948204c0b177ad2e244ded83b4f7c45d.ppt
- Количество слайдов: 25
 Review: Can you take the Heat How much heat is required to heat 100 g of water at 15 o. C to water at 75 o. C. q = m C △T q= 100 g(4. 18 J/go. C)(75 -15) q= 25080 J
Review: Can you take the Heat How much heat is required to heat 100 g of water at 15 o. C to water at 75 o. C. q = m C △T q= 100 g(4. 18 J/go. C)(75 -15) q= 25080 J
 More review 2)How many moles of Zinc oxide are in 81. 4 grams of Zinc oxide? 3) How many liters of N 2 O 4 gas have a mass of 100. 0 g at STP?
More review 2)How many moles of Zinc oxide are in 81. 4 grams of Zinc oxide? 3) How many liters of N 2 O 4 gas have a mass of 100. 0 g at STP?
 Review Three isotopes of carbon are indicated below: n 12 C 13 C 14 C n 6 6 6 How are these isotopes alike? A They have the same number of protons and the same atomic mass. B They have the same number of neutrons and the same atomic mass. C They have the same number of protons and the same atomic number. D They have the same number of neutrons and the same atomic number. n
Review Three isotopes of carbon are indicated below: n 12 C 13 C 14 C n 6 6 6 How are these isotopes alike? A They have the same number of protons and the same atomic mass. B They have the same number of neutrons and the same atomic mass. C They have the same number of protons and the same atomic number. D They have the same number of neutrons and the same atomic number. n
 Review How many electrons are in the outermost energy level of an electrically neutral atom of aluminum? n A 13 n. B 8 n. C 3 n. D 2 n
Review How many electrons are in the outermost energy level of an electrically neutral atom of aluminum? n A 13 n. B 8 n. C 3 n. D 2 n
 Review Which transition occurs when light with a wavelength of 434 nm is emitted by a n hydrogen atom? n A The electron jumps from n = 2 to n = 4. n B The electron jumps from n = 2 to n = 5. n C The electron falls from n = 4 to n = 2. n D The electron falls from n = 5 to n = 2. n
Review Which transition occurs when light with a wavelength of 434 nm is emitted by a n hydrogen atom? n A The electron jumps from n = 2 to n = 4. n B The electron jumps from n = 2 to n = 5. n C The electron falls from n = 4 to n = 2. n D The electron falls from n = 5 to n = 2. n
 Review Which best represents the electron configuration for an atom of iron? n A 1 s 22 p 63 s 23 p 64 s 23 d 6 1 n B 1 s 21 p 62 s 22 p 63 s 23 p 64 s 2 1 n C 1 s 22 p 63 s 23 p 63 d 8 1 n D 1 s 22 p 63 s 23 p 64 s 24 d 6 1 n
Review Which best represents the electron configuration for an atom of iron? n A 1 s 22 p 63 s 23 p 64 s 23 d 6 1 n B 1 s 21 p 62 s 22 p 63 s 23 p 64 s 2 1 n C 1 s 22 p 63 s 23 p 63 d 8 1 n D 1 s 22 p 63 s 23 p 64 s 24 d 6 1 n
 Review n How many formula units of calcium carbonate are in 200. 0 grams of calcium carbonate?
Review n How many formula units of calcium carbonate are in 200. 0 grams of calcium carbonate?
 Review n How many molecules of water are in 200. 0 grams of water?
Review n How many molecules of water are in 200. 0 grams of water?
 Review What color of light is produced if an electron falls from n = 3 to n = 2 in a hydrogen atom? a) blue b) green c) violet d) red n
Review What color of light is produced if an electron falls from n = 3 to n = 2 in a hydrogen atom? a) blue b) green c) violet d) red n
 Which combination of elements will most likely form an ionic compound? n hydrogen and oxygen n carbon and chlorine n sodium and fluorine n silicon and sulfur n
Which combination of elements will most likely form an ionic compound? n hydrogen and oxygen n carbon and chlorine n sodium and fluorine n silicon and sulfur n
 n What is the frequency of violet light with wavelength of 4. 1 X 10 -7 meters? C=
n What is the frequency of violet light with wavelength of 4. 1 X 10 -7 meters? C=
 Review n Which of the following elements has the highest electronegativity? 1) I, Br, Cl, F 2) K, Mn, Ga, Se
Review n Which of the following elements has the highest electronegativity? 1) I, Br, Cl, F 2) K, Mn, Ga, Se
 Review n What is the boiling point of water in kelvin?
Review n What is the boiling point of water in kelvin?
 Review Which statement correctly compares an atom of boron-11 and an atom of carbon-14? n A An atom of boron-11 has one fewer proton and two fewer neutrons than an atom of carbon-14. n B An atom of boron-11 has one fewer neutron and two fewer protons than an atom of carbon-14. n C An atom of boron-11 has one fewer proton and three fewer neutrons than an atom of carbon-14. n D An atom of boron-11 has one fewer neutron and three fewer protons than an atom of carbon-14 n
Review Which statement correctly compares an atom of boron-11 and an atom of carbon-14? n A An atom of boron-11 has one fewer proton and two fewer neutrons than an atom of carbon-14. n B An atom of boron-11 has one fewer neutron and two fewer protons than an atom of carbon-14. n C An atom of boron-11 has one fewer proton and three fewer neutrons than an atom of carbon-14. n D An atom of boron-11 has one fewer neutron and three fewer protons than an atom of carbon-14 n
 Review When aluminum and sulfur react, which compound is produced? n A Al 2 S 3 n B Al 3 S 2 n C Al. S 2 n D Al. S n
Review When aluminum and sulfur react, which compound is produced? n A Al 2 S 3 n B Al 3 S 2 n C Al. S 2 n D Al. S n
 An atom of which element has the strongest attraction for electrons? n A Ba n B Cs n. CO n. DF n
An atom of which element has the strongest attraction for electrons? n A Ba n B Cs n. CO n. DF n
 Review Which pair of elements is both malleable and able to conduct heat? A bromine and silver B iodine and neon C iron and bromine D silver and iron n
Review Which pair of elements is both malleable and able to conduct heat? A bromine and silver B iodine and neon C iron and bromine D silver and iron n
 Review Which group includes elements with the most similar properties? A) N, O, and F B) O, S, and Se C) Cr, Pb, and Xe D) Br, Ga, and Hg n
Review Which group includes elements with the most similar properties? A) N, O, and F B) O, S, and Se C) Cr, Pb, and Xe D) Br, Ga, and Hg n
 Review n Why does it require 5, 511 J of heat energy to melt 16. 5 g of ice? A) 2, 260 J/g of heat energy is absorbed by the ice as it is converted from a solid to a liquid. B) 334 J/g of heat energy is absorbed by the ice as it is converted from a solid to a liquid. C) 4. 18 J/g°C of heat energy is required as ice is converted from a solid to a liquid. D) 2. 05 J/g°C of heat energy is required as ice is converted from a solid to a liquid.
Review n Why does it require 5, 511 J of heat energy to melt 16. 5 g of ice? A) 2, 260 J/g of heat energy is absorbed by the ice as it is converted from a solid to a liquid. B) 334 J/g of heat energy is absorbed by the ice as it is converted from a solid to a liquid. C) 4. 18 J/g°C of heat energy is required as ice is converted from a solid to a liquid. D) 2. 05 J/g°C of heat energy is required as ice is converted from a solid to a liquid.
 Review What is the IUPAC name for the chemical formula Pb. O 2? n A lead oxide n B lead(II) oxide n C lead(IV) oxide n D lead dioxide n
Review What is the IUPAC name for the chemical formula Pb. O 2? n A lead oxide n B lead(II) oxide n C lead(IV) oxide n D lead dioxide n
 Which is true about the melting points of ionic and molecular compounds? A The melting points of ionic and molecular compounds are similar. B The melting points of ionic compounds are lower than the melting points of molecular compounds. C The melting points of ionic and molecular compounds increase with the number of atoms present in the compound. D The melting points of ionic compounds are higher than the melting points of molecular compounds. n
Which is true about the melting points of ionic and molecular compounds? A The melting points of ionic and molecular compounds are similar. B The melting points of ionic compounds are lower than the melting points of molecular compounds. C The melting points of ionic and molecular compounds increase with the number of atoms present in the compound. D The melting points of ionic compounds are higher than the melting points of molecular compounds. n
 Review How many moles of nitrogen gas are in 135 L of nitrogen gas at Standard Temperature and Pressure (STP)? A 4. 82 moles of N 2 B 5. 53 moles of N 2 C 6. 02 moles of N 2 D 9. 64 moles of N 2
Review How many moles of nitrogen gas are in 135 L of nitrogen gas at Standard Temperature and Pressure (STP)? A 4. 82 moles of N 2 B 5. 53 moles of N 2 C 6. 02 moles of N 2 D 9. 64 moles of N 2
 A mixture of gases (NO 2, CO 2, SO 2) is collected in a bottle. The partial pressure of NO 2 is 1. 25 atm, and the partial pressure of CO 2 is 2. 63 atm. If the total pressure of the gases is 11. 20 atm, what is the partial pressure of SO 2? A 2. 89 atm B 7. 32 atm C 9. 23 atm D 11. 20 atm
A mixture of gases (NO 2, CO 2, SO 2) is collected in a bottle. The partial pressure of NO 2 is 1. 25 atm, and the partial pressure of CO 2 is 2. 63 atm. If the total pressure of the gases is 11. 20 atm, what is the partial pressure of SO 2? A 2. 89 atm B 7. 32 atm C 9. 23 atm D 11. 20 atm
 Review When Ag. NO 3 (aq) is mixed with Na. Cl (aq), ( ( which type of reaction will occur? A single replacement B synthesis C decomposition D double replacement
Review When Ag. NO 3 (aq) is mixed with Na. Cl (aq), ( ( which type of reaction will occur? A single replacement B synthesis C decomposition D double replacement
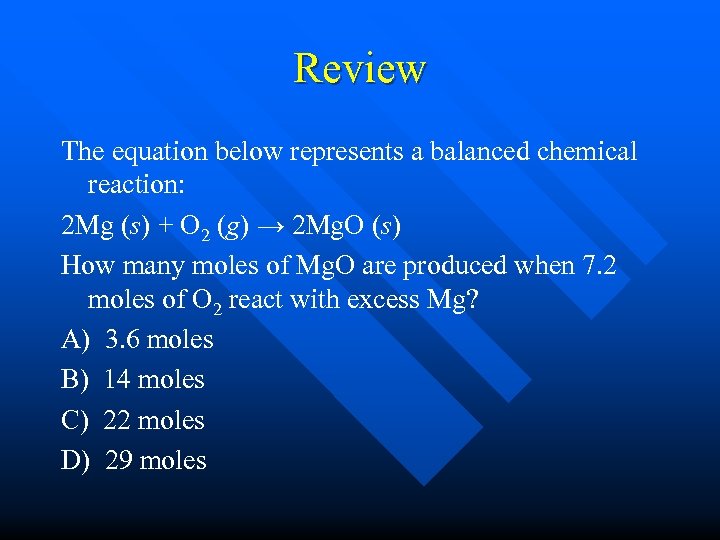 Review The equation below represents a balanced chemical reaction: 2 Mg (s) + O 2 (g) → 2 Mg. O (s) How many moles of Mg. O are produced when 7. 2 moles of O 2 react with excess Mg? A) 3. 6 moles B) 14 moles C) 22 moles D) 29 moles
Review The equation below represents a balanced chemical reaction: 2 Mg (s) + O 2 (g) → 2 Mg. O (s) How many moles of Mg. O are produced when 7. 2 moles of O 2 react with excess Mg? A) 3. 6 moles B) 14 moles C) 22 moles D) 29 moles


