c4dbd1d19d19f98092e8ca443630320a.ppt
- Количество слайдов: 97
 Research Approvals- Ethical and Institutional Considerations/Requirements ONE UNIVERSITY. MANY FUTURES.
Research Approvals- Ethical and Institutional Considerations/Requirements ONE UNIVERSITY. MANY FUTURES.
 Objectives • To define and identify what constitutes human participant research • Understand the rationale behind the regulatory aspects of research involving humans and the role of the researcher in the process • Understand the role and jurisdiction of the U of Manitoba REB • Understand the guiding ethical principles and the REB’s application of these principals • Understand the REB submission requirements to conduct a research study ONE UNIVERSITY. MANY FUTURES.
Objectives • To define and identify what constitutes human participant research • Understand the rationale behind the regulatory aspects of research involving humans and the role of the researcher in the process • Understand the role and jurisdiction of the U of Manitoba REB • Understand the guiding ethical principles and the REB’s application of these principals • Understand the REB submission requirements to conduct a research study ONE UNIVERSITY. MANY FUTURES.
 History of Research Ethics The ethics of research is born from the cauldron of horror. ONE UNIVERSITY. MANY FUTURES.
History of Research Ethics The ethics of research is born from the cauldron of horror. ONE UNIVERSITY. MANY FUTURES.
 and it continued … • Tuskegee Syphilis Study(1932 -1972) • Jewish Chronic Hospital • Willowbrook Hospital • Thalidomide drug approval – 1960’s ONE UNIVERSITY. MANY FUTURES.
and it continued … • Tuskegee Syphilis Study(1932 -1972) • Jewish Chronic Hospital • Willowbrook Hospital • Thalidomide drug approval – 1960’s ONE UNIVERSITY. MANY FUTURES.
 More recently … ONE UNIVERSITY. MANY FUTURES.
More recently … ONE UNIVERSITY. MANY FUTURES.
 Other influences…. . ONE UNIVERSITY. MANY FUTURES.
Other influences…. . ONE UNIVERSITY. MANY FUTURES.
 Continued…. . ONE UNIVERSITY. MANY FUTURES.
Continued…. . ONE UNIVERSITY. MANY FUTURES.
 International Ethical Documents • Nuremberg Code • Declaration of Helsinki • CIOMS – International Ethical Guidelines for Biomedical Research – Epidemiological • Belmont Report • Common Rule ONE UNIVERSITY. MANY FUTURES.
International Ethical Documents • Nuremberg Code • Declaration of Helsinki • CIOMS – International Ethical Guidelines for Biomedical Research – Epidemiological • Belmont Report • Common Rule ONE UNIVERSITY. MANY FUTURES.
 CANADA’s Ethical document Tri-Council Policy Statement(TCPS) 2: Ethical Conduct for Research Involving Humans” Constitutes the joint policy document for Canada’s three federal research agencies 1. 2. 3. Canadian Institutes of Health Research (CIHR) Natural Sciences and Engineering Research Council of Canada (NSERC) Social Sciences and Humanities Research Council of Canada (SSHRC) ONE UNIVERSITY. MANY FUTURES.
CANADA’s Ethical document Tri-Council Policy Statement(TCPS) 2: Ethical Conduct for Research Involving Humans” Constitutes the joint policy document for Canada’s three federal research agencies 1. 2. 3. Canadian Institutes of Health Research (CIHR) Natural Sciences and Engineering Research Council of Canada (NSERC) Social Sciences and Humanities Research Council of Canada (SSHRC) ONE UNIVERSITY. MANY FUTURES.
 History of TCPS • Each granting agency had an ethical document • Underlying ethical principles common across all disciplines • Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans(1998) A living document … • Adopted Canadian wide for all research • TCPS 2 – first comprehensive revision ONE UNIVERSITY. MANY FUTURES.
History of TCPS • Each granting agency had an ethical document • Underlying ethical principles common across all disciplines • Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans(1998) A living document … • Adopted Canadian wide for all research • TCPS 2 – first comprehensive revision ONE UNIVERSITY. MANY FUTURES.
 Privacy Documents • Personal Health Information Act of Manitoba (PHIA) • The Manitoba Freedom of Information and Protection of Privacy (FIPPA) • Personal Information Protection and Electronic Documents Act (PIPEDA) • The Health Insurance and Portability Act (HIPPA)– US • CIHR Best Practices for Protecting Privacy In Health Research (Sept 2005) ONE UNIVERSITY. MANY FUTURES.
Privacy Documents • Personal Health Information Act of Manitoba (PHIA) • The Manitoba Freedom of Information and Protection of Privacy (FIPPA) • Personal Information Protection and Electronic Documents Act (PIPEDA) • The Health Insurance and Portability Act (HIPPA)– US • CIHR Best Practices for Protecting Privacy In Health Research (Sept 2005) ONE UNIVERSITY. MANY FUTURES.
 Regulatory Guidelines and Legislation • • • University of Manitoba’s Policy (2011) Health Canada- Medical Device Regulations (1998) Health Canada – Food and Drug Regulations (2001) Health Canada - Natural Health Product Regulations (2004) FDA guidelines(1991) ICH -GCP Guidelines(2000) Federal Wide Assurance FWA (2002) Mental Health Act Health Care Directive Act Tissue Act Etc. ONE UNIVERSITY. MANY FUTURES.
Regulatory Guidelines and Legislation • • • University of Manitoba’s Policy (2011) Health Canada- Medical Device Regulations (1998) Health Canada – Food and Drug Regulations (2001) Health Canada - Natural Health Product Regulations (2004) FDA guidelines(1991) ICH -GCP Guidelines(2000) Federal Wide Assurance FWA (2002) Mental Health Act Health Care Directive Act Tissue Act Etc. ONE UNIVERSITY. MANY FUTURES.
 Regulatory approval - Health Canada • When the study involves the use of investigational drugs/devices/natural health products or the use of marketed drugs/devices/ (including natural health products) outside of their approved indication(s)(e. g. new age group, new disease entity or new dose range). • REB will require the “Letter of No Objection” from Health Canada prior to final approval. ONE UNIVERSITY. MANY FUTURES. 13
Regulatory approval - Health Canada • When the study involves the use of investigational drugs/devices/natural health products or the use of marketed drugs/devices/ (including natural health products) outside of their approved indication(s)(e. g. new age group, new disease entity or new dose range). • REB will require the “Letter of No Objection” from Health Canada prior to final approval. ONE UNIVERSITY. MANY FUTURES. 13
 Is this approval required for Investigator Initiated Trials? Health Canada keen on auditing these type of trials during 2006 REB audit. Clinical Trial Application(CTA) – Similar requirements to sponsor initiated trials – Investigator has responsibility to have monitoring in place ONE UNIVERSITY. MANY FUTURES. 14
Is this approval required for Investigator Initiated Trials? Health Canada keen on auditing these type of trials during 2006 REB audit. Clinical Trial Application(CTA) – Similar requirements to sponsor initiated trials – Investigator has responsibility to have monitoring in place ONE UNIVERSITY. MANY FUTURES. 14
 Clinical Trial Registration “All clinical trials shall be registered before recruitment of the first trial participant in a recognized and easily web-accessible public registry. ” Article 11. 3 ONE UNIVERSITY. MANY FUTURES.
Clinical Trial Registration “All clinical trials shall be registered before recruitment of the first trial participant in a recognized and easily web-accessible public registry. ” Article 11. 3 ONE UNIVERSITY. MANY FUTURES.
 Clinical Trials Definition “ A form of clinical research (also known as patient-oriented research), is any investigation involving participants that evaluates the effects of one or more health-related interventions on health outcomes. Interventions include, but are not restricted to, drugs, radiopharmaceuticals, cells and other biological products, surgical procedures, radiologic procedures, devices, genetic therapies, natural health products, process-of-care changes, preventive care, manual therapies and psychotherapies. Clinical trials may also include questions that are not directly related to therapeutic goals – for example, drug metabolism – in addition to those that directly evaluate the treatment of participants. ” ONE UNIVERSITY. MANY FUTURES.
Clinical Trials Definition “ A form of clinical research (also known as patient-oriented research), is any investigation involving participants that evaluates the effects of one or more health-related interventions on health outcomes. Interventions include, but are not restricted to, drugs, radiopharmaceuticals, cells and other biological products, surgical procedures, radiologic procedures, devices, genetic therapies, natural health products, process-of-care changes, preventive care, manual therapies and psychotherapies. Clinical trials may also include questions that are not directly related to therapeutic goals – for example, drug metabolism – in addition to those that directly evaluate the treatment of participants. ” ONE UNIVERSITY. MANY FUTURES.
 Why and Where of Registration? • The collective goal is to reduce publication bias, and reduce publication bias prevent the suppression of data in clinical research. • In a Registry compliant with the criteria set by the with the criteria World Health Organization (WHO)2 or International Committee of Medical Journal Editors (ICMJE) (ICMJE • Researchers shall provide the REB with the number assigned to the trial upon registration. • Done by Lead Investigators or Pharmaceutical sponsors ONE UNIVERSITY. MANY FUTURES.
Why and Where of Registration? • The collective goal is to reduce publication bias, and reduce publication bias prevent the suppression of data in clinical research. • In a Registry compliant with the criteria set by the with the criteria World Health Organization (WHO)2 or International Committee of Medical Journal Editors (ICMJE) (ICMJE • Researchers shall provide the REB with the number assigned to the trial upon registration. • Done by Lead Investigators or Pharmaceutical sponsors ONE UNIVERSITY. MANY FUTURES.
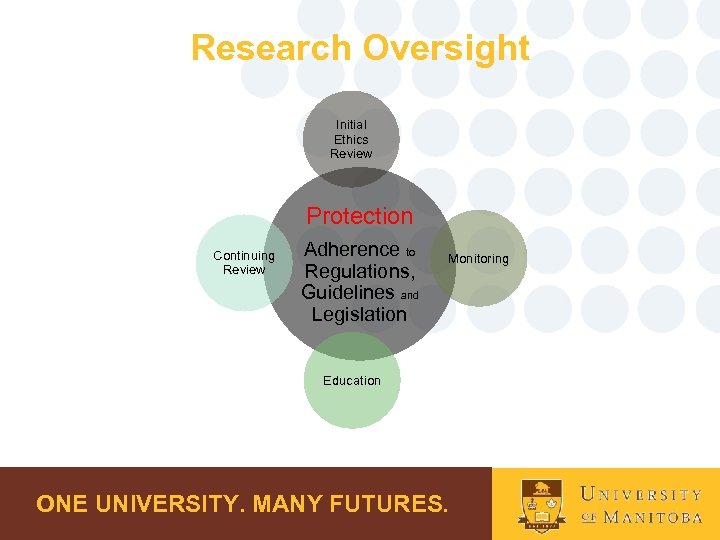 Research Oversight Initial Ethics Review Protection Continuing Review Adherence to Regulations, Guidelines and Legislation Monitoring Education ONE UNIVERSITY. MANY FUTURES.
Research Oversight Initial Ethics Review Protection Continuing Review Adherence to Regulations, Guidelines and Legislation Monitoring Education ONE UNIVERSITY. MANY FUTURES.
 Roles of Research Ethics Boards • To protect the rights, safety, and well being of research participants • Independent/Multidisciplinary review • To protect the rights of researchers • To ensure accountability to society to meet the highest ethical and scientific standards • To serve the research community as a consultant body - EDUCATION of research ethics ONE UNIVERSITY. MANY FUTURES.
Roles of Research Ethics Boards • To protect the rights, safety, and well being of research participants • Independent/Multidisciplinary review • To protect the rights of researchers • To ensure accountability to society to meet the highest ethical and scientific standards • To serve the research community as a consultant body - EDUCATION of research ethics ONE UNIVERSITY. MANY FUTURES.
 Investigator Responsibilities • • Primary responsibility for the protection of participants Obtaining “initial” and “ongoing” approvals Ensuring staff are properly trained Ensuring the confidentiality of participant data Conduct scientifically valid research Abide by decisions of REB Promptly report to Chair of REB any injuries, unanticipated problems, non-compliance with requirements or procedures stipulated by REB • Etc. ONE UNIVERSITY. MANY FUTURES.
Investigator Responsibilities • • Primary responsibility for the protection of participants Obtaining “initial” and “ongoing” approvals Ensuring staff are properly trained Ensuring the confidentiality of participant data Conduct scientifically valid research Abide by decisions of REB Promptly report to Chair of REB any injuries, unanticipated problems, non-compliance with requirements or procedures stipulated by REB • Etc. ONE UNIVERSITY. MANY FUTURES.
 When is Ethics Review Required? “Research” involving “human participants” “research” is defined as an undertaking intended to extend knowledge through a disciplined inquiry or systematic investigation. “human participants” are those individuals whose data, or responses to interventions, stimuli or questions by the researcher, are relevant to answering the research questions. ONE UNIVERSITY. MANY FUTURES.
When is Ethics Review Required? “Research” involving “human participants” “research” is defined as an undertaking intended to extend knowledge through a disciplined inquiry or systematic investigation. “human participants” are those individuals whose data, or responses to interventions, stimuli or questions by the researcher, are relevant to answering the research questions. ONE UNIVERSITY. MANY FUTURES.
 Research involving Human Participants includes …. • Information collected through interventions or interaction with living individuals • Human biological materials(organs, tissues, body fluids), human embryos, fetal tissues, reproductive material and stem cells (including those acquired incidentally or left over) from living and deceased individuals • Written/recorded information derived from the individuals • Secondary use of data ONE UNIVERSITY. MANY FUTURES.
Research involving Human Participants includes …. • Information collected through interventions or interaction with living individuals • Human biological materials(organs, tissues, body fluids), human embryos, fetal tissues, reproductive material and stem cells (including those acquired incidentally or left over) from living and deceased individuals • Written/recorded information derived from the individuals • Secondary use of data ONE UNIVERSITY. MANY FUTURES.
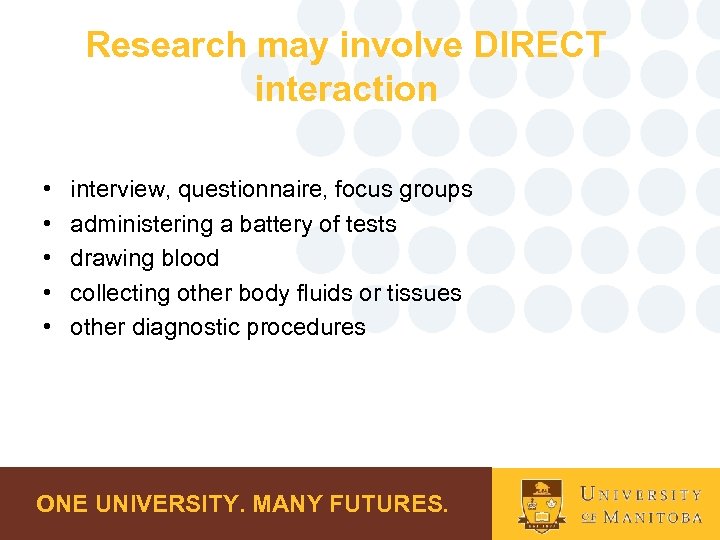 Research may involve DIRECT interaction • • • interview, questionnaire, focus groups administering a battery of tests drawing blood collecting other body fluids or tissues other diagnostic procedures ONE UNIVERSITY. MANY FUTURES.
Research may involve DIRECT interaction • • • interview, questionnaire, focus groups administering a battery of tests drawing blood collecting other body fluids or tissues other diagnostic procedures ONE UNIVERSITY. MANY FUTURES.
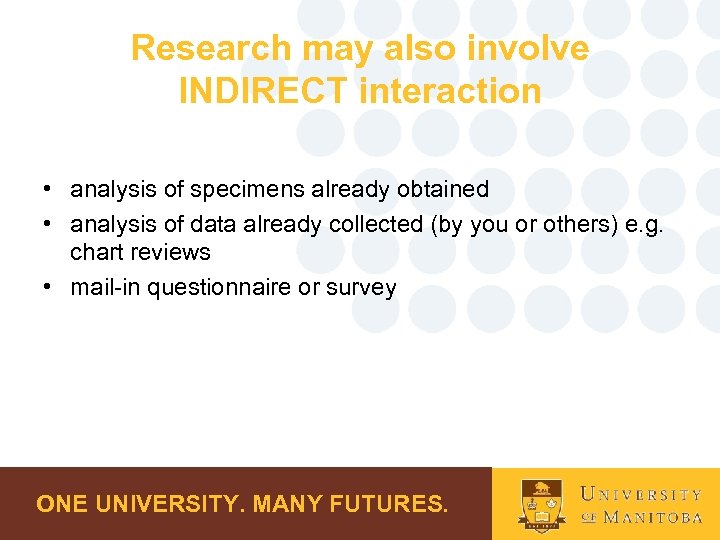 Research may also involve INDIRECT interaction • analysis of specimens already obtained • analysis of data already collected (by you or others) e. g. chart reviews • mail-in questionnaire or survey ONE UNIVERSITY. MANY FUTURES.
Research may also involve INDIRECT interaction • analysis of specimens already obtained • analysis of data already collected (by you or others) e. g. chart reviews • mail-in questionnaire or survey ONE UNIVERSITY. MANY FUTURES.
 What is Human Research to the UM? “Human Research refers to any project that involves the collection of specimens, data or information from persons, through interventions or otherwise. Included are procedures that have low degree of invasiveness (e. g. surveys, interviews…. examination of patient records) as well as more invasive procedures (e. g. blood sampling, insertion of cannula administration of a substance). ” University of Manitoba Policy ONE UNIVERSITY. MANY FUTURES.
What is Human Research to the UM? “Human Research refers to any project that involves the collection of specimens, data or information from persons, through interventions or otherwise. Included are procedures that have low degree of invasiveness (e. g. surveys, interviews…. examination of patient records) as well as more invasive procedures (e. g. blood sampling, insertion of cannula administration of a substance). ” University of Manitoba Policy ONE UNIVERSITY. MANY FUTURES.
 Is it Research or not? • Research versus practice? • Quality assurance or program evaluation studies do not need formal ethics approval but. . . • Is there an “intent” to produce generalizable knowledge beyond your practice or institution? Is there an intent to publish? • Distinguishing Research from Case/Professional Skill Development • Researchers responsibility to contact the Research Ethics Board if there is any doubt (written request). ONE UNIVERSITY. MANY FUTURES.
Is it Research or not? • Research versus practice? • Quality assurance or program evaluation studies do not need formal ethics approval but. . . • Is there an “intent” to produce generalizable knowledge beyond your practice or institution? Is there an intent to publish? • Distinguishing Research from Case/Professional Skill Development • Researchers responsibility to contact the Research Ethics Board if there is any doubt (written request). ONE UNIVERSITY. MANY FUTURES.
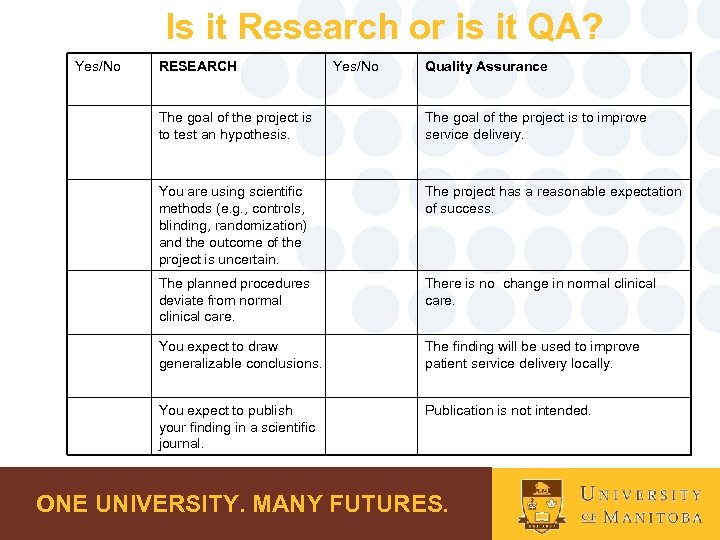 Is it Research or is it QA? Yes/No RESEARCH Yes/No Quality Assurance The goal of the project is to test an hypothesis. The goal of the project is to improve service delivery. You are using scientific methods (e. g. , controls, blinding, randomization) and the outcome of the project is uncertain. The project has a reasonable expectation of success. The planned procedures deviate from normal clinical care. There is no change in normal clinical care. You expect to draw generalizable conclusions. The finding will be used to improve patient service delivery locally. You expect to publish your finding in a scientific journal. Publication is not intended. ONE UNIVERSITY. MANY FUTURES.
Is it Research or is it QA? Yes/No RESEARCH Yes/No Quality Assurance The goal of the project is to test an hypothesis. The goal of the project is to improve service delivery. You are using scientific methods (e. g. , controls, blinding, randomization) and the outcome of the project is uncertain. The project has a reasonable expectation of success. The planned procedures deviate from normal clinical care. There is no change in normal clinical care. You expect to draw generalizable conclusions. The finding will be used to improve patient service delivery locally. You expect to publish your finding in a scientific journal. Publication is not intended. ONE UNIVERSITY. MANY FUTURES.
 If in Doubt…. • If in doubt, about whether the REB needs to review something, or whether consent needs to be obtained from participants. ASK, don't assume. • Outline protocol summary in e-mail or written summary to REB coordinator ONE UNIVERSITY. MANY FUTURES.
If in Doubt…. • If in doubt, about whether the REB needs to review something, or whether consent needs to be obtained from participants. ASK, don't assume. • Outline protocol summary in e-mail or written summary to REB coordinator ONE UNIVERSITY. MANY FUTURES.
 What about CASE Studies? • Our general approach to case reports is to request that authors obtain the patient’s consent whenever possible. , write up the case study, and then submit it to the Health Research Ethics Board (HREB) before sending if off for publication. • We review the case report largely to assess the risk that patient identity may be inadvertently revealed by the author(s) in the write-up. This is our primary concern in this situation. If the probability of inadvertent identification of an individual based on the write-up is judged to be low we generally approve it. ONE UNIVERSITY. MANY FUTURES.
What about CASE Studies? • Our general approach to case reports is to request that authors obtain the patient’s consent whenever possible. , write up the case study, and then submit it to the Health Research Ethics Board (HREB) before sending if off for publication. • We review the case report largely to assess the risk that patient identity may be inadvertently revealed by the author(s) in the write-up. This is our primary concern in this situation. If the probability of inadvertent identification of an individual based on the write-up is judged to be low we generally approve it. ONE UNIVERSITY. MANY FUTURES.
 CASE Studies continued…. • If we think the probability of individuals identification is unacceptably high (e. g. very rare condition, a lot of demographic data is provided in the write up, etc. ) we will request changes (that hopefully don’t result in the loss of critical information) designed to lower the risk of inadvertent individual identification from the publication of the case report. • In cases in which patient/family consent is either impossible or extremely difficult (and thus quite impractical to obtain) as well as in cases in which attempting to obtain consent from a living or from the family of deceased patient would be too traumatic for those involved we may waive the requirement for consent on a case by case basis. ONE UNIVERSITY. MANY FUTURES.
CASE Studies continued…. • If we think the probability of individuals identification is unacceptably high (e. g. very rare condition, a lot of demographic data is provided in the write up, etc. ) we will request changes (that hopefully don’t result in the loss of critical information) designed to lower the risk of inadvertent individual identification from the publication of the case report. • In cases in which patient/family consent is either impossible or extremely difficult (and thus quite impractical to obtain) as well as in cases in which attempting to obtain consent from a living or from the family of deceased patient would be too traumatic for those involved we may waive the requirement for consent on a case by case basis. ONE UNIVERSITY. MANY FUTURES.
 U of M REB Jurisdiction • Individuals with an affiliation with the University of Manitoba* • U of MB REB approval must be obtained for research conducted anywhere in the world • WRHA researchers • *This includes trainees, employees, GFT's, individuals with academic appointments, visiting professors, students, etc. ONE UNIVERSITY. MANY FUTURES.
U of M REB Jurisdiction • Individuals with an affiliation with the University of Manitoba* • U of MB REB approval must be obtained for research conducted anywhere in the world • WRHA researchers • *This includes trainees, employees, GFT's, individuals with academic appointments, visiting professors, students, etc. ONE UNIVERSITY. MANY FUTURES.
 U of M Research Ethics Boards • Bannatyne Campus – Health Research Ethics Board – Biomedical Research Ethics Board • Fort Gary Campus – Education and Nursing Research Ethics Board – Joint Faculty Research Ethics Board – Psychology and Sociology Research Ethics Board ONE UNIVERSITY. MANY FUTURES.
U of M Research Ethics Boards • Bannatyne Campus – Health Research Ethics Board – Biomedical Research Ethics Board • Fort Gary Campus – Education and Nursing Research Ethics Board – Joint Faculty Research Ethics Board – Psychology and Sociology Research Ethics Board ONE UNIVERSITY. MANY FUTURES.
 Who can submit to Bannatyne Campus? • Affiliation with U of M – – Faculty of Medicine Faculty of Pharmacy Faculty of Dentistry School of Med Rehab • WRHA researcher – Indemnification agreement between U of M and WRHA ONE UNIVERSITY. MANY FUTURES.
Who can submit to Bannatyne Campus? • Affiliation with U of M – – Faculty of Medicine Faculty of Pharmacy Faculty of Dentistry School of Med Rehab • WRHA researcher – Indemnification agreement between U of M and WRHA ONE UNIVERSITY. MANY FUTURES.
 Which REB to submit to? Biomedical Research Ethics Board (BREB) Research protocols involving clinical trials and other biomedical research interventions. Health Research Ethics Board(HREB) Research protocols involving behavioral sciences, databases, surveys, registries, specimens collection/banking, examination of medical records and protocols of generally lesser risk. ONE UNIVERSITY. MANY FUTURES.
Which REB to submit to? Biomedical Research Ethics Board (BREB) Research protocols involving clinical trials and other biomedical research interventions. Health Research Ethics Board(HREB) Research protocols involving behavioral sciences, databases, surveys, registries, specimens collection/banking, examination of medical records and protocols of generally lesser risk. ONE UNIVERSITY. MANY FUTURES.
 Determining Level of Review “In keeping with a proportionate approach to research ethics review, the selection of the level of REB review shall be determined by the level of foreseeable risks to participants…” TCPS 2 - Article 6. 12 ONE UNIVERSITY. MANY FUTURES.
Determining Level of Review “In keeping with a proportionate approach to research ethics review, the selection of the level of REB review shall be determined by the level of foreseeable risks to participants…” TCPS 2 - Article 6. 12 ONE UNIVERSITY. MANY FUTURES.
 Two Levels of Review Full Review • Review by the full committee is the default requirement Delegated Review • Review delegated to Chair or select REB members for minimal risk studies • Departmental review for student course based projects –Terms of Reference approval required by main REB. • Minimal Risk criteria set by board • Chair may request Full board ONE UNIVERSITY. MANY FUTURES.
Two Levels of Review Full Review • Review by the full committee is the default requirement Delegated Review • Review delegated to Chair or select REB members for minimal risk studies • Departmental review for student course based projects –Terms of Reference approval required by main REB. • Minimal Risk criteria set by board • Chair may request Full board ONE UNIVERSITY. MANY FUTURES.
 Criteria for initial Delegated Review • Analysis of existing anonymous, anonymized or deidentified data/records or biological samples in custody of a well established tumor/tissues bank (previous consent for such research was sought). • Retrospective records reviews (database and charts). • Most Mailed returned survey – if questions are not unduly alarming or sensitive. • Secondary analysis of data from a previously approved study. • Request to create a permission to contact for future research database where no clinical information is collected. • On case by case basis – minimal risk studies for participants that are not considered vulnerable ONE UNIVERSITY. MANY FUTURES.
Criteria for initial Delegated Review • Analysis of existing anonymous, anonymized or deidentified data/records or biological samples in custody of a well established tumor/tissues bank (previous consent for such research was sought). • Retrospective records reviews (database and charts). • Most Mailed returned survey – if questions are not unduly alarming or sensitive. • Secondary analysis of data from a previously approved study. • Request to create a permission to contact for future research database where no clinical information is collected. • On case by case basis – minimal risk studies for participants that are not considered vulnerable ONE UNIVERSITY. MANY FUTURES.
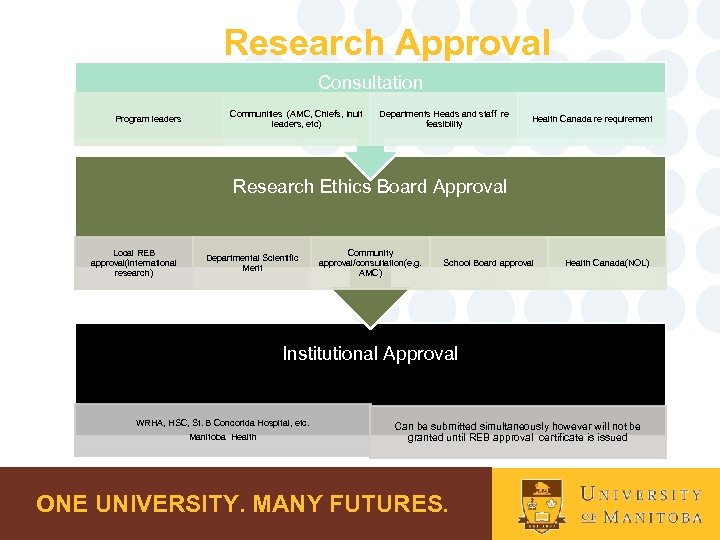 Research Approval Consultation Program leaders Communities (AMC, Chiefs, Inuit leaders, etc) Departments Heads and staff re feasibility Health Canada re requirement Research Ethics Board Approval Local REB approval(international research) Departmental Scientific Merit Community approval/consultation(e. g. AMC) School Board approval Health Canada(NOL) Institutional Approval WRHA, HSC, St. B Concorida Hospital, etc. Manitoba Health Can be submitted simultaneously however will not be granted until REB approval certificate is issued ONE UNIVERSITY. MANY FUTURES.
Research Approval Consultation Program leaders Communities (AMC, Chiefs, Inuit leaders, etc) Departments Heads and staff re feasibility Health Canada re requirement Research Ethics Board Approval Local REB approval(international research) Departmental Scientific Merit Community approval/consultation(e. g. AMC) School Board approval Health Canada(NOL) Institutional Approval WRHA, HSC, St. B Concorida Hospital, etc. Manitoba Health Can be submitted simultaneously however will not be granted until REB approval certificate is issued ONE UNIVERSITY. MANY FUTURES.
 I want to conduct Research – what do I need to do? • Design ethical and scientifically valid protocol • Collaborate with agencies or groups to assist with logistics of conducting research – E. g. Is Health Canada approval required? – Is aboriginal consultation required? • Check out submission deadlines • Prepare ethics submission • Prepare institutional approval ONE UNIVERSITY. MANY FUTURES.
I want to conduct Research – what do I need to do? • Design ethical and scientifically valid protocol • Collaborate with agencies or groups to assist with logistics of conducting research – E. g. Is Health Canada approval required? – Is aboriginal consultation required? • Check out submission deadlines • Prepare ethics submission • Prepare institutional approval ONE UNIVERSITY. MANY FUTURES.
 Research Ethics Board Approval Where to begin? Submission requirements on Bannatyne Campus website: – – – – – Cover page – listing all documents(date all documents) REB submission form(Main or Retrospective review form) Protocol Informed Consent or Disclosure of Consent statement for survey research Recruitment scripts, letters of invitation Questionnaires, survey, instruments, etc. CV template required each calendar year (Do not submit full CV) Investigators Brochure(if applicable) Health Canada approval letter (if applicable) Core Tutorial – required as of September 1, 2011 ONE UNIVERSITY. MANY FUTURES.
Research Ethics Board Approval Where to begin? Submission requirements on Bannatyne Campus website: – – – – – Cover page – listing all documents(date all documents) REB submission form(Main or Retrospective review form) Protocol Informed Consent or Disclosure of Consent statement for survey research Recruitment scripts, letters of invitation Questionnaires, survey, instruments, etc. CV template required each calendar year (Do not submit full CV) Investigators Brochure(if applicable) Health Canada approval letter (if applicable) Core Tutorial – required as of September 1, 2011 ONE UNIVERSITY. MANY FUTURES.
 U of M Educational Requirements ONE UNIVERSITY. MANY FUTURES.
U of M Educational Requirements ONE UNIVERSITY. MANY FUTURES.
 REB Submission Form – WHY? Purpose • study registration • summary for all members • describe details not found in all protocols – recruitment/consent issues – PHIA and privacy issues – budget – conflict of interest – etc. • guidance notes for researcher • direct links to Bannatyne Campus guidelines and policies ONE UNIVERSITY. MANY FUTURES.
REB Submission Form – WHY? Purpose • study registration • summary for all members • describe details not found in all protocols – recruitment/consent issues – PHIA and privacy issues – budget – conflict of interest – etc. • guidance notes for researcher • direct links to Bannatyne Campus guidelines and policies ONE UNIVERSITY. MANY FUTURES.
 Guiding Ethical Principals – TCPS 2 • Respect for Persons • Concern for Welfare • Justice ONE UNIVERSITY. MANY FUTURES.
Guiding Ethical Principals – TCPS 2 • Respect for Persons • Concern for Welfare • Justice ONE UNIVERSITY. MANY FUTURES.
 Principals into Practice The Research Ethics Board Must Assess: • • • Quality of Science Risks and Potential Benefits Informed Consent Recruitment and Retention of Participants Confidentiality and Privacy Qualifications of Investigator Budget Potential Conflicts of Interest Compliance with Ethical, Privacy Legislation and Regulatory Guidelines ONE UNIVERSITY. MANY FUTURES.
Principals into Practice The Research Ethics Board Must Assess: • • • Quality of Science Risks and Potential Benefits Informed Consent Recruitment and Retention of Participants Confidentiality and Privacy Qualifications of Investigator Budget Potential Conflicts of Interest Compliance with Ethical, Privacy Legislation and Regulatory Guidelines ONE UNIVERSITY. MANY FUTURES.
 Quality of Science Two Different Questions: • Is the design capable of answering the research questions? • Is the research question worthwhile? What needs to be assessed: • Rational and Background • Design (power, sample size, monitoring) • Clinical equipoise ONE UNIVERSITY. MANY FUTURES.
Quality of Science Two Different Questions: • Is the design capable of answering the research questions? • Is the research question worthwhile? What needs to be assessed: • Rational and Background • Design (power, sample size, monitoring) • Clinical equipoise ONE UNIVERSITY. MANY FUTURES.
 Risks and Potential Benefits • Identifying and minimizing risks • Physical, psychological, social, etc. • Individual, family and community risks • Maximizing benefits • Comparing risks and potential benefits • Quantifying risks by likelihood of occurrence thus ensuring patients/participants are well informed. • Not less than “Standard Care” • Placebos • Are they justified? Ethical and Scientific justifications. ONE UNIVERSITY. MANY FUTURES.
Risks and Potential Benefits • Identifying and minimizing risks • Physical, psychological, social, etc. • Individual, family and community risks • Maximizing benefits • Comparing risks and potential benefits • Quantifying risks by likelihood of occurrence thus ensuring patients/participants are well informed. • Not less than “Standard Care” • Placebos • Are they justified? Ethical and Scientific justifications. ONE UNIVERSITY. MANY FUTURES.
 Monitoring Safety and Reporting New Information “Researchers shall provide the REB with an acceptable plan for monitoring the safety of participants, including a plan for the tabulation, analysis and reporting of safety data, and the sharing of other new information in a form that permits REBs to interpret and respond appropriately. ” Article 11. 7 – Researchers need to develop well defined procedures in protocols ONE UNIVERSITY. MANY FUTURES.
Monitoring Safety and Reporting New Information “Researchers shall provide the REB with an acceptable plan for monitoring the safety of participants, including a plan for the tabulation, analysis and reporting of safety data, and the sharing of other new information in a form that permits REBs to interpret and respond appropriately. ” Article 11. 7 – Researchers need to develop well defined procedures in protocols ONE UNIVERSITY. MANY FUTURES.
 Monitoring Safety and Reporting New Information “REBs shall develop procedures to review safety reports and other new information arising from clinical trials that may affect the welfare or consent of participants, and to take appropriate steps in response. ” Article 11. 9 – Educational audit identified appropriate SOPS however the processes of review at UM REB review were not well documented – therefore new forms developed ONE UNIVERSITY. MANY FUTURES.
Monitoring Safety and Reporting New Information “REBs shall develop procedures to review safety reports and other new information arising from clinical trials that may affect the welfare or consent of participants, and to take appropriate steps in response. ” Article 11. 9 – Educational audit identified appropriate SOPS however the processes of review at UM REB review were not well documented – therefore new forms developed ONE UNIVERSITY. MANY FUTURES.
 Recruitment Who is being recruited? • Inclusion/exclusion criteria • Vulnerability of participants How is recruitment done and by whom? • From the researchers own clinical practice/workplace/classroom? – undue influence • By referral from other clinicians? – Confidentiality of clinical records, etc. and first contact – Does researcher have access to eligibility and contact information? • Advertising – ? incentives – undue influence ONE UNIVERSITY. MANY FUTURES.
Recruitment Who is being recruited? • Inclusion/exclusion criteria • Vulnerability of participants How is recruitment done and by whom? • From the researchers own clinical practice/workplace/classroom? – undue influence • By referral from other clinicians? – Confidentiality of clinical records, etc. and first contact – Does researcher have access to eligibility and contact information? • Advertising – ? incentives – undue influence ONE UNIVERSITY. MANY FUTURES.
 Informed Consent. A document or a process? Free and informed consent lies at the heart of ethical research involving human subjects. It encompasses a process that begins with the initial contact and carries through to the end of the involvement of research subjects in the project……refers to the dialogue, information sharing and general process through which prospective participants choose to participate in research involving themselves. Tri-Council Policy Statement ONE UNIVERSITY. MANY FUTURES.
Informed Consent. A document or a process? Free and informed consent lies at the heart of ethical research involving human subjects. It encompasses a process that begins with the initial contact and carries through to the end of the involvement of research subjects in the project……refers to the dialogue, information sharing and general process through which prospective participants choose to participate in research involving themselves. Tri-Council Policy Statement ONE UNIVERSITY. MANY FUTURES.
 Informed Consent- Questions we ask? • Is the request for a waiver of consent justified? • Is the participant competent? • Is the participant informed? – Required elements of informed consent – Confirm understanding • Is it voluntary? – Potential for therapeutic misconception – REB informed consent guidelines ONE UNIVERSITY. MANY FUTURES.
Informed Consent- Questions we ask? • Is the request for a waiver of consent justified? • Is the participant competent? • Is the participant informed? – Required elements of informed consent – Confirm understanding • Is it voluntary? – Potential for therapeutic misconception – REB informed consent guidelines ONE UNIVERSITY. MANY FUTURES.
 Confidentiality and Privacy The REB plays an important role in balancing the need for research against infringements of privacy and minimizing the necessary invasions of privacy. Individuals should be protected from harm caused by unauthorized use of personal information in which they believed they had an expectation of privacy and the benefit of confidentiality. Tri-Council Policy Statement ONE UNIVERSITY. MANY FUTURES.
Confidentiality and Privacy The REB plays an important role in balancing the need for research against infringements of privacy and minimizing the necessary invasions of privacy. Individuals should be protected from harm caused by unauthorized use of personal information in which they believed they had an expectation of privacy and the benefit of confidentiality. Tri-Council Policy Statement ONE UNIVERSITY. MANY FUTURES.
 Confidentiality and Privacy- the Questions we ask? • Necessity of Personal Data? • Consideration of Individuals’ Expectations • What are the safeguards for security and confidentiality? – Organizational, Technological, Physical • Provisions of confidentiality of data? • Adequate consent? • Anticipated secondary use of identifiable data? • Any data linkage? ONE UNIVERSITY. MANY FUTURES.
Confidentiality and Privacy- the Questions we ask? • Necessity of Personal Data? • Consideration of Individuals’ Expectations • What are the safeguards for security and confidentiality? – Organizational, Technological, Physical • Provisions of confidentiality of data? • Adequate consent? • Anticipated secondary use of identifiable data? • Any data linkage? ONE UNIVERSITY. MANY FUTURES.
 Types of Biological Materials (and DATA) • Identified human biological materials – the materials are labelled with a direct identifier (e. g. , name, personal health number). • Coded human biological materials – direct identifiers are removed from the materials and replaced with a code. • Anonymized human biological materials – the materials are irrevocably stripped of direct identifiers, a code is not kept to allow future re-linkage, … • Anonymous human biological materials – the materials never had identifiers attached to them and risk of identification of individuals is low or very low. ONE UNIVERSITY. MANY FUTURES.
Types of Biological Materials (and DATA) • Identified human biological materials – the materials are labelled with a direct identifier (e. g. , name, personal health number). • Coded human biological materials – direct identifiers are removed from the materials and replaced with a code. • Anonymized human biological materials – the materials are irrevocably stripped of direct identifiers, a code is not kept to allow future re-linkage, … • Anonymous human biological materials – the materials never had identifiers attached to them and risk of identification of individuals is low or very low. ONE UNIVERSITY. MANY FUTURES.
 Institutional Responsibilities “Institutions or organizations where research data are held have a responsibility to establish appropriate institutional security safeguards. ” Article 5. 4 • additional to researchers duties to protect data, safeguards must be in place at the institutional or organizational level – physical, administrative and technical measures, and should address the full life cycle of information. – E. g. PHIA pledges, firewalls, etc. • more emphasis on privacy impact assessment. – Data sharing agreements ONE UNIVERSITY. MANY FUTURES.
Institutional Responsibilities “Institutions or organizations where research data are held have a responsibility to establish appropriate institutional security safeguards. ” Article 5. 4 • additional to researchers duties to protect data, safeguards must be in place at the institutional or organizational level – physical, administrative and technical measures, and should address the full life cycle of information. – E. g. PHIA pledges, firewalls, etc. • more emphasis on privacy impact assessment. – Data sharing agreements ONE UNIVERSITY. MANY FUTURES.
 Why review Budgets? • Issues of recruitment and retention. – Do payments to investigators give incentive for undue influence? – Higher payments for final visits? • Issues of quality of science – Is there sufficient funds to complete the study? – Do payments give incentive to stretch the inclusion criteria? (ie recruitment bonuses, finders fees) • Are any of the costs of research being shifted into public health care system? ONE UNIVERSITY. MANY FUTURES.
Why review Budgets? • Issues of recruitment and retention. – Do payments to investigators give incentive for undue influence? – Higher payments for final visits? • Issues of quality of science – Is there sufficient funds to complete the study? – Do payments give incentive to stretch the inclusion criteria? (ie recruitment bonuses, finders fees) • Are any of the costs of research being shifted into public health care system? ONE UNIVERSITY. MANY FUTURES.
 Financial Conflicts of Interest “REBs shall ensure that clinical trial budgets are reviewed to ensure that conflicts of interest are identified and minimized, or otherwise managed. ” Article 11. 11 – More clear guidance that budget require review…but may be delegated. – US Regulations require often separate committees and detailed disclosures from researchers. Trend appearing in Canada. ONE UNIVERSITY. MANY FUTURES.
Financial Conflicts of Interest “REBs shall ensure that clinical trial budgets are reviewed to ensure that conflicts of interest are identified and minimized, or otherwise managed. ” Article 11. 11 – More clear guidance that budget require review…but may be delegated. – US Regulations require often separate committees and detailed disclosures from researchers. Trend appearing in Canada. ONE UNIVERSITY. MANY FUTURES.
 Conflict of Interest “A conflict between private interest and the official responsibilities of a person in a position of trust. ” • Apparent or real • Institutional or Individual • Issue of power in relationships • Clinician/patient • Teacher/student • Employer/employee • Financial • Personal/academic recognition ONE UNIVERSITY. MANY FUTURES.
Conflict of Interest “A conflict between private interest and the official responsibilities of a person in a position of trust. ” • Apparent or real • Institutional or Individual • Issue of power in relationships • Clinician/patient • Teacher/student • Employer/employee • Financial • Personal/academic recognition ONE UNIVERSITY. MANY FUTURES.
 Common Submission Issues • • Failure to answer submission form questions Provision of inconsistent responses to questions Confusing submission with explanatory gaps Consent form language that is too technical/jargon Failure to follow HREB/BREB consent form template guide Sloppy submission (grammar, spelling errors, etc. ) Inconsistency between consent and submission forms in relation to the objectives of the project • Failure to understand PHIA and other privacy laws ONE UNIVERSITY. MANY FUTURES.
Common Submission Issues • • Failure to answer submission form questions Provision of inconsistent responses to questions Confusing submission with explanatory gaps Consent form language that is too technical/jargon Failure to follow HREB/BREB consent form template guide Sloppy submission (grammar, spelling errors, etc. ) Inconsistency between consent and submission forms in relation to the objectives of the project • Failure to understand PHIA and other privacy laws ONE UNIVERSITY. MANY FUTURES.
 Other approvals may be required…. . • Regulatory approval(i. e. Health Canada) • Institutional Impact Approval – e. g. HSC, St. Boniface, WRHA, etc. • Health Information Privacy Committee (HIPC) • School Board Approval • Local REB Approval - National or international sites • Community Approval– Band approval, Assembly of Manitoba Chiefs • Clinical Trial Registry - prior to enrolment of first participant to be considered for publication by major journals ONE UNIVERSITY. MANY FUTURES.
Other approvals may be required…. . • Regulatory approval(i. e. Health Canada) • Institutional Impact Approval – e. g. HSC, St. Boniface, WRHA, etc. • Health Information Privacy Committee (HIPC) • School Board Approval • Local REB Approval - National or international sites • Community Approval– Band approval, Assembly of Manitoba Chiefs • Clinical Trial Registry - prior to enrolment of first participant to be considered for publication by major journals ONE UNIVERSITY. MANY FUTURES.
 Institutional Approvals: Purpose and Items to Consider • Impact on the institution’s resources: space, staff, equipment, diagnostics – Need to know ‘standard of care’ to determine if ‘over and above usual patient care • Funds to be administered where? • Lab services, diagnostic services, pharmacy services, costing? ? Medical information services, etc. ONE UNIVERSITY. MANY FUTURES.
Institutional Approvals: Purpose and Items to Consider • Impact on the institution’s resources: space, staff, equipment, diagnostics – Need to know ‘standard of care’ to determine if ‘over and above usual patient care • Funds to be administered where? • Lab services, diagnostic services, pharmacy services, costing? ? Medical information services, etc. ONE UNIVERSITY. MANY FUTURES.
 Following Initial REB Review • Full approval- Certificate of final approval granted. • Conditional approval – letter of conditional approval. Researcher must respond and wait for certificate of final approval prior to starting research. • Tabled – Study must be resubmitted to full board review subject to conditions in letter. • Not approved – Rational will be provided in letter of response. ONE UNIVERSITY. MANY FUTURES.
Following Initial REB Review • Full approval- Certificate of final approval granted. • Conditional approval – letter of conditional approval. Researcher must respond and wait for certificate of final approval prior to starting research. • Tabled – Study must be resubmitted to full board review subject to conditions in letter. • Not approved – Rational will be provided in letter of response. ONE UNIVERSITY. MANY FUTURES.
 Written approval is required? Initial and Continuing Review • Before starting to collect data or recruit participants, you must obtain a REB Certificate of approval for each research project. • Amendments - only minor administrative changes (e. g. telephone #, spelling corrections)can be made without REB written approval • Annual approval ONE UNIVERSITY. MANY FUTURES.
Written approval is required? Initial and Continuing Review • Before starting to collect data or recruit participants, you must obtain a REB Certificate of approval for each research project. • Amendments - only minor administrative changes (e. g. telephone #, spelling corrections)can be made without REB written approval • Annual approval ONE UNIVERSITY. MANY FUTURES.
 Continuing Review “Following initial REB review and approval, research ethics review shall continue through the life of the project in accordance with Article 6. 14” TCPS 2 – Article 2. 8 “…at minimum, continuing research ethics review shall consist of an annual status report(for multi-year research projects) and an end of study report(projects lasting less than one year). TCPS 2 – Article 6. 14 “Researchers shall report to the REB any unanticipated issue or event that may increase the level of risk to participants, or has other ethical implications that my affect participants welfare. ” TCPS 2 – Article 6. 14 ONE UNIVERSITY. MANY FUTURES.
Continuing Review “Following initial REB review and approval, research ethics review shall continue through the life of the project in accordance with Article 6. 14” TCPS 2 – Article 2. 8 “…at minimum, continuing research ethics review shall consist of an annual status report(for multi-year research projects) and an end of study report(projects lasting less than one year). TCPS 2 – Article 6. 14 “Researchers shall report to the REB any unanticipated issue or event that may increase the level of risk to participants, or has other ethical implications that my affect participants welfare. ” TCPS 2 – Article 6. 14 ONE UNIVERSITY. MANY FUTURES.
 Continuing Research Ethics Review at U of M • Annual approval – Funds withheld as required by CIHR – More substantive review required • Amendments – More substantive review required • Protocol Deviations and Serious Adverse Events • Monitoring – More detail required in protocols re assessing safety and monitoring within study – Audits – Health Canada requirements – Quality Assurance office at U of Manitoba ONE UNIVERSITY. MANY FUTURES.
Continuing Research Ethics Review at U of M • Annual approval – Funds withheld as required by CIHR – More substantive review required • Amendments – More substantive review required • Protocol Deviations and Serious Adverse Events • Monitoring – More detail required in protocols re assessing safety and monitoring within study – Audits – Health Canada requirements – Quality Assurance office at U of Manitoba ONE UNIVERSITY. MANY FUTURES.
 Protocol Deviations • Minor – Identify and record at site – reporting to REB annually • Major – Report to REB ASAP on Bannatyne forms – Inclusion/exclusion deviations – Errors in drug administration or study procedures, etc. ONE UNIVERSITY. MANY FUTURES.
Protocol Deviations • Minor – Identify and record at site – reporting to REB annually • Major – Report to REB ASAP on Bannatyne forms – Inclusion/exclusion deviations – Errors in drug administration or study procedures, etc. ONE UNIVERSITY. MANY FUTURES.
 Monitoring Safety and Reporting New Information “Researchers shall promptly report new information that may affect the welfare or consent of participants, to the REB, and to other appropriate regulatory or advisory bodies. When new information is relevant to participants’ welfare, researchers shall promptly inform all participants to whom the information applies (including former participants). Researchers shall work with their REB to determine which participants must be informed, and how the information should be conveyed. “ Article 11. 8 – New reporting procedures to REB ONE UNIVERSITY. MANY FUTURES.
Monitoring Safety and Reporting New Information “Researchers shall promptly report new information that may affect the welfare or consent of participants, to the REB, and to other appropriate regulatory or advisory bodies. When new information is relevant to participants’ welfare, researchers shall promptly inform all participants to whom the information applies (including former participants). Researchers shall work with their REB to determine which participants must be informed, and how the information should be conveyed. “ Article 11. 8 – New reporting procedures to REB ONE UNIVERSITY. MANY FUTURES.
 PI responsibilities for Adverse Event Reporting • Protocol development – Must include a section on how adverse events and unanticipated problems are to be defined, collected, recorded and reported to sponsor, REB and regulatory authorities in compliance with local REB reporting requirements and regulatory authorities – Monitoring of study- who, how and when – Data Safety Monitoring plan – Specific triggers or stopping rules • Review all local and external adverse reports and unanticipated problems that meet reporting requirements • Report appropriate Adverse Events to REB and regulatory authorities ONE UNIVERSITY. MANY FUTURES.
PI responsibilities for Adverse Event Reporting • Protocol development – Must include a section on how adverse events and unanticipated problems are to be defined, collected, recorded and reported to sponsor, REB and regulatory authorities in compliance with local REB reporting requirements and regulatory authorities – Monitoring of study- who, how and when – Data Safety Monitoring plan – Specific triggers or stopping rules • Review all local and external adverse reports and unanticipated problems that meet reporting requirements • Report appropriate Adverse Events to REB and regulatory authorities ONE UNIVERSITY. MANY FUTURES.
 Definitions An adverse event (AE) is defined as any occurrence in the health or well being of a research participant who is administered an investigational product (drug, natural health product, or device) or any other procedure(s) involved in the research or participants in a research activity that may or may not be caused by the administration of an investigational product or any procedure(s) involved in research. Adverse events occur in interventional as well as noninterventional studies. ONE UNIVERSITY. MANY FUTURES.
Definitions An adverse event (AE) is defined as any occurrence in the health or well being of a research participant who is administered an investigational product (drug, natural health product, or device) or any other procedure(s) involved in the research or participants in a research activity that may or may not be caused by the administration of an investigational product or any procedure(s) involved in research. Adverse events occur in interventional as well as noninterventional studies. ONE UNIVERSITY. MANY FUTURES.
 Definitions Unanticipated Problem: any incident, experience, or outcome that meets all of the following criteria: – Unexpected (in terms of nature, severity, or frequency) given (a) the research procedures that are described in the protocol-related documents, such as the REB-approved research protocol and informed consent document, or the Investigator Brochure; and (b) the characteristics of the research participant population being studied; and – Related or possibly related to participation in the research, (possibly related means there is a reasonable possibility that the incident, experience, or outcome may have been caused by the investigational product(s) or procedures involved in the research); and – Suggests that the research places research participants or others at a greater risk of harm (including physical, psychological, economic, or social harm) than was previously known or recognized. ONE UNIVERSITY. MANY FUTURES.
Definitions Unanticipated Problem: any incident, experience, or outcome that meets all of the following criteria: – Unexpected (in terms of nature, severity, or frequency) given (a) the research procedures that are described in the protocol-related documents, such as the REB-approved research protocol and informed consent document, or the Investigator Brochure; and (b) the characteristics of the research participant population being studied; and – Related or possibly related to participation in the research, (possibly related means there is a reasonable possibility that the incident, experience, or outcome may have been caused by the investigational product(s) or procedures involved in the research); and – Suggests that the research places research participants or others at a greater risk of harm (including physical, psychological, economic, or social harm) than was previously known or recognized. ONE UNIVERSITY. MANY FUTURES.
 Definitions Serious Adverse Event/Experience (SAE) or Reaction: any untoward medical occurrence that: – Results in death – Is life-threatening – Requires inpatient hospitalization or prolongation of existing hospitalization – Results in persistent or significant disability/incapacity – Results in congenital anomaly/birth defect – Any other adverse event that, based upon appropriate medical judgment, is an important medical event that may jeopardize the health of the research participant or may require medical intervention to prevent one of the outcome listed above. ONE UNIVERSITY. MANY FUTURES.
Definitions Serious Adverse Event/Experience (SAE) or Reaction: any untoward medical occurrence that: – Results in death – Is life-threatening – Requires inpatient hospitalization or prolongation of existing hospitalization – Results in persistent or significant disability/incapacity – Results in congenital anomaly/birth defect – Any other adverse event that, based upon appropriate medical judgment, is an important medical event that may jeopardize the health of the research participant or may require medical intervention to prevent one of the outcome listed above. ONE UNIVERSITY. MANY FUTURES.
 Definitions Local (Internal) adverse event: – local adverse events are those adverse events experienced by research participants enrolled by the investigator(s) at one or more centers under the jurisdiction of the University of Manitoba REB. In context of a single-centre clinical trial, all adverse events would be considered local adverse events. External (non-local) adverse event (EAE): – from the perspective of the REB overseeing one or more centres engaged in a multi-centre clinical trial, external adverse events are those adverse events experienced by research participants enrolled by investigator(s) at other centres/institutions outside the REB’s jurisdiction. ONE UNIVERSITY. MANY FUTURES.
Definitions Local (Internal) adverse event: – local adverse events are those adverse events experienced by research participants enrolled by the investigator(s) at one or more centers under the jurisdiction of the University of Manitoba REB. In context of a single-centre clinical trial, all adverse events would be considered local adverse events. External (non-local) adverse event (EAE): – from the perspective of the REB overseeing one or more centres engaged in a multi-centre clinical trial, external adverse events are those adverse events experienced by research participants enrolled by investigator(s) at other centres/institutions outside the REB’s jurisdiction. ONE UNIVERSITY. MANY FUTURES.
 Reporting Criteria of Local Events Only report adverse events that are unanticipated problems Any incident, experience, or outcome that meets all of the following criteria: – Unexpected; and – Related or possibly related to participation in the research, and – Suggests that the research places research participants or others at a greater risk of harm (including physical, psychological, economic, or social harm) than was previously known or recognized. Major Change in Criteria for reporting – – Previously the Bannatyne Campus required reporting of serious adverse whether or not they were related ONE UNIVERSITY. MANY FUTURES.
Reporting Criteria of Local Events Only report adverse events that are unanticipated problems Any incident, experience, or outcome that meets all of the following criteria: – Unexpected; and – Related or possibly related to participation in the research, and – Suggests that the research places research participants or others at a greater risk of harm (including physical, psychological, economic, or social harm) than was previously known or recognized. Major Change in Criteria for reporting – – Previously the Bannatyne Campus required reporting of serious adverse whether or not they were related ONE UNIVERSITY. MANY FUTURES.
 Examples ONE UNIVERSITY. MANY FUTURES.
Examples ONE UNIVERSITY. MANY FUTURES.
 Unanticipated problem that should be reported to local REB An investigator conducting behavioral research collects individually identifiable sensitive information about illicit drug use and other illegal behaviors by surveying college students. The data are stored on a laptop computer without encryption, and the laptop computer is stolen from the investigator’s car on the way home from work. This is an unanticipated problem that must be reported because the incident was: – (a) unexpected (i. e. , the investigators did not anticipate theft); – (b) related to participation in the research; and – (c) placed the subjects at a greater risk of psychological and social harm from the breach in confidentiality of the study data than was previously known or recognized. ONE UNIVERSITY. MANY FUTURES.
Unanticipated problem that should be reported to local REB An investigator conducting behavioral research collects individually identifiable sensitive information about illicit drug use and other illegal behaviors by surveying college students. The data are stored on a laptop computer without encryption, and the laptop computer is stolen from the investigator’s car on the way home from work. This is an unanticipated problem that must be reported because the incident was: – (a) unexpected (i. e. , the investigators did not anticipate theft); – (b) related to participation in the research; and – (c) placed the subjects at a greater risk of psychological and social harm from the breach in confidentiality of the study data than was previously known or recognized. ONE UNIVERSITY. MANY FUTURES.
 Unanticipated problem that should be reported to REB As a result of a processing error by a pharmacy technician, a subject enrolled in a multicenter clinical trial receives a dose of an experimental agent that is 10 -times higher than the dose dictated by the IRB-approved protocol. While the dosing error increased the risk of toxic manifestations of the experimental agent, the subject experienced no detectable harm or adverse effect after an appropriate period of careful observation. Nevertheless, this constitutes an unanticipated problem for the institution where the dosing error occurred that must be reported to the IRB, appropriate institutional officials, and OHRP because the incident was – (a) unexpected; – (b) related to participation in the research; and – (c) placed subject at a greater risk of physical harm than was previously known or recognized. ONE UNIVERSITY. MANY FUTURES.
Unanticipated problem that should be reported to REB As a result of a processing error by a pharmacy technician, a subject enrolled in a multicenter clinical trial receives a dose of an experimental agent that is 10 -times higher than the dose dictated by the IRB-approved protocol. While the dosing error increased the risk of toxic manifestations of the experimental agent, the subject experienced no detectable harm or adverse effect after an appropriate period of careful observation. Nevertheless, this constitutes an unanticipated problem for the institution where the dosing error occurred that must be reported to the IRB, appropriate institutional officials, and OHRP because the incident was – (a) unexpected; – (b) related to participation in the research; and – (c) placed subject at a greater risk of physical harm than was previously known or recognized. ONE UNIVERSITY. MANY FUTURES.
 Unanticipated problem that should be reported to the REB Subjects with cancer are enrolled in a phase 2 clinical trial evaluating an investigational biologic product derived from human sera. After several subjects are enrolled and receive the investigational product, a study audit reveals that the investigational product administered to subjects was obtained from donors who were not appropriately screened and tested for several potential viral contaminants, including the human immunodeficiency virus and the hepatitis B virus. This constitutes an unanticipated problem that must be reported because the incident was: – (a) unexpected; – (b) related to participation in the research; and – (c) placed subjects and others at a greater risk of physical harm than was previously known or recognized. ONE UNIVERSITY. MANY FUTURES.
Unanticipated problem that should be reported to the REB Subjects with cancer are enrolled in a phase 2 clinical trial evaluating an investigational biologic product derived from human sera. After several subjects are enrolled and receive the investigational product, a study audit reveals that the investigational product administered to subjects was obtained from donors who were not appropriately screened and tested for several potential viral contaminants, including the human immunodeficiency virus and the hepatitis B virus. This constitutes an unanticipated problem that must be reported because the incident was: – (a) unexpected; – (b) related to participation in the research; and – (c) placed subjects and others at a greater risk of physical harm than was previously known or recognized. ONE UNIVERSITY. MANY FUTURES.
 Further rational why the last three examples are reportable. The events described in the above examples were unexpected in nature, related to participation in the research, and resulted in new circumstances that increased the risk of harm to subjects. In all of these examples, the unanticipated problems warranted consideration of substantive changes in the research protocol or informed consent process/document or other corrective actions in order to protect the safety, welfare, or rights of subjects. In addition, the third example may have presented unanticipated risks to others (e. g. , the sexual partners of the subjects) in addition to the subjects. In each of these examples, while these events may not have caused any detectable harm or adverse effect to subjects or others, they nevertheless represent unanticipated problems and should be promptly reported to the IRB, appropriate institutional officials, the supporting agency head and OHRP in accordance with HHS regulations at 45 CFR 46. 103(a) and 46. 103(b)(5). ONE UNIVERSITY. MANY FUTURES.
Further rational why the last three examples are reportable. The events described in the above examples were unexpected in nature, related to participation in the research, and resulted in new circumstances that increased the risk of harm to subjects. In all of these examples, the unanticipated problems warranted consideration of substantive changes in the research protocol or informed consent process/document or other corrective actions in order to protect the safety, welfare, or rights of subjects. In addition, the third example may have presented unanticipated risks to others (e. g. , the sexual partners of the subjects) in addition to the subjects. In each of these examples, while these events may not have caused any detectable harm or adverse effect to subjects or others, they nevertheless represent unanticipated problems and should be promptly reported to the IRB, appropriate institutional officials, the supporting agency head and OHRP in accordance with HHS regulations at 45 CFR 46. 103(a) and 46. 103(b)(5). ONE UNIVERSITY. MANY FUTURES.
 Adverse events that are unanticipated problems that require reporting A subject with chronic gastroesophageal reflux disease enrolls in a randomized, placebo- controlled, double-blind, phase 3 clinical trial evaluating a new investigational agent that blocks acid release in the stomach. Two weeks after being randomized and started on the study intervention the subject develops acute kidney failure as evidenced by an increase in serum creatinine from 1. 0 mg/dl prerandomization to 5. 0 mg/dl. The known risk profile of the investigational agent does not include renal toxicity, and the IRB-approved protocol and informed consent document for the study does not identify kidney damage as a risk of the research. Evaluation of the subject reveals no other obvious cause for acute renal failure. The investigator concludes that the episode of acute renal failure probably was due to the investigational agent. This is an example of an unanticipated problem that must be reported because the subject’s acute renal failure was: (a) unexpected in nature, (b) related to participation in the research, and (c) serious. ONE UNIVERSITY. MANY FUTURES.
Adverse events that are unanticipated problems that require reporting A subject with chronic gastroesophageal reflux disease enrolls in a randomized, placebo- controlled, double-blind, phase 3 clinical trial evaluating a new investigational agent that blocks acid release in the stomach. Two weeks after being randomized and started on the study intervention the subject develops acute kidney failure as evidenced by an increase in serum creatinine from 1. 0 mg/dl prerandomization to 5. 0 mg/dl. The known risk profile of the investigational agent does not include renal toxicity, and the IRB-approved protocol and informed consent document for the study does not identify kidney damage as a risk of the research. Evaluation of the subject reveals no other obvious cause for acute renal failure. The investigator concludes that the episode of acute renal failure probably was due to the investigational agent. This is an example of an unanticipated problem that must be reported because the subject’s acute renal failure was: (a) unexpected in nature, (b) related to participation in the research, and (c) serious. ONE UNIVERSITY. MANY FUTURES.
 Adverse events that are unanticipated problems that require reporting Subjects with essential hypertension are enrolled in a phase 2, non-randomized clinical trial testing a new investigational antihypertensive drug. At the time the clinical trial is initiated, there is no documented evidence of gastroesophageal reflux disease (GERD) associated with the investigational drug, and the IRB-approved protocol and informed consent document do not describe GERD as a risk of the research. Three of the first ten subjects are noted by the investigator to have severe GERD symptoms that began within one week of starting the investigational drug and resolved a few days after the drug was discontinued. The investigator determines that the GERD symptoms were most likely caused by the investigational drug and warrant modification of the informed consent document to include a description of GERD as a risk of the research. This is an example of an adverse event that, although not serious, represents an unanticipated problem that must be reported because it was: (a) unexpected in nature; (b) possibly related to participation in the research; and (c) suggested that the research placed subjects at a greater risk of physical harm than was previously known or recognized. ONE UNIVERSITY. MANY FUTURES.
Adverse events that are unanticipated problems that require reporting Subjects with essential hypertension are enrolled in a phase 2, non-randomized clinical trial testing a new investigational antihypertensive drug. At the time the clinical trial is initiated, there is no documented evidence of gastroesophageal reflux disease (GERD) associated with the investigational drug, and the IRB-approved protocol and informed consent document do not describe GERD as a risk of the research. Three of the first ten subjects are noted by the investigator to have severe GERD symptoms that began within one week of starting the investigational drug and resolved a few days after the drug was discontinued. The investigator determines that the GERD symptoms were most likely caused by the investigational drug and warrant modification of the informed consent document to include a description of GERD as a risk of the research. This is an example of an adverse event that, although not serious, represents an unanticipated problem that must be reported because it was: (a) unexpected in nature; (b) possibly related to participation in the research; and (c) suggested that the research placed subjects at a greater risk of physical harm than was previously known or recognized. ONE UNIVERSITY. MANY FUTURES.
 Adverse events that are unanticipated problems that require reporting A behavioral researcher conducts a study in college students that involves completion of a detailed survey asking questions about early childhood experiences. The research was judged to involve no more than minimal risk and was approved by the IRB chairperson under an expedited review procedure. During the completion of the survey, one student subject has a transient psychological reaction manifested by intense sadness and depressed mood that resolved without intervention after a few hours. The protocol and informed consent document for the research did not describe any risk of such negative psychological reactions. Upon further evaluation, the investigator determines that the subject’s negative psychological reaction resulted from certain survey questions that triggered repressed memories of physical abuse as a child. The investigator had not expected that such reactions would be triggered by the survey questions. This is an example of an unanticipated problem that must be reported in the context of social and behavioral research because, although not serious, the adverse event was: ( a) unexpected; (b) related to participation in the research; and (c) suggested that the research places subjects at a greater risk of psychological harm than was previously known or recognized. ONE UNIVERSITY. MANY FUTURES.
Adverse events that are unanticipated problems that require reporting A behavioral researcher conducts a study in college students that involves completion of a detailed survey asking questions about early childhood experiences. The research was judged to involve no more than minimal risk and was approved by the IRB chairperson under an expedited review procedure. During the completion of the survey, one student subject has a transient psychological reaction manifested by intense sadness and depressed mood that resolved without intervention after a few hours. The protocol and informed consent document for the research did not describe any risk of such negative psychological reactions. Upon further evaluation, the investigator determines that the subject’s negative psychological reaction resulted from certain survey questions that triggered repressed memories of physical abuse as a child. The investigator had not expected that such reactions would be triggered by the survey questions. This is an example of an unanticipated problem that must be reported in the context of social and behavioral research because, although not serious, the adverse event was: ( a) unexpected; (b) related to participation in the research; and (c) suggested that the research places subjects at a greater risk of psychological harm than was previously known or recognized. ONE UNIVERSITY. MANY FUTURES.
 Adverse Events that are not Unanticipated problems A subject participating in a phase 3, randomized, double-blind, controlled clinical trial comparing the relative safety and efficacy of a new chemotherapy agent combined with the current standard chemotherapy regimen, versus placebo combined with the current standard chemotherapy regimen, for the management of multiple myeloma develops neutropenia and sepsis. The subject subsequently develops multi-organ failure and dies. Prolonged bone marrow suppression resulting in neutropenia and risk of life-threatening infections is a known complication of the chemotherapy regimens being tested in this clinical trial and these risks are described in the IRB-approved protocol and informed consent document. The investigators conclude that the subject’s infection and death are directly related to the research interventions. A review of data on all subjects enrolled so far reveals that the incidence of severe neutropenia, infection, and death are within the expected frequency. This example is not an unanticipated problem because the occurrence of severe infections and death – in terms of nature, severity, and frequency – was expected. ONE UNIVERSITY. MANY FUTURES.
Adverse Events that are not Unanticipated problems A subject participating in a phase 3, randomized, double-blind, controlled clinical trial comparing the relative safety and efficacy of a new chemotherapy agent combined with the current standard chemotherapy regimen, versus placebo combined with the current standard chemotherapy regimen, for the management of multiple myeloma develops neutropenia and sepsis. The subject subsequently develops multi-organ failure and dies. Prolonged bone marrow suppression resulting in neutropenia and risk of life-threatening infections is a known complication of the chemotherapy regimens being tested in this clinical trial and these risks are described in the IRB-approved protocol and informed consent document. The investigators conclude that the subject’s infection and death are directly related to the research interventions. A review of data on all subjects enrolled so far reveals that the incidence of severe neutropenia, infection, and death are within the expected frequency. This example is not an unanticipated problem because the occurrence of severe infections and death – in terms of nature, severity, and frequency – was expected. ONE UNIVERSITY. MANY FUTURES.
 Adverse Events that are not Unanticipated problems A subject enrolled in a phase 3, randomized, double-blind, placebocontrolled clinical trial evaluating the safety and efficacy of a new investigational anti-inflammatory agent for management of osteoarthritis develops severe abdominal pain and nausea one month after randomization. Subsequent medical evaluation reveals gastric ulcers. The IRB-approved protocol and informed consent document for the study indicated that there was a 10% chance of developing mild to moderate gastritis and a 2% chance of developing gastric ulcers for subjects assigned to the active investigational agent. The investigator concludes that the subject’s gastric ulcers resulted from the research intervention and withdraws the subject from the study. A review of data on all subjects enrolled so far reveals that the incidence of gastritis and gastric ulcer are within the expected frequency. This example is not an unanticipated problem because the occurrence of gastric ulcers – in terms of nature, severity, and frequency – was expected. ONE UNIVERSITY. MANY FUTURES.
Adverse Events that are not Unanticipated problems A subject enrolled in a phase 3, randomized, double-blind, placebocontrolled clinical trial evaluating the safety and efficacy of a new investigational anti-inflammatory agent for management of osteoarthritis develops severe abdominal pain and nausea one month after randomization. Subsequent medical evaluation reveals gastric ulcers. The IRB-approved protocol and informed consent document for the study indicated that there was a 10% chance of developing mild to moderate gastritis and a 2% chance of developing gastric ulcers for subjects assigned to the active investigational agent. The investigator concludes that the subject’s gastric ulcers resulted from the research intervention and withdraws the subject from the study. A review of data on all subjects enrolled so far reveals that the incidence of gastritis and gastric ulcer are within the expected frequency. This example is not an unanticipated problem because the occurrence of gastric ulcers – in terms of nature, severity, and frequency – was expected. ONE UNIVERSITY. MANY FUTURES.
 Adverse Events that are not Unanticipated problems An investigator is conducting a psychology study evaluating the factors that affect reaction times in response to auditory stimuli. In order to perform the reaction time measurements, subjects are placed in a small, windowless soundproof booth and asked to wear headphones. The IRB-approved protocol and informed consent document describe claustrophobic reactions as one of the risks of the research. The twentieth subject enrolled in the research experiences significant claustrophobia, resulting in the subject withdrawing from the research. This example is not an unanticipated problem because the occurrence of the claustrophobic reactions – in terms of nature, severity, and frequency – was expected. ONE UNIVERSITY. MANY FUTURES.
Adverse Events that are not Unanticipated problems An investigator is conducting a psychology study evaluating the factors that affect reaction times in response to auditory stimuli. In order to perform the reaction time measurements, subjects are placed in a small, windowless soundproof booth and asked to wear headphones. The IRB-approved protocol and informed consent document describe claustrophobic reactions as one of the risks of the research. The twentieth subject enrolled in the research experiences significant claustrophobia, resulting in the subject withdrawing from the research. This example is not an unanticipated problem because the occurrence of the claustrophobic reactions – in terms of nature, severity, and frequency – was expected. ONE UNIVERSITY. MANY FUTURES.
 Did you do what you said you were going to do? Monitoring by the U of Manitoba, Sponsor and Regulatory bodies • Educational monitoring site visits • Review of research records for compliance with REB approved protocol • Review of informed consent process • Interview with participants • Several reviews by Health Canada at HSC and St. B • U of M Research Services– audited by CIHR(to ensure ethical documents are adhered to and financial records follow guidelines) ONE UNIVERSITY. MANY FUTURES.
Did you do what you said you were going to do? Monitoring by the U of Manitoba, Sponsor and Regulatory bodies • Educational monitoring site visits • Review of research records for compliance with REB approved protocol • Review of informed consent process • Interview with participants • Several reviews by Health Canada at HSC and St. B • U of M Research Services– audited by CIHR(to ensure ethical documents are adhered to and financial records follow guidelines) ONE UNIVERSITY. MANY FUTURES.
 There is MORE to consider? ONE UNIVERSITY. MANY FUTURES.
There is MORE to consider? ONE UNIVERSITY. MANY FUTURES.
 Key Concepts in Aboriginal TCPS 2 Chapter • Aboriginal Peoples – distinct groups – First Nations, – Inuit – Metis • Community – organizational or territorial • • • Community customs, codes of research practice Community engagement First Nations, Inuit and Metis Land Indigenous people and knowledge Traditional knowledge ONE UNIVERSITY. MANY FUTURES.
Key Concepts in Aboriginal TCPS 2 Chapter • Aboriginal Peoples – distinct groups – First Nations, – Inuit – Metis • Community – organizational or territorial • • • Community customs, codes of research practice Community engagement First Nations, Inuit and Metis Land Indigenous people and knowledge Traditional knowledge ONE UNIVERSITY. MANY FUTURES.
 First Nation, Inuit and Metis Peoples of Canada Chapter • framework for the ethical conduct of research involving framework Aboriginal peoples. • offered in a spirit of respect. • not intended to override or replace ethical guidance offered by Aboriginal peoples themselves. • to ensure, to the extent possible, that research involving Aboriginal peoples is premised on respectful relationships • encourages collaboration and engagement between collaboration engagement researchers and participants ONE UNIVERSITY. MANY FUTURES.
First Nation, Inuit and Metis Peoples of Canada Chapter • framework for the ethical conduct of research involving framework Aboriginal peoples. • offered in a spirit of respect. • not intended to override or replace ethical guidance offered by Aboriginal peoples themselves. • to ensure, to the extent possible, that research involving Aboriginal peoples is premised on respectful relationships • encourages collaboration and engagement between collaboration engagement researchers and participants ONE UNIVERSITY. MANY FUTURES.
 Community Engagement in Aboriginal Research “Where the research is likely to affect the welfare of an Aboriginal community, or communities, to which prospective participants belong, researchers shall seek engagement with the relevant community. The conditions under which engagement is required include, but are not limited to: • • • (a) research conducted on First Nations, Inuit or Métis lands; (b) recruitment criteria that include Aboriginal identity as a factor for the recruitment criteria entire study or for a subgroup in the study; (c) research that seeks input from participants regarding a community’s cultural heritage, artefacts, traditional knowledge or unique characteristics; (d) research in which Aboriginal identity or membership in an Aboriginal community is used as a variable for the purpose of analysis of the variable for the purpose of analysis research data; and (e) interpretation of research results that will refer to Aboriginal interpretation of research results communities, peoples, language, history or culture. ” Article 9. 1 ONE UNIVERSITY. MANY FUTURES.
Community Engagement in Aboriginal Research “Where the research is likely to affect the welfare of an Aboriginal community, or communities, to which prospective participants belong, researchers shall seek engagement with the relevant community. The conditions under which engagement is required include, but are not limited to: • • • (a) research conducted on First Nations, Inuit or Métis lands; (b) recruitment criteria that include Aboriginal identity as a factor for the recruitment criteria entire study or for a subgroup in the study; (c) research that seeks input from participants regarding a community’s cultural heritage, artefacts, traditional knowledge or unique characteristics; (d) research in which Aboriginal identity or membership in an Aboriginal community is used as a variable for the purpose of analysis of the variable for the purpose of analysis research data; and (e) interpretation of research results that will refer to Aboriginal interpretation of research results communities, peoples, language, history or culture. ” Article 9. 1 ONE UNIVERSITY. MANY FUTURES.
 Nature and Extent of Community Engagement “The nature and extent of community engagement in a project shall be determined jointly by the researcher and the relevant community, and shall be appropriate to community characteristics and the nature of the research. ” Article 9. 2 ONE UNIVERSITY. MANY FUTURES.
Nature and Extent of Community Engagement “The nature and extent of community engagement in a project shall be determined jointly by the researcher and the relevant community, and shall be appropriate to community characteristics and the nature of the research. ” Article 9. 2 ONE UNIVERSITY. MANY FUTURES.
 Manitoba Perspective • Manitoba Assembly of Chiefs – Distinct populations – OCAP principles • Ownership • Control • Access • Possession – Enigok – Health Information Research Governance Committee(HIRGC) • Manitoba Metis Federation • Advisory groups ONE UNIVERSITY. MANY FUTURES.
Manitoba Perspective • Manitoba Assembly of Chiefs – Distinct populations – OCAP principles • Ownership • Control • Access • Possession – Enigok – Health Information Research Governance Committee(HIRGC) • Manitoba Metis Federation • Advisory groups ONE UNIVERSITY. MANY FUTURES.
 Reporting Incidental Findings “Researchers have an obligation to disclose to the participant any material incidental findings discovered in the course of research. ” Article 3. 4 • • Greater likelihood in medical and genetic research E. g. Genetic risks, questionnaires disclosing severe depression, harming oneself, etc. Potential risks outlined in consent REB review on case by case basis ONE UNIVERSITY. MANY FUTURES.
Reporting Incidental Findings “Researchers have an obligation to disclose to the participant any material incidental findings discovered in the course of research. ” Article 3. 4 • • Greater likelihood in medical and genetic research E. g. Genetic risks, questionnaires disclosing severe depression, harming oneself, etc. Potential risks outlined in consent REB review on case by case basis ONE UNIVERSITY. MANY FUTURES.
 Research Directives “Where individuals have signed a research directive indicating their preferences about future participation in research in the event that they lose capacity or upon death, researchers and authorized third parties should be guided by these directives during the consent process. ” Article 3. 11 • • • More emphasis on need for directives Manitoba’s Health Care Directive –Health Care Directive Act requires research to be mentioned explicitly otherwise authorized individual cannot consent for research U of M REB’s seeking further legal opinion ONE UNIVERSITY. MANY FUTURES.
Research Directives “Where individuals have signed a research directive indicating their preferences about future participation in research in the event that they lose capacity or upon death, researchers and authorized third parties should be guided by these directives during the consent process. ” Article 3. 11 • • • More emphasis on need for directives Manitoba’s Health Care Directive –Health Care Directive Act requires research to be mentioned explicitly otherwise authorized individual cannot consent for research U of M REB’s seeking further legal opinion ONE UNIVERSITY. MANY FUTURES.
 Emerging Issues • Internet research – As a tool – Area of research – Confidentiality and consent issues • Health Care Directive Act – Consent issues for research ONE UNIVERSITY. MANY FUTURES.
Emerging Issues • Internet research – As a tool – Area of research – Confidentiality and consent issues • Health Care Directive Act – Consent issues for research ONE UNIVERSITY. MANY FUTURES.
 ONE UNIVERSITY. MANY FUTURES.
ONE UNIVERSITY. MANY FUTURES.
 The Researcher’s perspective is that we are too difficult…. hopefully not! ONE UNIVERSITY. MANY FUTURES.
The Researcher’s perspective is that we are too difficult…. hopefully not! ONE UNIVERSITY. MANY FUTURES.
 Thank you. Shelly Rempel-Rossum shelly. rempel-rossum@med. umcanitoba. ca Phone: (204) 789 -3389 (204) 789 -3255 ONE UNIVERSITY. MANY FUTURES.
Thank you. Shelly Rempel-Rossum shelly. rempel-rossum@med. umcanitoba. ca Phone: (204) 789 -3389 (204) 789 -3255 ONE UNIVERSITY. MANY FUTURES.


