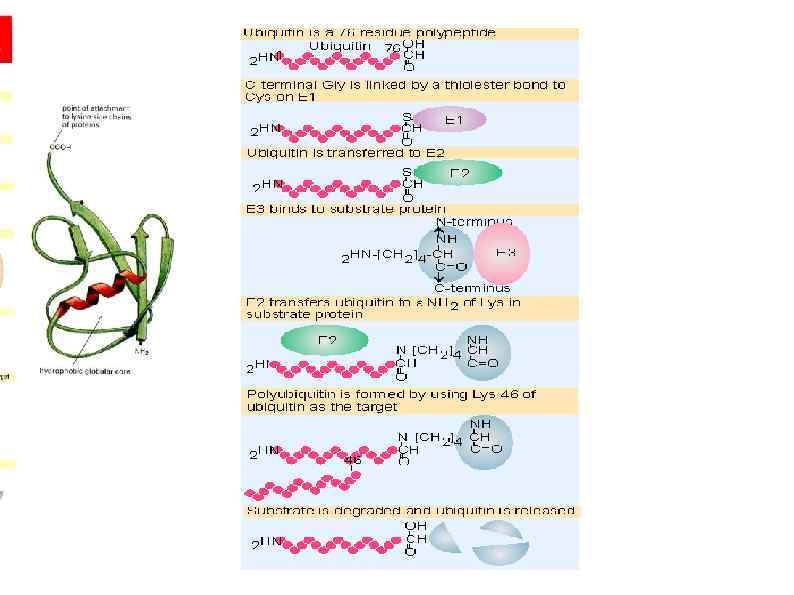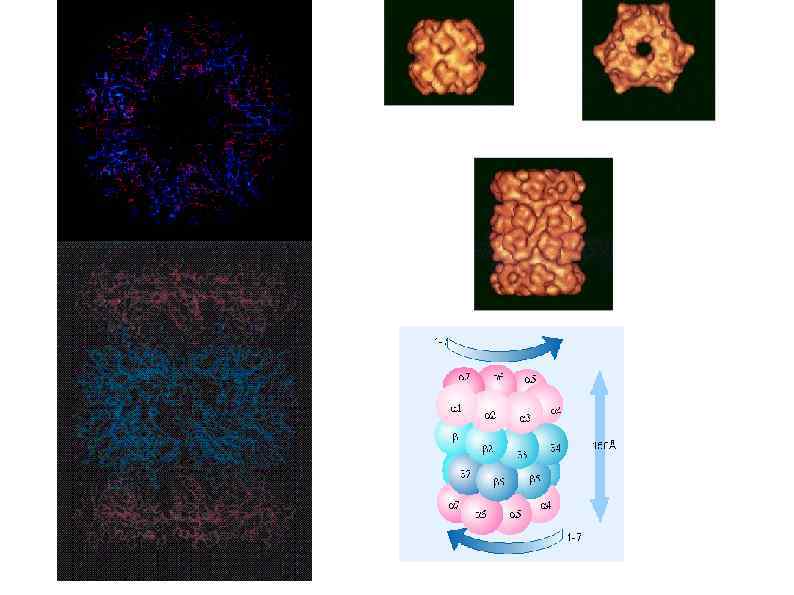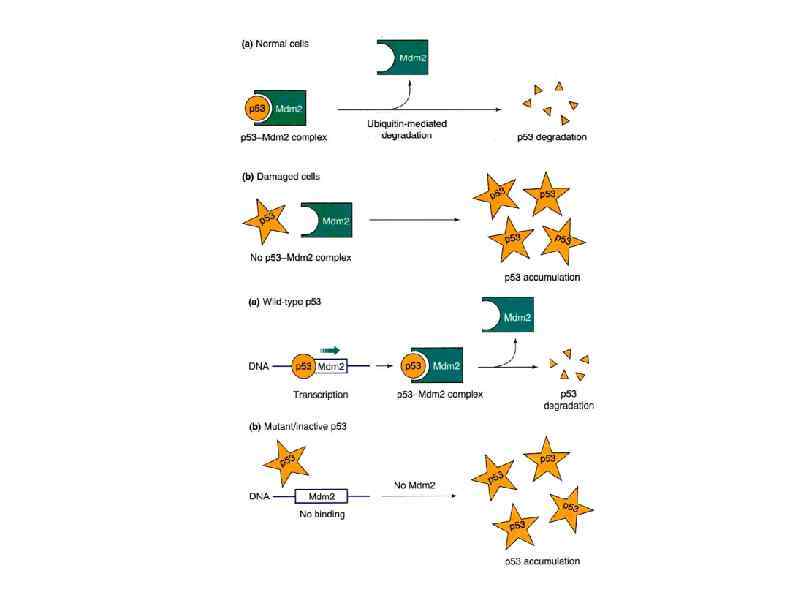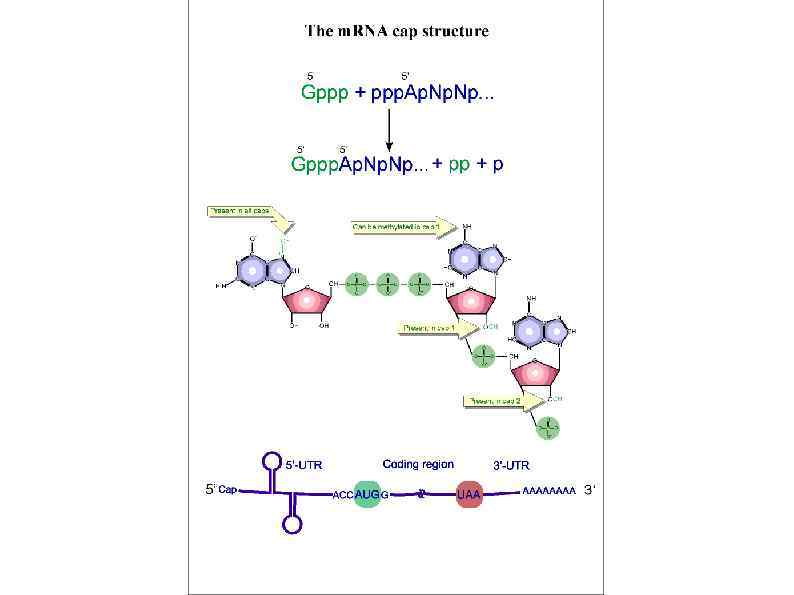
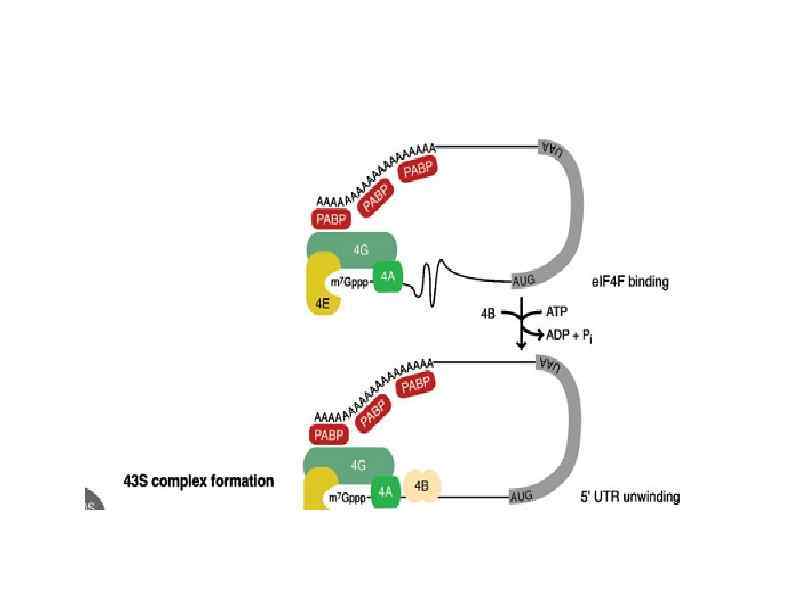
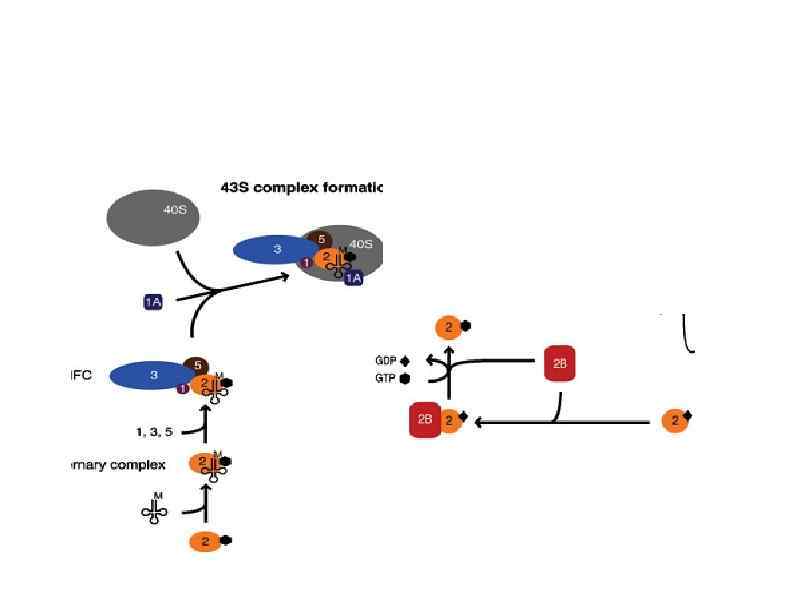
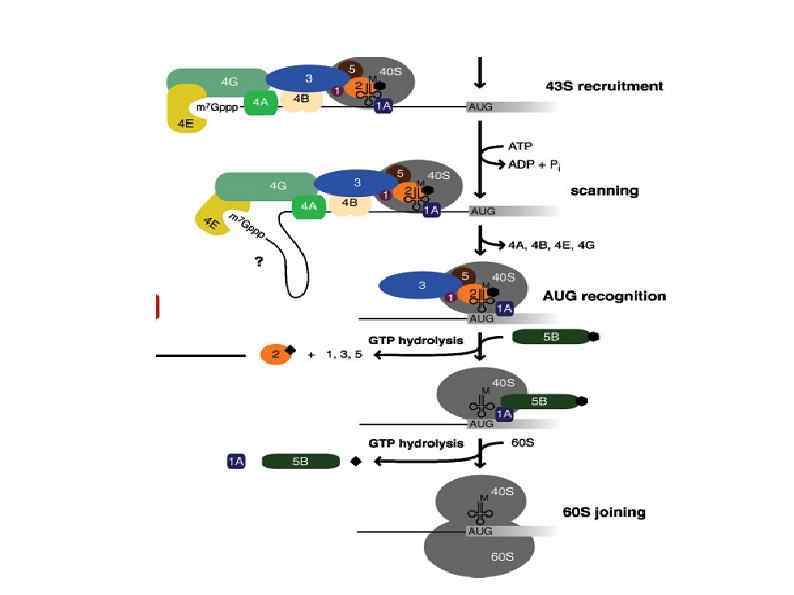
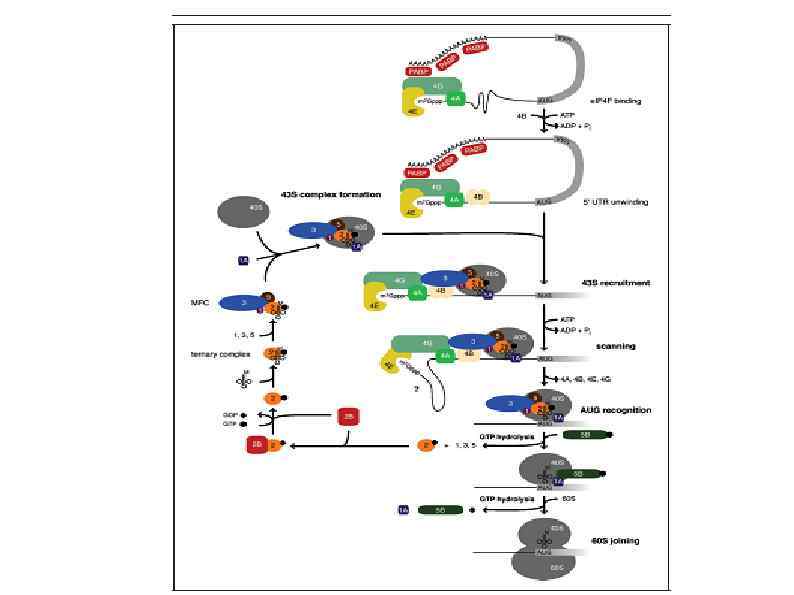
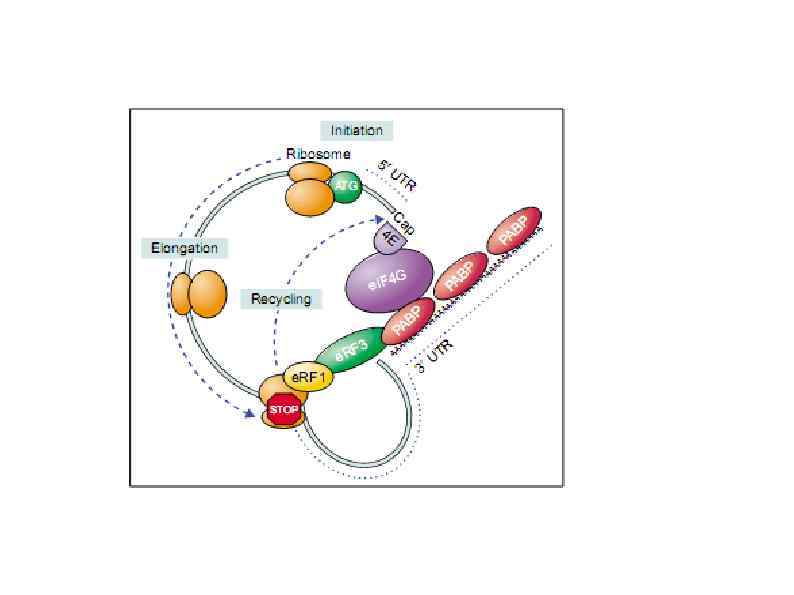
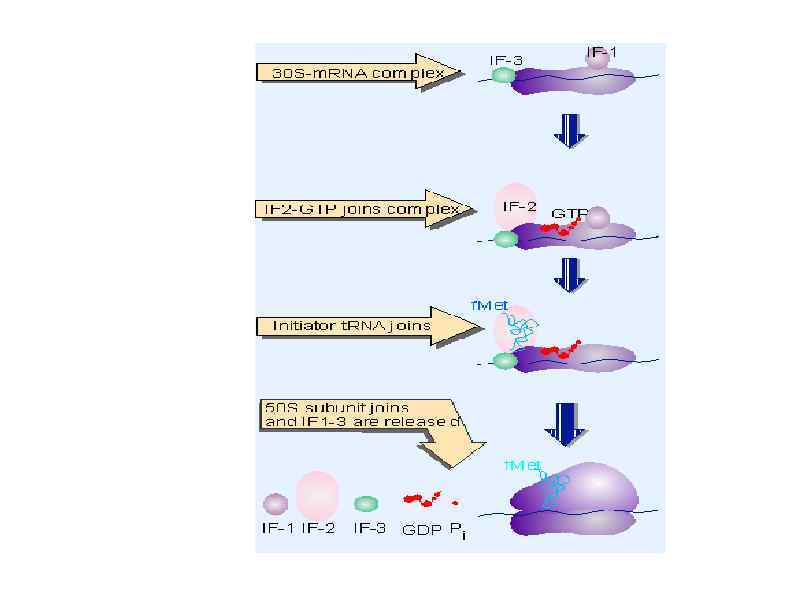
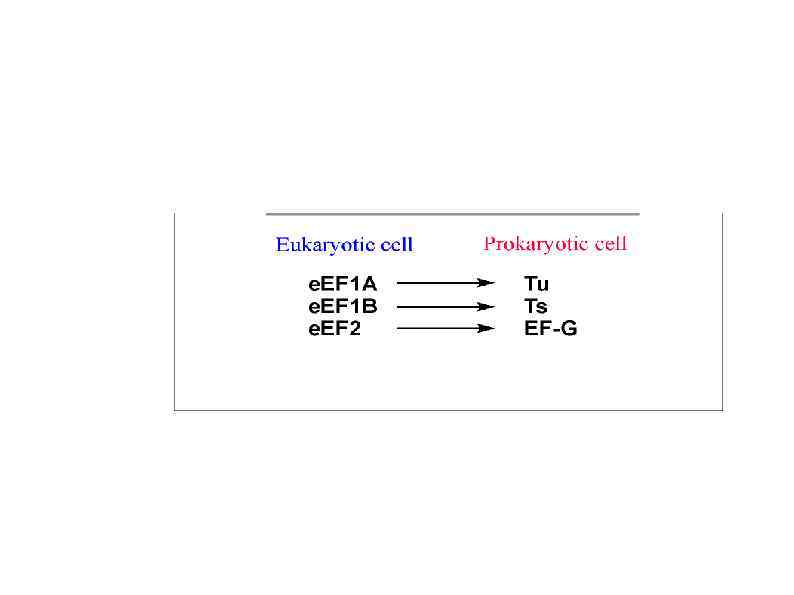
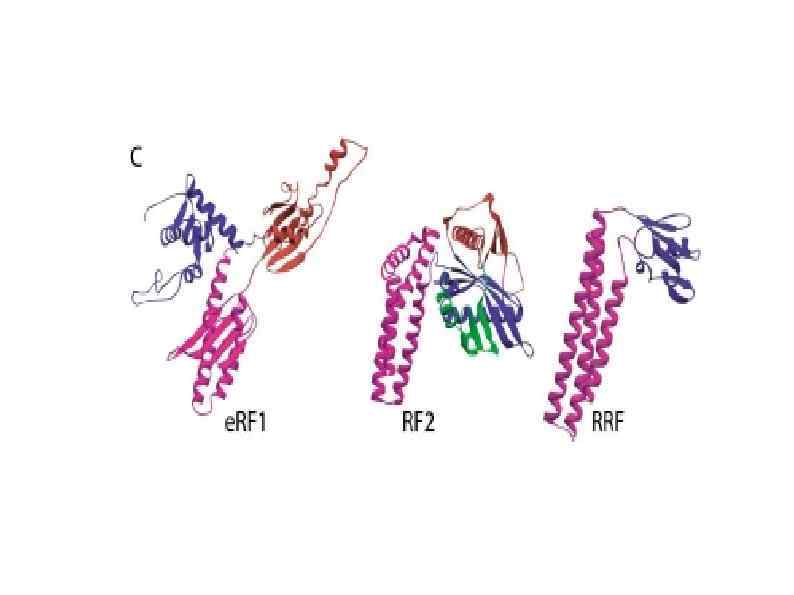
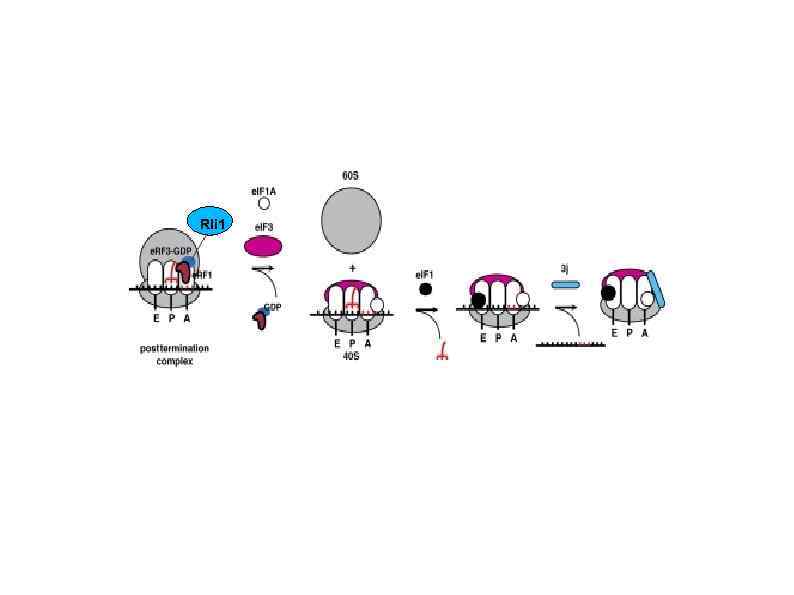
 Rli 1
Rli 1
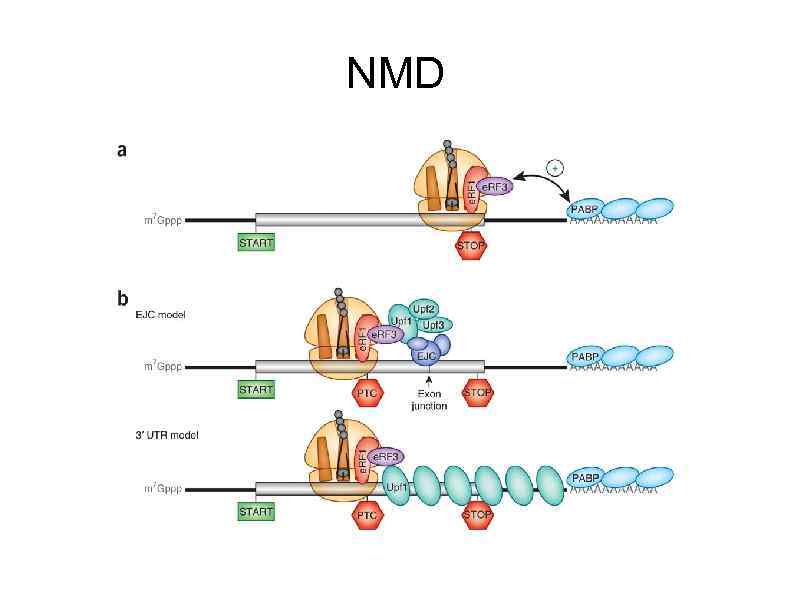 NMD
NMD
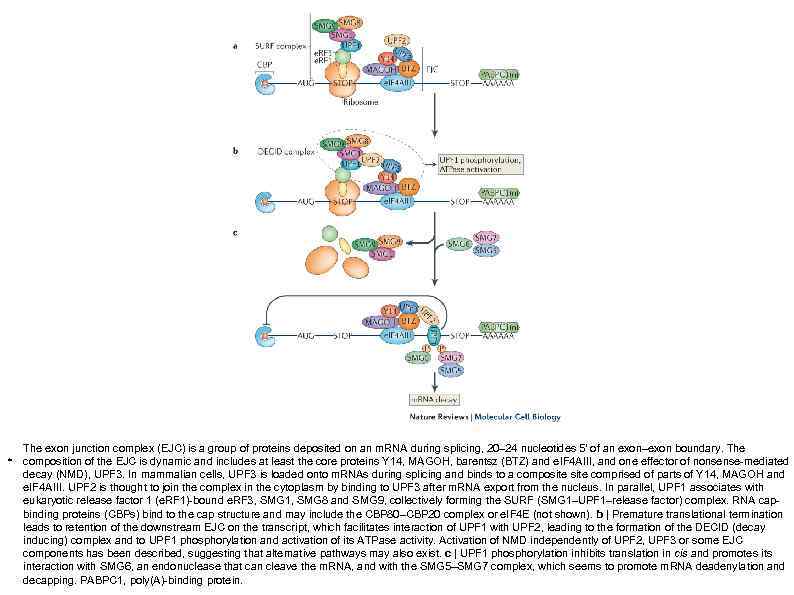 . The exon junction complex (EJC) is a group of proteins deposited on an m. RNA during splicing, 20– 24 nucleotides 5′ of an exon–exon boundary. The composition of the EJC is dynamic and includes at least the core proteins Y 14, MAGOH, barentsz (BTZ) and e. IF 4 AIII, and one effector of nonsense-mediated decay (NMD), UPF 3. In mammalian cells, UPF 3 is loaded onto m. RNAs during splicing and binds to a composite comprised of parts of Y 14, MAGOH and e. IF 4 AIII. UPF 2 is thought to join the complex in the cytoplasm by binding to UPF 3 after m. RNA export from the nucleus. In parallel, UPF 1 associates with eukaryotic release factor 1 (e. RF 1)-bound e. RF 3, SMG 1, SMG 8 and SMG 9, collectively forming the SURF (SMG 1–UPF 1–release factor) complex. RNA capbinding proteins (CBPs) bind to the cap structure and may include the CBP 80–CBP 20 complex or e. IF 4 E (not shown). b | Premature translational termination leads to retention of the downstream EJC on the transcript, which facilitates interaction of UPF 1 with UPF 2, leading to the formation of the DECID (decay inducing) complex and to UPF 1 phosphorylation and activation of its ATPase activity. Activation of NMD independently of UPF 2, UPF 3 or some EJC components has been described, suggesting that alternative pathways may also exist. c | UPF 1 phosphorylation inhibits translation in cis and promotes its interaction with SMG 6, an endonuclease that can cleave the m. RNA, and with the SMG 5–SMG 7 complex, which seems to promote m. RNA deadenylation and decapping. PABPC 1, poly(A)-binding protein.
. The exon junction complex (EJC) is a group of proteins deposited on an m. RNA during splicing, 20– 24 nucleotides 5′ of an exon–exon boundary. The composition of the EJC is dynamic and includes at least the core proteins Y 14, MAGOH, barentsz (BTZ) and e. IF 4 AIII, and one effector of nonsense-mediated decay (NMD), UPF 3. In mammalian cells, UPF 3 is loaded onto m. RNAs during splicing and binds to a composite comprised of parts of Y 14, MAGOH and e. IF 4 AIII. UPF 2 is thought to join the complex in the cytoplasm by binding to UPF 3 after m. RNA export from the nucleus. In parallel, UPF 1 associates with eukaryotic release factor 1 (e. RF 1)-bound e. RF 3, SMG 1, SMG 8 and SMG 9, collectively forming the SURF (SMG 1–UPF 1–release factor) complex. RNA capbinding proteins (CBPs) bind to the cap structure and may include the CBP 80–CBP 20 complex or e. IF 4 E (not shown). b | Premature translational termination leads to retention of the downstream EJC on the transcript, which facilitates interaction of UPF 1 with UPF 2, leading to the formation of the DECID (decay inducing) complex and to UPF 1 phosphorylation and activation of its ATPase activity. Activation of NMD independently of UPF 2, UPF 3 or some EJC components has been described, suggesting that alternative pathways may also exist. c | UPF 1 phosphorylation inhibits translation in cis and promotes its interaction with SMG 6, an endonuclease that can cleave the m. RNA, and with the SMG 5–SMG 7 complex, which seems to promote m. RNA deadenylation and decapping. PABPC 1, poly(A)-binding protein.
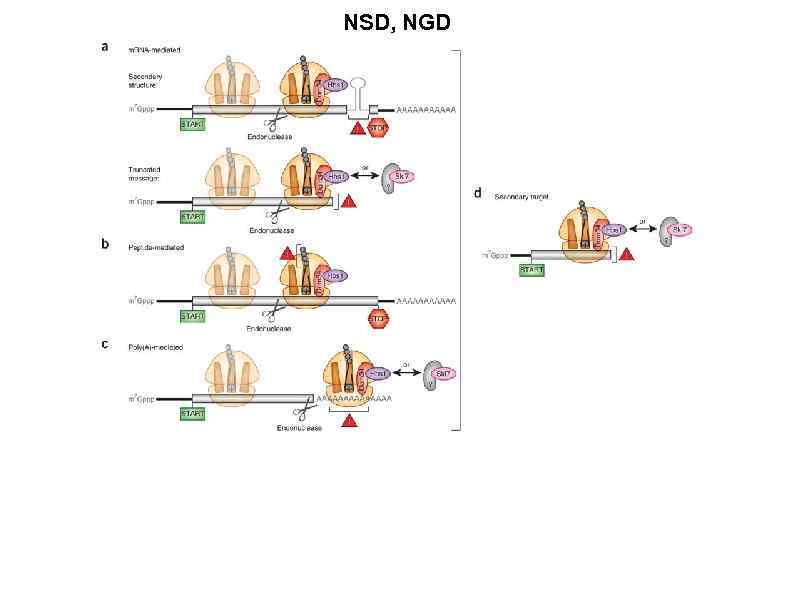 NSD, NGD and NSD both involve the recognition of stalled ribosome complexes. These stalls can arise through multiple mechanisms. (a) m. RNAmediated targets. Inhibitory m. RNA secondary structures stall ribosomes at internal loci (top), whereas truncated m. RNAs result in terminal stalls (bottom). Although the former and latter m. RNAs are classically distinguished as NGD and NSD targets, respectively, increasing evidence suggests that such distinctions belie common mechanistic features. (b) Peptide-mediated targets. Inhibitory peptide sequences lead to internally stalled ribosomes, classically defined as NGD substrates. (c) Poly(A)-mediated targets. Translation of the poly(A) tail, originally considered to mimic a truncated m. RNA and invoke NSD, probably induces ribosome stalling before its arrival at the end of the m. RNA. As such, the distinction between NGD and NSD under these conditions is ambiguous in the absence of further experimentation. In all cases detailed in a–c, endonucleolytic cleavage occurs upstream of the stalled ribosome, potentially stimulated by Dom 34 and Hbs 1. Following cleavage, the trailing ribosome (shown in a lighter shade) elongates to the point of cleavage, generating a secondary target (d) for Dom 34–Hbs 1 (or Ski 7) recognition. At present, no Dom 34 -like factor has been identified that interacts with Ski 7.
NSD, NGD and NSD both involve the recognition of stalled ribosome complexes. These stalls can arise through multiple mechanisms. (a) m. RNAmediated targets. Inhibitory m. RNA secondary structures stall ribosomes at internal loci (top), whereas truncated m. RNAs result in terminal stalls (bottom). Although the former and latter m. RNAs are classically distinguished as NGD and NSD targets, respectively, increasing evidence suggests that such distinctions belie common mechanistic features. (b) Peptide-mediated targets. Inhibitory peptide sequences lead to internally stalled ribosomes, classically defined as NGD substrates. (c) Poly(A)-mediated targets. Translation of the poly(A) tail, originally considered to mimic a truncated m. RNA and invoke NSD, probably induces ribosome stalling before its arrival at the end of the m. RNA. As such, the distinction between NGD and NSD under these conditions is ambiguous in the absence of further experimentation. In all cases detailed in a–c, endonucleolytic cleavage occurs upstream of the stalled ribosome, potentially stimulated by Dom 34 and Hbs 1. Following cleavage, the trailing ribosome (shown in a lighter shade) elongates to the point of cleavage, generating a secondary target (d) for Dom 34–Hbs 1 (or Ski 7) recognition. At present, no Dom 34 -like factor has been identified that interacts with Ski 7.
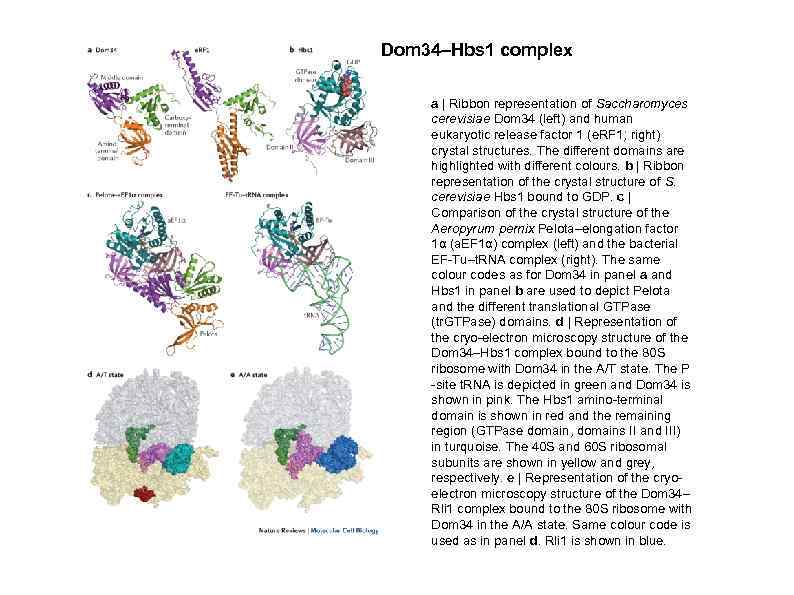 Dom 34–Hbs 1 complex a | Ribbon representation of Saccharomyces cerevisiae Dom 34 (left) and human eukaryotic release factor 1 (e. RF 1; right) crystal structures. The different domains are highlighted with different colours. b | Ribbon representation of the crystal structure of S. cerevisiae Hbs 1 bound to GDP. c | Comparison of the crystal structure of the Aeropyrum pernix Pelota–elongation factor 1α (a. EF 1α) complex (left) and the bacterial EF-Tu–t. RNA complex (right). The same colour codes as for Dom 34 in panel a and Hbs 1 in panel b are used to depict Pelota and the different translational GTPase (tr. GTPase) domains. d | Representation of the cryo-electron microscopy structure of the Dom 34–Hbs 1 complex bound to the 80 S ribosome with Dom 34 in the A/T state. The P -site t. RNA is depicted in green and Dom 34 is shown in pink. The Hbs 1 amino-terminal domain is shown in red and the remaining region (GTPase domain, domains II and III) in turquoise. The 40 S and 60 S ribosomal subunits are shown in yellow and grey, respectively. e | Representation of the cryoelectron microscopy structure of the Dom 34– Rli 1 complex bound to the 80 S ribosome with Dom 34 in the A/A state. Same colour code is used as in panel d. Rli 1 is shown in blue.
Dom 34–Hbs 1 complex a | Ribbon representation of Saccharomyces cerevisiae Dom 34 (left) and human eukaryotic release factor 1 (e. RF 1; right) crystal structures. The different domains are highlighted with different colours. b | Ribbon representation of the crystal structure of S. cerevisiae Hbs 1 bound to GDP. c | Comparison of the crystal structure of the Aeropyrum pernix Pelota–elongation factor 1α (a. EF 1α) complex (left) and the bacterial EF-Tu–t. RNA complex (right). The same colour codes as for Dom 34 in panel a and Hbs 1 in panel b are used to depict Pelota and the different translational GTPase (tr. GTPase) domains. d | Representation of the cryo-electron microscopy structure of the Dom 34–Hbs 1 complex bound to the 80 S ribosome with Dom 34 in the A/T state. The P -site t. RNA is depicted in green and Dom 34 is shown in pink. The Hbs 1 amino-terminal domain is shown in red and the remaining region (GTPase domain, domains II and III) in turquoise. The 40 S and 60 S ribosomal subunits are shown in yellow and grey, respectively. e | Representation of the cryoelectron microscopy structure of the Dom 34– Rli 1 complex bound to the 80 S ribosome with Dom 34 in the A/A state. Same colour code is used as in panel d. Rli 1 is shown in blue.
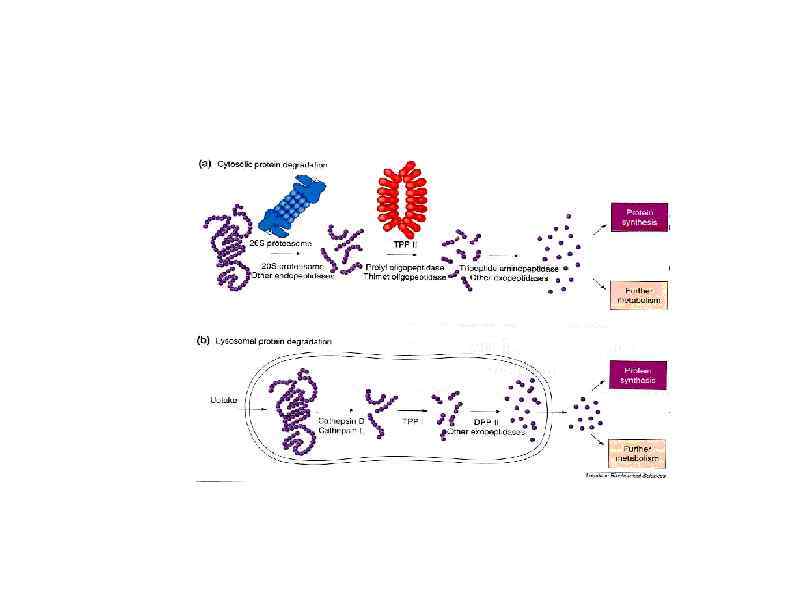
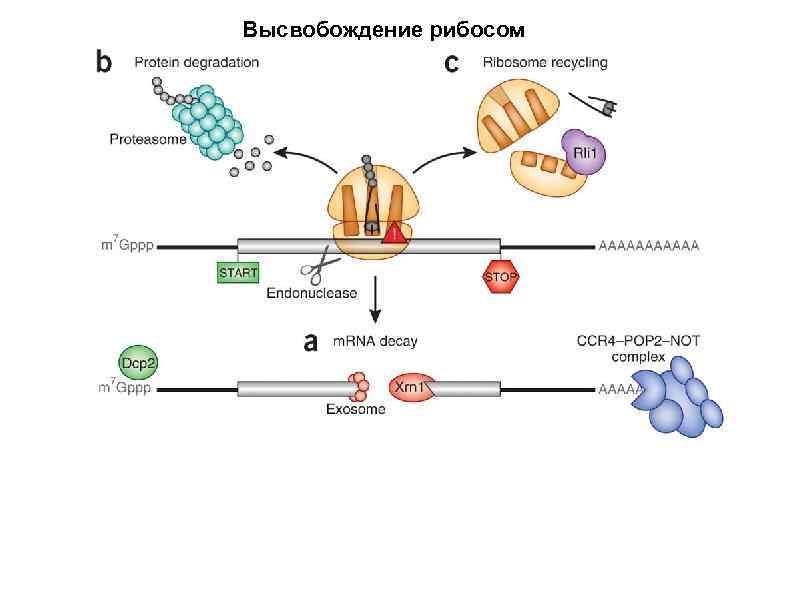 Высвобождение рибосом Following the recognition of NMD, NGD or NSD ribosome complexes, at least three discrete salvage pathways are invoked: m. RNA decay, protein degradation and ribosome recycling. (a) m. RNA decay (see Box 1 for more details). Endonucleolytic cleavage subverts the need for deadenylation by the CCR 4–POP 2–NOT complex and decapping by Dcp 2, before m. RNA decay. Rapid m. RNA degradation then proceeds through canonical means, including 5′– 3′ degradation by Xrn 1 and 3′– 5′ degradation by the exosome. (b) Protein degradation. Targeted degradation of aberrant peptides occurs via the ubiquitin-proteasome system. Several E 3 ligases have been implicated in this process, but the molecular features of substrate recognition remain to be determined. (c) Ribosome recycling. Dom 34 and Hbs 1 are known to exploit the canonical recycling activities of Rli 1 to effect ribosome recycling during NGD and NSD. Recycling of ribosome complexes during NMD is less well-characterized, but may involve Upf 1.
Высвобождение рибосом Following the recognition of NMD, NGD or NSD ribosome complexes, at least three discrete salvage pathways are invoked: m. RNA decay, protein degradation and ribosome recycling. (a) m. RNA decay (see Box 1 for more details). Endonucleolytic cleavage subverts the need for deadenylation by the CCR 4–POP 2–NOT complex and decapping by Dcp 2, before m. RNA decay. Rapid m. RNA degradation then proceeds through canonical means, including 5′– 3′ degradation by Xrn 1 and 3′– 5′ degradation by the exosome. (b) Protein degradation. Targeted degradation of aberrant peptides occurs via the ubiquitin-proteasome system. Several E 3 ligases have been implicated in this process, but the molecular features of substrate recognition remain to be determined. (c) Ribosome recycling. Dom 34 and Hbs 1 are known to exploit the canonical recycling activities of Rli 1 to effect ribosome recycling during NGD and NSD. Recycling of ribosome complexes during NMD is less well-characterized, but may involve Upf 1.
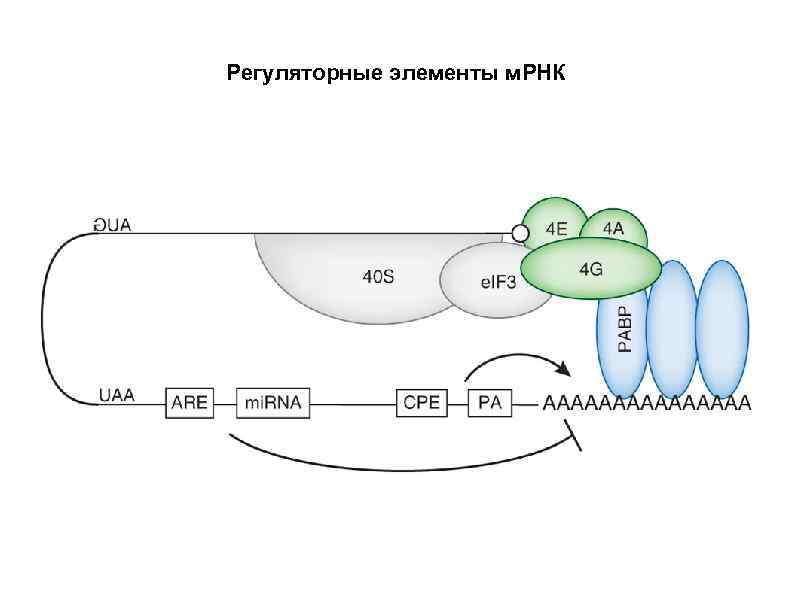 Регуляторные элементы м. РНК
Регуляторные элементы м. РНК
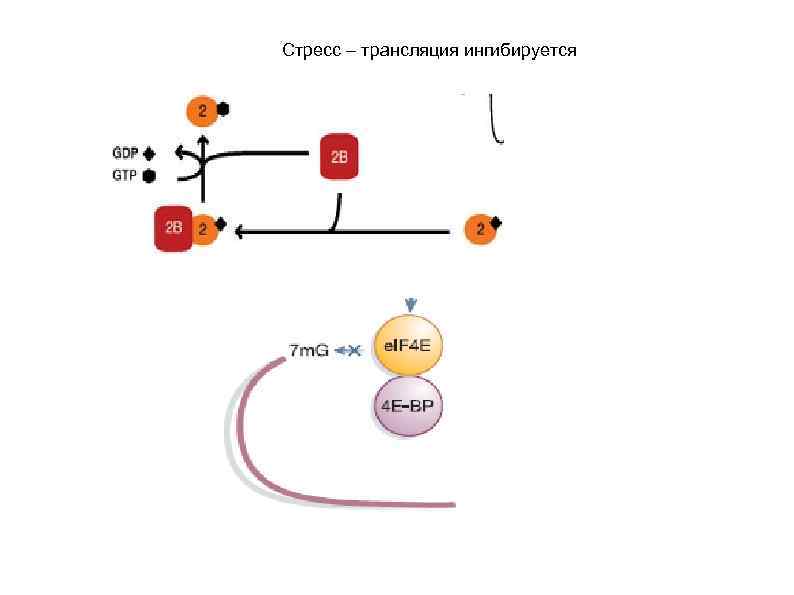 Стресс – трансляция ингибируется
Стресс – трансляция ингибируется
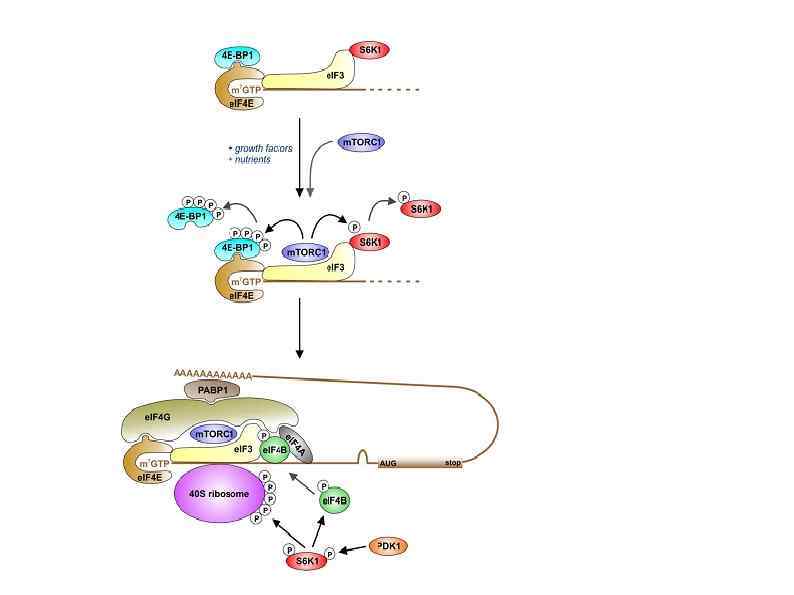
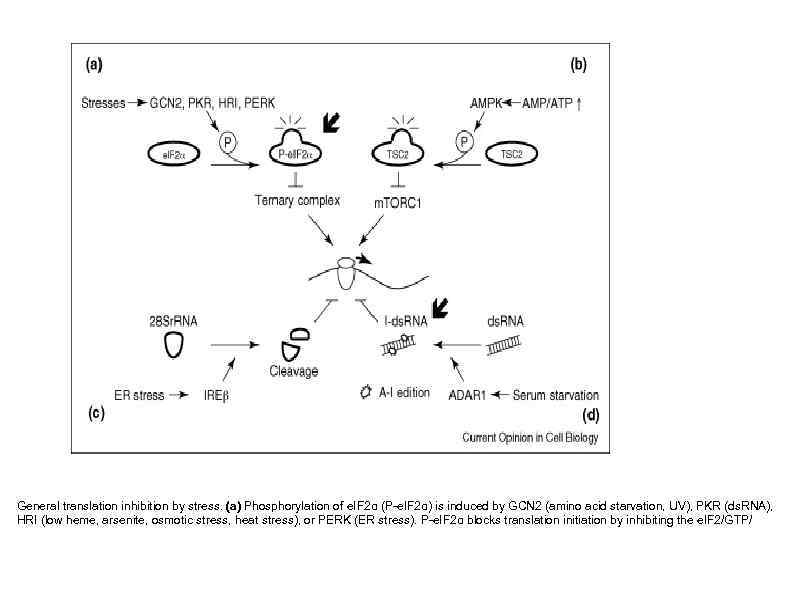 General translation inhibition by stress. (a) Phosphorylation of e. IF 2α (P-e. IF 2α) is induced by GCN 2 (amino acid starvation, UV), PKR (ds. RNA), HRI (low heme, arsenite, osmotic stress, heat stress), or PERK (ER stress). P-e. IF 2α blocks translation initiation by inhibiting the e. IF 2/GTP/
General translation inhibition by stress. (a) Phosphorylation of e. IF 2α (P-e. IF 2α) is induced by GCN 2 (amino acid starvation, UV), PKR (ds. RNA), HRI (low heme, arsenite, osmotic stress, heat stress), or PERK (ER stress). P-e. IF 2α blocks translation initiation by inhibiting the e. IF 2/GTP/
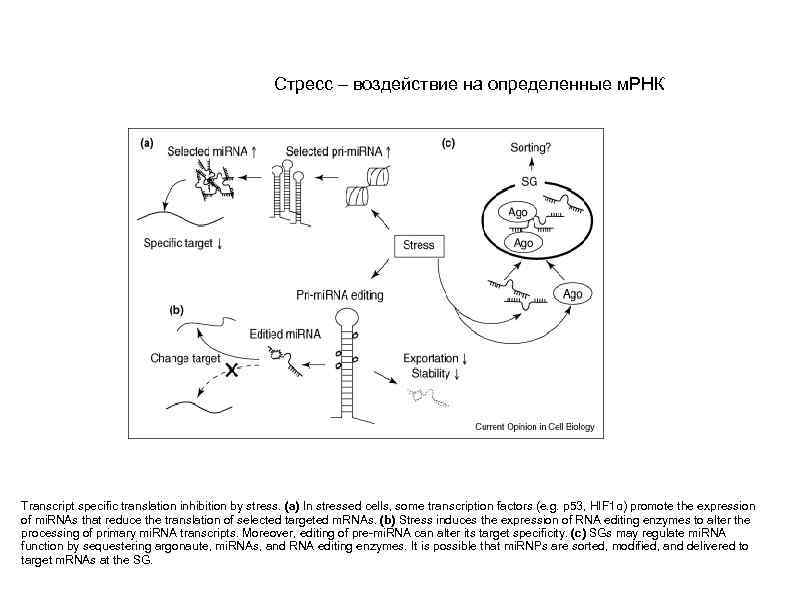 Стресс – воздействие на определенные м. РНК Transcript specific translation inhibition by stress. (a) In stressed cells, some transcription factors (e. g. p 53, HIF 1α) promote the expression of mi. RNAs that reduce the translation of selected targeted m. RNAs. (b) Stress induces the expression of RNA editing enzymes to alter the processing of primary mi. RNA transcripts. Moreover, editing of pre-mi. RNA can alter its target specificity. (c) SGs may regulate mi. RNA function by sequestering argonaute, mi. RNAs, and RNA editing enzymes. It is possible that mi. RNPs are sorted, modified, and delivered to target m. RNAs at the SG.
Стресс – воздействие на определенные м. РНК Transcript specific translation inhibition by stress. (a) In stressed cells, some transcription factors (e. g. p 53, HIF 1α) promote the expression of mi. RNAs that reduce the translation of selected targeted m. RNAs. (b) Stress induces the expression of RNA editing enzymes to alter the processing of primary mi. RNA transcripts. Moreover, editing of pre-mi. RNA can alter its target specificity. (c) SGs may regulate mi. RNA function by sequestering argonaute, mi. RNAs, and RNA editing enzymes. It is possible that mi. RNPs are sorted, modified, and delivered to target m. RNAs at the SG.

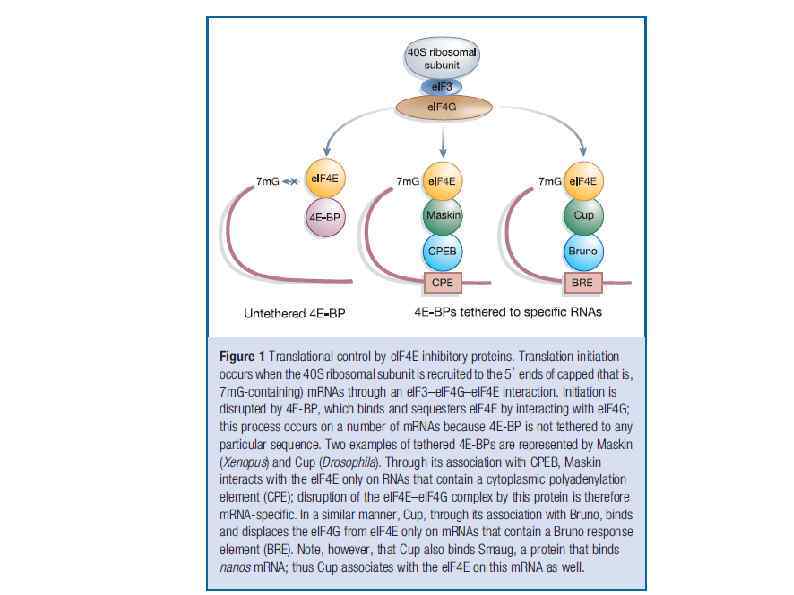
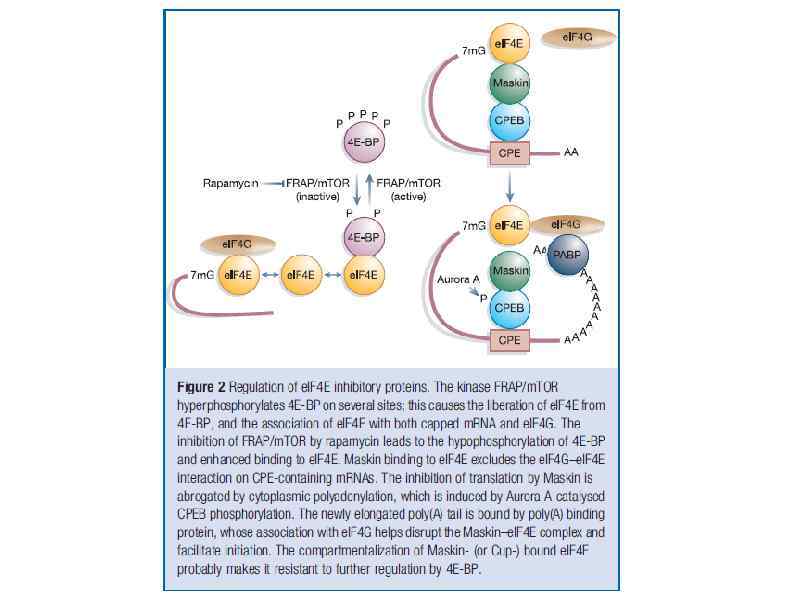
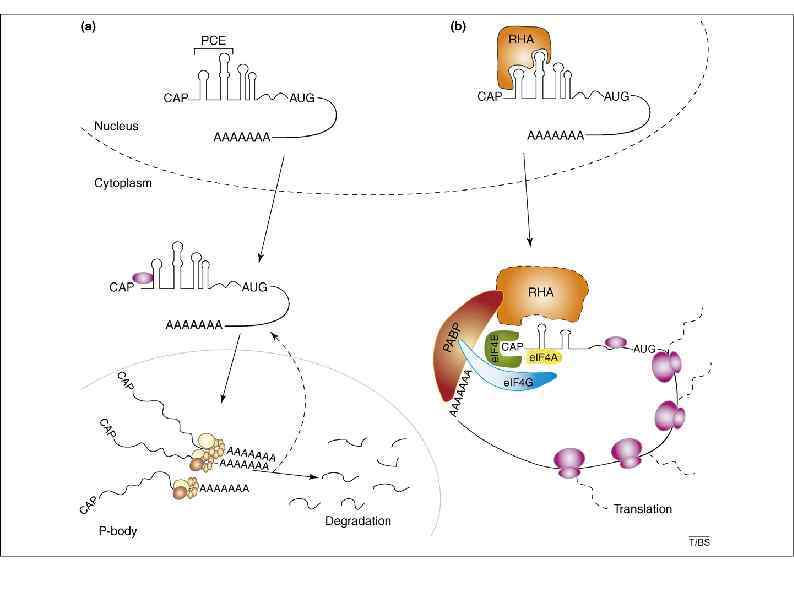
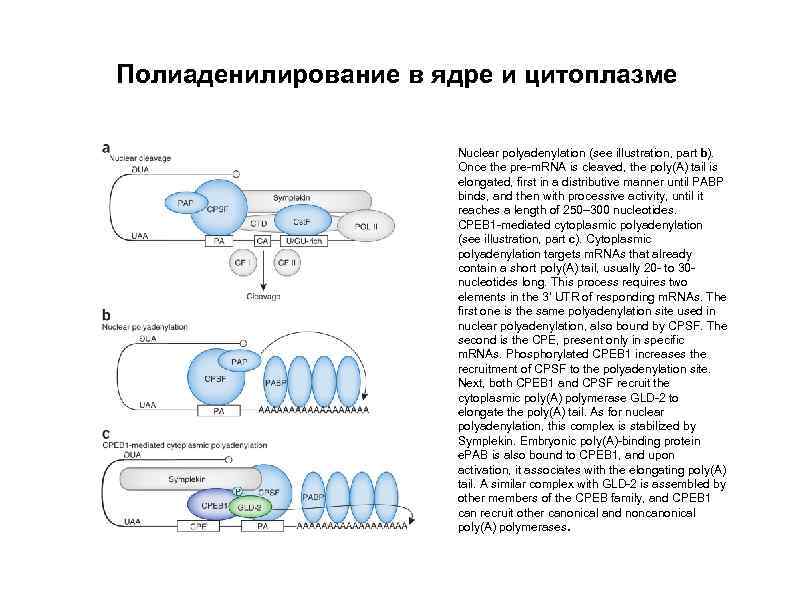 Полиаденилирование в ядре и цитоплазме Nuclear polyadenylation (see illustration, part b). Once the pre-m. RNA is cleaved, the poly(A) tail is elongated, first in a distributive manner until PABP binds, and then with processive activity, until it reaches a length of 250– 300 nucleotides. CPEB 1 -mediated cytoplasmic polyadenylation (see illustration, part c). Cytoplasmic polyadenylation targets m. RNAs that already contain a short poly(A) tail, usually 20 - to 30 nucleotides long. This process requires two elements in the 3′ UTR of responding m. RNAs. The first one is the same polyadenylation site used in nuclear polyadenylation, also bound by CPSF. The second is the CPE, present only in specific m. RNAs. Phosphorylated CPEB 1 increases the recruitment of CPSF to the polyadenylation site. Next, both CPEB 1 and CPSF recruit the cytoplasmic poly(A) polymerase GLD-2 to elongate the poly(A) tail. As for nuclear polyadenylation, this complex is stabilized by Symplekin. Embryonic poly(A)-binding protein e. PAB is also bound to CPEB 1, and upon activation, it associates with the elongating poly(A) tail. A similar complex with GLD-2 is assembled by other members of the CPEB family, and CPEB 1 can recruit other canonical and noncanonical poly(A) polymerases.
Полиаденилирование в ядре и цитоплазме Nuclear polyadenylation (see illustration, part b). Once the pre-m. RNA is cleaved, the poly(A) tail is elongated, first in a distributive manner until PABP binds, and then with processive activity, until it reaches a length of 250– 300 nucleotides. CPEB 1 -mediated cytoplasmic polyadenylation (see illustration, part c). Cytoplasmic polyadenylation targets m. RNAs that already contain a short poly(A) tail, usually 20 - to 30 nucleotides long. This process requires two elements in the 3′ UTR of responding m. RNAs. The first one is the same polyadenylation site used in nuclear polyadenylation, also bound by CPSF. The second is the CPE, present only in specific m. RNAs. Phosphorylated CPEB 1 increases the recruitment of CPSF to the polyadenylation site. Next, both CPEB 1 and CPSF recruit the cytoplasmic poly(A) polymerase GLD-2 to elongate the poly(A) tail. As for nuclear polyadenylation, this complex is stabilized by Symplekin. Embryonic poly(A)-binding protein e. PAB is also bound to CPEB 1, and upon activation, it associates with the elongating poly(A) tail. A similar complex with GLD-2 is assembled by other members of the CPEB family, and CPEB 1 can recruit other canonical and noncanonical poly(A) polymerases.
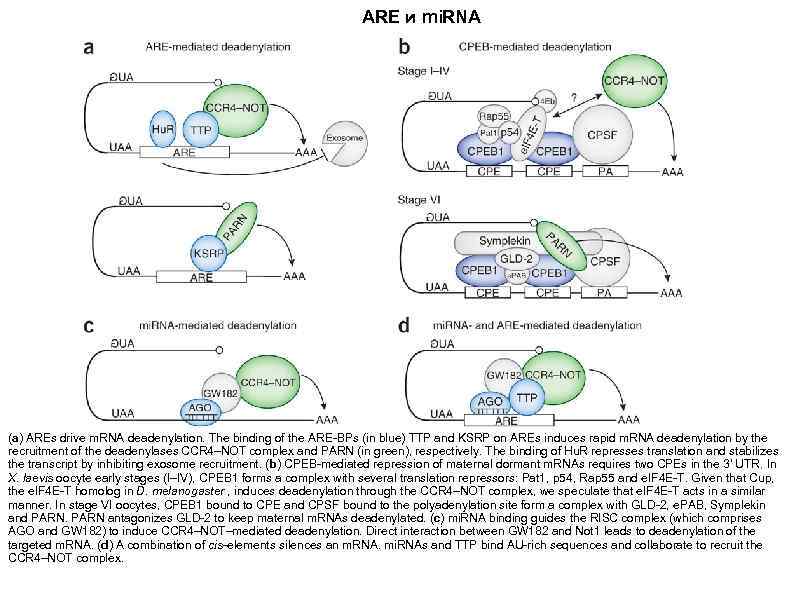 АRЕ и mi. RNA (a) AREs drive m. RNA deadenylation. The binding of the ARE-BPs (in blue) TTP and KSRP on AREs induces rapid m. RNA deadenylation by the recruitment of the deadenylases CCR 4–NOT complex and PARN (in green), respectively. The binding of Hu. R represses translation and stabilizes the transcript by inhibiting exosome recruitment. (b) CPEB-mediated repression of maternal dormant m. RNAs requires two CPEs in the 3′ UTR. In X. laevis oocyte early stages (I–IV), CPEB 1 forms a complex with several translation repressors: Pat 1, p 54, Rap 55 and e. IF 4 E-T. Given that Cup, the e. IF 4 E-T homolog in D. melanogaster , induces deadenylation through the CCR 4–NOT complex, we speculate that e. IF 4 E-T acts in a similar manner. In stage VI oocytes, CPEB 1 bound to CPE and CPSF bound to the polyadenylation site form a complex with GLD-2, e. PAB, Symplekin and PARN antagonizes GLD-2 to keep maternal m. RNAs deadenylated. (c) mi. RNA binding guides the RISC complex (which comprises AGO and GW 182) to induce CCR 4–NOT–mediated deadenylation. Direct interaction between GW 182 and Not 1 leads to deadenylation of the targeted m. RNA. (d) A combination of cis-elements silences an m. RNA. mi. RNAs and TTP bind AU-rich sequences and collaborate to recruit the CCR 4–NOT complex.
АRЕ и mi. RNA (a) AREs drive m. RNA deadenylation. The binding of the ARE-BPs (in blue) TTP and KSRP on AREs induces rapid m. RNA deadenylation by the recruitment of the deadenylases CCR 4–NOT complex and PARN (in green), respectively. The binding of Hu. R represses translation and stabilizes the transcript by inhibiting exosome recruitment. (b) CPEB-mediated repression of maternal dormant m. RNAs requires two CPEs in the 3′ UTR. In X. laevis oocyte early stages (I–IV), CPEB 1 forms a complex with several translation repressors: Pat 1, p 54, Rap 55 and e. IF 4 E-T. Given that Cup, the e. IF 4 E-T homolog in D. melanogaster , induces deadenylation through the CCR 4–NOT complex, we speculate that e. IF 4 E-T acts in a similar manner. In stage VI oocytes, CPEB 1 bound to CPE and CPSF bound to the polyadenylation site form a complex with GLD-2, e. PAB, Symplekin and PARN antagonizes GLD-2 to keep maternal m. RNAs deadenylated. (c) mi. RNA binding guides the RISC complex (which comprises AGO and GW 182) to induce CCR 4–NOT–mediated deadenylation. Direct interaction between GW 182 and Not 1 leads to deadenylation of the targeted m. RNA. (d) A combination of cis-elements silences an m. RNA. mi. RNAs and TTP bind AU-rich sequences and collaborate to recruit the CCR 4–NOT complex.

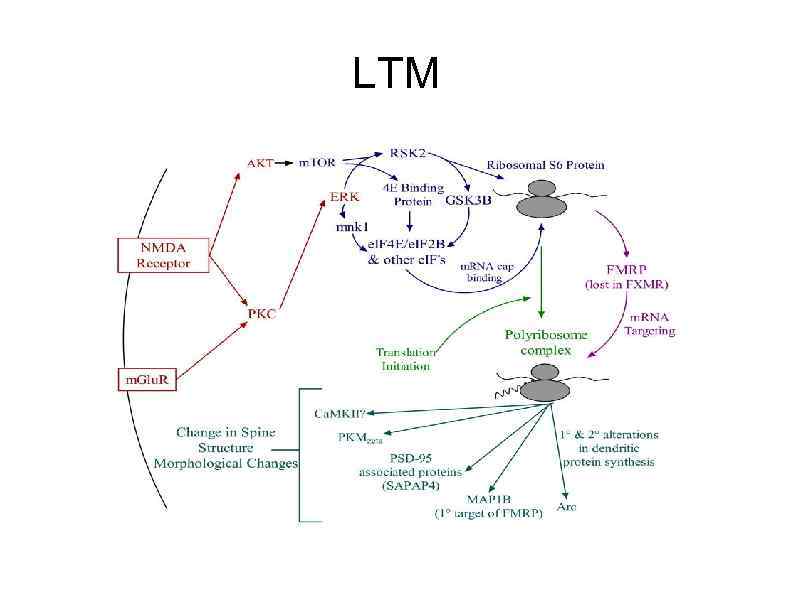 LTM
LTM
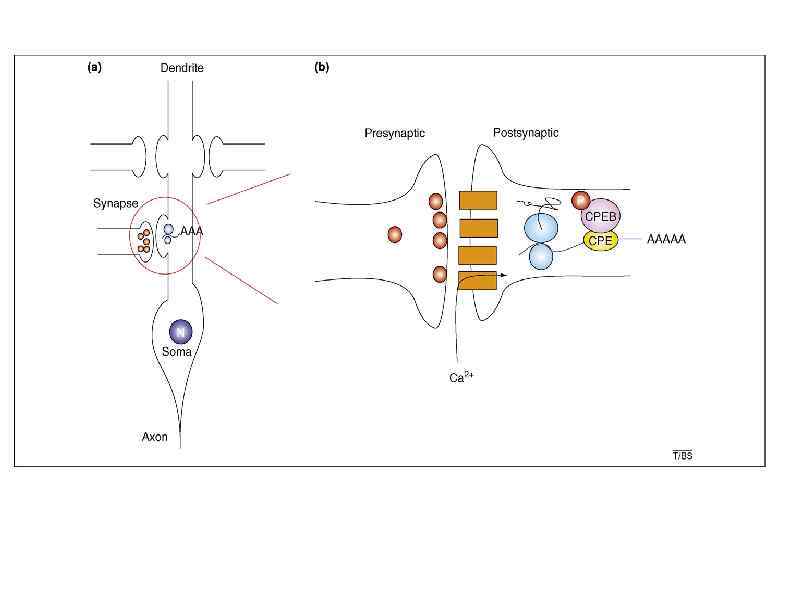
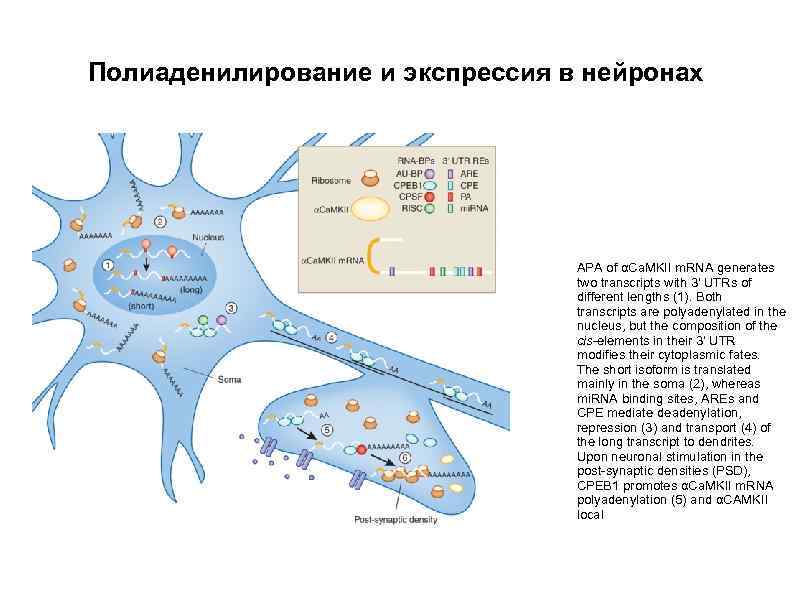 Полиаденилирование и экспрессия в нейронах APA of αCa. MKII m. RNA generates two transcripts with 3′ UTRs of different lengths (1). Both transcripts are polyadenylated in the nucleus, but the composition of the cis-elements in their 3′ UTR modifies their cytoplasmic fates. The short isoform is translated mainly in the soma (2), whereas mi. RNA binding sites, AREs and CPE mediate deadenylation, repression (3) and transport (4) of the long transcript to dendrites. Upon neuronal stimulation in the post-synaptic densities (PSD), CPEB 1 promotes αCa. MKII m. RNA polyadenylation (5) and αCAMKII local translation (6).
Полиаденилирование и экспрессия в нейронах APA of αCa. MKII m. RNA generates two transcripts with 3′ UTRs of different lengths (1). Both transcripts are polyadenylated in the nucleus, but the composition of the cis-elements in their 3′ UTR modifies their cytoplasmic fates. The short isoform is translated mainly in the soma (2), whereas mi. RNA binding sites, AREs and CPE mediate deadenylation, repression (3) and transport (4) of the long transcript to dendrites. Upon neuronal stimulation in the post-synaptic densities (PSD), CPEB 1 promotes αCa. MKII m. RNA polyadenylation (5) and αCAMKII local translation (6).
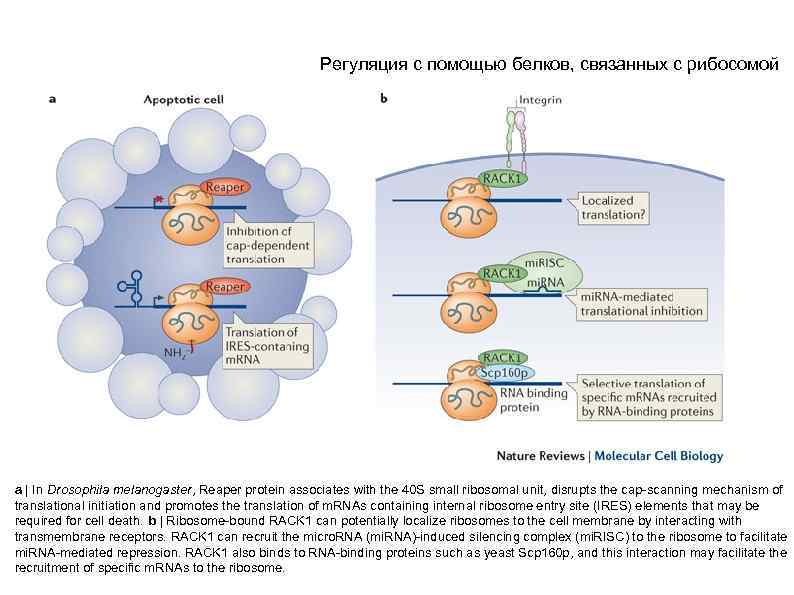 Регуляция с помощью белков, связанных с рибосомой a | In Drosophila melanogaster, Reaper protein associates with the 40 S small ribosomal unit, disrupts the cap-scanning mechanism of translational initiation and promotes the translation of m. RNAs containing internal ribosome entry site (IRES) elements that may be required for cell death. b | Ribosome-bound RACK 1 can potentially localize ribosomes to the cell membrane by interacting with transmembrane receptors. RACK 1 can recruit the micro. RNA (mi. RNA)-induced silencing complex (mi. RISC) to the ribosome to facilitate mi. RNA-mediated repression. RACK 1 also binds to RNA-binding proteins such as yeast Scp 160 p, and this interaction may facilitate the recruitment of specific m. RNAs to the ribosome.
Регуляция с помощью белков, связанных с рибосомой a | In Drosophila melanogaster, Reaper protein associates with the 40 S small ribosomal unit, disrupts the cap-scanning mechanism of translational initiation and promotes the translation of m. RNAs containing internal ribosome entry site (IRES) elements that may be required for cell death. b | Ribosome-bound RACK 1 can potentially localize ribosomes to the cell membrane by interacting with transmembrane receptors. RACK 1 can recruit the micro. RNA (mi. RNA)-induced silencing complex (mi. RISC) to the ribosome to facilitate mi. RNA-mediated repression. RACK 1 also binds to RNA-binding proteins such as yeast Scp 160 p, and this interaction may facilitate the recruitment of specific m. RNAs to the ribosome.
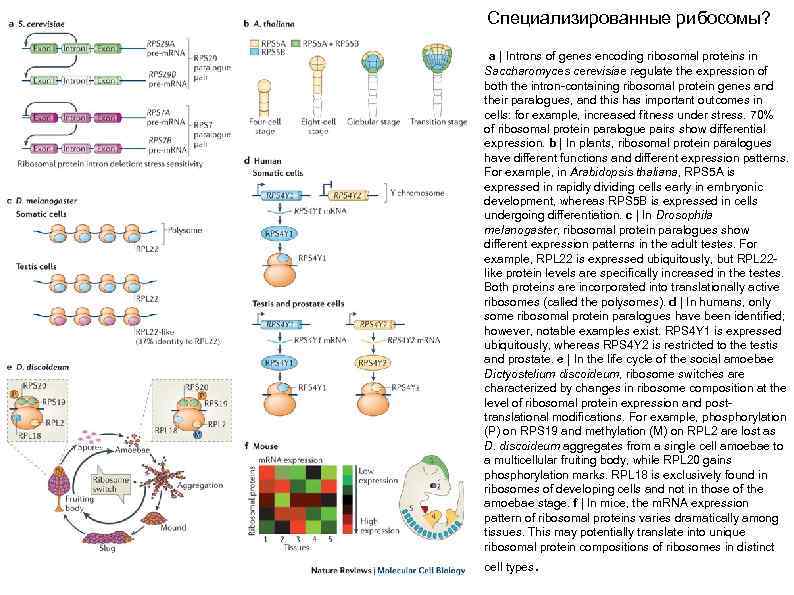 Специализированные рибосомы? a | Introns of genes encoding ribosomal proteins in Saccharomyces cerevisiae regulate the expression of both the intron-containing ribosomal protein genes and their paralogues, and this has important outcomes in cells: for example, increased fitness under stress. 70% of ribosomal protein paralogue pairs show differential expression. b | In plants, ribosomal protein paralogues have different functions and different expression patterns. For example, in Arabidopsis thaliana, RPS 5 A is expressed in rapidly dividing cells early in embryonic development, whereas RPS 5 B is expressed in cells undergoing differentiation. c | In Drosophila melanogaster, ribosomal protein paralogues show different expression patterns in the adult testes. For example, RPL 22 is expressed ubiquitously, but RPL 22 like protein levels are specifically increased in the testes. Both proteins are incorporated into translationally active ribosomes (called the polysomes). d | In humans, only some ribosomal protein paralogues have been identified; however, notable examples exist. RPS 4 Y 1 is expressed ubiquitously, whereas RPS 4 Y 2 is restricted to the testis and prostate. e | In the life cycle of the social amoebae Dictyostelium discoideum, ribosome switches are characterized by changes in ribosome composition at the level of ribosomal protein expression and posttranslational modifications. For example, phosphorylation (P) on RPS 19 and methylation (M) on RPL 2 are lost as D. discoideum aggregates from a single cell amoebae to a multicellular fruiting body, while RPL 20 gains phosphorylation marks. RPL 18 is exclusively found in ribosomes of developing cells and not in those of the amoebae stage. f | In mice, the m. RNA expression pattern of ribosomal proteins varies dramatically among tissues. This may potentially translate into unique ribosomal protein compositions of ribosomes in distinct cell types.
Специализированные рибосомы? a | Introns of genes encoding ribosomal proteins in Saccharomyces cerevisiae regulate the expression of both the intron-containing ribosomal protein genes and their paralogues, and this has important outcomes in cells: for example, increased fitness under stress. 70% of ribosomal protein paralogue pairs show differential expression. b | In plants, ribosomal protein paralogues have different functions and different expression patterns. For example, in Arabidopsis thaliana, RPS 5 A is expressed in rapidly dividing cells early in embryonic development, whereas RPS 5 B is expressed in cells undergoing differentiation. c | In Drosophila melanogaster, ribosomal protein paralogues show different expression patterns in the adult testes. For example, RPL 22 is expressed ubiquitously, but RPL 22 like protein levels are specifically increased in the testes. Both proteins are incorporated into translationally active ribosomes (called the polysomes). d | In humans, only some ribosomal protein paralogues have been identified; however, notable examples exist. RPS 4 Y 1 is expressed ubiquitously, whereas RPS 4 Y 2 is restricted to the testis and prostate. e | In the life cycle of the social amoebae Dictyostelium discoideum, ribosome switches are characterized by changes in ribosome composition at the level of ribosomal protein expression and posttranslational modifications. For example, phosphorylation (P) on RPS 19 and methylation (M) on RPL 2 are lost as D. discoideum aggregates from a single cell amoebae to a multicellular fruiting body, while RPL 20 gains phosphorylation marks. RPL 18 is exclusively found in ribosomes of developing cells and not in those of the amoebae stage. f | In mice, the m. RNA expression pattern of ribosomal proteins varies dramatically among tissues. This may potentially translate into unique ribosomal protein compositions of ribosomes in distinct cell types.
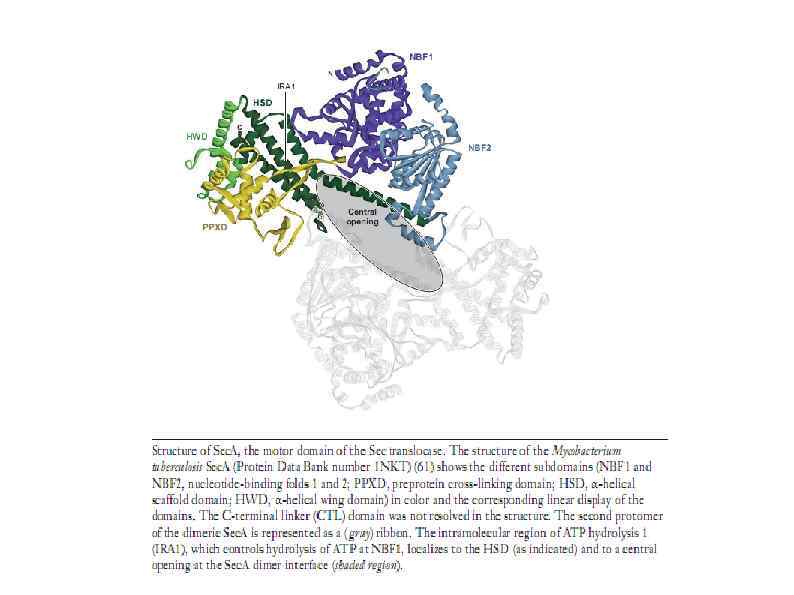
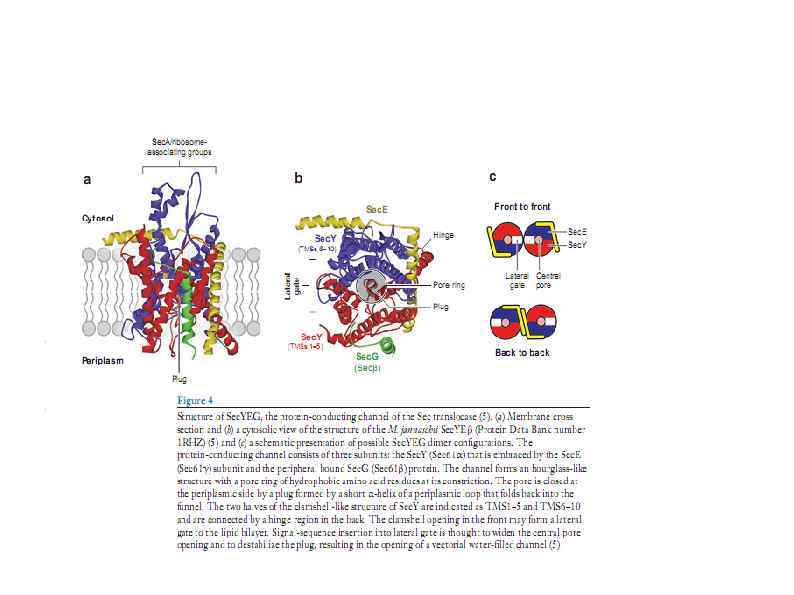
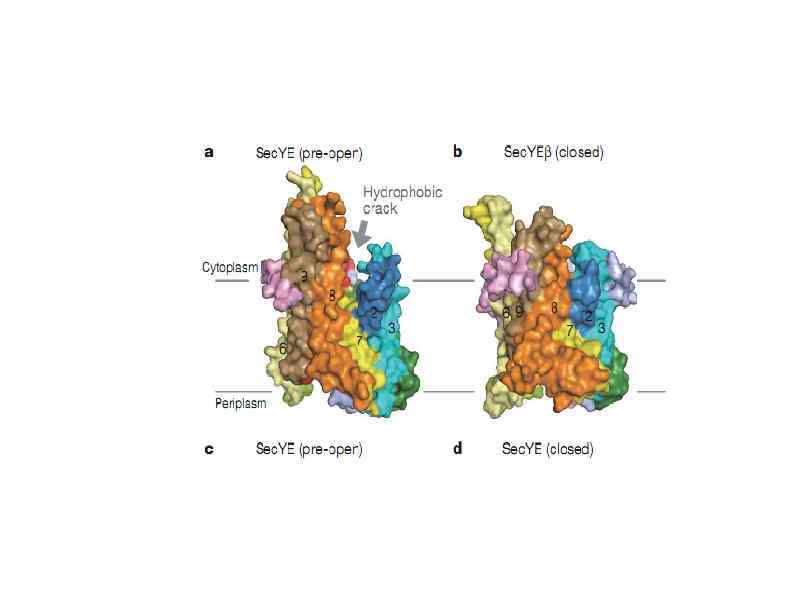
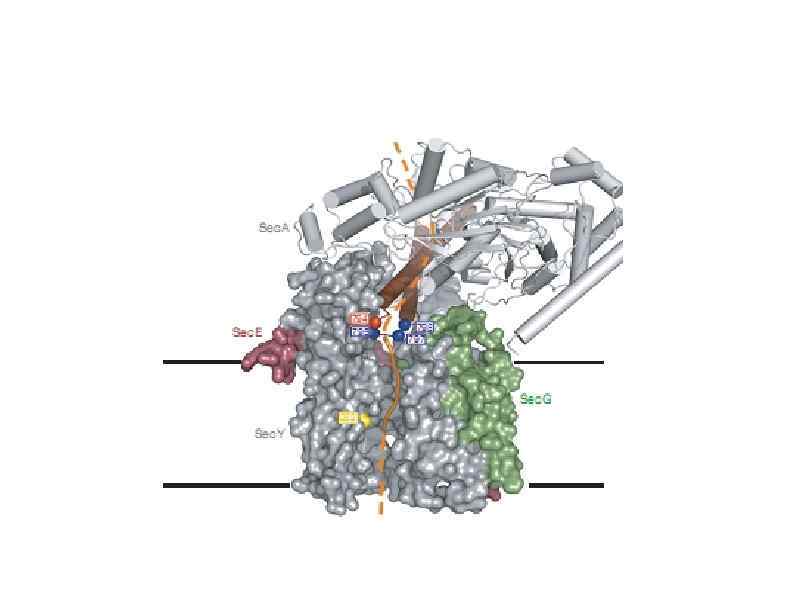

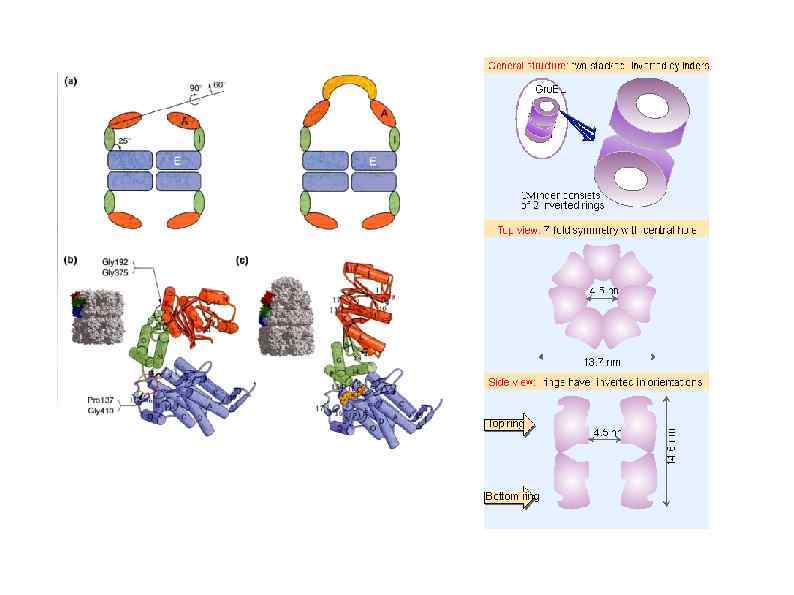
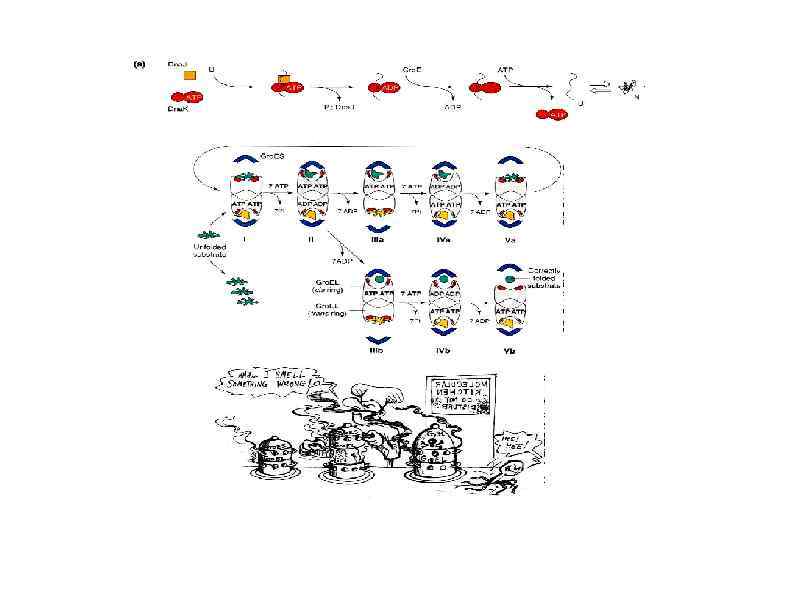
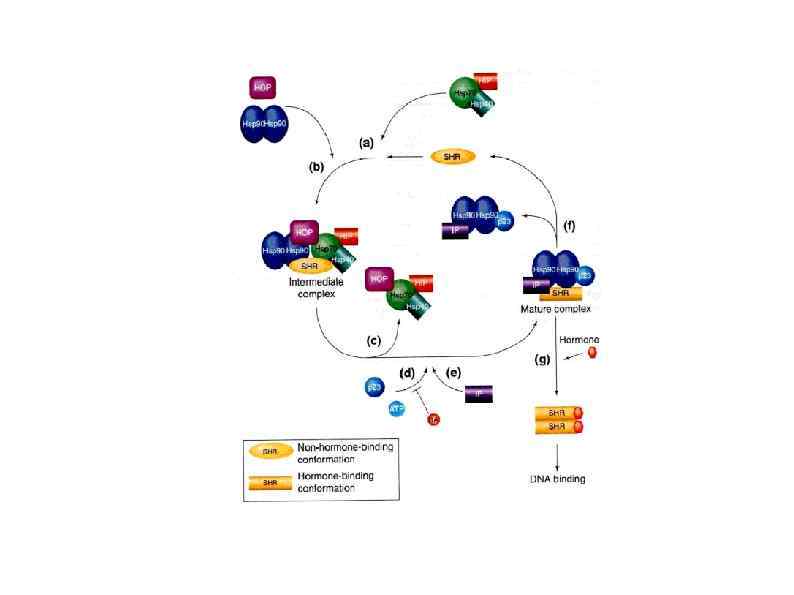
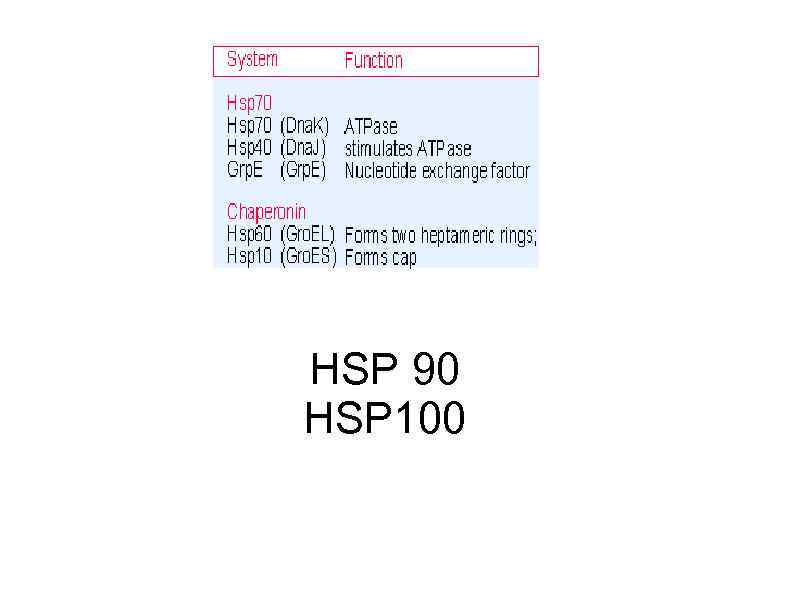 HSP 90 HSP 100
HSP 90 HSP 100

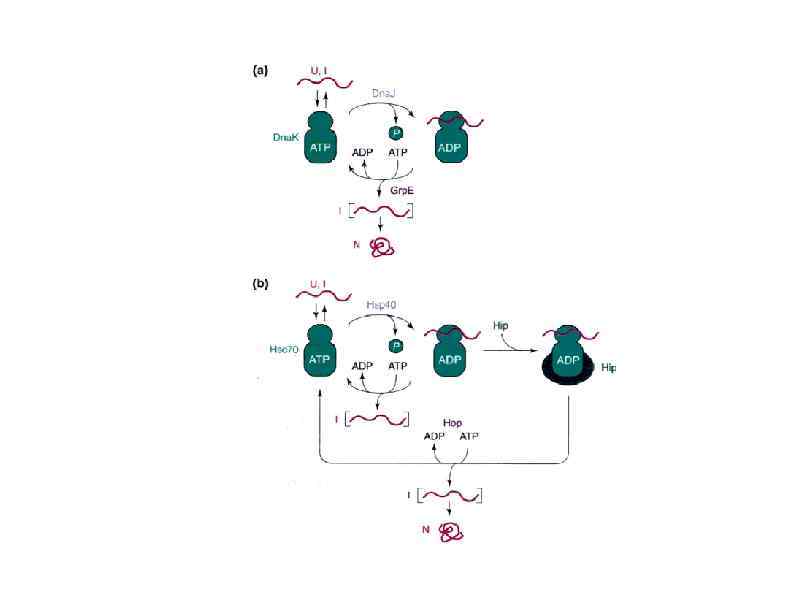
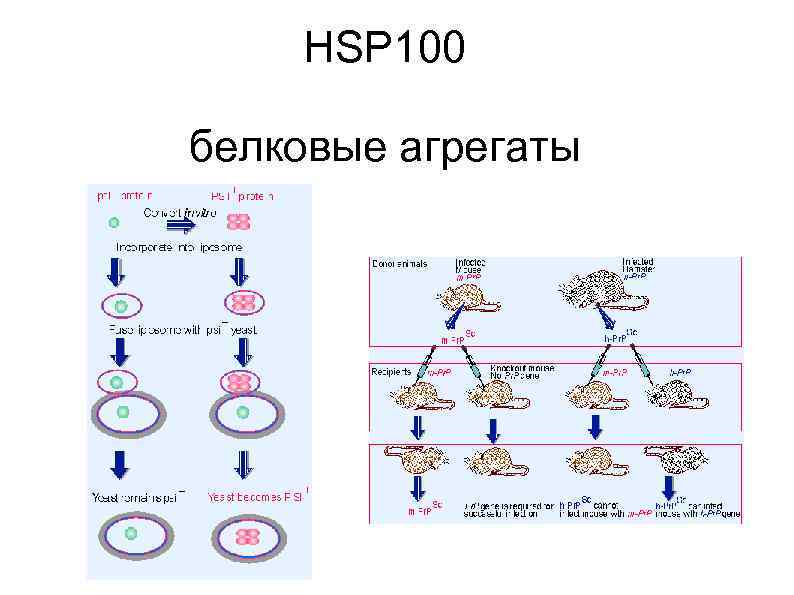 HSP 100 белковые агрегаты
HSP 100 белковые агрегаты