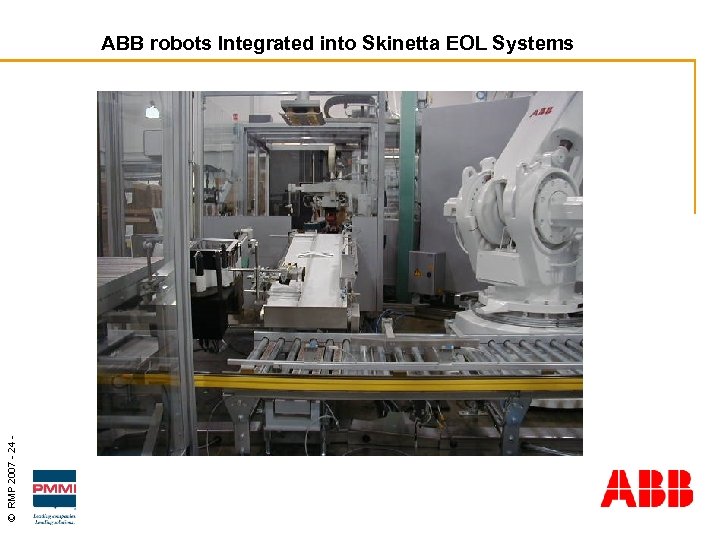
© RMP 2007 - 24 - ABB robots Integrated into Skinetta EOL Systems

© RMP 2007 - 25 - ABB in Skinetta Systems
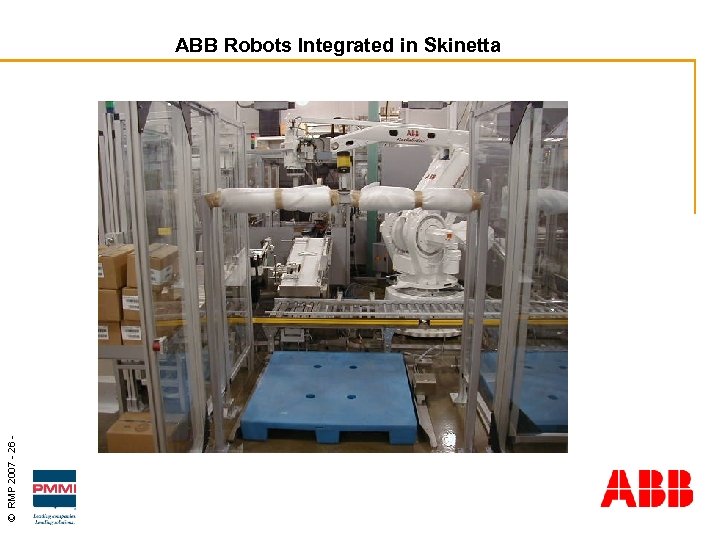
© RMP 2007 - 26 - ABB Robots Integrated in Skinetta

© RMP 2007 - 27 - Overland Park Kansas

© RMP 2007 - 28 -
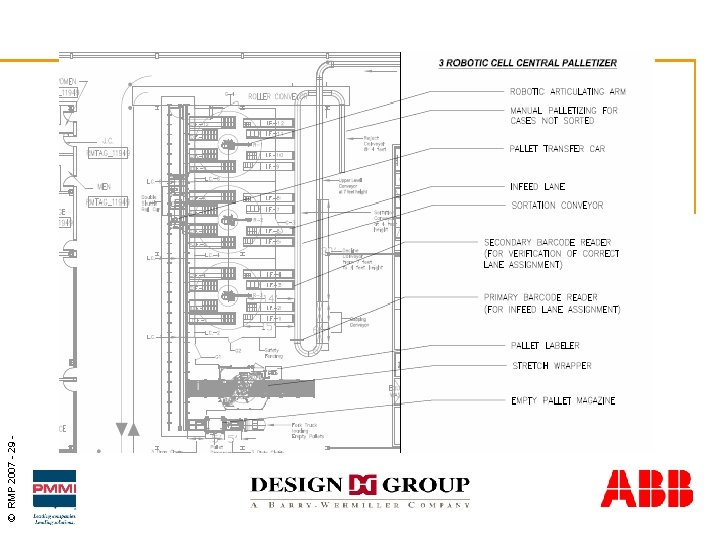
© RMP 2007 - 29 -
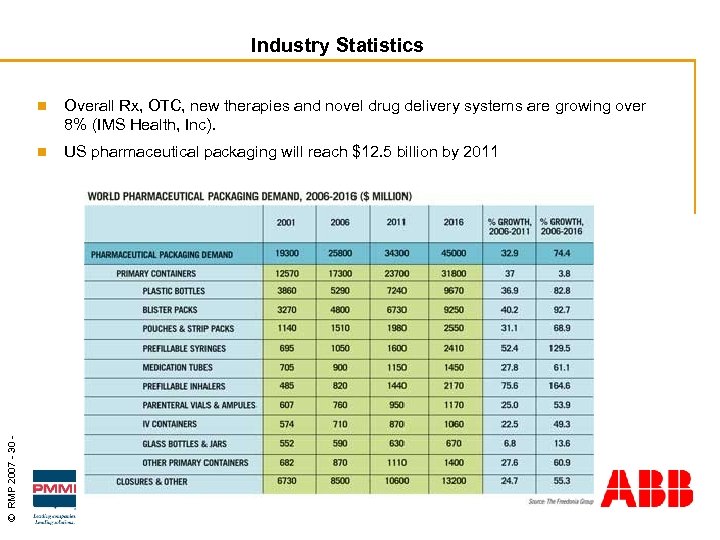
Industry Statistics Overall Rx, OTC, new therapies and novel drug delivery systems are growing over 8% (IMS Health, Inc). n © RMP 2007 - 30 - n US pharmaceutical packaging will reach $12. 5 billion by 2011

© RMP 2007 - 31 - Thank You

Biography BCM Group President William Cairns has more than three decades of experience in the global life sciences industry and a track-record of successfully building strong businesses. Prior to forming BCM Group, he served as President, Romaco USA, Inc. , where he was responsible for the integration of the sales, marketing and manufacturing and service teams for North America. Prior to joining Romaco, Mr. Cairns held senior sales and business development positions at Sig. Pack Systems, Bosch Packaging, West Pharmaceutical Services and Eisai USA, American Hospital Supply and Bristol Myers Squibb. He has a Bachelors degree for Norwich University. He has served on numerous committees including the Packaging Machinery Manufacturers Institute Global Marketing Committee and is a member of the International Society of Pharmaceutical Engineers and the New Jersey Technology. © RMP 2007 - 32 - Council.
16ee185a3eb69a181e03bde107839220.ppt