52e1ea238b9669ec12a84c2b93e1a64d.ppt
- Количество слайдов: 23
 Results of ARTS-HF: finerenone versus eplerenone in patients with worsening chronic heart failure and diabetes and/or chronic kidney disease Gerasimos Filippatos Stefan D Anker, Michael Böhm, Mihai Gheorghiade, Lars Køber, Henry Krum, Aldo P Maggioni, Piotr Ponikowski, Adriaan A Voors, Faiez Zannad, So. Young Kim, Christina Nowack, Giovanni Palombo, Peter Kolkhof, Nina Kimmeskamp. Kirschbaum, Alexander Pieper and Bertram Pitt, for the Miner. Alocorticoid Receptor An. Tagonist Study In Heart Failure (ARTS-HF) Committees and Investigators
Results of ARTS-HF: finerenone versus eplerenone in patients with worsening chronic heart failure and diabetes and/or chronic kidney disease Gerasimos Filippatos Stefan D Anker, Michael Böhm, Mihai Gheorghiade, Lars Køber, Henry Krum, Aldo P Maggioni, Piotr Ponikowski, Adriaan A Voors, Faiez Zannad, So. Young Kim, Christina Nowack, Giovanni Palombo, Peter Kolkhof, Nina Kimmeskamp. Kirschbaum, Alexander Pieper and Bertram Pitt, for the Miner. Alocorticoid Receptor An. Tagonist Study In Heart Failure (ARTS-HF) Committees and Investigators
 Study sponsor and presenter conflict of interest statement • • Clinical. Trials. gov Identifier: NCT 01807221 This study was funded by Bayer Healthcare AG G Filippatos has been adviser for Bayer Health. Care AG Conflicts of interest for all other authors are listed in the full manuscript
Study sponsor and presenter conflict of interest statement • • Clinical. Trials. gov Identifier: NCT 01807221 This study was funded by Bayer Healthcare AG G Filippatos has been adviser for Bayer Health. Care AG Conflicts of interest for all other authors are listed in the full manuscript
 Background • Mineralocorticoid receptor antagonists (MRAs) such as eplerenone and spironolactone reduce mortality and hospitalizations in patients with heart failure with reduced ejection fraction (HFr. EF) and are recommended by treatment guidelines 1, 2 • Spironolactone is not specific for the mineralocorticoid receptor and eplerenone is less tightly bound to the MR than spironolactone • Underuse of MRAs may be due to fear of inducing hyperkalemia or worsening renal function in high-risk patients • Despite current treatment mortality and morbidity remains high, especially after hospitalization for worsening heart failure 1. Mc. Murray JJ et al. Eur J Heart Fail 2012; 14: 803– 69; 2. Yancy CW et al. 2013 Circulation 2013; 128: 1810– 52
Background • Mineralocorticoid receptor antagonists (MRAs) such as eplerenone and spironolactone reduce mortality and hospitalizations in patients with heart failure with reduced ejection fraction (HFr. EF) and are recommended by treatment guidelines 1, 2 • Spironolactone is not specific for the mineralocorticoid receptor and eplerenone is less tightly bound to the MR than spironolactone • Underuse of MRAs may be due to fear of inducing hyperkalemia or worsening renal function in high-risk patients • Despite current treatment mortality and morbidity remains high, especially after hospitalization for worsening heart failure 1. Mc. Murray JJ et al. Eur J Heart Fail 2012; 14: 803– 69; 2. Yancy CW et al. 2013 Circulation 2013; 128: 1810– 52
 Study objective • Finerenone (BAY 94 -8862) is a novel non-steroidal MRA that has greater receptor selectivity than spironolactone and better receptor affinity than eplerenone in vitro 1 Study objective: to compare the safety and efficacy of different once-daily oral doses of finerenone with eplerenone in patients who presented in emergency departments with worsening chronic HFr. EF with type 2 diabetes mellitus and/or chronic kidney disease (CKD) 1. Pitt B et al. Eur Heart J 2013; 34: 2453– 63
Study objective • Finerenone (BAY 94 -8862) is a novel non-steroidal MRA that has greater receptor selectivity than spironolactone and better receptor affinity than eplerenone in vitro 1 Study objective: to compare the safety and efficacy of different once-daily oral doses of finerenone with eplerenone in patients who presented in emergency departments with worsening chronic HFr. EF with type 2 diabetes mellitus and/or chronic kidney disease (CKD) 1. Pitt B et al. Eur Heart J 2013; 34: 2453– 63
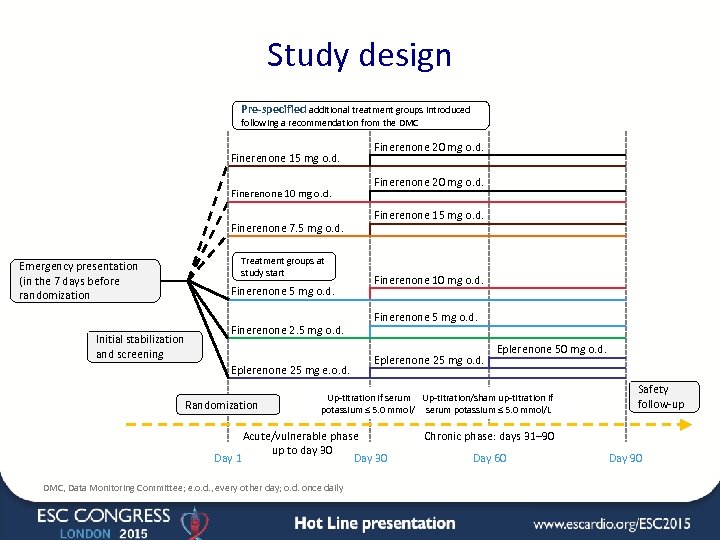 Study design Pre-specified additional treatment groups introduced following a recommendation from the DMC Finerenone 15 mg o. d. Finerenone 10 mg o. d. Finerenone 7. 5 mg o. d. Treatment groups at study start Emergency presentation (in the 7 days before randomization Finerenone 5 mg o. d. Initial stabilization and screening Finerenone 2. 5 mg o. d. Eplerenone 25 mg e. o. d. Randomization Finerenone 20 mg o. d. Finerenone 15 mg o. d. Finerenone 10 mg o. d. Finerenone 5 mg o. d. Eplerenone 25 mg o. d. Eplerenone 50 mg o. d. Up-titration if serum Up-titration/sham up-titration if potassium ≤ 5. 0 mmol/L serum potassium ≤ 5. 0 mmol/L Acute/vulnerable phase up to day 30 Day 1 Day 30 DMC, Data Monitoring Committee; e. o. d. , every other day; o. d. once daily Safety follow-up Chronic phase: days 31– 90 Day 60 Day 90
Study design Pre-specified additional treatment groups introduced following a recommendation from the DMC Finerenone 15 mg o. d. Finerenone 10 mg o. d. Finerenone 7. 5 mg o. d. Treatment groups at study start Emergency presentation (in the 7 days before randomization Finerenone 5 mg o. d. Initial stabilization and screening Finerenone 2. 5 mg o. d. Eplerenone 25 mg e. o. d. Randomization Finerenone 20 mg o. d. Finerenone 15 mg o. d. Finerenone 10 mg o. d. Finerenone 5 mg o. d. Eplerenone 25 mg o. d. Eplerenone 50 mg o. d. Up-titration if serum Up-titration/sham up-titration if potassium ≤ 5. 0 mmol/L serum potassium ≤ 5. 0 mmol/L Acute/vulnerable phase up to day 30 Day 1 Day 30 DMC, Data Monitoring Committee; e. o. d. , every other day; o. d. once daily Safety follow-up Chronic phase: days 31– 90 Day 60 Day 90
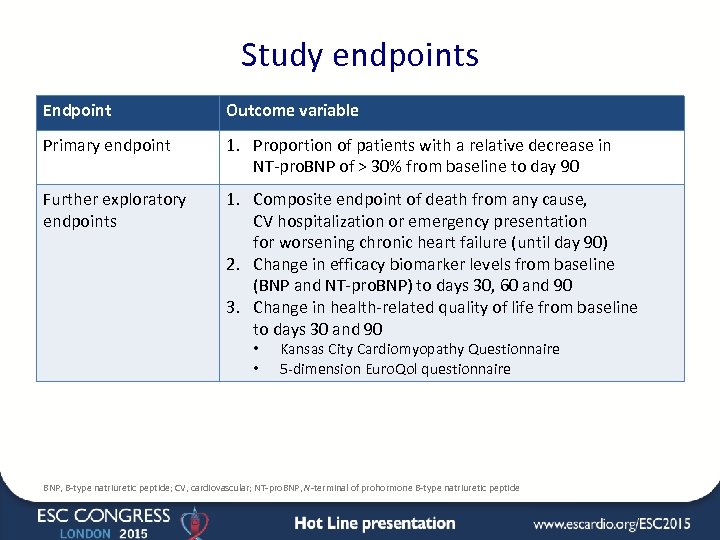 Study endpoints Endpoint Outcome variable Primary endpoint 1. Proportion of patients with a relative decrease in NT-pro. BNP of > 30% from baseline to day 90 Further exploratory endpoints 1. Composite endpoint of death from any cause, CV hospitalization or emergency presentation for worsening chronic heart failure (until day 90) 2. Change in efficacy biomarker levels from baseline (BNP and NT-pro. BNP) to days 30, 60 and 90 3. Change in health-related quality of life from baseline to days 30 and 90 • • Kansas City Cardiomyopathy Questionnaire 5 -dimension Euro. Qol questionnaire BNP, B-type natriuretic peptide; CV, cardiovascular; NT-pro. BNP, N-terminal of prohormone B-type natriuretic peptide
Study endpoints Endpoint Outcome variable Primary endpoint 1. Proportion of patients with a relative decrease in NT-pro. BNP of > 30% from baseline to day 90 Further exploratory endpoints 1. Composite endpoint of death from any cause, CV hospitalization or emergency presentation for worsening chronic heart failure (until day 90) 2. Change in efficacy biomarker levels from baseline (BNP and NT-pro. BNP) to days 30, 60 and 90 3. Change in health-related quality of life from baseline to days 30 and 90 • • Kansas City Cardiomyopathy Questionnaire 5 -dimension Euro. Qol questionnaire BNP, B-type natriuretic peptide; CV, cardiovascular; NT-pro. BNP, N-terminal of prohormone B-type natriuretic peptide
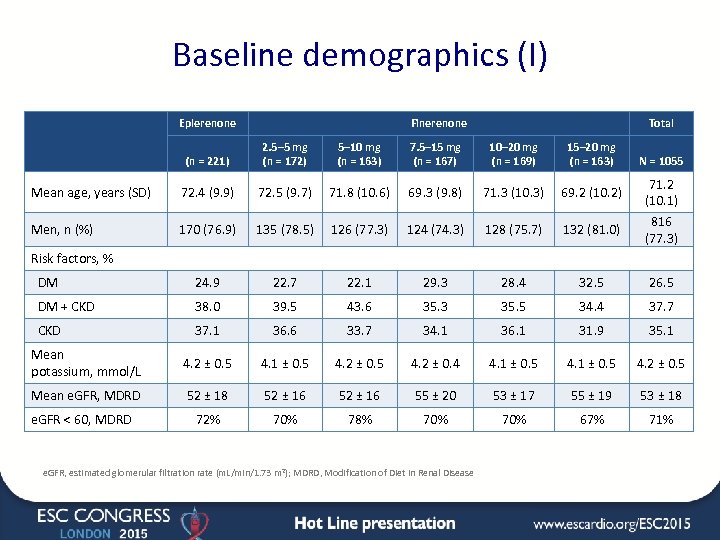 Baseline demographics (I) Eplerenone Finerenone Total (n = 221) 2. 5– 5 mg (n = 172) 5– 10 mg (n = 163) 7. 5– 15 mg (n = 167) 10– 20 mg (n = 169) 15– 20 mg (n = 163) Mean age, years (SD) 72. 4 (9. 9) 72. 5 (9. 7) 71. 8 (10. 6) 69. 3 (9. 8) 71. 3 (10. 3) 69. 2 (10. 2) Men, n (%) 170 (76. 9) 135 (78. 5) 126 (77. 3) 124 (74. 3) 128 (75. 7) 132 (81. 0) DM 24. 9 22. 7 22. 1 29. 3 28. 4 32. 5 26. 5 DM + CKD 38. 0 39. 5 43. 6 35. 3 35. 5 34. 4 37. 7 CKD 37. 1 36. 6 33. 7 34. 1 36. 1 31. 9 35. 1 Mean potassium, mmol/L 4. 2 ± 0. 5 4. 1 ± 0. 5 4. 2 ± 0. 4 4. 1 ± 0. 5 4. 2 ± 0. 5 Mean e. GFR, MDRD 52 ± 18 52 ± 16 55 ± 20 53 ± 17 55 ± 19 53 ± 18 72% 70% 78% 70% 67% 71% N = 1055 71. 2 (10. 1) 816 (77. 3) Risk factors, % e. GFR < 60, MDRD e. GFR, estimated glomerular filtration rate (m. L/min/1. 73 m 2); MDRD, Modification of Diet in Renal Disease
Baseline demographics (I) Eplerenone Finerenone Total (n = 221) 2. 5– 5 mg (n = 172) 5– 10 mg (n = 163) 7. 5– 15 mg (n = 167) 10– 20 mg (n = 169) 15– 20 mg (n = 163) Mean age, years (SD) 72. 4 (9. 9) 72. 5 (9. 7) 71. 8 (10. 6) 69. 3 (9. 8) 71. 3 (10. 3) 69. 2 (10. 2) Men, n (%) 170 (76. 9) 135 (78. 5) 126 (77. 3) 124 (74. 3) 128 (75. 7) 132 (81. 0) DM 24. 9 22. 7 22. 1 29. 3 28. 4 32. 5 26. 5 DM + CKD 38. 0 39. 5 43. 6 35. 3 35. 5 34. 4 37. 7 CKD 37. 1 36. 6 33. 7 34. 1 36. 1 31. 9 35. 1 Mean potassium, mmol/L 4. 2 ± 0. 5 4. 1 ± 0. 5 4. 2 ± 0. 4 4. 1 ± 0. 5 4. 2 ± 0. 5 Mean e. GFR, MDRD 52 ± 18 52 ± 16 55 ± 20 53 ± 17 55 ± 19 53 ± 18 72% 70% 78% 70% 67% 71% N = 1055 71. 2 (10. 1) 816 (77. 3) Risk factors, % e. GFR < 60, MDRD e. GFR, estimated glomerular filtration rate (m. L/min/1. 73 m 2); MDRD, Modification of Diet in Renal Disease
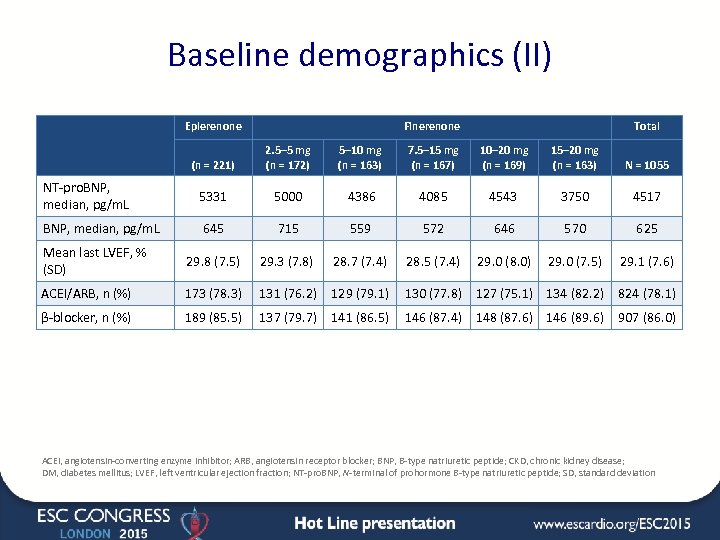 Baseline demographics (II) Eplerenone Finerenone Total (n = 221) 2. 5– 5 mg (n = 172) 5– 10 mg (n = 163) 7. 5– 15 mg (n = 167) 10– 20 mg (n = 169) 15– 20 mg (n = 163) N = 1055 NT-pro. BNP, median, pg/m. L 5331 5000 4386 4085 4543 3750 4517 BNP, median, pg/m. L 645 715 559 572 646 570 625 Mean last LVEF, % (SD) 29. 8 (7. 5) 29. 3 (7. 8) 28. 7 (7. 4) 28. 5 (7. 4) 29. 0 (8. 0) 29. 0 (7. 5) 29. 1 (7. 6) ACEI/ARB, n (%) 173 (78. 3) 131 (76. 2) 129 (79. 1) 130 (77. 8) 127 (75. 1) 134 (82. 2) 824 (78. 1) β-blocker, n (%) 189 (85. 5) 137 (79. 7) 141 (86. 5) 146 (87. 4) 148 (87. 6) 146 (89. 6) 907 (86. 0) ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BNP, B-type natriuretic peptide; CKD, chronic kidney disease; DM, diabetes mellitus; LVEF, left ventricular ejection fraction; NT-pro. BNP, N-terminal of prohormone B-type natriuretic peptide; SD, standard deviation
Baseline demographics (II) Eplerenone Finerenone Total (n = 221) 2. 5– 5 mg (n = 172) 5– 10 mg (n = 163) 7. 5– 15 mg (n = 167) 10– 20 mg (n = 169) 15– 20 mg (n = 163) N = 1055 NT-pro. BNP, median, pg/m. L 5331 5000 4386 4085 4543 3750 4517 BNP, median, pg/m. L 645 715 559 572 646 570 625 Mean last LVEF, % (SD) 29. 8 (7. 5) 29. 3 (7. 8) 28. 7 (7. 4) 28. 5 (7. 4) 29. 0 (8. 0) 29. 0 (7. 5) 29. 1 (7. 6) ACEI/ARB, n (%) 173 (78. 3) 131 (76. 2) 129 (79. 1) 130 (77. 8) 127 (75. 1) 134 (82. 2) 824 (78. 1) β-blocker, n (%) 189 (85. 5) 137 (79. 7) 141 (86. 5) 146 (87. 4) 148 (87. 6) 146 (89. 6) 907 (86. 0) ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BNP, B-type natriuretic peptide; CKD, chronic kidney disease; DM, diabetes mellitus; LVEF, left ventricular ejection fraction; NT-pro. BNP, N-terminal of prohormone B-type natriuretic peptide; SD, standard deviation
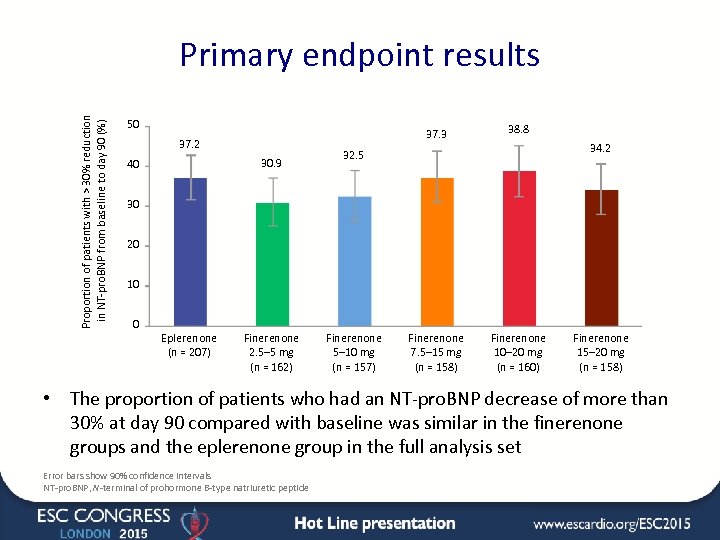 Proportion of patients with > 30% reduction in NT-pro. BNP from baseline to day 90 (%) Primary endpoint results 50 37. 3 37. 2 30. 9 40 38. 8 Finerenone 7. 5– 15 mg (n = 158) Finerenone 10– 20 mg (n = 160) 34. 2 32. 5 30 20 10 0 Eplerenone (n = 207) Finerenone 2. 5– 5 mg (n = 162) Finerenone 5– 10 mg (n = 157) Finerenone 15– 20 mg (n = 158) • The proportion of patients who had an NT-pro. BNP decrease of more than 30% at day 90 compared with baseline was similar in the finerenone groups and the eplerenone group in the full analysis set Error bars show 90% confidence intervals NT-pro. BNP, N-terminal of prohormone B-type natriuretic peptide
Proportion of patients with > 30% reduction in NT-pro. BNP from baseline to day 90 (%) Primary endpoint results 50 37. 3 37. 2 30. 9 40 38. 8 Finerenone 7. 5– 15 mg (n = 158) Finerenone 10– 20 mg (n = 160) 34. 2 32. 5 30 20 10 0 Eplerenone (n = 207) Finerenone 2. 5– 5 mg (n = 162) Finerenone 5– 10 mg (n = 157) Finerenone 15– 20 mg (n = 158) • The proportion of patients who had an NT-pro. BNP decrease of more than 30% at day 90 compared with baseline was similar in the finerenone groups and the eplerenone group in the full analysis set Error bars show 90% confidence intervals NT-pro. BNP, N-terminal of prohormone B-type natriuretic peptide
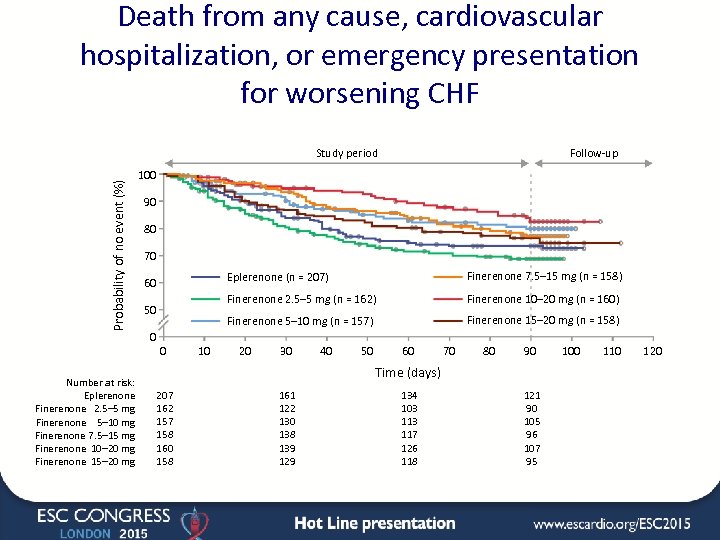 Death from any cause, cardiovascular hospitalization, or emergency presentation for worsening CHF Probability of no event (%) Study period Number at risk: Eplerenone Finerenone 2. 5– 5 mg Finerenone 5– 10 mg Finerenone 7. 5– 15 mg Finerenone 10– 20 mg Finerenone 15– 20 mg Follow-up 100 90 80 70 Eplerenone (n = 207) 0 0 10 Finerenone 10– 20 mg (n = 160) Finerenone 5– 10 mg (n = 157) 50 Finerenone 7. 5– 15 mg (n = 158) Finerenone 2. 5– 5 mg (n = 162) 60 Finerenone 15– 20 mg (n = 158) 20 30 40 50 60 70 80 90 Time (days) 207 162 157 158 160 158 161 122 130 138 139 129 134 103 117 126 118 121 90 105 96 107 95 100 110 120
Death from any cause, cardiovascular hospitalization, or emergency presentation for worsening CHF Probability of no event (%) Study period Number at risk: Eplerenone Finerenone 2. 5– 5 mg Finerenone 5– 10 mg Finerenone 7. 5– 15 mg Finerenone 10– 20 mg Finerenone 15– 20 mg Follow-up 100 90 80 70 Eplerenone (n = 207) 0 0 10 Finerenone 10– 20 mg (n = 160) Finerenone 5– 10 mg (n = 157) 50 Finerenone 7. 5– 15 mg (n = 158) Finerenone 2. 5– 5 mg (n = 162) 60 Finerenone 15– 20 mg (n = 158) 20 30 40 50 60 70 80 90 Time (days) 207 162 157 158 160 158 161 122 130 138 139 129 134 103 117 126 118 121 90 105 96 107 95 100 110 120
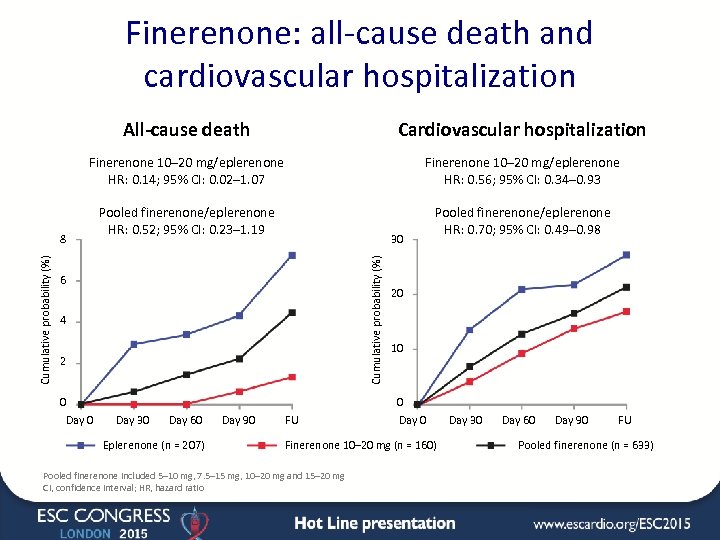 Finerenone: all-cause death and cardiovascular hospitalization All-cause death Cardiovascular hospitalization Finerenone 10– 20 mg/eplerenone HR: 0. 14; 95% CI: 0. 02– 1. 07 Finerenone 10– 20 mg/eplerenone HR: 0. 56; 95% CI: 0. 34– 0. 93 Pooled finerenone/eplerenone HR: 0. 52; 95% CI: 0. 23– 1. 19 Pooled finerenone/eplerenone HR: 0. 70; 95% CI: 0. 49– 0. 98 30 Cumulative probability (%) 8 6 4 2 0 Day 30 Day 60 Eplerenone (n = 207) Day 90 FU 20 10 0 Day 0 Finerenone 10– 20 mg (n = 160) Pooled finerenone included 5– 10 mg, 7. 5– 15 mg, 10– 20 mg and 15– 20 mg CI, confidence interval; HR, hazard ratio Day 30 Day 60 Day 90 FU Pooled finerenone (n = 633)
Finerenone: all-cause death and cardiovascular hospitalization All-cause death Cardiovascular hospitalization Finerenone 10– 20 mg/eplerenone HR: 0. 14; 95% CI: 0. 02– 1. 07 Finerenone 10– 20 mg/eplerenone HR: 0. 56; 95% CI: 0. 34– 0. 93 Pooled finerenone/eplerenone HR: 0. 52; 95% CI: 0. 23– 1. 19 Pooled finerenone/eplerenone HR: 0. 70; 95% CI: 0. 49– 0. 98 30 Cumulative probability (%) 8 6 4 2 0 Day 30 Day 60 Eplerenone (n = 207) Day 90 FU 20 10 0 Day 0 Finerenone 10– 20 mg (n = 160) Pooled finerenone included 5– 10 mg, 7. 5– 15 mg, 10– 20 mg and 15– 20 mg CI, confidence interval; HR, hazard ratio Day 30 Day 60 Day 90 FU Pooled finerenone (n = 633)
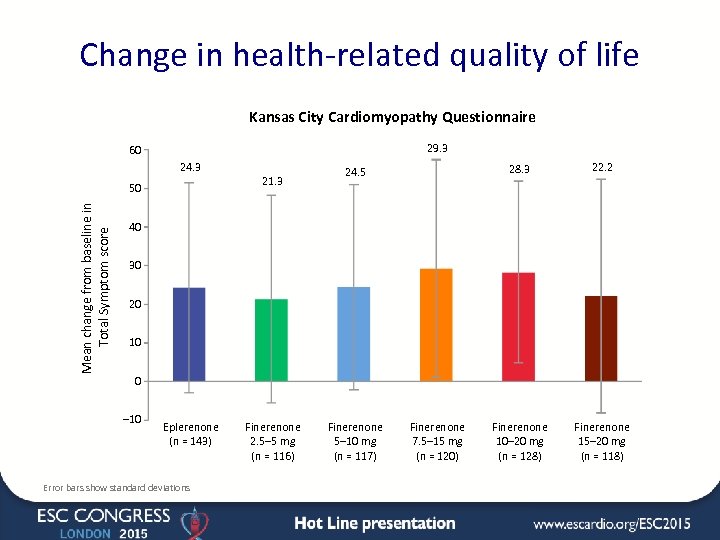 Change in health-related quality of life Kansas City Cardiomyopathy Questionnaire 29. 3 60 24. 3 21. 3 Mean change from baseline in Total Symptom score 50 28. 3 24. 5 22. 2 Finerenone 10– 20 mg (n = 128) Finerenone 15– 20 mg (n = 118) 40 30 20 10 0 – 10 Eplerenone (n = 143) Error bars show standard deviations Finerenone 2. 5– 5 mg (n = 116) Finerenone 5– 10 mg (n = 117) Finerenone 7. 5– 15 mg (n = 120)
Change in health-related quality of life Kansas City Cardiomyopathy Questionnaire 29. 3 60 24. 3 21. 3 Mean change from baseline in Total Symptom score 50 28. 3 24. 5 22. 2 Finerenone 10– 20 mg (n = 128) Finerenone 15– 20 mg (n = 118) 40 30 20 10 0 – 10 Eplerenone (n = 143) Error bars show standard deviations Finerenone 2. 5– 5 mg (n = 116) Finerenone 5– 10 mg (n = 117) Finerenone 7. 5– 15 mg (n = 120)
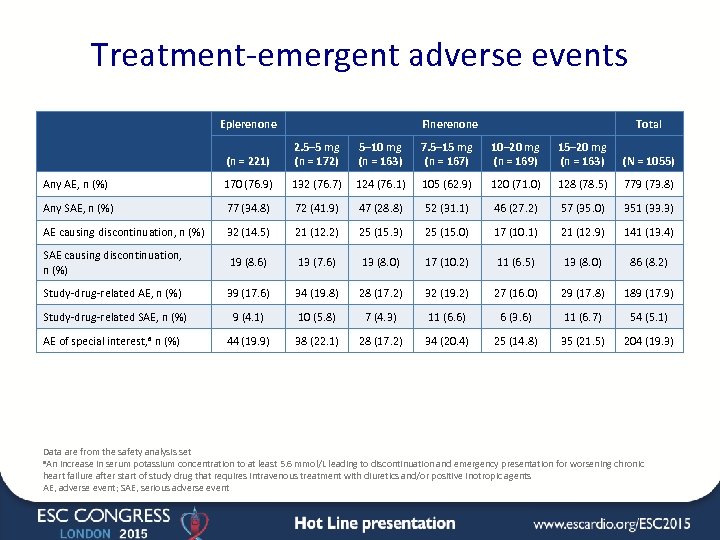 Treatment-emergent adverse events Eplerenone Finerenone Total (n = 221) 2. 5– 5 mg (n = 172) 5– 10 mg (n = 163) 7. 5– 15 mg (n = 167) 10– 20 mg (n = 169) 15– 20 mg (n = 163) (N = 1055) Any AE, n (%) 170 (76. 9) 132 (76. 7) 124 (76. 1) 105 (62. 9) 120 (71. 0) 128 (78. 5) 779 (73. 8) Any SAE, n (%) 77 (34. 8) 72 (41. 9) 47 (28. 8) 52 (31. 1) 46 (27. 2) 57 (35. 0) 351 (33. 3) AE causing discontinuation, n (%) 32 (14. 5) 21 (12. 2) 25 (15. 3) 25 (15. 0) 17 (10. 1) 21 (12. 9) 141 (13. 4) SAE causing discontinuation, n (%) 19 (8. 6) 13 (7. 6) 13 (8. 0) 17 (10. 2) 11 (6. 5) 13 (8. 0) 86 (8. 2) Study-drug-related AE, n (%) 39 (17. 6) 34 (19. 8) 28 (17. 2) 32 (19. 2) 27 (16. 0) 29 (17. 8) 189 (17. 9) Study-drug-related SAE, n (%) 9 (4. 1) 10 (5. 8) 7 (4. 3) 11 (6. 6) 6 (3. 6) 11 (6. 7) 54 (5. 1) AE of special interest, a n (%) 44 (19. 9) 38 (22. 1) 28 (17. 2) 34 (20. 4) 25 (14. 8) 35 (21. 5) 204 (19. 3) Data are from the safety analysis set a. An increase in serum potassium concentration to at least 5. 6 mmol/L leading to discontinuation and emergency presentation for worsening chronic heart failure after start of study drug that requires intravenous treatment with diuretics and/or positive inotropic agents AE, adverse event; SAE, serious adverse event
Treatment-emergent adverse events Eplerenone Finerenone Total (n = 221) 2. 5– 5 mg (n = 172) 5– 10 mg (n = 163) 7. 5– 15 mg (n = 167) 10– 20 mg (n = 169) 15– 20 mg (n = 163) (N = 1055) Any AE, n (%) 170 (76. 9) 132 (76. 7) 124 (76. 1) 105 (62. 9) 120 (71. 0) 128 (78. 5) 779 (73. 8) Any SAE, n (%) 77 (34. 8) 72 (41. 9) 47 (28. 8) 52 (31. 1) 46 (27. 2) 57 (35. 0) 351 (33. 3) AE causing discontinuation, n (%) 32 (14. 5) 21 (12. 2) 25 (15. 3) 25 (15. 0) 17 (10. 1) 21 (12. 9) 141 (13. 4) SAE causing discontinuation, n (%) 19 (8. 6) 13 (7. 6) 13 (8. 0) 17 (10. 2) 11 (6. 5) 13 (8. 0) 86 (8. 2) Study-drug-related AE, n (%) 39 (17. 6) 34 (19. 8) 28 (17. 2) 32 (19. 2) 27 (16. 0) 29 (17. 8) 189 (17. 9) Study-drug-related SAE, n (%) 9 (4. 1) 10 (5. 8) 7 (4. 3) 11 (6. 6) 6 (3. 6) 11 (6. 7) 54 (5. 1) AE of special interest, a n (%) 44 (19. 9) 38 (22. 1) 28 (17. 2) 34 (20. 4) 25 (14. 8) 35 (21. 5) 204 (19. 3) Data are from the safety analysis set a. An increase in serum potassium concentration to at least 5. 6 mmol/L leading to discontinuation and emergency presentation for worsening chronic heart failure after start of study drug that requires intravenous treatment with diuretics and/or positive inotropic agents AE, adverse event; SAE, serious adverse event
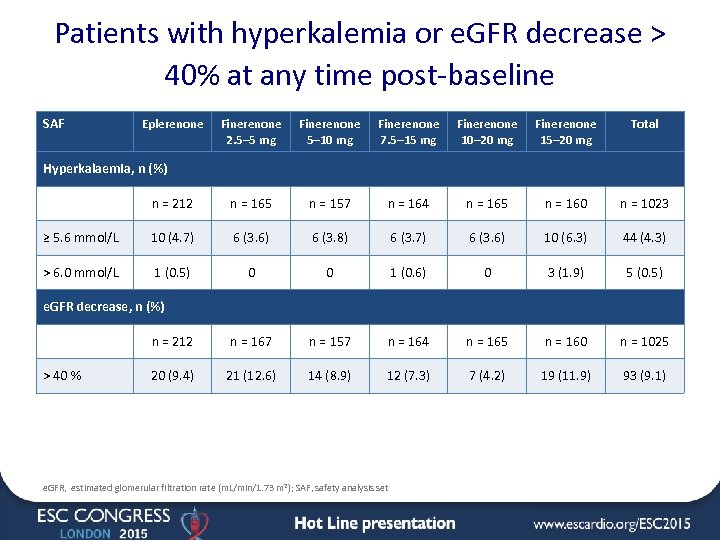 Patients with hyperkalemia or e. GFR decrease > 40% at any time post-baseline SAF Eplerenone Finerenone 2. 5– 5 mg Finerenone 5– 10 mg Finerenone 7. 5– 15 mg Finerenone 10– 20 mg Finerenone 15– 20 mg Total n = 212 n = 165 n = 157 n = 164 n = 165 n = 160 n = 1023 ≥ 5. 6 mmol/L 10 (4. 7) 6 (3. 6) 6 (3. 8) 6 (3. 7) 6 (3. 6) 10 (6. 3) 44 (4. 3) > 6. 0 mmol/L 1 (0. 5) 0 0 1 (0. 6) 0 3 (1. 9) 5 (0. 5) n = 212 n = 167 n = 157 n = 164 n = 165 n = 160 n = 1025 20 (9. 4) 21 (12. 6) 14 (8. 9) 12 (7. 3) 7 (4. 2) 19 (11. 9) 93 (9. 1) Hyperkalaemia, n (%) e. GFR decrease, n (%) > 40 % e. GFR, estimated glomerular filtration rate (m. L/min/1. 73 m 2); SAF, safety analysis set
Patients with hyperkalemia or e. GFR decrease > 40% at any time post-baseline SAF Eplerenone Finerenone 2. 5– 5 mg Finerenone 5– 10 mg Finerenone 7. 5– 15 mg Finerenone 10– 20 mg Finerenone 15– 20 mg Total n = 212 n = 165 n = 157 n = 164 n = 165 n = 160 n = 1023 ≥ 5. 6 mmol/L 10 (4. 7) 6 (3. 6) 6 (3. 8) 6 (3. 7) 6 (3. 6) 10 (6. 3) 44 (4. 3) > 6. 0 mmol/L 1 (0. 5) 0 0 1 (0. 6) 0 3 (1. 9) 5 (0. 5) n = 212 n = 167 n = 157 n = 164 n = 165 n = 160 n = 1025 20 (9. 4) 21 (12. 6) 14 (8. 9) 12 (7. 3) 7 (4. 2) 19 (11. 9) 93 (9. 1) Hyperkalaemia, n (%) e. GFR decrease, n (%) > 40 % e. GFR, estimated glomerular filtration rate (m. L/min/1. 73 m 2); SAF, safety analysis set
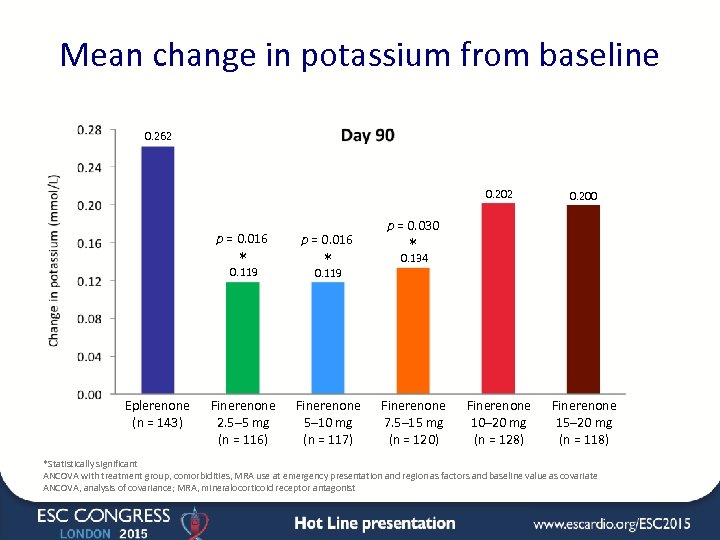 Mean change in potassium from baseline 0. 262 0. 202 p = 0. 016 * p = 0. 016 Finerenone 10– 20 mg (n = 128) Finerenone 15– 20 mg (n = 118) p = 0. 030 * 0. 119 Eplerenone (n = 143) 0. 200 * 0. 134 0. 119 Finerenone 2. 5– 5 mg (n = 116) Finerenone 5– 10 mg (n = 117) Finerenone 7. 5– 15 mg (n = 120) *Statistically significant ANCOVA with treatment group, comorbidities, MRA use at emergency presentation and region as factors and baseline value as covariate ANCOVA, analysis of covariance; MRA, mineralocorticoid receptor antagonist
Mean change in potassium from baseline 0. 262 0. 202 p = 0. 016 * p = 0. 016 Finerenone 10– 20 mg (n = 128) Finerenone 15– 20 mg (n = 118) p = 0. 030 * 0. 119 Eplerenone (n = 143) 0. 200 * 0. 134 0. 119 Finerenone 2. 5– 5 mg (n = 116) Finerenone 5– 10 mg (n = 117) Finerenone 7. 5– 15 mg (n = 120) *Statistically significant ANCOVA with treatment group, comorbidities, MRA use at emergency presentation and region as factors and baseline value as covariate ANCOVA, analysis of covariance; MRA, mineralocorticoid receptor antagonist
 Summary I • In patients hospitalized for worsening chronic HFr. EF with type 2 diabetes mellitus and/or CKD, the proportion of patients with a relative decrease in NT-pro. BNP of more than 30% from baseline to day 90 was similar in the eplerenone group and the finerenone groups • The incidence of the clinical composite endpoint (all-cause death, cardiovascular hospitalization or emergency presentation for worsening chronic heart failure) at day 90 was lower with all finerenone doses (except 2. 5– 5 mg) than with eplerenone, with the lowest incidence observed in the finerenone 10– 20 mg dose group
Summary I • In patients hospitalized for worsening chronic HFr. EF with type 2 diabetes mellitus and/or CKD, the proportion of patients with a relative decrease in NT-pro. BNP of more than 30% from baseline to day 90 was similar in the eplerenone group and the finerenone groups • The incidence of the clinical composite endpoint (all-cause death, cardiovascular hospitalization or emergency presentation for worsening chronic heart failure) at day 90 was lower with all finerenone doses (except 2. 5– 5 mg) than with eplerenone, with the lowest incidence observed in the finerenone 10– 20 mg dose group
 Summary II • All doses of finerenone were well tolerated, with a similar incidence of treatment-emergent adverse events in the eplerenone group and finerenone groups • Hyperkalaemia (serum potassium ≥ 5. 6 mmol/L) was observed in 44 patients (4. 3%) at any time post-baseline
Summary II • All doses of finerenone were well tolerated, with a similar incidence of treatment-emergent adverse events in the eplerenone group and finerenone groups • Hyperkalaemia (serum potassium ≥ 5. 6 mmol/L) was observed in 44 patients (4. 3%) at any time post-baseline
 Trial Committees • Data Monitoring Committee members – Marco Metra – Chairman (Italy), Barry Greenberg (USA), Meinhard Kieser (Germany), Eberhard Ritz (Germany), Pantelis Sarafidis (Greece), Guntram Schernthaner (Austria) • Steering Committee members – Bertram Pitt – Chairman (USA), Gerasimos Filippatos – Chairman (Greece), Stefan D Anker (Germany), Michael Böhm (Germany), Mihai Gheorghiade (USA), Lars Kober (Denmark), Henry Krum (Australia), Aldo Maggioni (Italy), Piotr Ponikowski (Poland), Adrian Voors (Netherlands), Faiez Zannad (France) • Adjudication Committee members – Stefan D Anker (Germany), Aldo Maggioni (Italy), Piotr Ponikowski (Poland)
Trial Committees • Data Monitoring Committee members – Marco Metra – Chairman (Italy), Barry Greenberg (USA), Meinhard Kieser (Germany), Eberhard Ritz (Germany), Pantelis Sarafidis (Greece), Guntram Schernthaner (Austria) • Steering Committee members – Bertram Pitt – Chairman (USA), Gerasimos Filippatos – Chairman (Greece), Stefan D Anker (Germany), Michael Böhm (Germany), Mihai Gheorghiade (USA), Lars Kober (Denmark), Henry Krum (Australia), Aldo Maggioni (Italy), Piotr Ponikowski (Poland), Adrian Voors (Netherlands), Faiez Zannad (France) • Adjudication Committee members – Stefan D Anker (Germany), Aldo Maggioni (Italy), Piotr Ponikowski (Poland)
 Investigators Austria Andrea Podczeck-Schweighofer, Uta Hoppe, Armin Böhmer, Peter Siostrzonek, Dieter Botegal, Friedrich Fruhwald, Gerhard Pölzl, Gabriele Jakl-Kotauschek, Giorgio Giacomini Australia Henry Krum, Carmine De. Pasquale, Andrew Sindone, Eugene Kotlyar, John Atherton, John Amerena Bulgaria Lyubomir Lyubenov, Yotov, Petyo Georgiev, Elina Blatadzhieva-Trendafilova, Temenuga Donova, Valentina Mincheva, Mariana Konteva Canada Gordon Moe, Serge Lepage, Haissam Haddad, Richard Sheppard, Mark Liszkowski, Daniel Savard, Debra Isaac, Sebastien Bergeron, Dominique Auger, Imad Nadra, Sean Virani, Simon Kou Czech Republic Filip Malek, Radim Kryza, Lubomir Ballek, Jan Macha, Tomas Brabec Germany Johann Bauersachs, Stefan Anker, Michael Böhm, Jürgen vom Dahl, Harald Lapp, Rolf Wachter, Stefan Stoerk, Olaf Oldenburg, Sebastian Philipp, Holger Eggebrecht, Stephan Steiner, A Mügge, T Gori, A Sandek Denmark Lars Køber, Gunnar Gislason, Olav. Wendelboe Nielsen, Niels Jørgen Frandsen, Knud. Skagen, Kenneth Egstrup, Jacob Eifer Møller, Tonny Nielsen, Jens Refsgaard, Ole Nyvad, Jørgen Jeppesen Spain Domingo A Pascual Figal, Julio Núñez, Luís Almenar Bonet, Juan Delgado, Enrique Galve Basilio, Sonia. Ruíz Bustillo, Josep Bisbe, Pablo García Pavía Finland Johan Lassus, Anna-Mari Hekkala, Heikki Ukkonen, Kai Nyman, Alexandre Hadjikov France Fabrice Bauer, Michel Galinier, Richard Isnard, Nathan Mewton, Philippe Le Corvoisier, Alain Cohen-Solal, Faïez Zannad, Pierre Gibelin Greece John Parissis, John Nanas, Apostolos Karavidas, Sotirios Patsilinakos, Filippos Triposkiadis Hungary Ebrahim Noori, Janos Tomcsanyi, Andras Vertes, Bela Merkely, Andras Matoltsy Israel Alon Marmor, Amos Katz, Tuvia Ben Gal, Sorel Goland, Avraham Shotan, Haim Shmilovich, Yoav Turgeman, Tanya Weitsman, Basil Lewis, Shaul Atar, Morris Mosseri, Zvi Vered Italy Michele Senni, Andrea Mortara, Gianfranco Parati, Matteo Di Biase, Maurizio Volterrani, Franco. Cosmi, Mario Marzilli, Gianfranco Alunni, Antonello Gavazzi South Korea Jae-Joong Kim, Hae-Young Lee, Byung Su Yoo, Sang-Hong Baek, Seok-Min Kang Lithuania Jelena Celutkiene, Ausra Kavoliuniene, Alfredas Bagdonas, Gintautas Gumbrevicius, Sigitas Stonkus, Sigute, Norkiene Netherlands Adriaan Voors, MA Brouwer, GL Bartels, C de Nooijer, RGEJ Groutars, PR Nierop, E Ronner, SHK The, NYY Al Windy Norway Stein Ørn, Jan Erik Otterstad, Roar Thorshaug, Aina Brekk Poland Joanna Szachniewicz, Ewa Mirek-Bryniarska, Aleksander Goch, Waldemar Krysiak, Marcin Gruchala, Janina Stepinska, Michal Tendera, Beata Wozakowska-Kaplon, Wlodzimierz Musial Portugal José Carlos Silva Cardoso, Pedro Moraes Sarmento, Hélder Pereira, Cândida Fonseca, Dulce Brito, Jorge Mimoso, Carlos Cotrim Sweden Maria Schaufelberger, Jens Olson, Inger Hagerman, Hans Persson, Thomas Kronvall, Stefan Berglund, Bengt Johansson Turkey Sadi Gulec, Bulent Boyaci, Cemil Gurgun, Sema Guneri, Ahmet Temizhan, Aytül Belgi Yildirim Taiwan Chern-En Chiang, Wen-Jone Chen, Kou-Gi Shyu, Kwo-Chang Ueng, Yen-Wen Wu USA Phillip Levy, David Lanfear, Robert Cole, Liviu Klein, Jalal Ghali, Frank Smart, Paul Mather, Stephen Gottlieb, Marc Klapholz, Jorge Silva Enciso, Douglas Chapman, Hector Ventura, Salman Khan, Steven Isserman, Suzanne Oparil, Lynne Wagoner, Joshua Larned South Africa Richard Siebert, Mohamed Sarvan, Naresh Ranjith, Johannes Engelbrecht, Louis van Zyl, Saleem Dawood, Fayzal Ahmed, Jan Saaiman, Hans Prozesky, Thomas Mabin
Investigators Austria Andrea Podczeck-Schweighofer, Uta Hoppe, Armin Böhmer, Peter Siostrzonek, Dieter Botegal, Friedrich Fruhwald, Gerhard Pölzl, Gabriele Jakl-Kotauschek, Giorgio Giacomini Australia Henry Krum, Carmine De. Pasquale, Andrew Sindone, Eugene Kotlyar, John Atherton, John Amerena Bulgaria Lyubomir Lyubenov, Yotov, Petyo Georgiev, Elina Blatadzhieva-Trendafilova, Temenuga Donova, Valentina Mincheva, Mariana Konteva Canada Gordon Moe, Serge Lepage, Haissam Haddad, Richard Sheppard, Mark Liszkowski, Daniel Savard, Debra Isaac, Sebastien Bergeron, Dominique Auger, Imad Nadra, Sean Virani, Simon Kou Czech Republic Filip Malek, Radim Kryza, Lubomir Ballek, Jan Macha, Tomas Brabec Germany Johann Bauersachs, Stefan Anker, Michael Böhm, Jürgen vom Dahl, Harald Lapp, Rolf Wachter, Stefan Stoerk, Olaf Oldenburg, Sebastian Philipp, Holger Eggebrecht, Stephan Steiner, A Mügge, T Gori, A Sandek Denmark Lars Køber, Gunnar Gislason, Olav. Wendelboe Nielsen, Niels Jørgen Frandsen, Knud. Skagen, Kenneth Egstrup, Jacob Eifer Møller, Tonny Nielsen, Jens Refsgaard, Ole Nyvad, Jørgen Jeppesen Spain Domingo A Pascual Figal, Julio Núñez, Luís Almenar Bonet, Juan Delgado, Enrique Galve Basilio, Sonia. Ruíz Bustillo, Josep Bisbe, Pablo García Pavía Finland Johan Lassus, Anna-Mari Hekkala, Heikki Ukkonen, Kai Nyman, Alexandre Hadjikov France Fabrice Bauer, Michel Galinier, Richard Isnard, Nathan Mewton, Philippe Le Corvoisier, Alain Cohen-Solal, Faïez Zannad, Pierre Gibelin Greece John Parissis, John Nanas, Apostolos Karavidas, Sotirios Patsilinakos, Filippos Triposkiadis Hungary Ebrahim Noori, Janos Tomcsanyi, Andras Vertes, Bela Merkely, Andras Matoltsy Israel Alon Marmor, Amos Katz, Tuvia Ben Gal, Sorel Goland, Avraham Shotan, Haim Shmilovich, Yoav Turgeman, Tanya Weitsman, Basil Lewis, Shaul Atar, Morris Mosseri, Zvi Vered Italy Michele Senni, Andrea Mortara, Gianfranco Parati, Matteo Di Biase, Maurizio Volterrani, Franco. Cosmi, Mario Marzilli, Gianfranco Alunni, Antonello Gavazzi South Korea Jae-Joong Kim, Hae-Young Lee, Byung Su Yoo, Sang-Hong Baek, Seok-Min Kang Lithuania Jelena Celutkiene, Ausra Kavoliuniene, Alfredas Bagdonas, Gintautas Gumbrevicius, Sigitas Stonkus, Sigute, Norkiene Netherlands Adriaan Voors, MA Brouwer, GL Bartels, C de Nooijer, RGEJ Groutars, PR Nierop, E Ronner, SHK The, NYY Al Windy Norway Stein Ørn, Jan Erik Otterstad, Roar Thorshaug, Aina Brekk Poland Joanna Szachniewicz, Ewa Mirek-Bryniarska, Aleksander Goch, Waldemar Krysiak, Marcin Gruchala, Janina Stepinska, Michal Tendera, Beata Wozakowska-Kaplon, Wlodzimierz Musial Portugal José Carlos Silva Cardoso, Pedro Moraes Sarmento, Hélder Pereira, Cândida Fonseca, Dulce Brito, Jorge Mimoso, Carlos Cotrim Sweden Maria Schaufelberger, Jens Olson, Inger Hagerman, Hans Persson, Thomas Kronvall, Stefan Berglund, Bengt Johansson Turkey Sadi Gulec, Bulent Boyaci, Cemil Gurgun, Sema Guneri, Ahmet Temizhan, Aytül Belgi Yildirim Taiwan Chern-En Chiang, Wen-Jone Chen, Kou-Gi Shyu, Kwo-Chang Ueng, Yen-Wen Wu USA Phillip Levy, David Lanfear, Robert Cole, Liviu Klein, Jalal Ghali, Frank Smart, Paul Mather, Stephen Gottlieb, Marc Klapholz, Jorge Silva Enciso, Douglas Chapman, Hector Ventura, Salman Khan, Steven Isserman, Suzanne Oparil, Lynne Wagoner, Joshua Larned South Africa Richard Siebert, Mohamed Sarvan, Naresh Ranjith, Johannes Engelbrecht, Louis van Zyl, Saleem Dawood, Fayzal Ahmed, Jan Saaiman, Hans Prozesky, Thomas Mabin
 Participating countries North America Europe Asia Others (Australia, Israel, South Africa)
Participating countries North America Europe Asia Others (Australia, Israel, South Africa)
 Back-up
Back-up
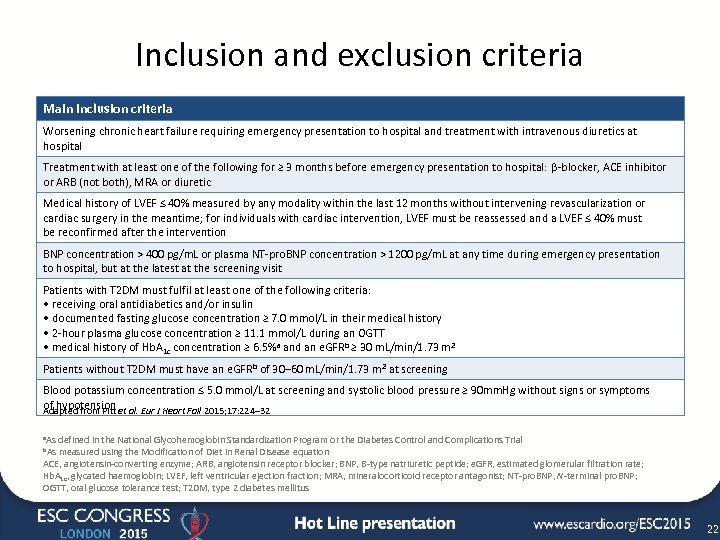 Inclusion and exclusion criteria Main inclusion criteria Worsening chronic heart failure requiring emergency presentation to hospital and treatment with intravenous diuretics at hospital Treatment with at least one of the following for ≥ 3 months before emergency presentation to hospital: β-blocker, ACE inhibitor or ARB (not both), MRA or diuretic Medical history of LVEF ≤ 40% measured by any modality within the last 12 months without intervening revascularization or cardiac surgery in the meantime; for individuals with cardiac intervention, LVEF must be reassessed and a LVEF ≤ 40% must be reconfirmed after the intervention BNP concentration > 400 pg/m. L or plasma NT-pro. BNP concentration > 1200 pg/m. L at any time during emergency presentation to hospital, but at the latest at the screening visit Patients with T 2 DM must fulfil at least one of the following criteria: • receiving oral antidiabetics and/or insulin • documented fasting glucose concentration ≥ 7. 0 mmol/L in their medical history • 2 -hour plasma glucose concentration ≥ 11. 1 mmol/L during an OGTT • medical history of Hb. A 1 c concentration ≥ 6. 5%a and an e. GFRb ≥ 30 m. L/min/1. 73 m 2 Patients without T 2 DM must have an e. GFR b of 30– 60 m. L/min/1. 73 m 2 at screening Blood potassium concentration ≤ 5. 0 mmol/L at screening and systolic blood pressure ≥ 90 mm. Hg without signs or symptoms of hypotension al. Eur J Heart Fail 2015; 17: 224– 32 Adapted from Pitt et a. As defined in the National Glycohemoglobin Standardization Program or the Diabetes Control and Complications Trial b. As measured using the Modification of Diet in Renal Disease equation ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; BNP, B-type natriuretic peptide; e. GFR, estimated glomerular filtration rate; Hb. A 1 c, glycated haemoglobin; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NT-pro. BNP, N-terminal pro. BNP; OGTT, oral glucose tolerance test; T 2 DM, type 2 diabetes mellitus 22
Inclusion and exclusion criteria Main inclusion criteria Worsening chronic heart failure requiring emergency presentation to hospital and treatment with intravenous diuretics at hospital Treatment with at least one of the following for ≥ 3 months before emergency presentation to hospital: β-blocker, ACE inhibitor or ARB (not both), MRA or diuretic Medical history of LVEF ≤ 40% measured by any modality within the last 12 months without intervening revascularization or cardiac surgery in the meantime; for individuals with cardiac intervention, LVEF must be reassessed and a LVEF ≤ 40% must be reconfirmed after the intervention BNP concentration > 400 pg/m. L or plasma NT-pro. BNP concentration > 1200 pg/m. L at any time during emergency presentation to hospital, but at the latest at the screening visit Patients with T 2 DM must fulfil at least one of the following criteria: • receiving oral antidiabetics and/or insulin • documented fasting glucose concentration ≥ 7. 0 mmol/L in their medical history • 2 -hour plasma glucose concentration ≥ 11. 1 mmol/L during an OGTT • medical history of Hb. A 1 c concentration ≥ 6. 5%a and an e. GFRb ≥ 30 m. L/min/1. 73 m 2 Patients without T 2 DM must have an e. GFR b of 30– 60 m. L/min/1. 73 m 2 at screening Blood potassium concentration ≤ 5. 0 mmol/L at screening and systolic blood pressure ≥ 90 mm. Hg without signs or symptoms of hypotension al. Eur J Heart Fail 2015; 17: 224– 32 Adapted from Pitt et a. As defined in the National Glycohemoglobin Standardization Program or the Diabetes Control and Complications Trial b. As measured using the Modification of Diet in Renal Disease equation ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; BNP, B-type natriuretic peptide; e. GFR, estimated glomerular filtration rate; Hb. A 1 c, glycated haemoglobin; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NT-pro. BNP, N-terminal pro. BNP; OGTT, oral glucose tolerance test; T 2 DM, type 2 diabetes mellitus 22
 Inclusion and exclusion criteria Main exclusion criteria Acute de novo heart failure or acute inflammatory heart disease or ACSa in the last 30 days before the screening visit Cardiogenic shock, Addison’s disease, valvular heart disease requiring surgical intervention during the course of the study, or patients with a left ventricular assistance device or those awaiting heart transplantation Stroke or TIA ≤ 3 months before the screening visit, known hypersensitivity to the study drug (active substance or excipients) or eplerenone, or acute infectious disease requiring intravenous antibiotics within the last 24 hours before randomization Dialysis for acute renal failure within the previous 3 months before emergency presentation to hospital, or hepatic insufficiency (Child–Pugh B or C) Requirement for any intravenous vasodilator, any intravenous natriuretic peptide ≤ 24 hours before randomization, or any intravenous positive inotrope ≤ 14 days before dosing with study drug Treatment with either spironolactone or eplerenone that cannot be discontinued 48 hours or 24 hours, respectively, before randomization and for the duration of the treatment period Treatment with any renin inhibitor or potassium-sparing diuretic that cannot be discontinued 24 hours before randomization and for the duration of the treatment period Treatment with high-dose acetylsalicylic acid (> 500 mg/day) or another NSAID Treatment with potent CYP 3 A 4 inhibitors or inducers, or strong CYP 2 C 8 inhibitors (all must be stopped ≥ 7 days before randomization) Adapted from Pitt et al. Eur J Heart Fail 2015; 17: 224– 32 a. As defined by the European Society of Cardiology and American College of Cardiology/American Heart Association guidelines ACS, acute coronary syndromes; CYP, cytochrome P 450; NSAID, non-steroidal anti-inflammatory drug; TIA, transient ischaemic attack 23
Inclusion and exclusion criteria Main exclusion criteria Acute de novo heart failure or acute inflammatory heart disease or ACSa in the last 30 days before the screening visit Cardiogenic shock, Addison’s disease, valvular heart disease requiring surgical intervention during the course of the study, or patients with a left ventricular assistance device or those awaiting heart transplantation Stroke or TIA ≤ 3 months before the screening visit, known hypersensitivity to the study drug (active substance or excipients) or eplerenone, or acute infectious disease requiring intravenous antibiotics within the last 24 hours before randomization Dialysis for acute renal failure within the previous 3 months before emergency presentation to hospital, or hepatic insufficiency (Child–Pugh B or C) Requirement for any intravenous vasodilator, any intravenous natriuretic peptide ≤ 24 hours before randomization, or any intravenous positive inotrope ≤ 14 days before dosing with study drug Treatment with either spironolactone or eplerenone that cannot be discontinued 48 hours or 24 hours, respectively, before randomization and for the duration of the treatment period Treatment with any renin inhibitor or potassium-sparing diuretic that cannot be discontinued 24 hours before randomization and for the duration of the treatment period Treatment with high-dose acetylsalicylic acid (> 500 mg/day) or another NSAID Treatment with potent CYP 3 A 4 inhibitors or inducers, or strong CYP 2 C 8 inhibitors (all must be stopped ≥ 7 days before randomization) Adapted from Pitt et al. Eur J Heart Fail 2015; 17: 224– 32 a. As defined by the European Society of Cardiology and American College of Cardiology/American Heart Association guidelines ACS, acute coronary syndromes; CYP, cytochrome P 450; NSAID, non-steroidal anti-inflammatory drug; TIA, transient ischaemic attack 23


