55e80f315020a3fac2482eddfaa5c454.ppt
- Количество слайдов: 42
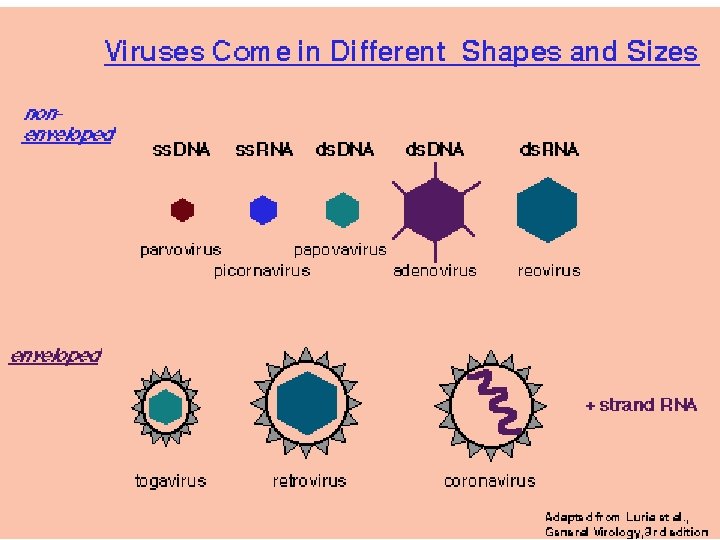
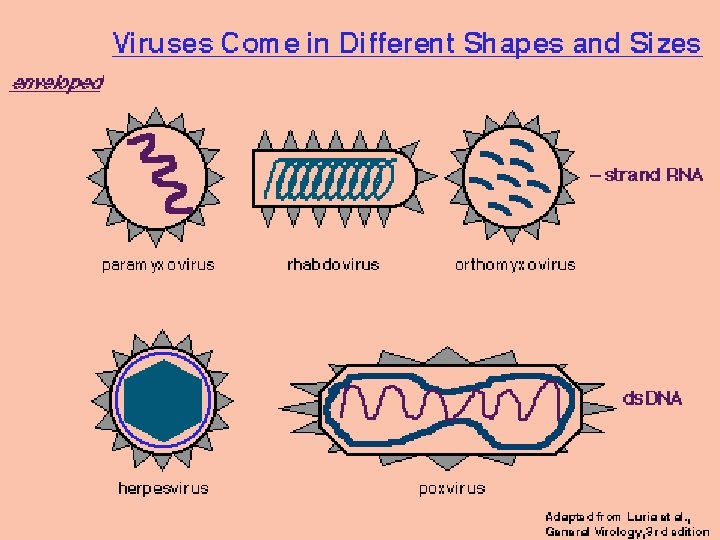
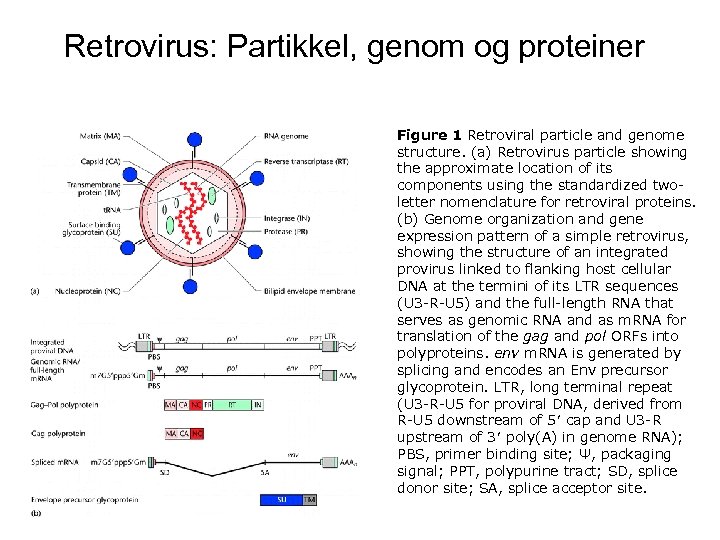 Retrovirus: Partikkel, genom og proteiner Figure 1 Retroviral particle and genome structure. (a) Retrovirus particle showing the approximate location of its components using the standardized twoletter nomenclature for retroviral proteins. (b) Genome organization and gene expression pattern of a simple retrovirus, showing the structure of an integrated provirus linked to flanking host cellular DNA at the termini of its LTR sequences (U 3 -R-U 5) and the full-length RNA that serves as genomic RNA and as m. RNA for translation of the gag and pol ORFs into polyproteins. env m. RNA is generated by splicing and encodes an Env precursor glycoprotein. LTR, long terminal repeat (U 3 -R-U 5 for proviral DNA, derived from R-U 5 downstream of 5′ cap and U 3 -R upstream of 3′ poly(A) in genome RNA); PBS, primer binding site; Ψ, packaging signal; PPT, polypurine tract; SD, splice donor site; SA, splice acceptor site.
Retrovirus: Partikkel, genom og proteiner Figure 1 Retroviral particle and genome structure. (a) Retrovirus particle showing the approximate location of its components using the standardized twoletter nomenclature for retroviral proteins. (b) Genome organization and gene expression pattern of a simple retrovirus, showing the structure of an integrated provirus linked to flanking host cellular DNA at the termini of its LTR sequences (U 3 -R-U 5) and the full-length RNA that serves as genomic RNA and as m. RNA for translation of the gag and pol ORFs into polyproteins. env m. RNA is generated by splicing and encodes an Env precursor glycoprotein. LTR, long terminal repeat (U 3 -R-U 5 for proviral DNA, derived from R-U 5 downstream of 5′ cap and U 3 -R upstream of 3′ poly(A) in genome RNA); PBS, primer binding site; Ψ, packaging signal; PPT, polypurine tract; SD, splice donor site; SA, splice acceptor site.
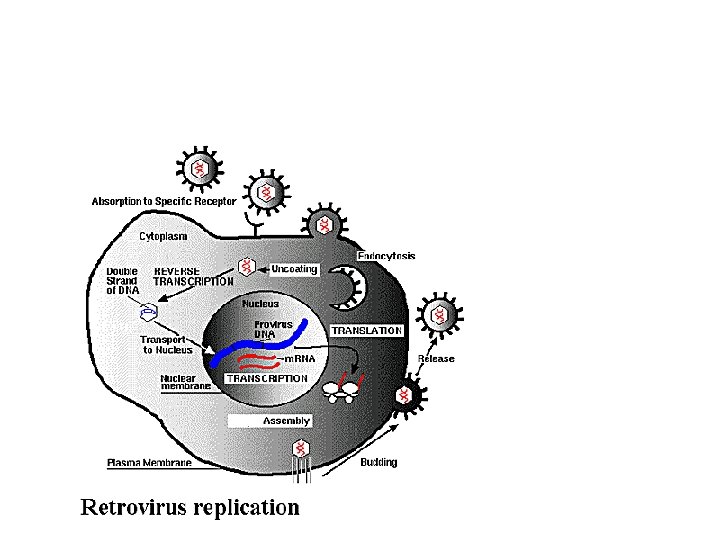
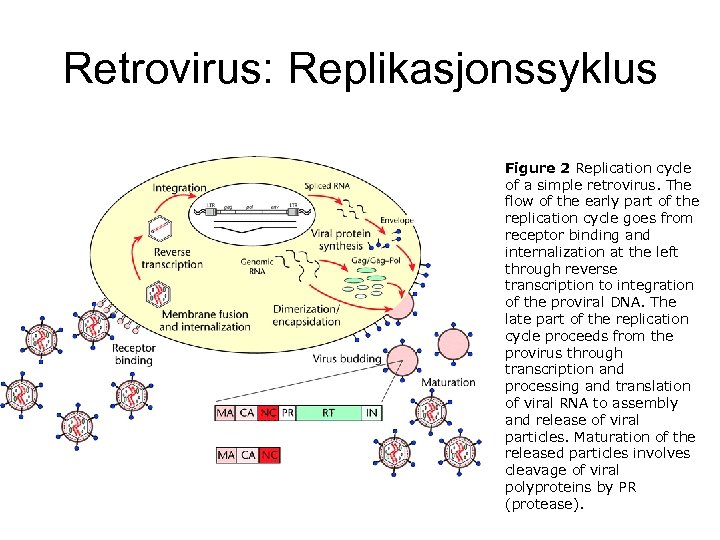 Retrovirus: Replikasjonssyklus Figure 2 Replication cycle of a simple retrovirus. The flow of the early part of the replication cycle goes from receptor binding and internalization at the left through reverse transcription to integration of the proviral DNA. The late part of the replication cycle proceeds from the provirus through transcription and processing and translation of viral RNA to assembly and release of viral particles. Maturation of the released particles involves cleavage of viral polyproteins by PR (protease).
Retrovirus: Replikasjonssyklus Figure 2 Replication cycle of a simple retrovirus. The flow of the early part of the replication cycle goes from receptor binding and internalization at the left through reverse transcription to integration of the proviral DNA. The late part of the replication cycle proceeds from the provirus through transcription and processing and translation of viral RNA to assembly and release of viral particles. Maturation of the released particles involves cleavage of viral polyproteins by PR (protease).
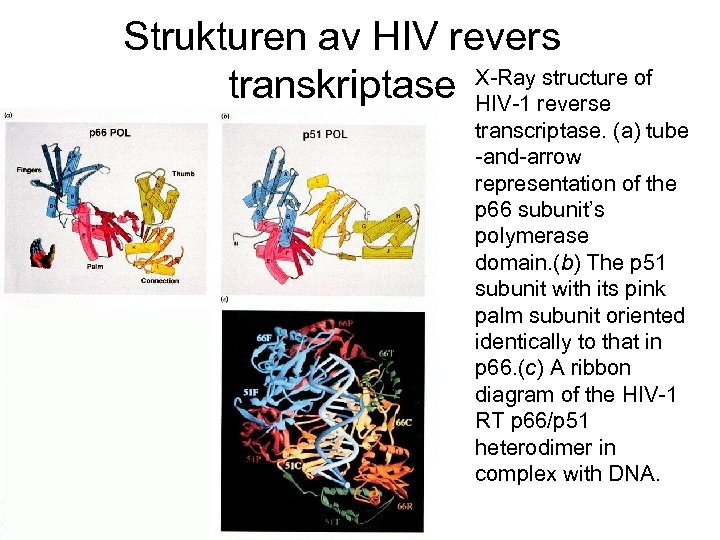 Strukturen av HIV revers structure transkriptase X-Rayreverse of HIV-1 transcriptase. (a) tube -and-arrow representation of the p 66 subunit’s polymerase domain. (b) The p 51 subunit with its pink palm subunit oriented identically to that in p 66. (c) A ribbon diagram of the HIV-1 RT p 66/p 51 heterodimer in complex with DNA.
Strukturen av HIV revers structure transkriptase X-Rayreverse of HIV-1 transcriptase. (a) tube -and-arrow representation of the p 66 subunit’s polymerase domain. (b) The p 51 subunit with its pink palm subunit oriented identically to that in p 66. (c) A ribbon diagram of the HIV-1 RT p 66/p 51 heterodimer in complex with DNA.
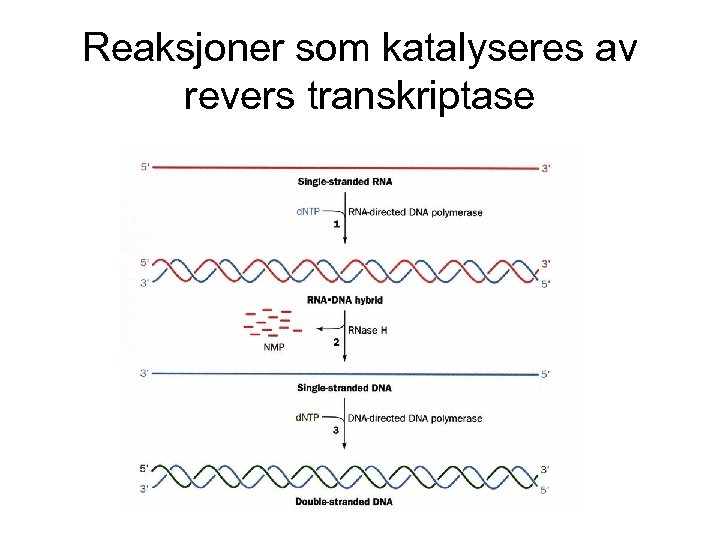 Reaksjoner som katalyseres av revers transkriptase
Reaksjoner som katalyseres av revers transkriptase
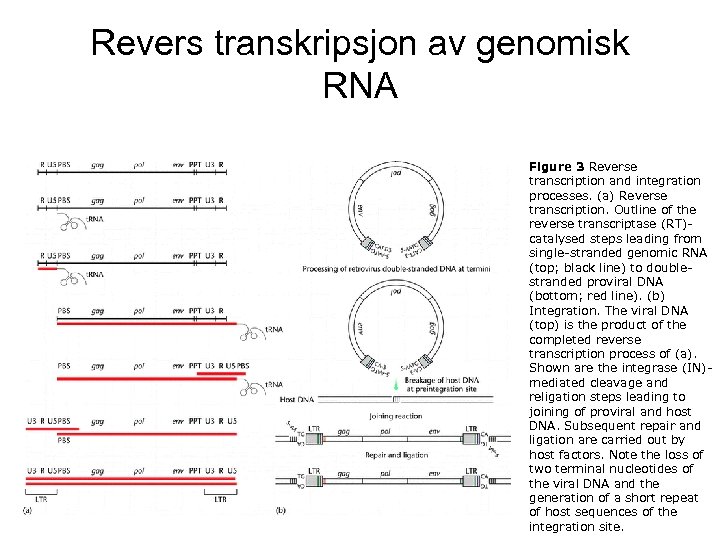 Revers transkripsjon av genomisk RNA Figure 3 Reverse transcription and integration processes. (a) Reverse transcription. Outline of the reverse transcriptase (RT)catalysed steps leading from single-stranded genomic RNA (top; black line) to doublestranded proviral DNA (bottom; red line). (b) Integration. The viral DNA (top) is the product of the completed reverse transcription process of (a). Shown are the integrase (IN)mediated cleavage and religation steps leading to joining of proviral and host DNA. Subsequent repair and ligation are carried out by host factors. Note the loss of two terminal nucleotides of the viral DNA and the generation of a short repeat of host sequences of the integration site.
Revers transkripsjon av genomisk RNA Figure 3 Reverse transcription and integration processes. (a) Reverse transcription. Outline of the reverse transcriptase (RT)catalysed steps leading from single-stranded genomic RNA (top; black line) to doublestranded proviral DNA (bottom; red line). (b) Integration. The viral DNA (top) is the product of the completed reverse transcription process of (a). Shown are the integrase (IN)mediated cleavage and religation steps leading to joining of proviral and host DNA. Subsequent repair and ligation are carried out by host factors. Note the loss of two terminal nucleotides of the viral DNA and the generation of a short repeat of host sequences of the integration site.
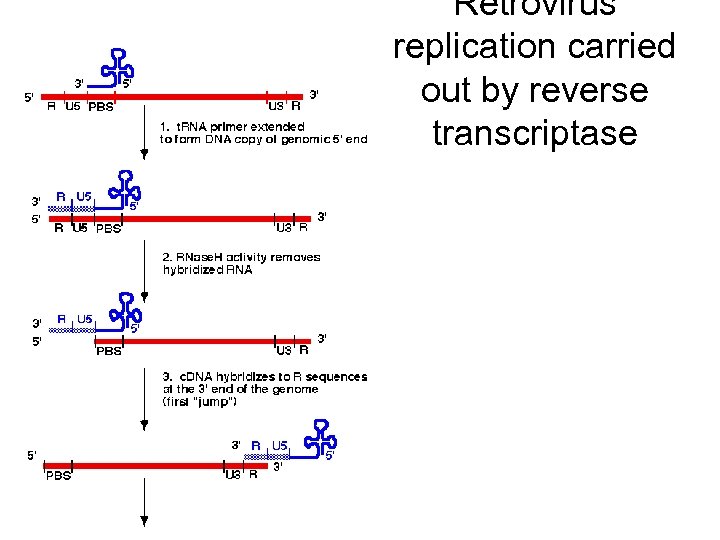 Retrovirus replication carried out by reverse transcriptase
Retrovirus replication carried out by reverse transcriptase
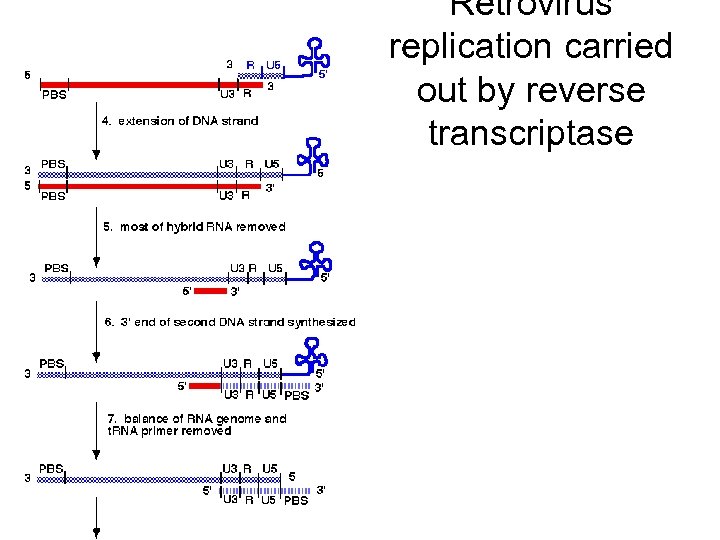 Retrovirus replication carried out by reverse transcriptase
Retrovirus replication carried out by reverse transcriptase
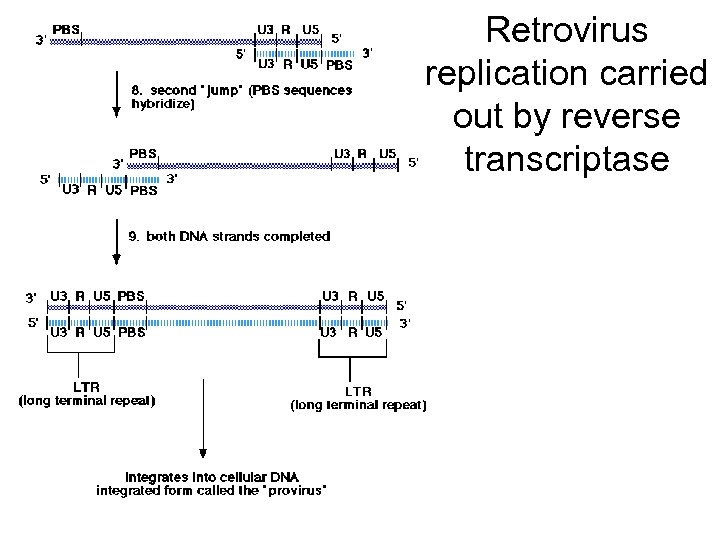 Retrovirus replication carried out by reverse transcriptase
Retrovirus replication carried out by reverse transcriptase
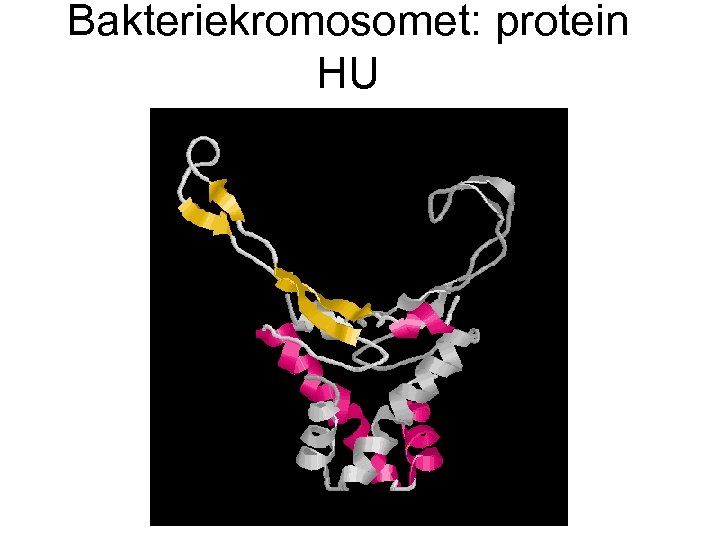 Bakteriekromosomet: protein HU
Bakteriekromosomet: protein HU
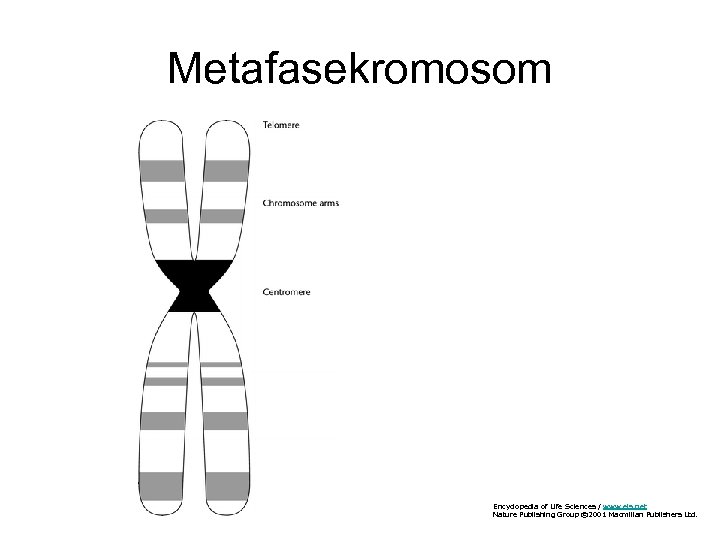 Metafasekromosom Encyclopedia of Life Sciences / www. els. net Nature Publishing Group © 2001 Macmillan Publishers Ltd.
Metafasekromosom Encyclopedia of Life Sciences / www. els. net Nature Publishing Group © 2001 Macmillan Publishers Ltd.
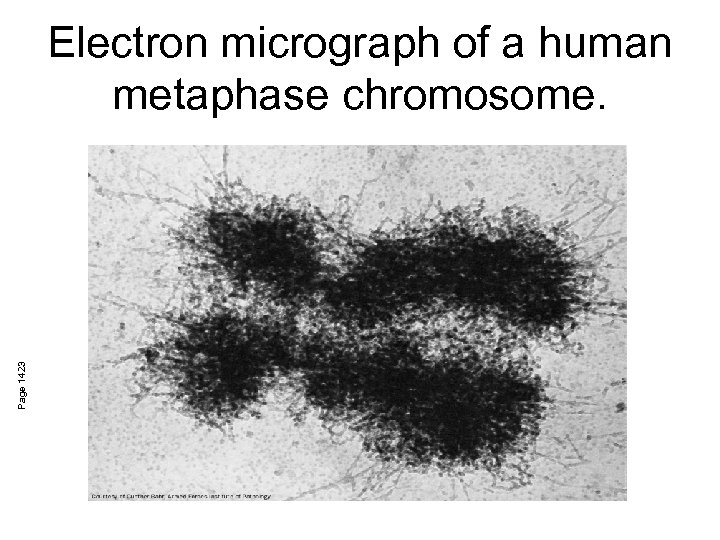 Page 1423 Electron micrograph of a human metaphase chromosome.
Page 1423 Electron micrograph of a human metaphase chromosome.
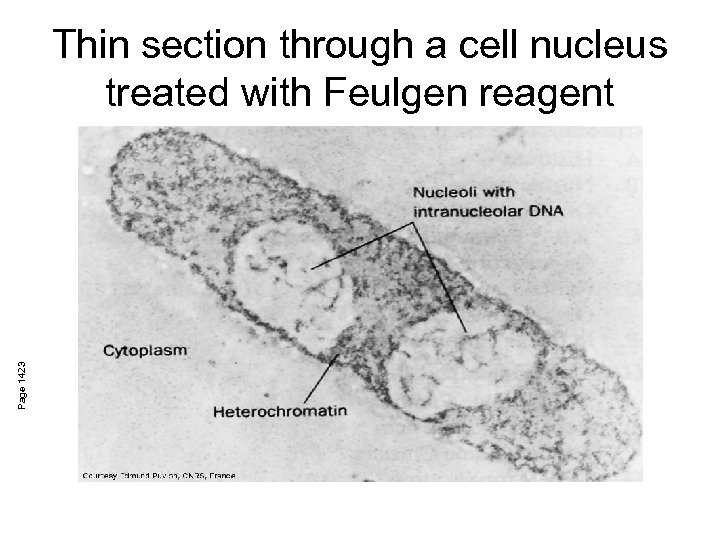 Page 1423 Thin section through a cell nucleus treated with Feulgen reagent
Page 1423 Thin section through a cell nucleus treated with Feulgen reagent
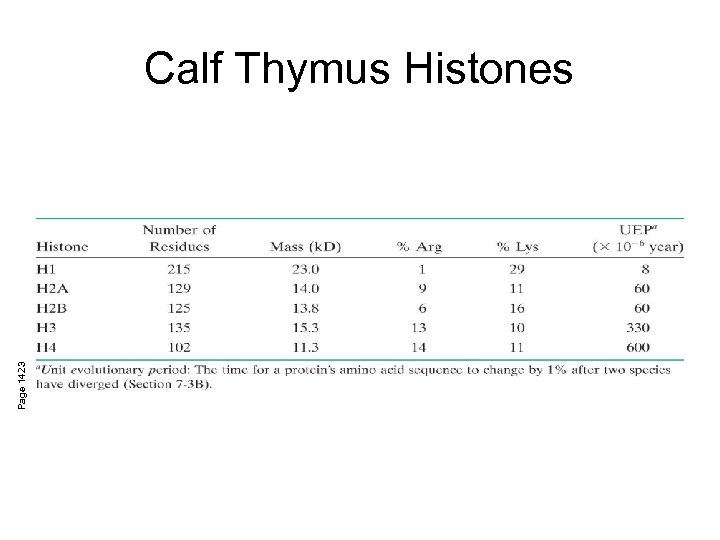 Page 1423 Calf Thymus Histones
Page 1423 Calf Thymus Histones
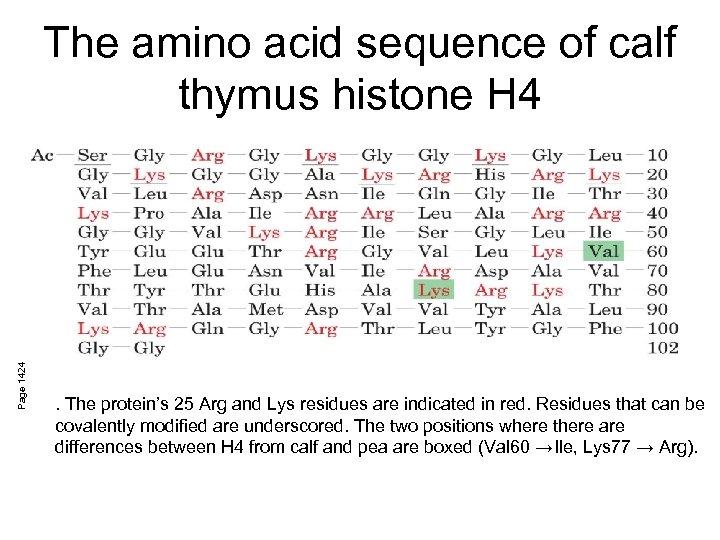 Page 1424 The amino acid sequence of calf thymus histone H 4 . The protein’s 25 Arg and Lys residues are indicated in red. Residues that can be covalently modified are underscored. The two positions where there are differences between H 4 from calf and pea are boxed (Val 60 →Ile, Lys 77 → Arg).
Page 1424 The amino acid sequence of calf thymus histone H 4 . The protein’s 25 Arg and Lys residues are indicated in red. Residues that can be covalently modified are underscored. The two positions where there are differences between H 4 from calf and pea are boxed (Val 60 →Ile, Lys 77 → Arg).
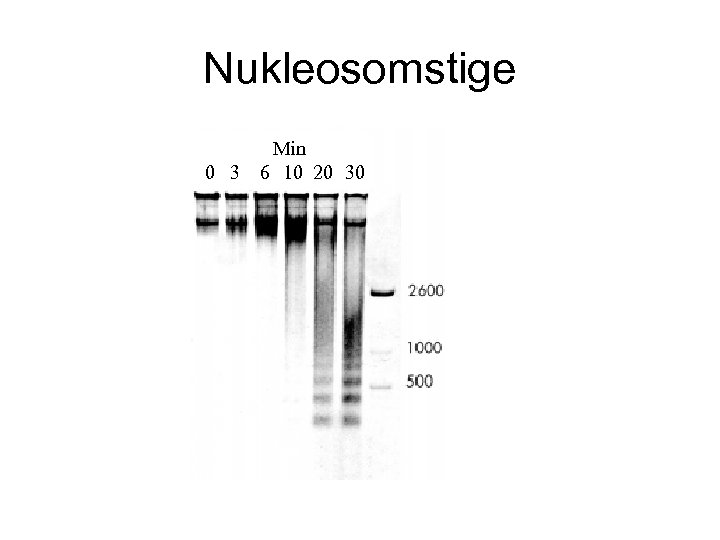 Nukleosomstige 0 3 Min 6 10 20 30
Nukleosomstige 0 3 Min 6 10 20 30
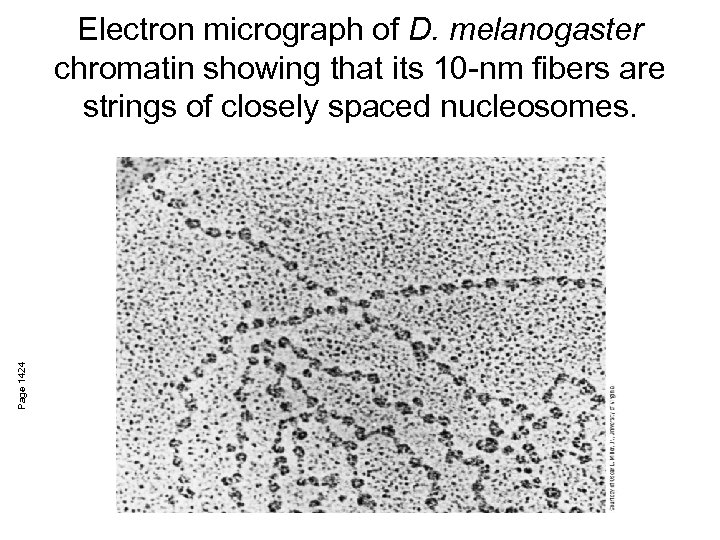 Page 1424 Electron micrograph of D. melanogaster chromatin showing that its 10 -nm fibers are strings of closely spaced nucleosomes.
Page 1424 Electron micrograph of D. melanogaster chromatin showing that its 10 -nm fibers are strings of closely spaced nucleosomes.
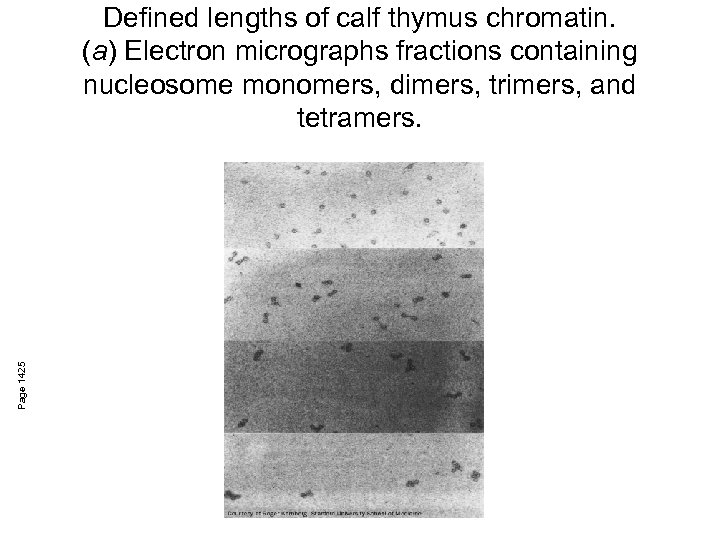 Page 1425 Defined lengths of calf thymus chromatin. (a) Electron micrographs fractions containing nucleosome monomers, dimers, trimers, and tetramers.
Page 1425 Defined lengths of calf thymus chromatin. (a) Electron micrographs fractions containing nucleosome monomers, dimers, trimers, and tetramers.
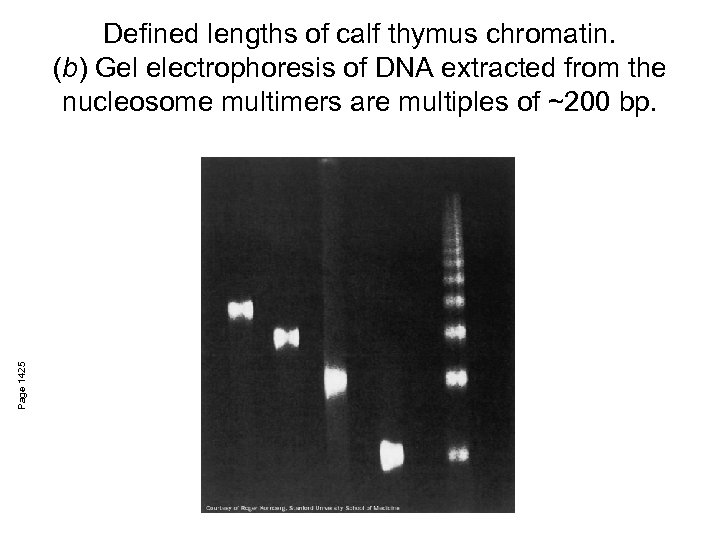 Page 1425 Defined lengths of calf thymus chromatin. (b) Gel electrophoresis of DNA extracted from the nucleosome multimers are multiples of ~200 bp.
Page 1425 Defined lengths of calf thymus chromatin. (b) Gel electrophoresis of DNA extracted from the nucleosome multimers are multiples of ~200 bp.
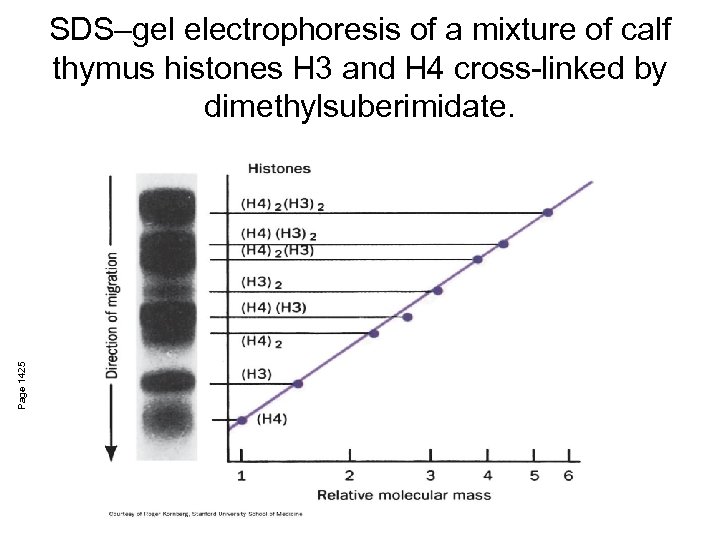 Page 1425 SDS–gel electrophoresis of a mixture of calf thymus histones H 3 and H 4 cross-linked by dimethylsuberimidate.
Page 1425 SDS–gel electrophoresis of a mixture of calf thymus histones H 3 and H 4 cross-linked by dimethylsuberimidate.
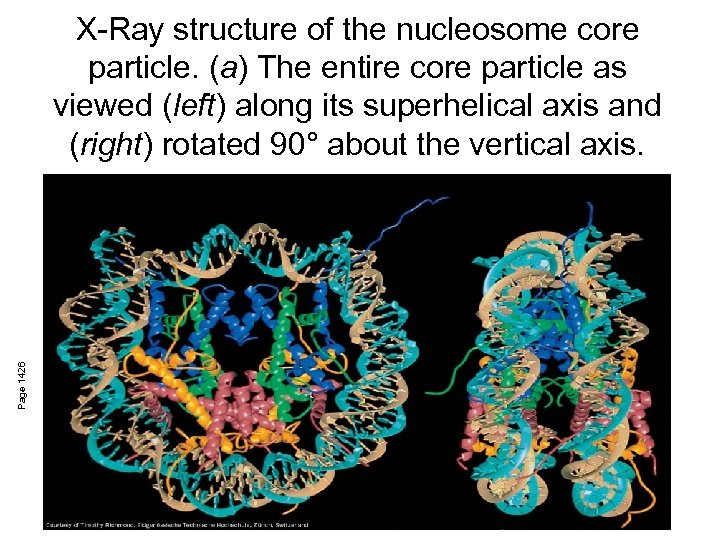 Page 1426 X-Ray structure of the nucleosome core particle. (a) The entire core particle as viewed (left) along its superhelical axis and (right) rotated 90° about the vertical axis.
Page 1426 X-Ray structure of the nucleosome core particle. (a) The entire core particle as viewed (left) along its superhelical axis and (right) rotated 90° about the vertical axis.
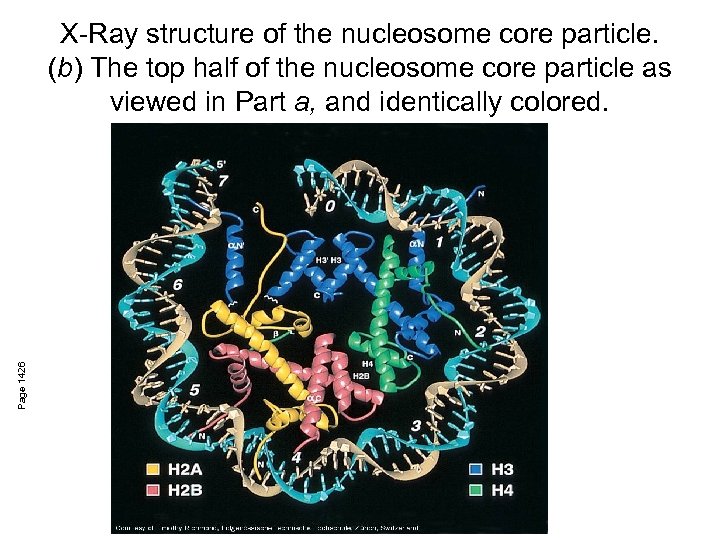 Page 1426 X-Ray structure of the nucleosome core particle. (b) The top half of the nucleosome core particle as viewed in Part a, and identically colored.
Page 1426 X-Ray structure of the nucleosome core particle. (b) The top half of the nucleosome core particle as viewed in Part a, and identically colored.
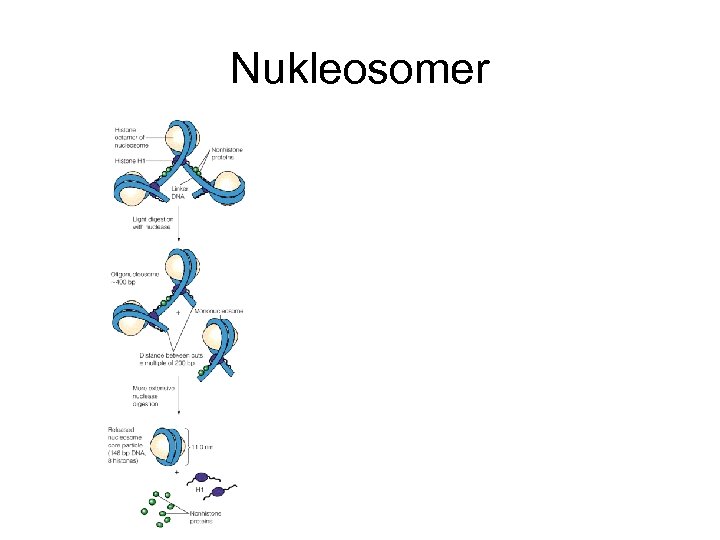 Nukleosomer
Nukleosomer
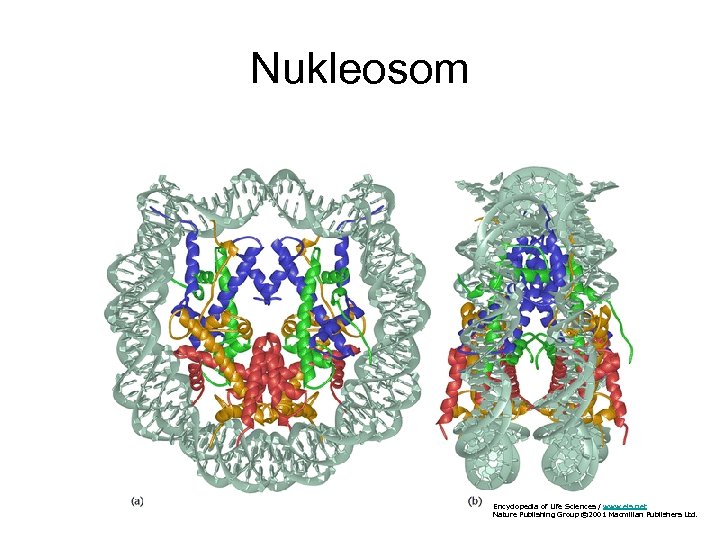 Nukleosom Encyclopedia of Life Sciences / www. els. net Nature Publishing Group © 2001 Macmillan Publishers Ltd.
Nukleosom Encyclopedia of Life Sciences / www. els. net Nature Publishing Group © 2001 Macmillan Publishers Ltd.
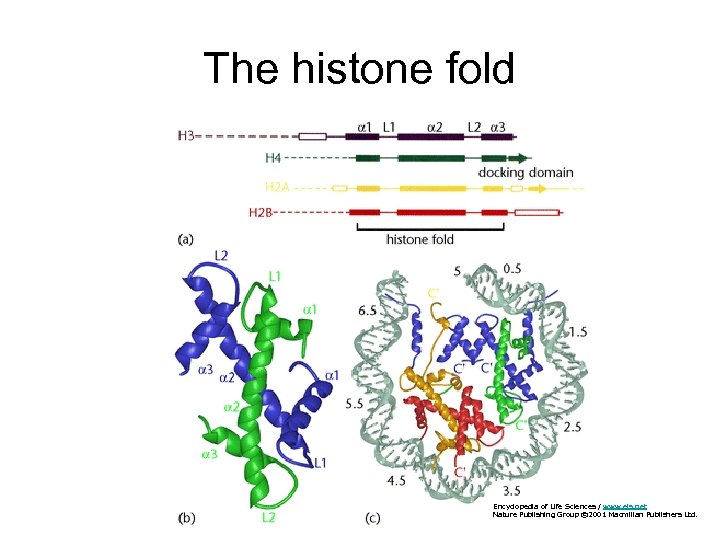 The histone fold Encyclopedia of Life Sciences / www. els. net Nature Publishing Group © 2001 Macmillan Publishers Ltd.
The histone fold Encyclopedia of Life Sciences / www. els. net Nature Publishing Group © 2001 Macmillan Publishers Ltd.
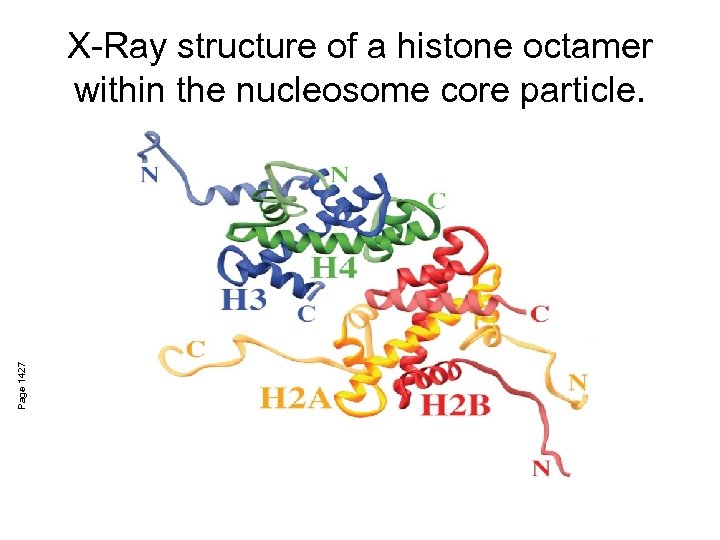 Page 1427 X-Ray structure of a histone octamer within the nucleosome core particle.
Page 1427 X-Ray structure of a histone octamer within the nucleosome core particle.
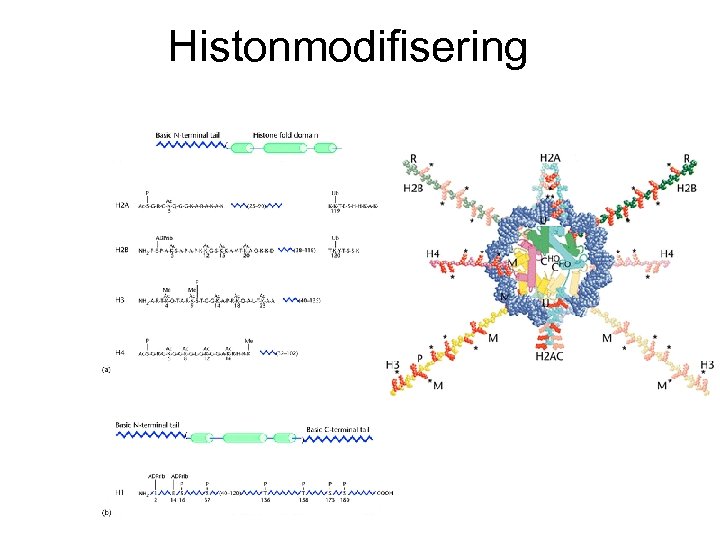 Histonmodifisering
Histonmodifisering
 Nukleosom og histonhaler Encyclopedia of Life Sciences / www. els. net Nature Publishing Group © 2001 Macmillan Publishers Ltd.
Nukleosom og histonhaler Encyclopedia of Life Sciences / www. els. net Nature Publishing Group © 2001 Macmillan Publishers Ltd.
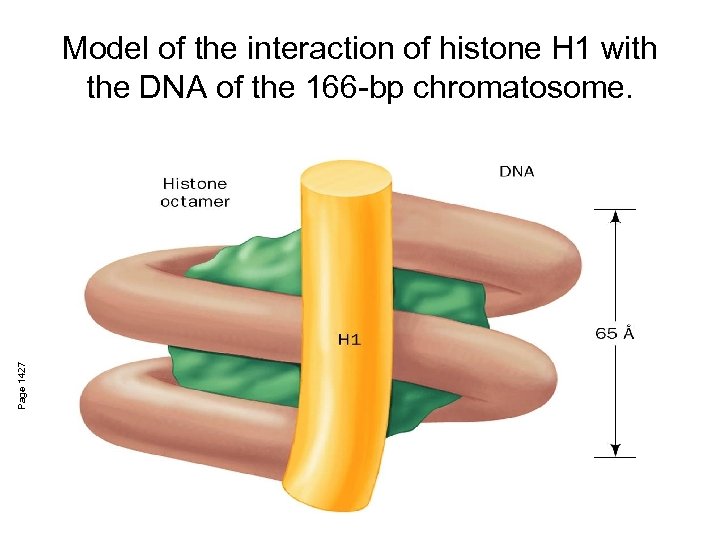 Page 1427 Model of the interaction of histone H 1 with the DNA of the 166 -bp chromatosome.
Page 1427 Model of the interaction of histone H 1 with the DNA of the 166 -bp chromatosome.
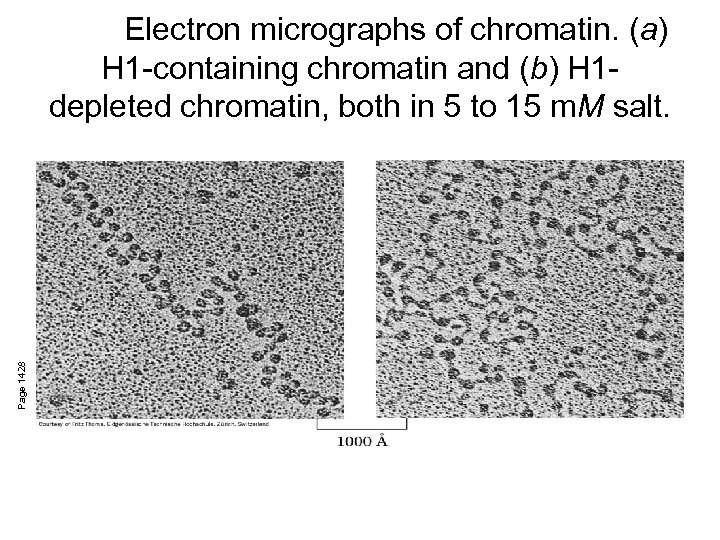 Page 1428 Electron micrographs of chromatin. (a) H 1 -containing chromatin and (b) H 1 depleted chromatin, both in 5 to 15 m. M salt.
Page 1428 Electron micrographs of chromatin. (a) H 1 -containing chromatin and (b) H 1 depleted chromatin, both in 5 to 15 m. M salt.
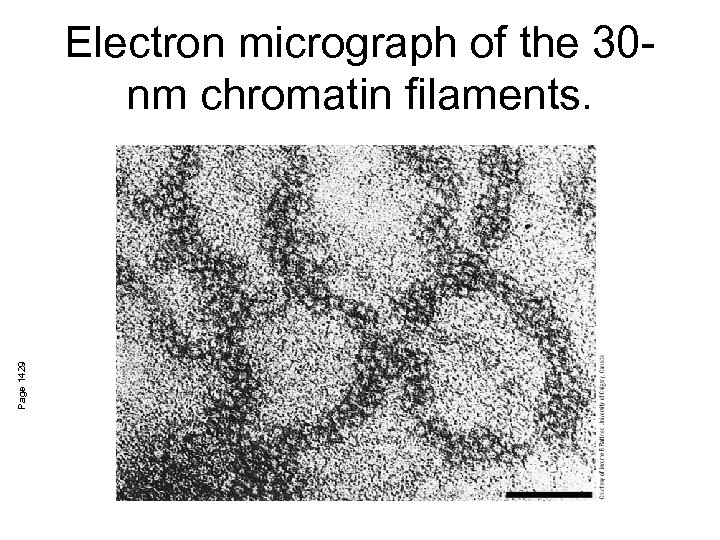 Page 1429 Electron micrograph of the 30 nm chromatin filaments.
Page 1429 Electron micrograph of the 30 nm chromatin filaments.
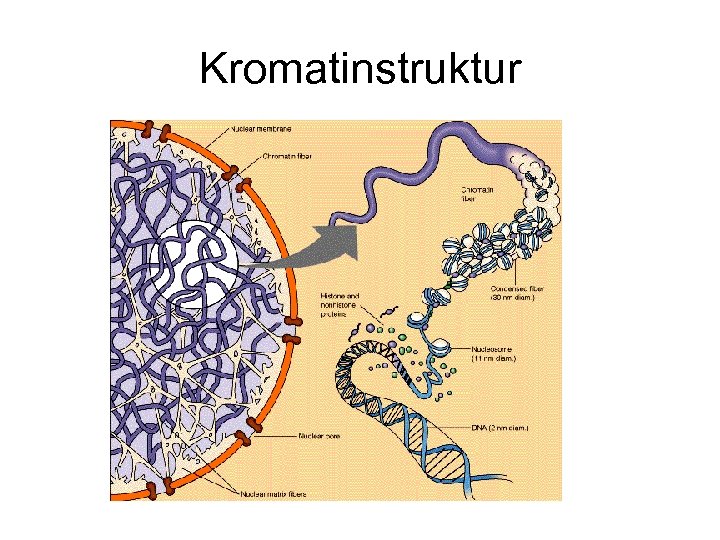 Kromatinstruktur
Kromatinstruktur
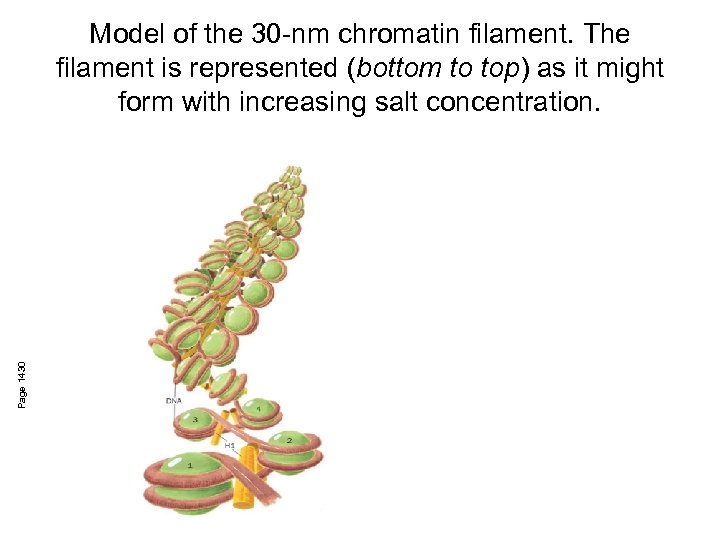 Page 1430 Model of the 30 -nm chromatin filament. The filament is represented (bottom to top) as it might form with increasing salt concentration.
Page 1430 Model of the 30 -nm chromatin filament. The filament is represented (bottom to top) as it might form with increasing salt concentration.
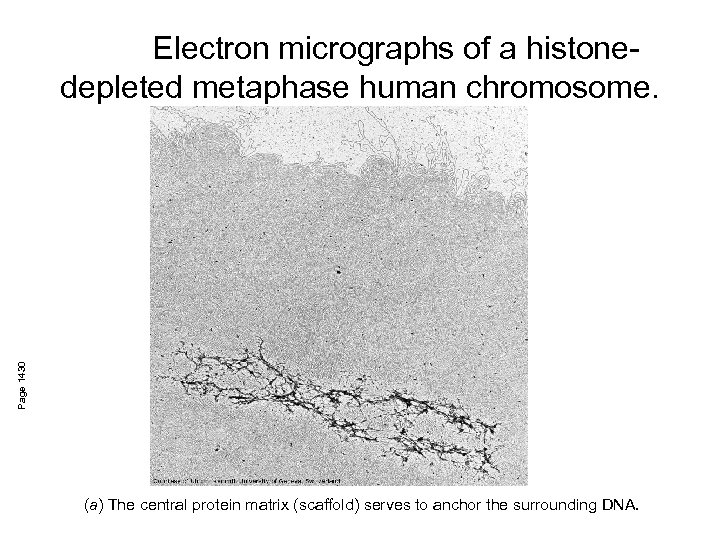 Page 1430 Electron micrographs of a histonedepleted metaphase human chromosome. (a) The central protein matrix (scaffold) serves to anchor the surrounding DNA.
Page 1430 Electron micrographs of a histonedepleted metaphase human chromosome. (a) The central protein matrix (scaffold) serves to anchor the surrounding DNA.
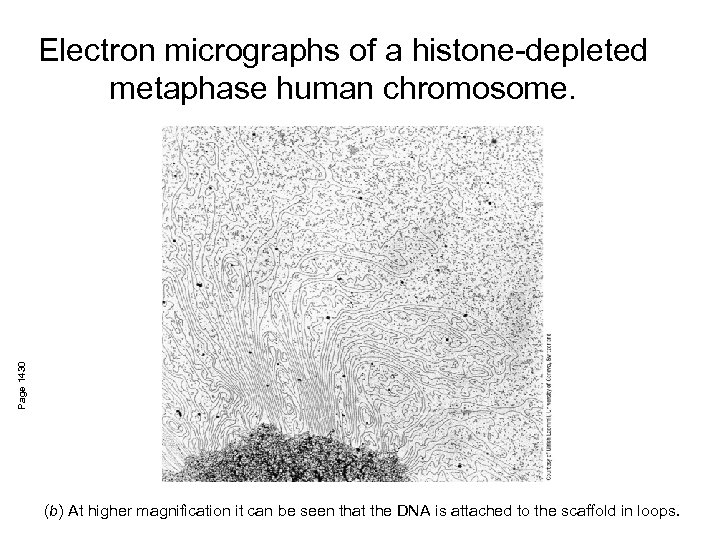 Page 1430 Electron micrographs of a histone-depleted metaphase human chromosome. (b) At higher magnification it can be seen that the DNA is attached to the scaffold in loops.
Page 1430 Electron micrographs of a histone-depleted metaphase human chromosome. (b) At higher magnification it can be seen that the DNA is attached to the scaffold in loops.
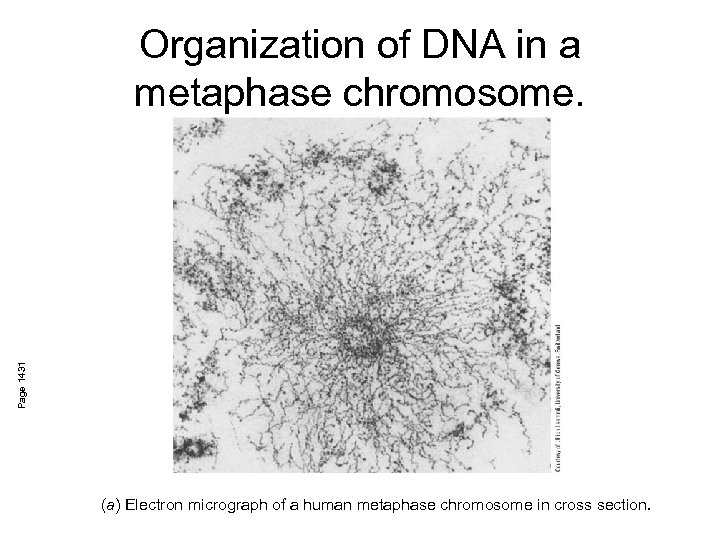 Page 1431 Organization of DNA in a metaphase chromosome. (a) Electron micrograph of a human metaphase chromosome in cross section.
Page 1431 Organization of DNA in a metaphase chromosome. (a) Electron micrograph of a human metaphase chromosome in cross section.
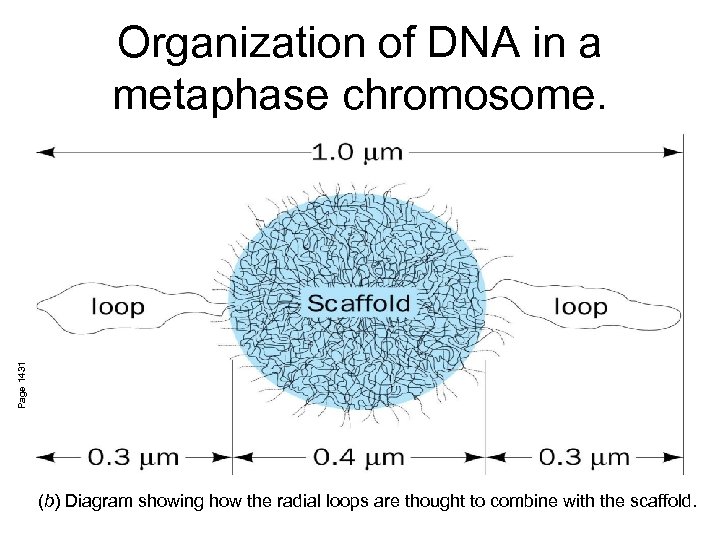 Page 1431 Organization of DNA in a metaphase chromosome. (b) Diagram showing how the radial loops are thought to combine with the scaffold.
Page 1431 Organization of DNA in a metaphase chromosome. (b) Diagram showing how the radial loops are thought to combine with the scaffold.
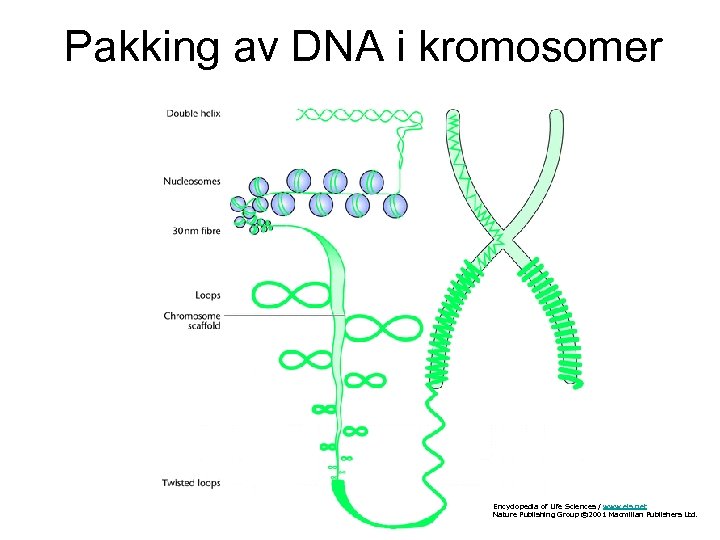 Pakking av DNA i kromosomer Encyclopedia of Life Sciences / www. els. net Nature Publishing Group © 2001 Macmillan Publishers Ltd.
Pakking av DNA i kromosomer Encyclopedia of Life Sciences / www. els. net Nature Publishing Group © 2001 Macmillan Publishers Ltd.
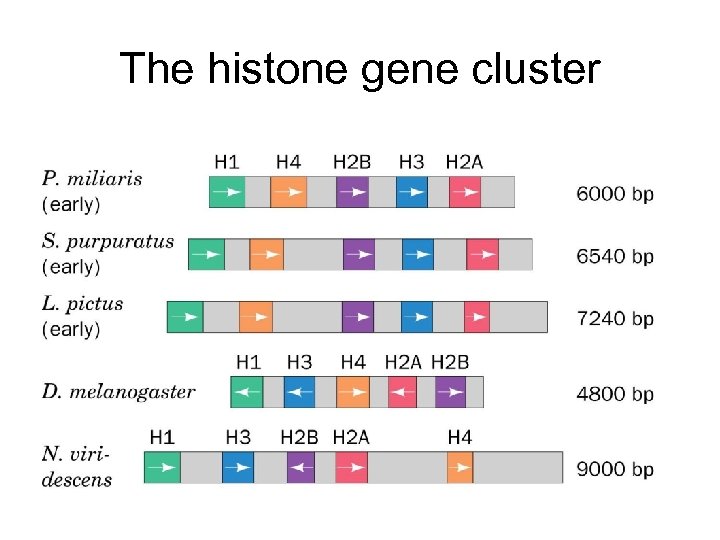 The histone gene cluster
The histone gene cluster
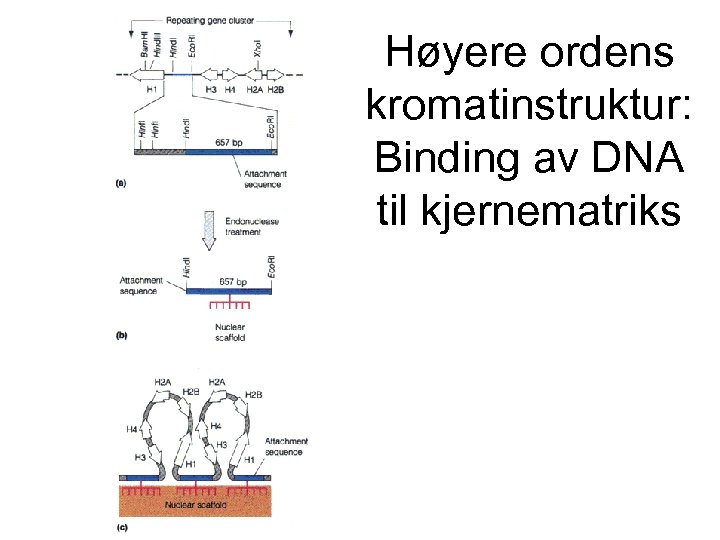 Høyere ordens kromatinstruktur: Binding av DNA til kjernematriks
Høyere ordens kromatinstruktur: Binding av DNA til kjernematriks


