8554ce35c5e30c7da8431b732fc38bfd.ppt
- Количество слайдов: 19
 Retooling Task Force
Retooling Task Force
 Checklist of Key Actions for Diagnostics Liquid Media for Culture and Drug Susceptibility Testing • Generic checklist for TB Diagnostics developed by Retooling Task Force adapted specifically for LMC-DST • Based on expert opinion, further refinement was made • Checklist was sent to 27 NTP managers with copies to WHO regional representatives soliciting their feedback and comments • Refined checklist finalized, to be widely disseminated Similar checklists for each upcoming new technology will be developed as they become available
Checklist of Key Actions for Diagnostics Liquid Media for Culture and Drug Susceptibility Testing • Generic checklist for TB Diagnostics developed by Retooling Task Force adapted specifically for LMC-DST • Based on expert opinion, further refinement was made • Checklist was sent to 27 NTP managers with copies to WHO regional representatives soliciting their feedback and comments • Refined checklist finalized, to be widely disseminated Similar checklists for each upcoming new technology will be developed as they become available
 Checklist of Key Actions for LMC-DST Dissemination - Global • • • • Stop TB Partnership groups Relevant WHO Depts, bodies, region/country offices Global Laboratory Initiative International organizations eg UNION, IOM Regulatory authorities Sponsoring agencies Technical agencies Professional associations National TB Control Programmes and Mo. Hs NGOs Patient community and civil society organizations Test manufacturers Test developers and evaluators Untargetted (available on RTF website)
Checklist of Key Actions for LMC-DST Dissemination - Global • • • • Stop TB Partnership groups Relevant WHO Depts, bodies, region/country offices Global Laboratory Initiative International organizations eg UNION, IOM Regulatory authorities Sponsoring agencies Technical agencies Professional associations National TB Control Programmes and Mo. Hs NGOs Patient community and civil society organizations Test manufacturers Test developers and evaluators Untargetted (available on RTF website)
 Checklist of Key Actions for LMC-DST Dissemination - National • • • • National TB Reference Laboratory NGOs Patient community and civil society organizations Test manufacturers/distributors/agents Test developers eg FIND country offices National regulatory authorities Laboratory equipment suppliers/service Professional associations Lab training institutions Procurement/equipment management services Authorities at main port of entry for country (delays) Health systems initiatives at Mo. H (eg SWAp) Untargetted (available on national website - NTP? )
Checklist of Key Actions for LMC-DST Dissemination - National • • • • National TB Reference Laboratory NGOs Patient community and civil society organizations Test manufacturers/distributors/agents Test developers eg FIND country offices National regulatory authorities Laboratory equipment suppliers/service Professional associations Lab training institutions Procurement/equipment management services Authorities at main port of entry for country (delays) Health systems initiatives at Mo. H (eg SWAp) Untargetted (available on national website - NTP? )

 Retooling Task Force
Retooling Task Force
 What should be in the diagnostics pipeline? What are the stages in the pipeline? How are candidate tests positioned along the pipeline? How is the appropriate health service delivery level or other setting specifications determined for particular candidate tests? What evidence is required at each stage to move forward?
What should be in the diagnostics pipeline? What are the stages in the pipeline? How are candidate tests positioned along the pipeline? How is the appropriate health service delivery level or other setting specifications determined for particular candidate tests? What evidence is required at each stage to move forward?
 Wide stakeholder agreement that there is a need for: • Inclusive pipeline • More, and more diverse, partners engaged in development and evaluation of new diagnostics (commercial and non-commercial) • A clearly-defined common pathway for TB diagnostics development and evaluation • Clear criteria for defining setting/HS delivery specs • Clear criteria for positioning tests in pipeline • Standards of evidence required for progression
Wide stakeholder agreement that there is a need for: • Inclusive pipeline • More, and more diverse, partners engaged in development and evaluation of new diagnostics (commercial and non-commercial) • A clearly-defined common pathway for TB diagnostics development and evaluation • Clear criteria for defining setting/HS delivery specs • Clear criteria for positioning tests in pipeline • Standards of evidence required for progression
 WG Challenges • Expanding development pipeline • Wide range of technological platforms • Increasing need for collaboration between test developers • Increasing need to consult with end-users Reorganization of Workgroup To promote efficiency in its work and create synergies with partners
WG Challenges • Expanding development pipeline • Wide range of technological platforms • Increasing need for collaboration between test developers • Increasing need to consult with end-users Reorganization of Workgroup To promote efficiency in its work and create synergies with partners
 Working Group Reorganization New constituency-based Core Group 8 Sub-Groups around technologies and relevant issues Revised Strategic Plan Work-plan for the biennium
Working Group Reorganization New constituency-based Core Group 8 Sub-Groups around technologies and relevant issues Revised Strategic Plan Work-plan for the biennium
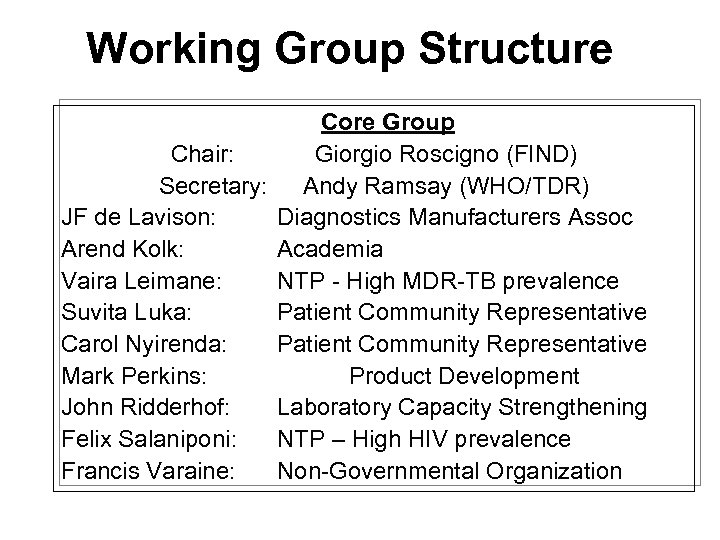 Working Group Structure Core Group Chair: Giorgio Roscigno (FIND) Secretary: Andy Ramsay (WHO/TDR) JF de Lavison: Diagnostics Manufacturers Assoc Arend Kolk: Academia Vaira Leimane: NTP - High MDR-TB prevalence Suvita Luka: Patient Community Representative Carol Nyirenda: Patient Community Representative Mark Perkins: Product Development John Ridderhof: Laboratory Capacity Strengthening Felix Salaniponi: NTP – High HIV prevalence Francis Varaine: Non-Governmental Organization
Working Group Structure Core Group Chair: Giorgio Roscigno (FIND) Secretary: Andy Ramsay (WHO/TDR) JF de Lavison: Diagnostics Manufacturers Assoc Arend Kolk: Academia Vaira Leimane: NTP - High MDR-TB prevalence Suvita Luka: Patient Community Representative Carol Nyirenda: Patient Community Representative Mark Perkins: Product Development John Ridderhof: Laboratory Capacity Strengthening Felix Salaniponi: NTP – High HIV prevalence Francis Varaine: Non-Governmental Organization
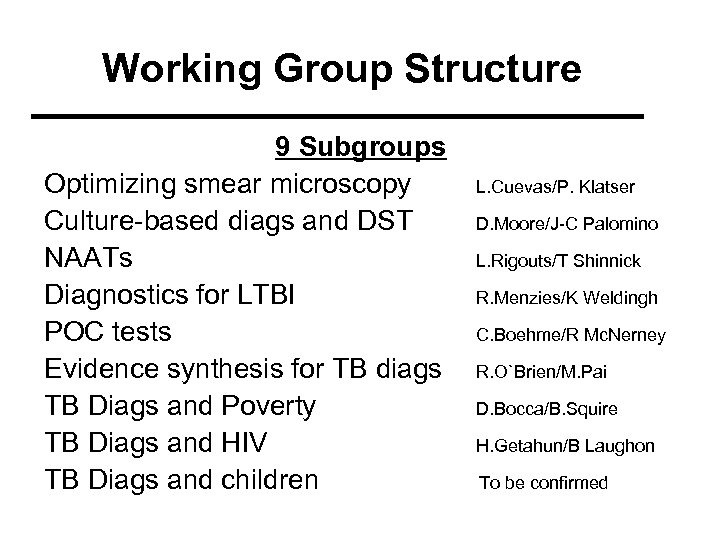 Working Group Structure 9 Subgroups Optimizing smear microscopy Culture-based diags and DST NAATs Diagnostics for LTBI POC tests Evidence synthesis for TB diags TB Diags and Poverty TB Diags and HIV TB Diags and children L. Cuevas/P. Klatser D. Moore/J-C Palomino L. Rigouts/T Shinnick R. Menzies/K Weldingh C. Boehme/R Mc. Nerney R. O`Brien/M. Pai D. Bocca/B. Squire H. Getahun/B Laughon To be confirmed
Working Group Structure 9 Subgroups Optimizing smear microscopy Culture-based diags and DST NAATs Diagnostics for LTBI POC tests Evidence synthesis for TB diags TB Diags and Poverty TB Diags and HIV TB Diags and children L. Cuevas/P. Klatser D. Moore/J-C Palomino L. Rigouts/T Shinnick R. Menzies/K Weldingh C. Boehme/R Mc. Nerney R. O`Brien/M. Pai D. Bocca/B. Squire H. Getahun/B Laughon To be confirmed
 Priorities in Work-plan • Intensify collaboration within and between WGs • To describe an inclusive pipeline • To develop criteria for describing the intended setting and use of TB diagnostics
Priorities in Work-plan • Intensify collaboration within and between WGs • To describe an inclusive pipeline • To develop criteria for describing the intended setting and use of TB diagnostics
 Priorities in Work-plan • To develop a Scientific Blueprint for TB diagnostics development and evaluation that will clearly lay out the stages in the pipeline leading to global implementation and the nature of the evidence required to move through these stages • To develop criteria to place developing technologies in the "value chain" and determine likely time-lines for availability. • To work closely with the Retooling Task Force and other partners to facilitate adoption, introduction and implementation of new and improved diagnostics
Priorities in Work-plan • To develop a Scientific Blueprint for TB diagnostics development and evaluation that will clearly lay out the stages in the pipeline leading to global implementation and the nature of the evidence required to move through these stages • To develop criteria to place developing technologies in the "value chain" and determine likely time-lines for availability. • To work closely with the Retooling Task Force and other partners to facilitate adoption, introduction and implementation of new and improved diagnostics

 Retooling Task Force
Retooling Task Force
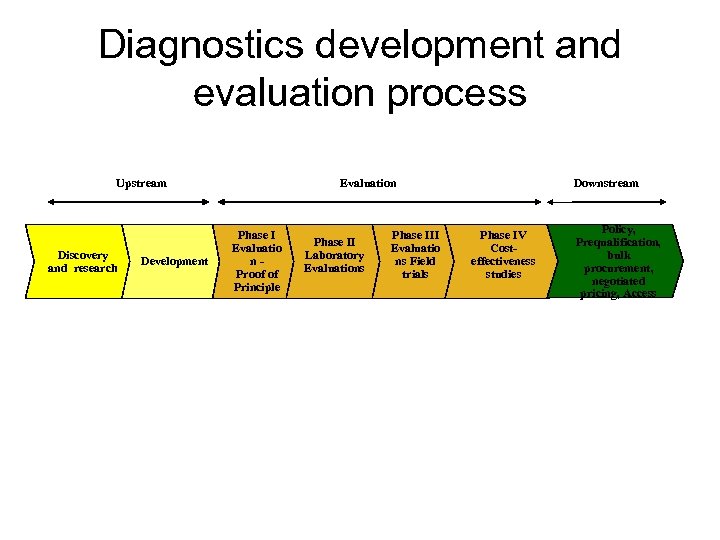 Diagnostics development and evaluation process Upstream Discovery and research Development Evaluation Phase I Evaluatio n. Proof of Principle Phase III Phase II Evaluatio Laboratory ns Field Evaluations focus FIND’s trials Downstream Phase IV Costeffectiveness studies Policy, Prequalification, bulk procurement, negotiated pricing, Access
Diagnostics development and evaluation process Upstream Discovery and research Development Evaluation Phase I Evaluatio n. Proof of Principle Phase III Phase II Evaluatio Laboratory ns Field Evaluations focus FIND’s trials Downstream Phase IV Costeffectiveness studies Policy, Prequalification, bulk procurement, negotiated pricing, Access
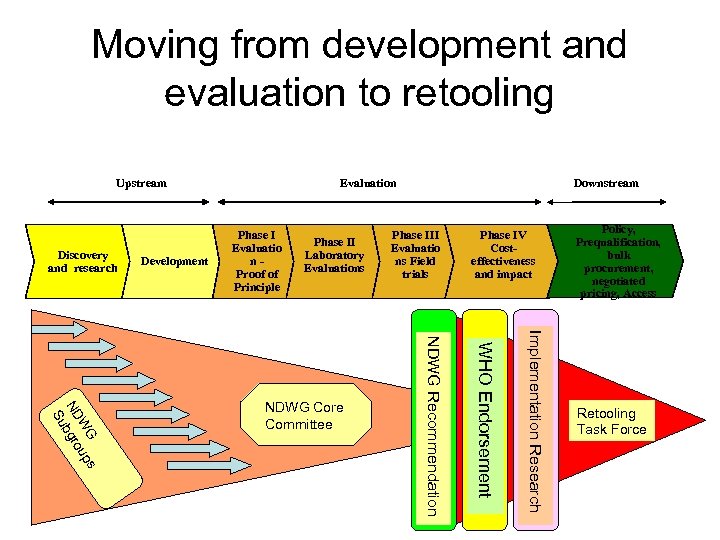 Moving from development and evaluation to retooling Upstream Discovery and research Development Evaluation Phase I Evaluatio n. Proof of Principle Downstream Phase III Phase II Evaluatio Laboratory ns Field Evaluations focus FIND’s trials Implementation Research WHO Endorsement NDWG Recommendation s G W up N D b g ro Su NDWG Core Committee Phase IV Costeffectiveness and impact Policy, Prequalification, bulk procurement, negotiated pricing, Access Retooling Task Force
Moving from development and evaluation to retooling Upstream Discovery and research Development Evaluation Phase I Evaluatio n. Proof of Principle Downstream Phase III Phase II Evaluatio Laboratory ns Field Evaluations focus FIND’s trials Implementation Research WHO Endorsement NDWG Recommendation s G W up N D b g ro Su NDWG Core Committee Phase IV Costeffectiveness and impact Policy, Prequalification, bulk procurement, negotiated pricing, Access Retooling Task Force
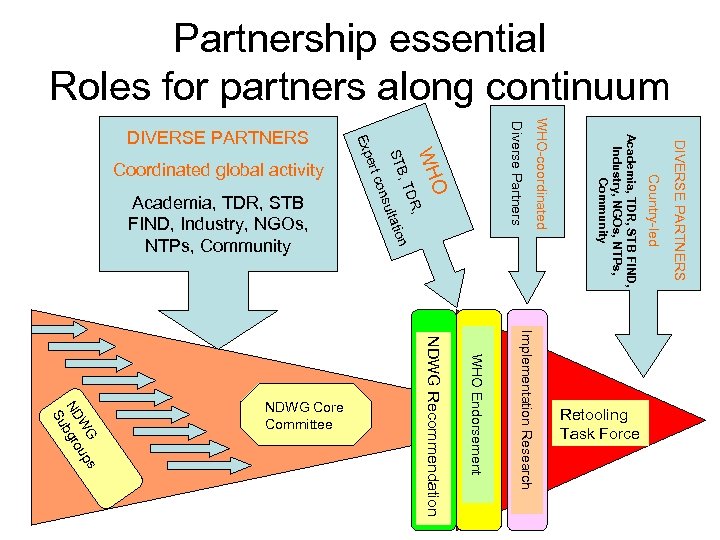 Partnership essential Roles for partners along continuum DIVERSE PARTNERS Country-led n Academia, TDR, STB FIND, Industry, NGOs, NTPs, Community WHO-coordinated at io Diverse Partners O R, sult , TD WH STB NDWG Recommendation WHO Endorsement Implementation Research Retooling Task Force s G W up N D b g ro Su NDWG Core Committee co n Academia, TDR, STB FIND, Industry, NGOs, NTPs, Community e rt Coordinated global activity Exp DIVERSE PARTNERS
Partnership essential Roles for partners along continuum DIVERSE PARTNERS Country-led n Academia, TDR, STB FIND, Industry, NGOs, NTPs, Community WHO-coordinated at io Diverse Partners O R, sult , TD WH STB NDWG Recommendation WHO Endorsement Implementation Research Retooling Task Force s G W up N D b g ro Su NDWG Core Committee co n Academia, TDR, STB FIND, Industry, NGOs, NTPs, Community e rt Coordinated global activity Exp DIVERSE PARTNERS


