 • Results from recent EDQM/OMCL PTS Studies on B 19 Virus DNA NAT Testing of Plasma Pools • So. GAT Meeting, Berne, 14 June 2006 M. Nübling (PEI, Germany) • K. H. Buchheit (EDQM, Co. E) K. H. Buchheit, So. GAT 14/6/06
• Results from recent EDQM/OMCL PTS Studies on B 19 Virus DNA NAT Testing of Plasma Pools • So. GAT Meeting, Berne, 14 June 2006 M. Nübling (PEI, Germany) • K. H. Buchheit (EDQM, Co. E) K. H. Buchheit, So. GAT 14/6/06
 Background Ph. Eur. Requirements • NAT testing of plasma pools for manufacture of – anti-D Ig: since 1/1/04 – human plasma (pooled and virus inactivated): since 1/7/04 • B 19 virus DNA maximum: 104 IU/ml • 104 IU/ml positive control included • PTS studies once per year (start 2004) K. H. Buchheit, So. GAT 14/6/06 2
Background Ph. Eur. Requirements • NAT testing of plasma pools for manufacture of – anti-D Ig: since 1/1/04 – human plasma (pooled and virus inactivated): since 1/7/04 • B 19 virus DNA maximum: 104 IU/ml • 104 IU/ml positive control included • PTS studies once per year (start 2004) K. H. Buchheit, So. GAT 14/6/06 2
 Organisation • Organised by EDQM on behalf of OMCL network • Scientific Advisor: M. Nübling (PEI) • Open to OMCLs & manufacturers – OMCLs 10 - 13 – Manufacturers: 6 - 12 – List of participants not disclosed K. H. Buchheit, So. GAT 14/6/06 3
Organisation • Organised by EDQM on behalf of OMCL network • Scientific Advisor: M. Nübling (PEI) • Open to OMCLs & manufacturers – OMCLs 10 - 13 – Manufacturers: 6 - 12 – List of participants not disclosed K. H. Buchheit, So. GAT 14/6/06 3
 Materials & Methods • Panel (1 provided): – 10 test samples: 1. 2 ml (frozen) – Negative samples – B 19 samples: 107 - 102 IU/ml – A 6 samples: ≈106, 102 IU/ml • not known to participants • obtained from J. Blümel (PEI) • NAT Methods: routine in-house – Roche and Artus kits used K. H. Buchheit, So. GAT 14/6/06 4
Materials & Methods • Panel (1 provided): – 10 test samples: 1. 2 ml (frozen) – Negative samples – B 19 samples: 107 - 102 IU/ml – A 6 samples: ≈106, 102 IU/ml • not known to participants • obtained from J. Blümel (PEI) • NAT Methods: routine in-house – Roche and Artus kits used K. H. Buchheit, So. GAT 14/6/06 4
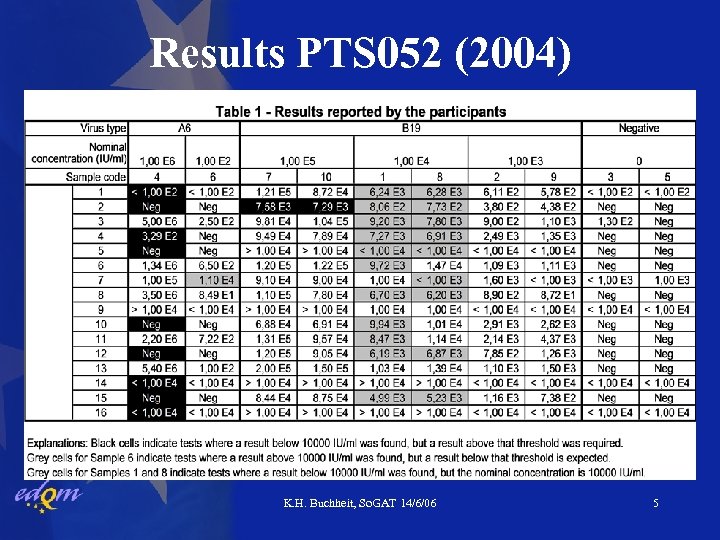 Results PTS 052 (2004) K. H. Buchheit, So. GAT 14/6/06 5
Results PTS 052 (2004) K. H. Buchheit, So. GAT 14/6/06 5
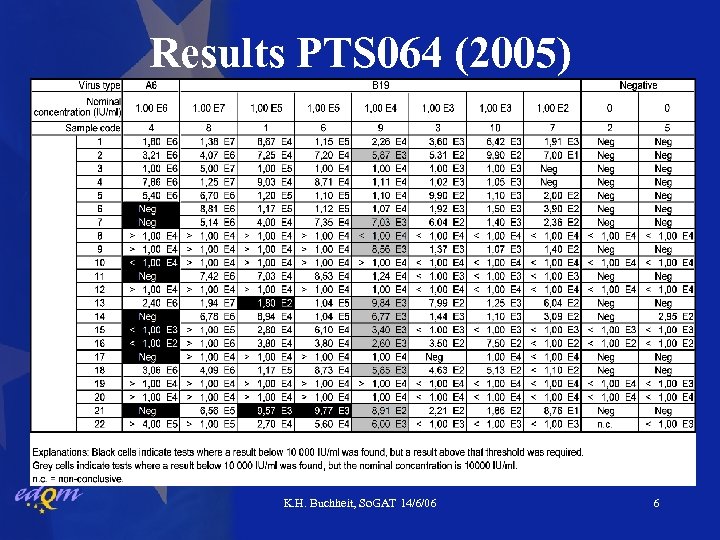 Results PTS 064 (2005) K. H. Buchheit, So. GAT 14/6/06 6
Results PTS 064 (2005) K. H. Buchheit, So. GAT 14/6/06 6
 Results PTS 052 & PTS 064 • Negative samples below 104 IU/ml by all labs • 103 IU/ml samples correctly classified “pass” by all • 1 -2 lab(s) underestimated 105 IU/ml samples • B 19 104 IU/ml samples tested “pass” in > 50% of tests (mind uncertainty about ‘true’ concentration) • 9 labs unable to detect A 6 variant virus at 106 IU/ml • Roche kit fails to amplify efficiently A 6 -variant • Artus kit does not have this problem K. H. Buchheit, So. GAT 14/6/06 7
Results PTS 052 & PTS 064 • Negative samples below 104 IU/ml by all labs • 103 IU/ml samples correctly classified “pass” by all • 1 -2 lab(s) underestimated 105 IU/ml samples • B 19 104 IU/ml samples tested “pass” in > 50% of tests (mind uncertainty about ‘true’ concentration) • 9 labs unable to detect A 6 variant virus at 106 IU/ml • Roche kit fails to amplify efficiently A 6 -variant • Artus kit does not have this problem K. H. Buchheit, So. GAT 14/6/06 7
 Conclusions • Majority of participating laboratories were not able to detect a sample containing 106 IU/ml of virus variant A 6 • For virus type B 19 performance is in general satisfactory (similar to HCVNAT PTS) K. H. Buchheit, So. GAT 14/6/06 8
Conclusions • Majority of participating laboratories were not able to detect a sample containing 106 IU/ml of virus variant A 6 • For virus type B 19 performance is in general satisfactory (similar to HCVNAT PTS) K. H. Buchheit, So. GAT 14/6/06 8