3dd007d91884e6baeb0f79e11eed5049.ppt
- Количество слайдов: 15

REPUBLIKA SLOVENIJA MINISTRSTVO ZA KMETIJSTVO, GOZDARSTVO IN PREHRANO VETERINARSKA UPRAVA REPUBLIKE SLOVENIJE Traceability of ABPs and derived products in EU trade Regulation (EC) No 1069/2009 Commission Regulation No 142/2011/EU TAIEX Workshop Beograd, 27 and 28 March, 2012 Urška Galjot, DVM Veterinary Aministration of the Republic of Slovenia 1

1. Why traceability 2. Who (starting point) 3. How (means to achieve it) 4. Until which point (end point) 5. Examples (PAPs, manure, untreated/treated wool) 2
1. Why traceability - to avoid risks (AH, PH); - to avoid frauds; - feed ban 3

2. Who (starting point) -1 As soon as operators generate animal by-products or derived products falling within the scope of this Regulation, they shall identify them and ensure that they are dealt with in accordance with this Regulation (starting point) : -collection; -treatment, processing, transformation; -storage; -placing on the market; -distribution; -use; -disposal. On farm, slaughterhouse, butchershops, supermarkets, petshops, restaurants, BIPs, veterinary clinic, airport, …. . Controls performed by competent authorities!!! 4
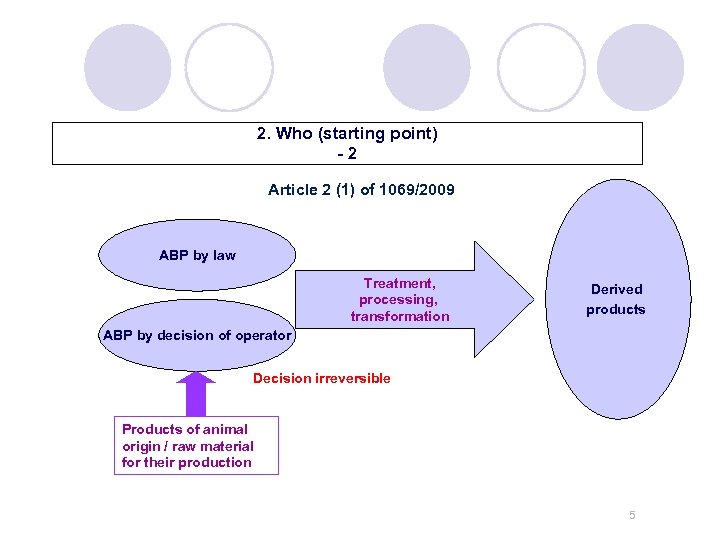
2. Who (starting point) -2 Article 2 (1) of 1069/2009 ABP by law Treatment, processing, transformation Derived products ABP by decision of operator Decision irreversible Products of animal origin / raw material for their production 5
3. How (means to achieve it) – (1) 3. 1 Categorised directy after they are “produced”, categories kept separate; 3. 2 Identification of products and means of transport; 3. 3 Marking; 3. 4 Commercial documents / certificates; 3. 5 Pre-authorisation, TRACES; 3. 6 Record keeping. Official controls! 6
3. How (means to achieve it) – (2) 3. 1 Categorised directy after they are “produced”, categories kept separate 3. 2 Identification of products and means of transport 3. 2. 1 Colour coding - colour coding between MSs (cat 1 – black, cat 2 - yellow (except manure and digestive tract content), cat 3 – green with high content of blue); - import – colour coding after entry to the EU; 3. 2. 2 Labelling - category; - prescribed wording (Chapter II, Annex VIII of Reg. 142/2011); CHAPTER II Traceability. doc - not necessary for certain cases in OF/SI, return of milk, milk based products and milk derived products to establishment of production (former foodstuffs), manure between two farms in same MS, compound feed manufactured from ABPs or DPs. 7
3. How (means to achieve it) – (3) 3. 3 Marking 3. 3. 1. Marking of certain derived products - DP from Cat 1 and 2 - glycerol triheptanoate (GTH) - addition during processing in concentration 250 mg /kg fat - homogenously - validation of procedure, monitoring and recording by the operator - not needed in certain cases : * liquid DPs for biogas/compost, or; * DPs for feeding fur animals, or; * biodiesel produced with 2 (D) method, or; * on-the-spot incineration / co-incineration / alternative method; * research purposes authorised by CA); * renewable fuels produce with 2 (J) method. - gas cromatography. 8
3. How (means to achieve it) – (4) 3. 4 Commercial documents / certificates (2 years) 3. 4. 1 Model commercial document in line with Chapter III, Annex VIII of Reg. 142/2011 - not necessary for: - DP from Cat 3 and OF/SI in the same MS by retailers to final users, or; - return of milk, milk based products and milk derived products to establishment of production (former foodstuffs), or; - compound feed manufactured from ABPs or DPs. - produced at least in triplicate (original + 2 copies); - duly completed with necessary data. Commercial document. jpg - for trade in unprocessed manure of species other than equidae , additional Health attestation signed by official veterinarian (Annex XI, Chapter I, Section 1, point 3) Health attestation manure. doc 3. 4. 2 Not necessary to use Model commercial document in the same MS (obligatory data in Point 6) 3. 4. 3 Certificate between MSs – only when required from AH reasons 9
3. How (means to achieve it) – (5) 3. 5 Pre-authorisation, TRACES 3. 5. 1 Pre-authorisation - art. 48 of Reg 1069/2009 for Cat 1 and 2, MBM, rendered fats from Cat 1 and 2; - application format Section 10 Chapter III of Annex XVI of Reg 142/2011; 3. 5. 1 TRACES notification for Cat 1 and 2, MBM, rendered fats from Cat 1 and 2, PAPs. CA at place of destination has to confirm arrival (Part 3 of TRACES, other means); 3. 6 Record keeping 3. 6. 1 General provisions: description of material, date in/date out, place of origin/place of destination. 3. 6. 2 Additional provisions for use of derogations, use of certain OF/SI, photogelatine … 10
4. Until which point (end point) 4. End point is determined for certain products which represent no more risk, due to: -treatment in line with ABP rules or other appliceable legislation; and -the concepts of : - safe sourcing, - safe treatment, and - safe end use are complied with. From that point on, no restrictions with ABP rules! 11
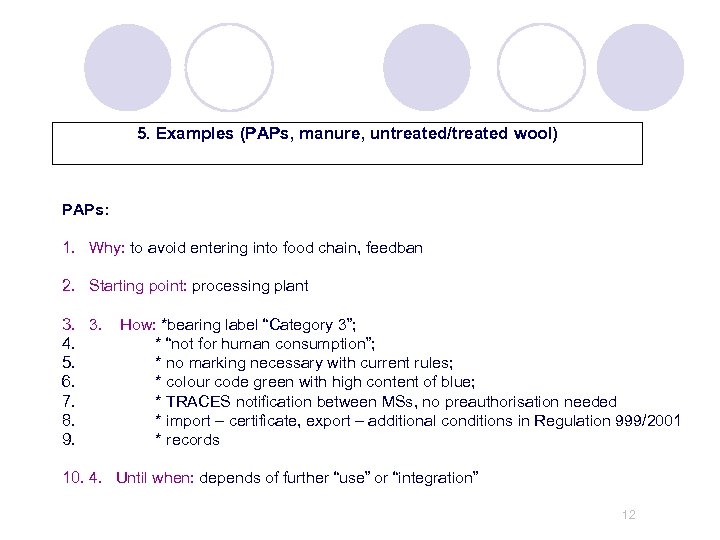
5. Examples (PAPs, manure, untreated/treated wool) PAPs: 1. Why: to avoid entering into food chain, feedban 2. Starting point: processing plant 3. 3. 4. 5. 6. 7. 8. 9. How: *bearing label “Category 3”; * “not for human consumption”; * no marking necessary with current rules; * colour code green with high content of blue; * TRACES notification between MSs, no preauthorisation needed * import – certificate, export – additional conditions in Regulation 999/2001 * records 10. 4. Until when: depends of further “use” or “integration” 12
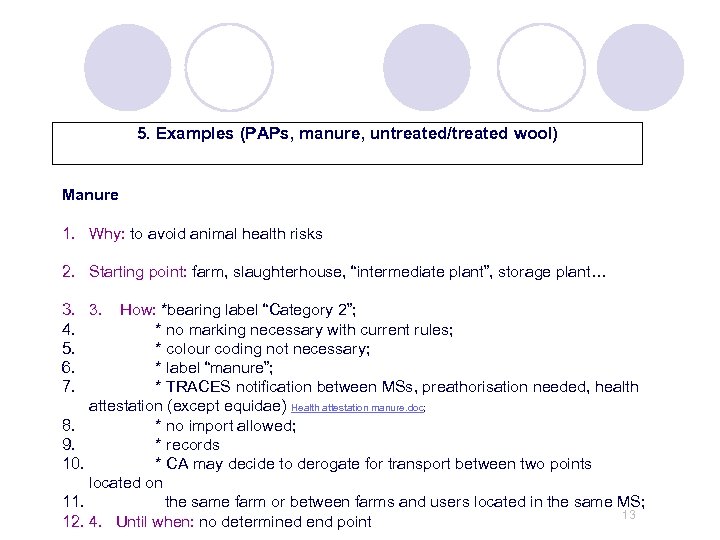
5. Examples (PAPs, manure, untreated/treated wool) Manure 1. Why: to avoid animal health risks 2. Starting point: farm, slaughterhouse, “intermediate plant”, storage plant… 3. 3. How: *bearing label “Category 2”; 4. * no marking necessary with current rules; 5. * colour coding not necessary; 6. * label “manure”; 7. * TRACES notification between MSs, preathorisation needed, health attestation (except equidae) Health attestation manure. doc; 8. * no import allowed; 9. * records 10. * CA may decide to derogate for transport between two points located on 11. the same farm or between farms and users located in the same MS; 13 12. 4. Until when: no determined end point
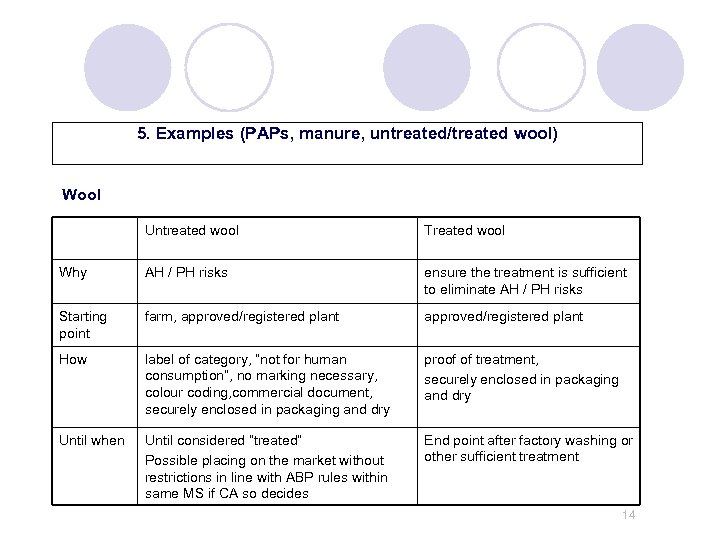
5. Examples (PAPs, manure, untreated/treated wool) Wool Untreated wool Treated wool Why AH / PH risks ensure the treatment is sufficient to eliminate AH / PH risks Starting point farm, approved/registered plant How label of category, “not for human consumption”, no marking necessary, colour coding, commercial document, securely enclosed in packaging and dry proof of treatment, securely enclosed in packaging and dry Until when Until considered “treated” Possible placing on the market without restrictions in line with ABP rules within same MS if CA so decides End point after factory washing or other sufficient treatment 14

QUESTIONS ? ? ? TAIEX Workshop on ABPs - March 2012 Beograd 15
3dd007d91884e6baeb0f79e11eed5049.ppt