2203cb92483fdb5f8a0528eee872aabc.ppt
- Количество слайдов: 29

Risk-Based CMC Review Moheb M. Nasr, Ph. D. Office of New Drug Chemistry (ONDC) OPS, CDER, FDA DIA Annual Conference 2004 Washington, D. C. June 16, 2004 1
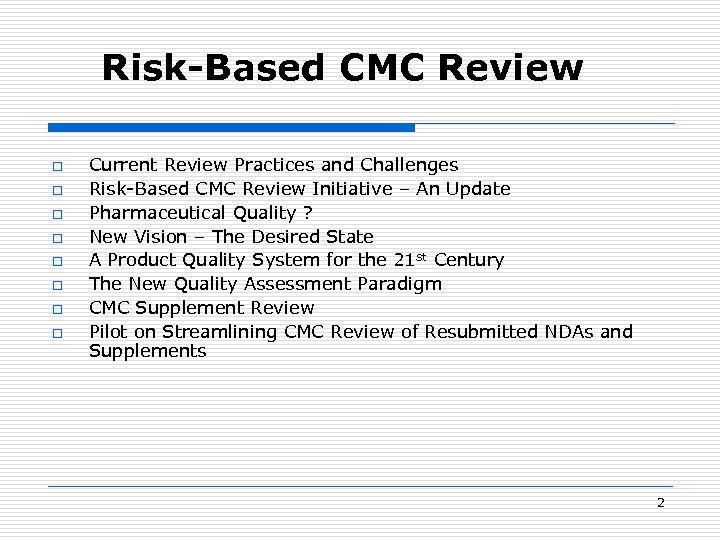
Risk-Based CMC Review o o o o Current Review Practices and Challenges Risk-Based CMC Review Initiative – An Update Pharmaceutical Quality ? New Vision – The Desired State A Product Quality System for the 21 st Century The New Quality Assessment Paradigm CMC Supplement Review Pilot on Streamlining CMC Review of Resubmitted NDAs and Supplements 2
3
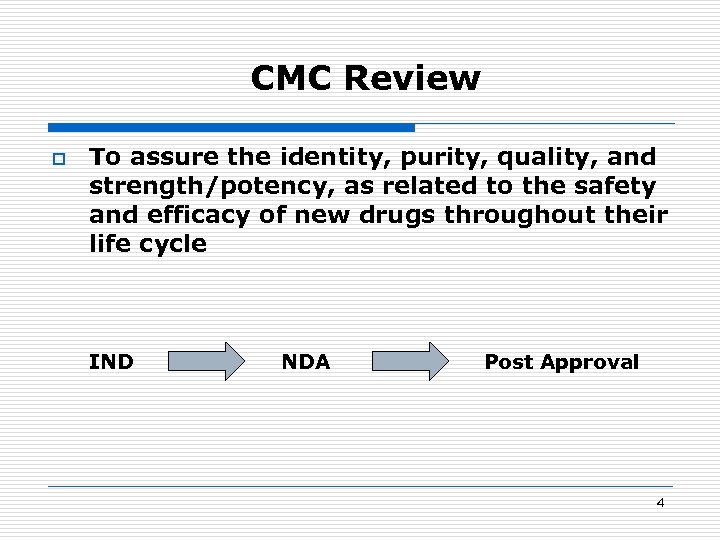
CMC Review o To assure the identity, purity, quality, and strength/potency, as related to the safety and efficacy of new drugs throughout their life cycle IND NDA Post Approval 4
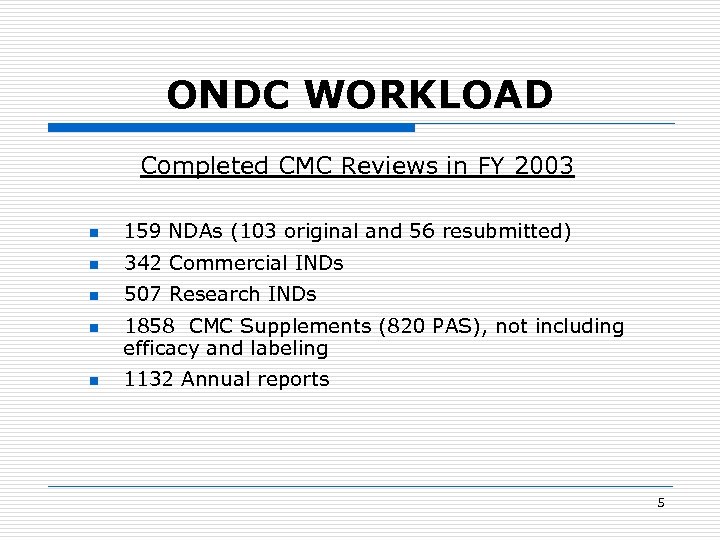
ONDC WORKLOAD Completed CMC Reviews in FY 2003 n 159 NDAs (103 original and 56 resubmitted) n 342 Commercial INDs n 507 Research INDs n n 1858 CMC Supplements (820 PAS), not including efficacy and labeling 1132 Annual reports 5
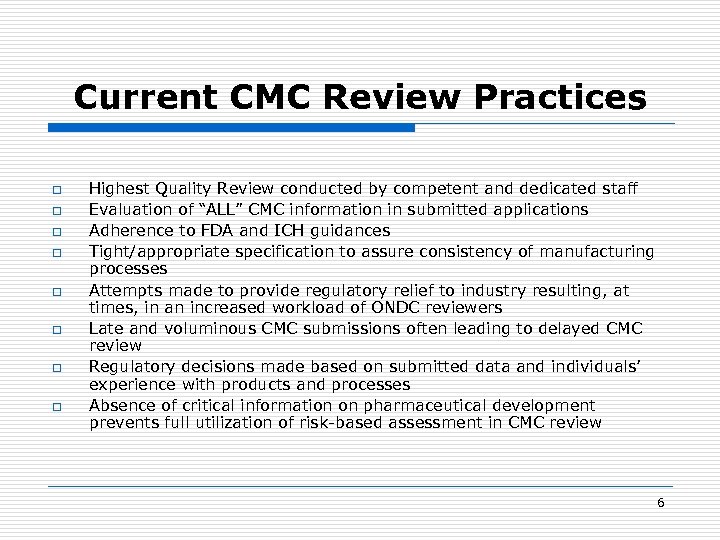
Current CMC Review Practices o o o o Highest Quality Review conducted by competent and dedicated staff Evaluation of “ALL” CMC information in submitted applications Adherence to FDA and ICH guidances Tight/appropriate specification to assure consistency of manufacturing processes Attempts made to provide regulatory relief to industry resulting, at times, in an increased workload of ONDC reviewers Late and voluminous CMC submissions often leading to delayed CMC review Regulatory decisions made based on submitted data and individuals’ experience with products and processes Absence of critical information on pharmaceutical development prevents full utilization of risk-based assessment in CMC review 6

Challenges o o o o Product Quality System for the 21 st Century – A new Paradigm for the regulation of Pharmaceutical Quality First Cycle Approval Work Load Consistency among 19 Chemistry Teams across CDER 15 clinical divisions Guidance and policy development Lack of expertise in some critical CMC areas Novel dosage forms and combination drug products New technologies 7

Risk-Based CMC Initiative – An Update o o Intended to provide regulatory relief by incorporating science-based risk assessment to CMC review Risk-Based CMC Initiative: n Evolved over the last few years n Multi-Tiered (establish attributes, propose and finalize a drug list before determining eligibility for regulatory relief) n Product Specific n Synthetic Drug Substances only n Characterization achieved by traditional analytical techniques n IR oral solids, oral solutions, non-sterile topical solutions and sterile solutions of simple salts Relevance in the new pharmaceutical quality paradigm? A Progressive and Expanded Approach will focus on quality assessment in entirety and associated regulatory processes 8

Pharmaceutical Quality: A product that meets customer’s needs o Old Paradigm n n o Establishment and enforcement of public/private standards to assure the integrity of interstate commerce (adulteration, counterfeit, etc. ) Relevance to safety and efficacy? New Paradigm (JW, September 17, 2003) n FDA stands in for the customer to establish & enforce quality standards generally in the realm of clinical performance (ultimate metric!) 9

Pharmaceutical Quality The New Paradigm (JW) o Clinical performance = delivery of efficacy and safety as described in the label and derived from the clinical trials o Fitness for use is a surrogate for clinical performance o A product that is “fit for use” meets established quality attributes (Purity, Potency/strength, Identity, Bioavailability/delivery, Labeling/packaging, Physical performance, etc. ) o Products must be made in compliance with c. GMP 10

Pharmaceutical Quality The New Paradigm (JW) o Need to examine and evaluate linkages between: n n n o Quality attributes and clinical performance? Values/specifications and safety and effectiveness? c. GMP compliance/inspection and safety and efficacy? Ultimate Goal: The availability to the patient of high quality safe and effective drugs at reasonable cost 11
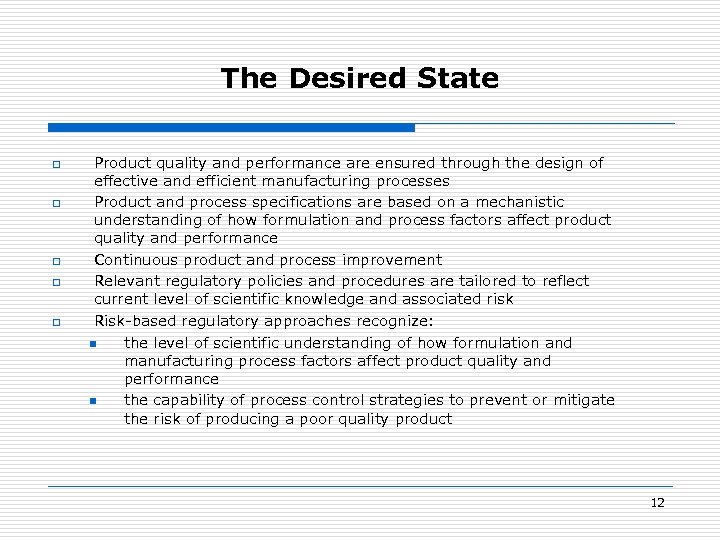
The Desired State o o o Product quality and performance are ensured through the design of effective and efficient manufacturing processes Product and process specifications are based on a mechanistic understanding of how formulation and process factors affect product quality and performance Continuous product and process improvement Relevant regulatory policies and procedures are tailored to reflect current level of scientific knowledge and associated risk Risk-based regulatory approaches recognize: n the level of scientific understanding of how formulation and manufacturing process factors affect product quality and performance n the capability of process control strategies to prevent or mitigate the risk of producing a poor quality product 12

A Product Quality System for the 21 st Century p Initiative focus on product quality and not just c. GMP p Major changes in pharmaceutical business over the years: p p Advances in pharmaceutical and manufacturing sciences p Applications of biotechnology p p Greater dependence on medicine in health care Globalization of industry Minor regulatory adjustments over the years: p 1978 c. GMP Revision p CDER-ORA agreement in early 90’s p SUPAC, BACPAC, FDAMA in late 90’s p Team Biologics 13

Problems and Issues in Current System q For FDA: q q Expensive and time-consuming litigation and legal actions q q Resource intensive Dealing with recalls and drug shortages For Industry: q q Regulatory burden, not value added q q Continuous improvement discouraged Consequences of non-compliance For Public: q High cost of drugs q Hostility, at times, towards industry and regulators 14

A Product Quality System for the 21 st Century - Fundamental Principles q Risk Management (priorities, resource allocation and setting regulatory requirements) q Science-based regulatory approaches (conduct scientific risk assessment and facilitate technological advances) q Strong public health focus q International cooperation q Assessment and implementation of appropriate quality management systems q Integrated product quality regulatory practice (review and inspection processes) 15

Risk-Based CMC Review Benefits of a Risk-Based System* o o Patients n Increased availability n Faster approval of new products n Continue to receive quality products FDA n More product and process knowledge shared by industry n More efficient resource allocation for review and inspection n Increased trust and understanding of industry decision making * FDA/PQRI Workshop, April 2003, Washington, D. C. 16

Risk-Based CMC Review Benefits of a Risk-Based System* o Industry n More efficient, science-based inspections resulting in increased consistency n Faster, more consistent reviews n Potential for reduced regulatory burden n Manage changes and nonconformance with less FDA oversight n Focuses resources on critical issues n Flexibility to focus on what should be done, not what can be done n Improves communication with FDA * FDA/PQRI Workshop, April 2003, Washington, D. C. 17

Risk-Based CMC Review o o This is not a single initiative to address one dimension of a multi-dimensional, often complex, quality assessment process (not just streamlining or reducing CMC supplements, etc. ) It is the new paradigm in quality assessment of new drug applications (entire pre and post marketing activities) 18
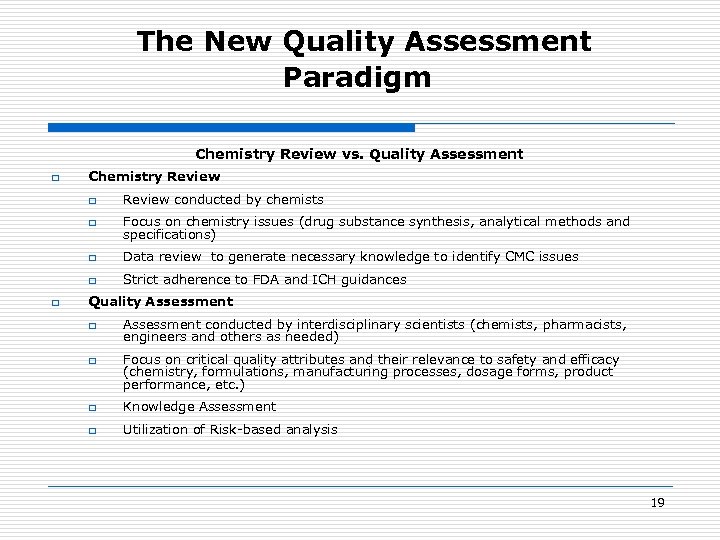
The New Quality Assessment Paradigm Chemistry Review vs. Quality Assessment q Chemistry Review q q Focus on chemistry issues (drug substance synthesis, analytical methods and specifications) q Data review to generate necessary knowledge to identify CMC issues q q Review conducted by chemists Strict adherence to FDA and ICH guidances Quality Assessment q q Assessment conducted by interdisciplinary scientists (chemists, pharmacists, engineers and others as needed) Focus on critical quality attributes and their relevance to safety and efficacy (chemistry, formulations, manufacturing processes, dosage forms, product performance, etc. ) q Knowledge Assessment q Utilization of Risk-based analysis 19

The New Quality Assessment Paradigm o o ONDC Vision: ONDC is a strong scientific organization that serves CDER, FDA, and the public through leadership in innovation and international collaboration ONDC Mission: ONDC assesses the critical quality attributes and manufacturing processes of new drugs, establishes quality standards to assure safety and efficacy, and facilitates new drug development 20

The New Quality Assessment Paradigm o o o CMC specifications to be based on: n Risk-based assessment n Clinical Relevance n Safety Considerations n Process Capabilities n Knowledge gained from Pharmaceutical Development Reports n Better utilization of modern statistical methodologies Assessment starts with a comprehensive Quality Overall Summary (QOS) Review practices based on good scientific principles (“GSP”) Increased emphasis on manufacturing science Peer/critical review of CMC evaluation by FDA scientists Integration of review and inspection (ICH Q 8, 9 & 10 ? ) 21

The New Quality Assessment Paradigm o o Regulatory relief based on: n Process understanding and control (pharmaceutical development reports) n Quality systems throughout manufacturing processes n Continuous improvement Pharmaceutical Development Reports may facilitate: n Meeting first cycle approval goal n Science–based specifications n Risk-based GMP inspection 22
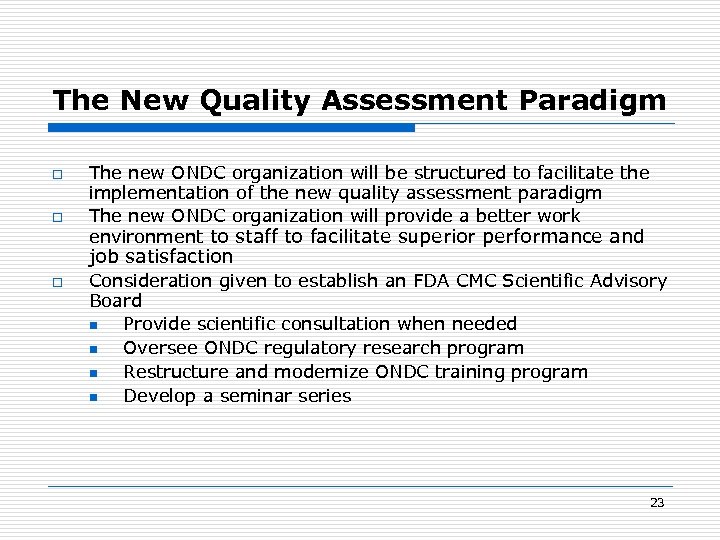
The New Quality Assessment Paradigm o o The new ONDC organization will be structured to facilitate the implementation of the new quality assessment paradigm The new ONDC organization will provide a better work environment to staff to facilitate superior performance and job satisfaction o Consideration given to establish an FDA CMC Scientific Advisory Board n Provide scientific consultation when needed n Oversee ONDC regulatory research program n Restructure and modernize ONDC training program n Develop a seminar series 23
The New Quality Assessment Paradigm o o o We developed new strategies to recruit, hire and train pharmaceutical quality assessors with expertise in drug discovery, analytical chemistry, pharmaceutical development/formulation and pharmaceutical engineering (HELP? ? ) We will build a strong and independent scientific organization to better serve the public and all internal (New Drugs, Compliance, Generic Drugs) and external (industry, scientific organizations and academic institutes) stakeholders We will reengineer the CMC review process at ONDC to: n Address problems identified by FDA, Public and Industry n Meet expectations of the new paradigm and to achieve the desired state n Establish a modern quality system with appropriate metrics to measure quality of CMC review and performance 24

Proposed ICH Q 10 “Life Cycle Management Systems” o o Systems for monitoring processes to demonstrate control and/or identify trends Systems for managing and rectifying undesirable occurrences Systems to facilitate process improvements Systems to manage change and to monitor the effect of change 25
CMC Supplement Review o o o Facilitate and encourage continuous improvements resulting in down-regulation and elimination of some CMC Supplements Comparability Protocols n Provides an opportunity to bridge current system with future paradigm n Quality by Design (Qb. D) Principles n Continuous Improvements n Expecting, understanding and managing changes n Evaluation of impact of proposed changes and developing strategies to address impact n Critical vs. non-critical changes n A Great Potential to down-regulate CMC supplements Future ONDC Organization to manage CMC supplement review more efficiently to facilitate continuous post-marketing product improvement 26
Pilot on Streamlining CMC Review of Resubmitted NDAs and Supplements o Initial assessment by a senior CMC reviewer n n o Forward initial assessment and protocol to primary reviewer Primary reviewer n o Complete preliminary assessment within 14 days Order referenced DMFs (not a trivial process) Prioritize issues by impact to approvability Development of an assessment protocol Review, identify, communicate, and resolve CMC approvability issues in a timely manner Streamlining resubmission and supplements review to provide adequate resources for new NDAs 27

CMC Review of NDAs There is no substitute for a quality submission, including a Comprehensive Quality Overall Summary, which anticipates and addresses all approvability issues 28
Acknowledgments o o o Janet Woodcock (CDER) and the GMP Steering Committee Helen Winkle (OPS) Ajaz Hussain (OPS) Chi-Wan Chen (ONDC) Guirag Poochikian (ONDC) 29
2203cb92483fdb5f8a0528eee872aabc.ppt