a9c6f63e809d9d2ec1ab4ffb1fca901b.ppt
- Количество слайдов: 91

Reproductive Health Advisory Committee September 30, 2003 NDA #21 -322 Pamela Williamson Joyce, RAC Vice President, Regulatory Affairs & Quality Assurance – US Serono, Inc. 1

Proposed Indication – Luveris® (lutropin alfa for injection) administered with follitropin alfa for injection, is indicated for stimulation of follicular development in infertile hypogonadotropic hypogonadal women with profound LH deficiency (LH < 1. 2 IU/L)* * The indication presented in the NDA was amended on August 21, 2003 to be consistent with the indication as currently approved for Luveris® in the European Union and other countries outside of the United States 2

Agenda Introduction and Regulatory History Need for and Role of LH: HH Women with Profound Gonadotropin Deficiency Jerome Strauss, M. D. , Ph. D. Luigi Mastroianni Jr. Professor Director, Center for Research on Reproduction and Women’s Health University of Pennsylvania Luveris® Clinical Development Program Paul Lammers, M. D. Chief Medical Officer Serono, Inc. Clinical Perspective and Risk/Benefit Assessment Nanette F. Santoro, M. D. Professor and Director, Division of Reproductive Endocrinology Department of Obstetrics and Gynecology and Women’s Health Albert Einstein College of Medicine Summary and Conclusions 3 Pamela Williamson Joyce, RAC Vice President, Regulatory Affairs & Quality Assurance – US Serono, Inc. Pamela Williamson Joyce, RAC Serono, Inc.

Luveris® (lutropin alfa for injection) • Luteinizing hormone produced by recombinant DNA technology • Common names – Recombinant human luteinizing hormone – r-h. LH • Lyophilized powder in 75 IU vials • Self-administered by subcutaneous injection 4

Currently Approved in 46 Countries Argentina Australia Austria Belgium Brazil Bulgaria Chile Colombia Costa Rica Czech Republic Denmark Dominican Republic Estonia Finland France Germany 5 Greece Guatemala Holland Hong Kong Hungary Iceland Ireland Israel Italy Latvia Lithuania Luxemburg Mauritius Mexico Norway Peru Poland Portugal Romania Singapore Slovak Republic Slovenia Spain Sri Lanka Sweden Switzerland Trinidad & Tobaggo United Kingdom Uruguay Venezuela

Orphan Drug Designation for Luveris® “For use in association with recombinant human follicle stimulating hormone for the treatment of women with chronic anovulation due to hypogonadotropic hypogonadism” Orphan Drug Provides incentive for the development Regulation of drugs to treat rare diseases and conditions Rare Disease Prevalence < 200, 000 patients with the or Condition disease in the US Prevalence * OOPD #94 -802 6 Hypogonadotropic Hypogonadal Women 2, 800 -5, 600 (1/50, 000 -1/25, 000)*
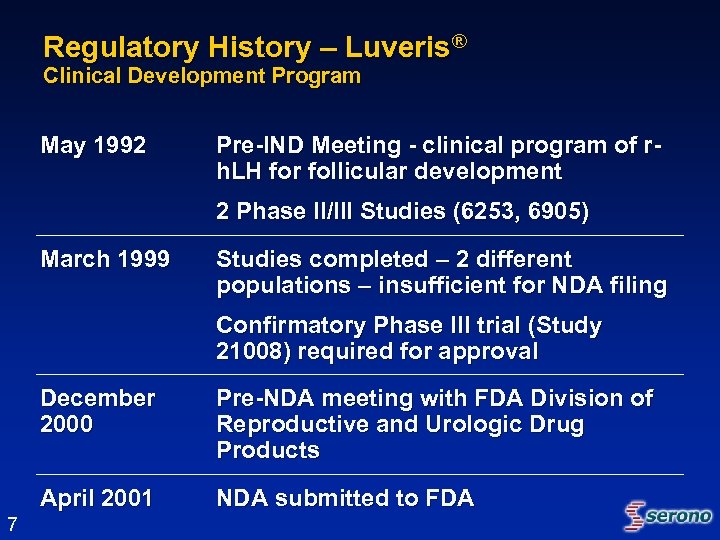
Regulatory History – Luveris® Clinical Development Program May 1992 Pre-IND Meeting - clinical program of rh. LH for follicular development 2 Phase II/III Studies (6253, 6905) March 1999 Studies completed – 2 different populations – insufficient for NDA filing Confirmatory Phase III trial (Study 21008) required for approval December 2000 April 2001 7 Pre-NDA meeting with FDA Division of Reproductive and Urologic Drug Products NDA submitted to FDA

Regulatory History – Luveris® (cont’d) Clinical Development Program March 2002 Not Approvable Letter • Insufficient evidence to support efficacy of the 75 IU/day dose • Conduct another Phase III study – Efficacy vs. Placebo – Ovulation induction – Dose ranging – Placebo, 75 IU and lower dose (50 or 25 IU) April 2002 8 Type A meeting – cycle cancellation due to Risk of OHSS January 2003 FDA will bring Luveris® before Advisory Committee

Regulatory History – Luveris® (cont’d) Clinical Development Program April 2003 August 2003 9 NDA amended to include results from extension study (21415) for confirmatory phase III trial NDA amended to revise indication

Topics for Discussion • Is there a need for recombinant luteinizing hormone? • Has the appropriate patient population been defined? • Has a safe and effective dose been identified? – 75 IU/day • Is the composite primary endpoint of follicular development an appropriate endpoint to assess efficacy in this patient population? 10

Topics for Discussion (cont’d) • Is the definition of treatment success appropriate? – Primary efficacy endpoint • Follicular development – Cancellation for the Risk of OHSS – Pregnancy • Serum -h. CG pregnancy test and/or ultrasound • Do the data support the safety and efficacy of Luveris® in the proposed indication? • Should another phase III double-blind placebo-controlled clinical trial be required for approval of Luveris® in patients with this rare condition? 11

External Consultant Experts Sarah L. Berga, MD • James Robert Mc. Cord Professor and Chair Department of Gynecology and Obstetrics Emory University School of Medicine Michael Diamond, MD • Kamram S. Moghissi Professor and Associate Chair of Obstetrics and Gynecology, Director, Division of Reproductive Endocrinology & Infertility Wayne State University (Detroit, MI) Gary Koch, Ph. D • Statistical Consultant Chapel Hill, NC 12

External Consultant Experts (cont’d) Bert Spilker, MD, Ph. D • Co-founder and former President Orphan Medical Adjunct Professor of Medicine; Clinical Professor of Pharmacy University of North Carolina (Chapel Hill, NC) Nanette F. Santoro, MD • Professor and Director, Division of Reproductive Endocrinology Department of Obstetrics and Gynecology and Women’s Health Albert Einstein College of Medicine (NY) Jerome Strauss, MD, Ph. D • Luigi Mastroianni Jr Professor Director, Center for Research on Reproduction and Women’s Health University of Pennsylvania Associate Chairman, Department of Obstetrics and Gynecology 13

Need for and Role of LH in HH Women with Profound Gonadotropin Deficiency Jerome F. Strauss III, MD, Ph. D Luigi Mastroianni Jr Professor Director, Center for Research on Reproduction & Women’s Health University of Pennsylvania Associate Chairman, Department of Obstetrics and Gynecology 14

Outline • Heterogeneity of pathophysiology and phenotype in Hypogonadotropic Hypogonadism (HH) • Consequences of profound LH deficiency • Current therapeutic options for HH • Unmet medical need 15
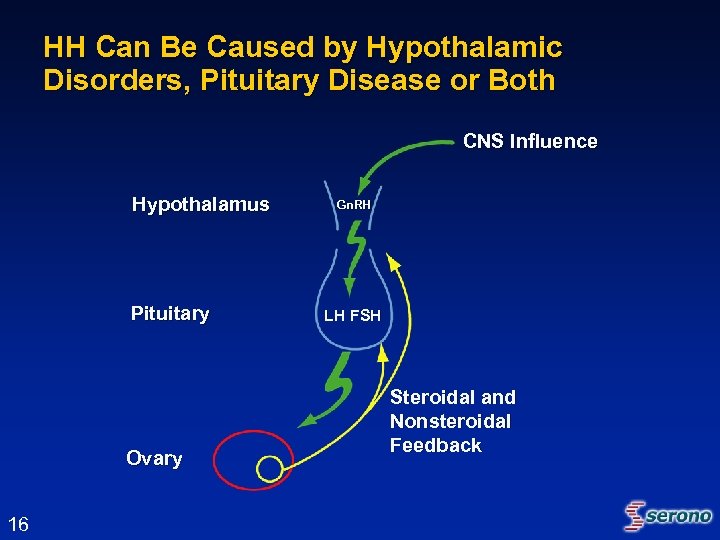
HH Can Be Caused by Hypothalamic Disorders, Pituitary Disease or Both CNS Influence Hypothalamus Pituitary Ovary 16 Gn. RH LH FSH Steroidal and Nonsteroidal Feedback
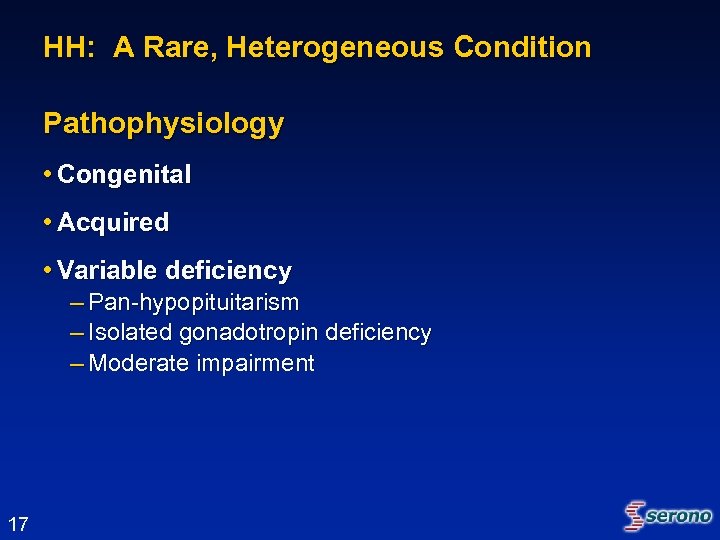
HH: A Rare, Heterogeneous Condition Pathophysiology • Congenital • Acquired • Variable deficiency – Pan-hypopituitarism – Isolated gonadotropin deficiency – Moderate impairment 17

Diagnosis of Profound LH Deficiency in HH • Serum LH levels < 1. 2 IU/L – Shoham Z et al. Fertil Steril 1991; 56: 1048 -1053 • Endocrine or functional evidence of estradiol deficiency – Estradiol level <30 pg/ml – Failed progestin challenge test 18

Outline • Heterogeneity of pathophysiology and phenotype in Hypogonadotropic Hypogonadism (HH) • Consequences of profound LH deficiency • Current therapeutic options for HH • Unmet medical need 19

Role of LH in Ovarian Function • Follicular steroidogenesis – Stimulation of androgen synthesis by theca cells • Follicular maturation – Can support terminal stages of follicular maturation • Ovulation – Resumption of meiosis – Ovulation – Luteinization • Maintenance of luteal function 20
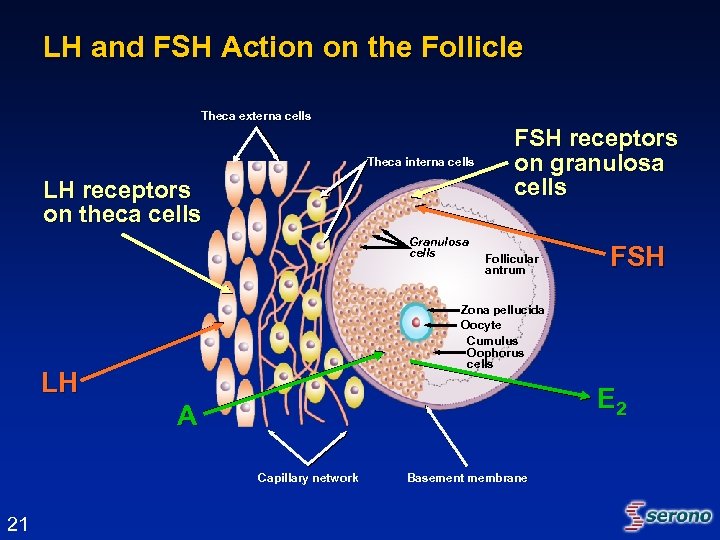
LH and FSH Action on the Follicle Theca externa cells Theca interna cells LH receptors on theca cells Granulosa cells FSH receptors on granulosa cells Follicular antrum Zona pellucida Oocyte Cumulus Oophorus cells LH E 2 A Capillary network 21 FSH Basement membrane
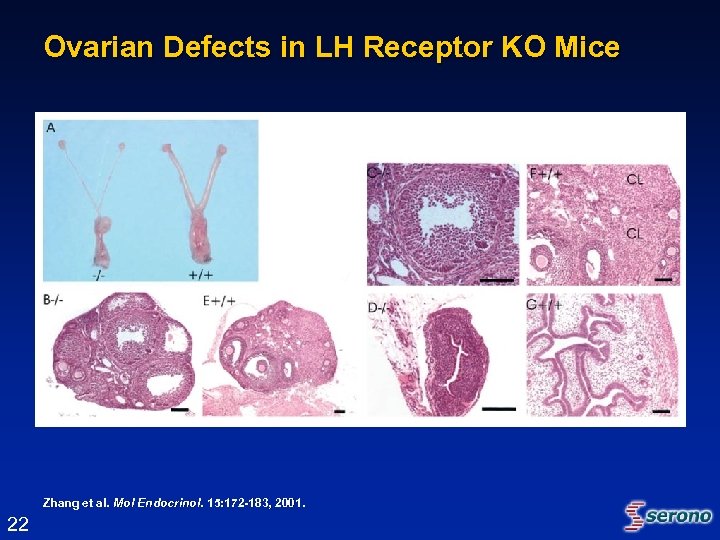
Ovarian Defects in LH Receptor KO Mice Zhang et al. Mol Endocrinol. 15: 172 -183, 2001. Endocrinol. 22
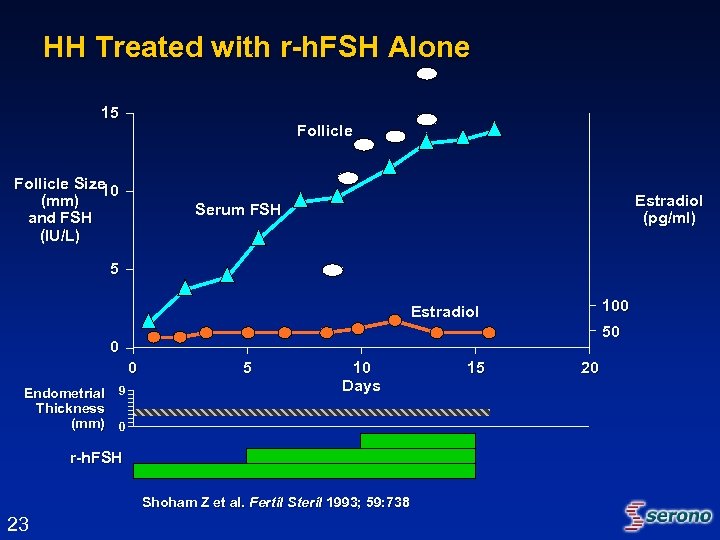
HH Treated with r-h. FSH Alone 15 Follicle Size 10 (mm) and FSH (IU/L) Estradiol (pg/ml) Serum FSH 5 100 Estradiol 50 0 0 Endometrial 9 Thickness (mm) 0 5 10 Days r-h. FSH Shoham Z et al. Fertil Steril 1993; 59: 738 23 15 20
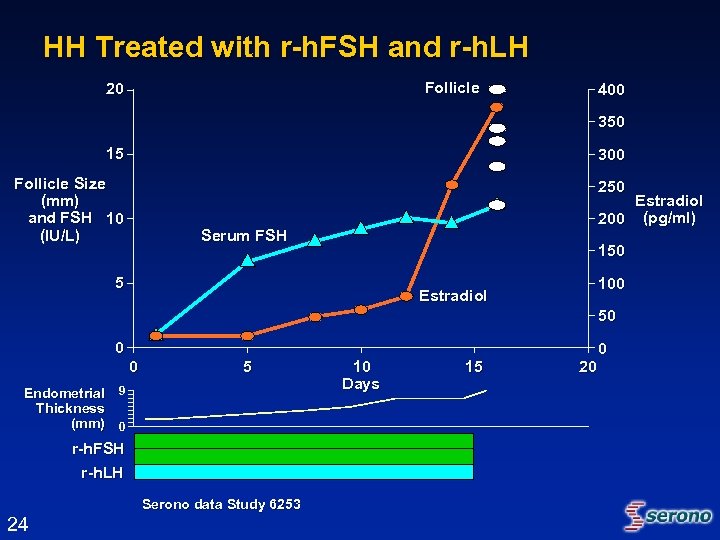
HH Treated with r-h. FSH and r-h. LH Follicle 20 400 350 15 300 Follicle Size (mm) and FSH 10 (IU/L) 250 Estradiol 200 (pg/ml) Serum FSH 150 5 100 Estradiol 50 0 0 5 Endometrial 9 Thickness (mm) 0 r-h. FSH r-h. LH Serono data Study 6253 24 10 Days 15 20 0
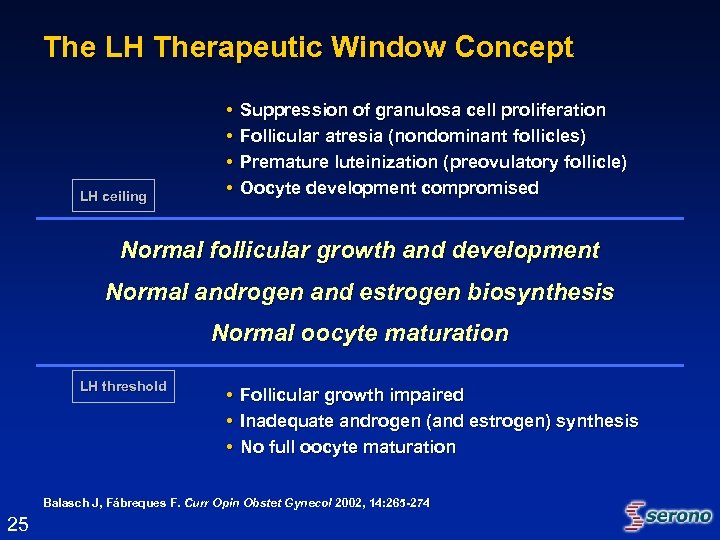
The LH Therapeutic Window Concept LH ceiling • • Suppression of granulosa cell proliferation Follicular atresia (nondominant follicles) Premature luteinization (preovulatory follicle) Oocyte development compromised Normal follicular growth and development Normal androgen and estrogen biosynthesis Normal oocyte maturation LH threshold • • • Follicular growth impaired Inadequate androgen (and estrogen) synthesis No full oocyte maturation Balasch J, Fábreques F. Curr Opin Obstet Gynecol 2002, 14: 265 -274 25

Outline • Heterogeneity of pathophysiology and phenotype in Hypogonadotropic Hypogonadism (HH) • Consequences of profound LH deficiency • Current therapeutic options for HH • Unmet medical need 26

Current Therapeutic Options for HH Gonadotropin Releasing Hormone (Gn. RH) • Pro – Effective in women with hypothalamic disease and intact pituitary – Monofollicular ovulation • Con – Approved but discontinued – Requires long-term pulsatile infusion 27
Current Therapeutic Options for HH Human Menopausal Gonadotropins (h. MG) • Pro – Reported to be effective in mostly small, uncontrolled series – Can be used in women with hypothalamic and/or pituitary disease • Con – Fixed ratio of FSH and LH – Ovarian Hyperstimulation Syndrome (OHSS) – Multiple gestation 28

Outline • Heterogeneity of pathophysiology and phenotype in Hypogonadotropic Hypogonadism (HH) • Consequences of profound LH deficiency • Current therapeutic options for HH • Unmet medical need 29

Unmet Medical Need • No FDA approved LH-only treatment for profoundly LH deficient patients • Individualization/titration – No options available for individualization of treatment • Recombinant Product – Purity – Consistency – S. C. administration 30

Conclusions • LH is required for follicular competency • HH is a very rare, heterogeneous disorder • HH women profoundly deficient in LH require exogenous LH for normal follicular function • Optimal therapy of HH is based on individualized treatment 31

Luveris® Clinical Development Program Paul Lammers, MD Chief Medical Officer Serono, Inc. 32

Presentation Outline • Luveris® (r-h. LH) Clinical Development Program – Study Design Considerations – Study Endpoints & Definition of Treatment Effect • Dose Finding Studies • Efficacy Overview – Confirmatory Phase III Study 21008 – Extension Study 21415 – Pregnancies & Pregnancy Outcomes • Safety Overview • Overall Conclusions 33
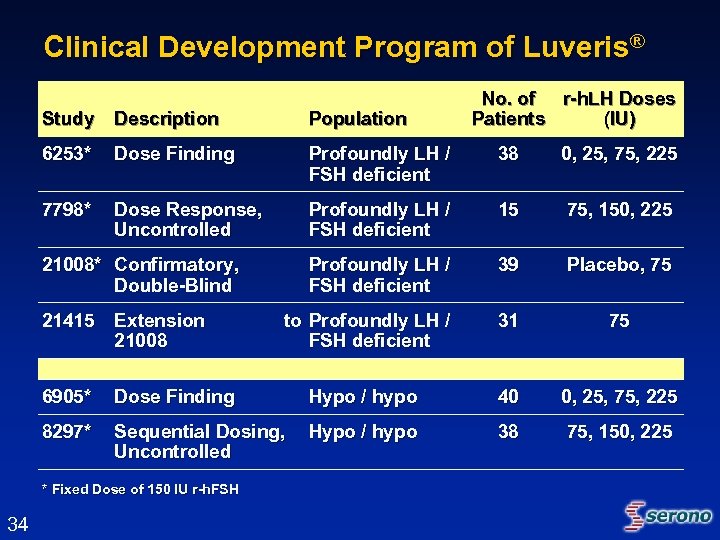
Clinical Development Program of Luveris® No. of r-h. LH Doses Patients (IU) Study Description Population 6253* Dose Finding Profoundly LH / FSH deficient 38 0, 25, 75, 225 7798* Dose Response, Uncontrolled Profoundly LH / FSH deficient 15 75, 150, 225 Profoundly LH / FSH deficient 39 Placebo, 75 to Profoundly LH / FSH deficient 31 75 21008* Confirmatory, Double-Blind 21415 Extension 21008 6905* Dose Finding Hypo / hypo 40 0, 25, 75, 225 8297* Sequential Dosing, Uncontrolled Hypo / hypo 38 75, 150, 225 * Fixed Dose of 150 IU r-h. FSH 34

Design Considerations Dose Finding Studies and Confirmatory Study 21008 • Study design considerations – Identify a clear dose response – Focus on effect of LH treatment only – Avoid potential confounder: change in FSH dose – Fix the dose of LH and FSH in each cycle • Clinical considerations – Absence of pre-existing data in this population – Provide adequate starting dose of FSH (150 IU) – Conservative criteria for discontinuing treatment for ovarian over-response (Risk of OHSS) 35
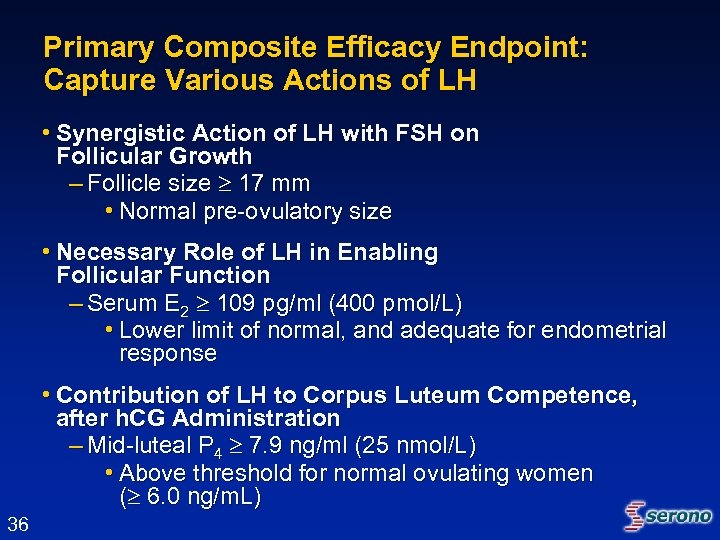
Primary Composite Efficacy Endpoint: Capture Various Actions of LH • Synergistic Action of LH with FSH on Follicular Growth – Follicle size 17 mm • Normal pre-ovulatory size • Necessary Role of LH in Enabling Follicular Function – Serum E 2 109 pg/ml (400 pmol/L) • Lower limit of normal, and adequate for endometrial response • Contribution of LH to Corpus Luteum Competence, after h. CG Administration – Mid-luteal P 4 7. 9 ng/ml (25 nmol/L) • Above threshold for normal ovulating women ( 6. 0 ng/m. L) 36
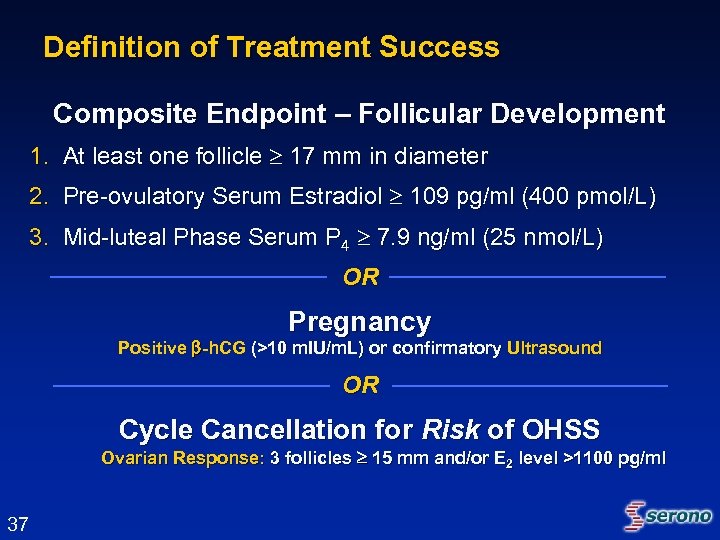
Definition of Treatment Success Composite Endpoint – Follicular Development 1. At least one follicle 17 mm in diameter 2. Pre-ovulatory Serum Estradiol 109 pg/ml (400 pmol/L) 3. Mid-luteal Phase Serum P 4 7. 9 ng/ml (25 nmol/L) OR Pregnancy Positive -h. CG (>10 m. IU/m. L) or confirmatory Ultrasound OR Cycle Cancellation for Risk of OHSS Ovarian Response: 3 follicles 15 mm and/or E 2 level >1100 pg/ml 37
Key Secondary Efficacy Endpoints • Estradiol (E 2) level • Endometrial thickness (mm) – Receptive uterine ‘milieu’ • Pregnancy Rate 38

Dose Finding Studies Study 6253 and Study 6905 39

Study 6253 Study Design • Controlled, parallel-designed, open-label, randomized, 3 cycle, dose-finding – Multinational study: September 1993 - April 1995 – 38 subjects enrolled in 10 centers in 4 countries • Standard dose-finding approach – r-h. FSH: 150 IU per day – r-h. LH: 0, 25, 75, and 225 IU per day (1: 1: 1: 1 in Cycle 1) – Protocol pre-specified an Armitage trend test to detect relationship between the LH dose and follicular development in Cycle 1 • Adequately powered at 85% 40

Study 6253 Study Design (cont’d) • Clinical Entry Criteria – Amenorrhea – Low gonadotropin levels • LH < 1. 2 IU/L and FSH < 5 IU/L (Profoundly LH-deficient) – Negative progestin challenge test as indicator of chronic low estrogen status • Treatment duration up to 14 days – If follicular response was ongoing (E 2 rise and follicle > 10 mm), treatment could continue beyond 14 days • Primary and secondary endpoints to be analyzed in cycle 1 • Pregnancy rates to be evaluated across all 3 cycles 41
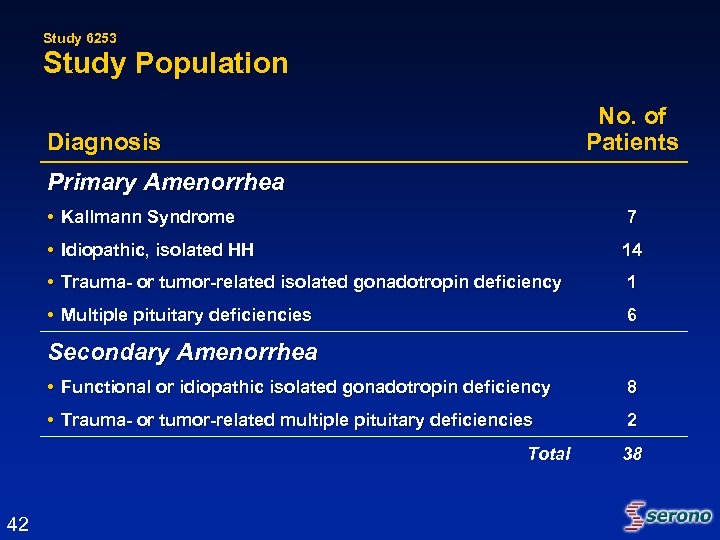
Study 6253 Study Population No. of Patients Diagnosis Primary Amenorrhea • Kallmann Syndrome 7 • Idiopathic, isolated HH 14 • Trauma- or tumor-related isolated gonadotropin deficiency 1 • Multiple pituitary deficiencies 6 Secondary Amenorrhea • Functional or idiopathic isolated gonadotropin deficiency 8 • Trauma- or tumor-related multiple pituitary deficiencies 2 Total 42 38
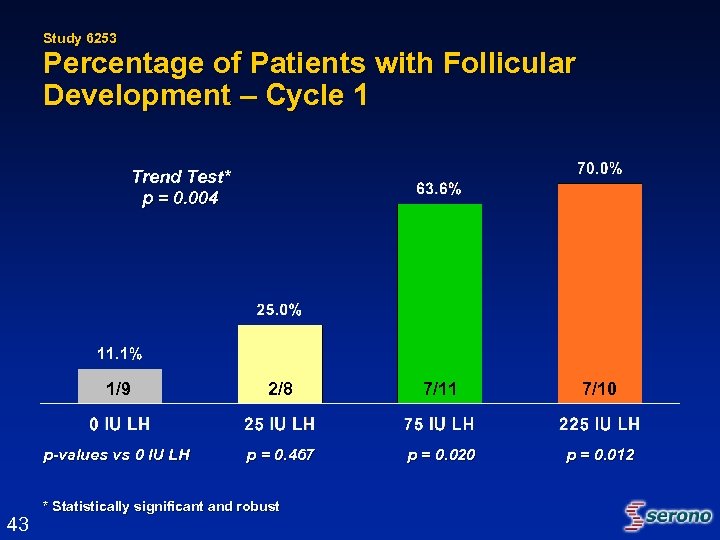
Study 6253 Percentage of Patients with Follicular Development – Cycle 1 Trend Test* p = 0. 004 1/9 7/11 7/10 p-values vs 0 IU LH 43 2/8 p = 0. 467 p = 0. 020 p = 0. 012 * Statistically significant and robust
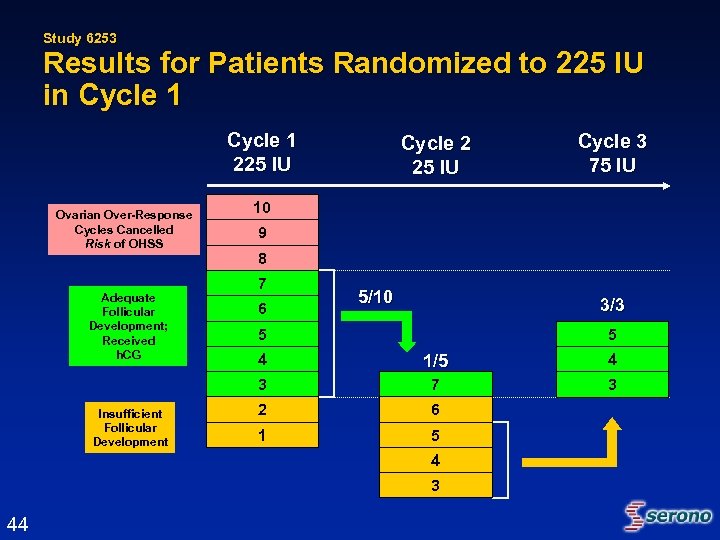
Study 6253 Results for Patients Randomized to 225 IU in Cycle 1 225 IU Ovarian Over-Response Cycles Cancelled Risk of OHSS Adequate Follicular Development; Received h. CG Cycle 2 25 IU Cycle 3 75 IU 10 9 8 7 6 5/10 3/3 5 5 1/5 4 3 Insufficient Follicular Development 4 7 3 2 6 1 5 4 3 44
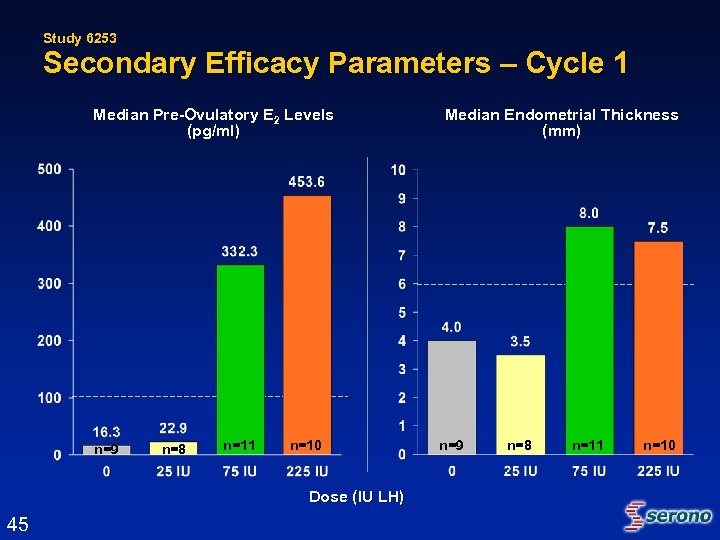
Study 6253 Secondary Efficacy Parameters – Cycle 1 Median Pre-Ovulatory E 2 Levels (pg/ml) n=9 n=8 n=11 n=10 Dose (IU LH) 45 Median Endometrial Thickness (mm) n=9 n=8 n=11 n=10
Study 6905 Study Design • Controlled, parallel-designed, open-label, randomized, 3 cycle, dose-finding study in US – July 1994 - July 1997 – 14 centers treated 40 subjects • Dose Groups: 0, 25, 75, and 225 IU LH co-administered with 150 IU FSH daily – Randomization Ratio - 1: 1: 1: 1 in cycle 1 • Fixed dose of LH and FSH within cycles 46
Study 6905 Study Design (cont’d) • Major Differences to Study 6253 – LH < 13 IU/L and FSH < 11 IU/L* • Broader HH population – No progestin challenge test; E 2 < 60 pg/m. L – Treatment duration up to 21 days • If follicular maturation was imminent, treatment could continue beyond 21 days 47 * <50 th percentile normal
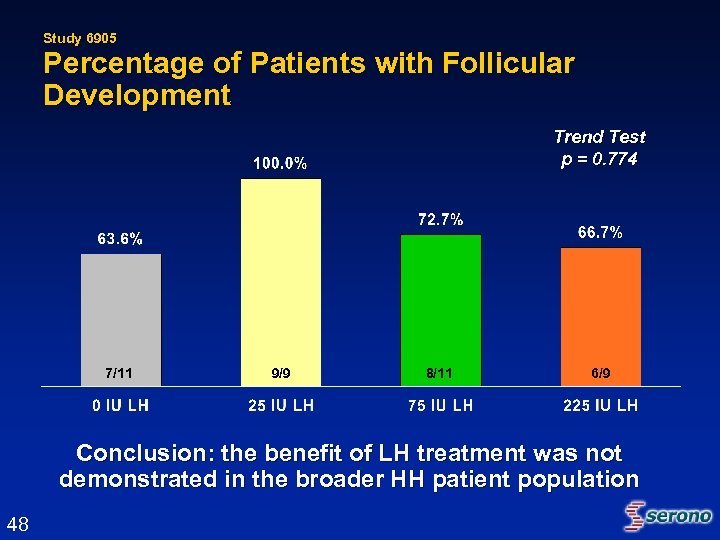
Study 6905 Percentage of Patients with Follicular Development Trend Test p = 0. 774 7/11 9/9 8/11 6/9 Conclusion: the benefit of LH treatment was not demonstrated in the broader HH patient population 48
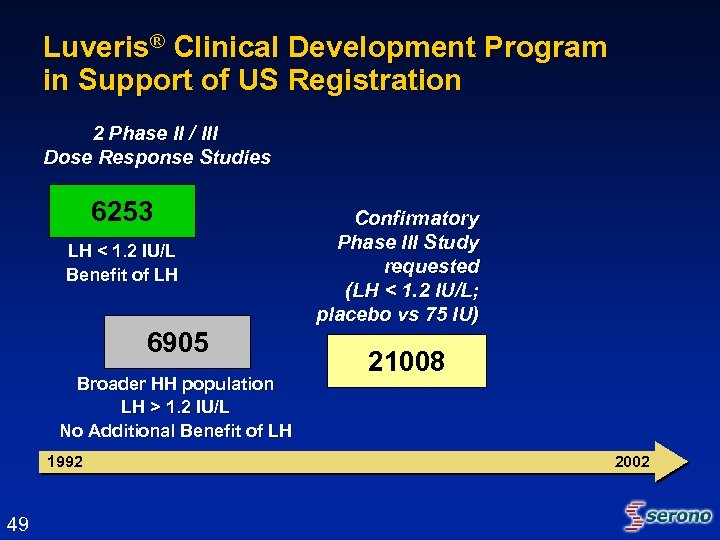
Luveris® Clinical Development Program in Support of US Registration 2 Phase II / III Dose Response Studies 6253 LH < 1. 2 IU/L Benefit of LH 6905 Broader HH population LH > 1. 2 IU/L No Additional Benefit of LH 1992 49 Confirmatory Phase III Study requested (LH < 1. 2 IU/L; placebo vs 75 IU) 21008 2002
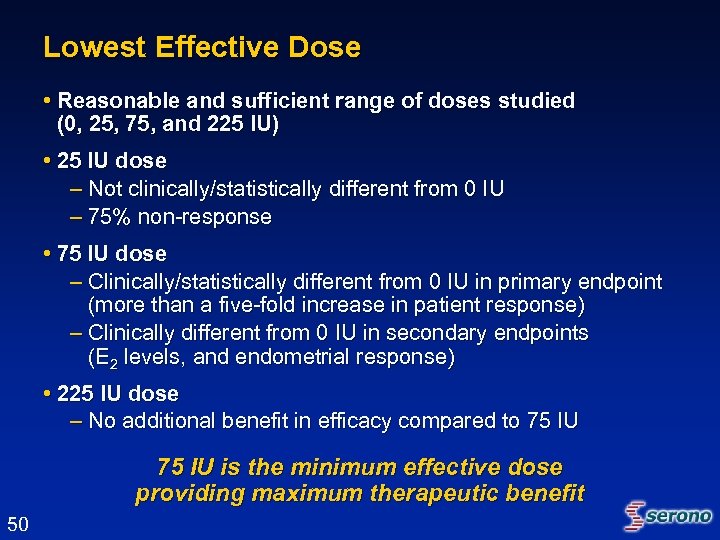
Lowest Effective Dose • Reasonable and sufficient range of doses studied (0, 25, 75, and 225 IU) • 25 IU dose – Not clinically/statistically different from 0 IU – 75% non-response • 75 IU dose – Clinically/statistically different from 0 IU in primary endpoint (more than a five-fold increase in patient response) – Clinically different from 0 IU in secondary endpoints (E 2 levels, and endometrial response) • 225 IU dose – No additional benefit in efficacy compared to 75 IU is the minimum effective dose providing maximum therapeutic benefit 50
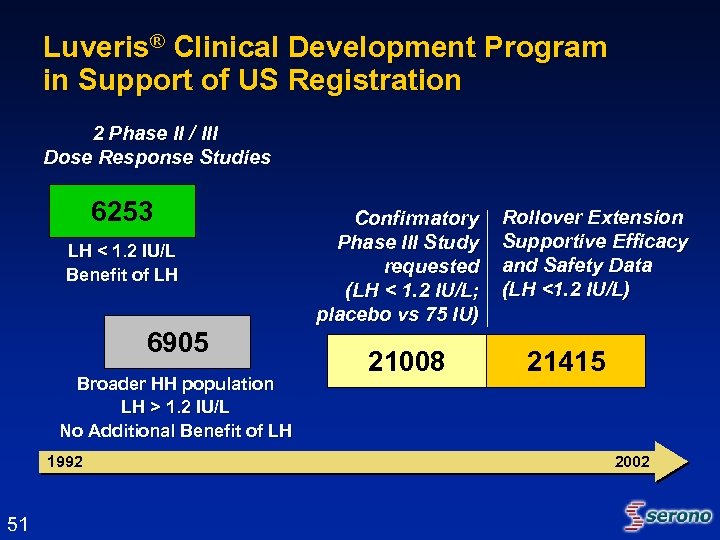
Luveris® Clinical Development Program in Support of US Registration 2 Phase II / III Dose Response Studies 6253 LH < 1. 2 IU/L Benefit of LH 6905 Broader HH population LH > 1. 2 IU/L No Additional Benefit of LH 1992 51 Confirmatory Phase III Study requested (LH < 1. 2 IU/L; placebo vs 75 IU) 21008 Rollover Extension Supportive Efficacy and Safety Data (LH <1. 2 IU/L) 21415 2002

Efficacy Overview Confirmatory Phase III Trial Study 21008 52

Study 21008 Study Design • Double-blind, randomized, placebo-controlled, multinational study in patients seeking pregnancy – 37 centers initiated; 25 centers enrolled 39 patients • Dose Groups: Placebo and 75 IU LH co-administered with 150 IU FSH daily – Randomization Ratio - 1: 2 • Fixed dose of LH and FSH 53

Study 21008 Study Design (cont’d) • Clinical Entry Criteria – Amenorrhea – Low gonadotropin levels • LH < 1. 2 IU/L and FSH < 5 IU/L (Profoundly LH-deficient) – Negative progestin challenge test as indicator of chronic low estrogen status • Treatment duration up to 14 days – If follicular maturation was imminent, treatment could continue beyond 14 days • Single cycle of treatment (roll-over possibility to extension Study 21415) 54
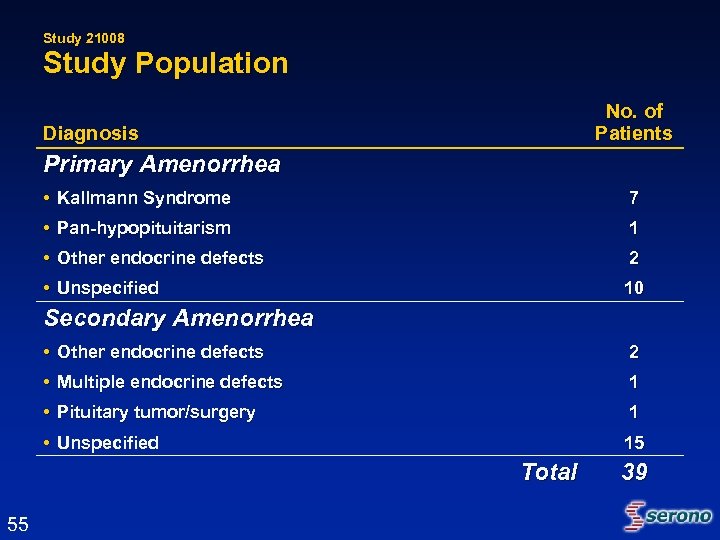
Study 21008 Study Population No. of Patients Diagnosis Primary Amenorrhea • Kallmann Syndrome 7 • Pan-hypopituitarism 1 • Other endocrine defects 2 • Unspecified 10 Secondary Amenorrhea • Other endocrine defects 2 • Multiple endocrine defects 1 • Pituitary tumor/surgery 1 • Unspecified 15 Total 55 39
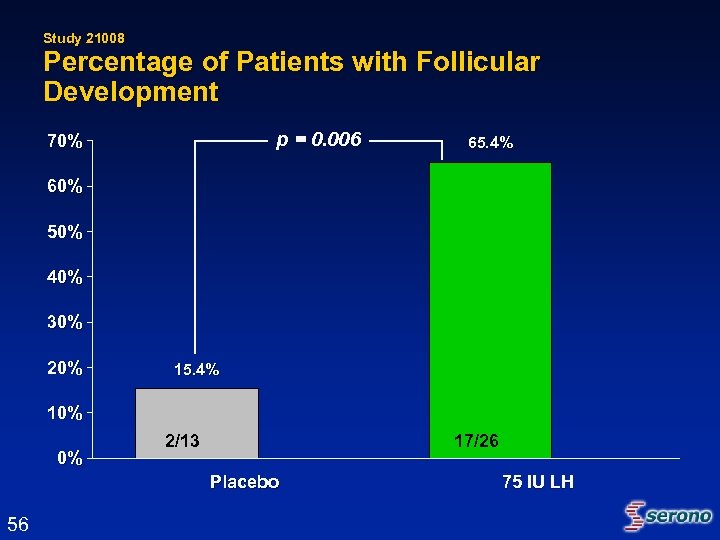
Study 21008 Percentage of Patients with Follicular Development p = 0. 006 70% 65. 4% 60% 50% 40% 30% 20% 15. 4% 10% 0% 2/13 17/26 Placebo 56 75 IU LH
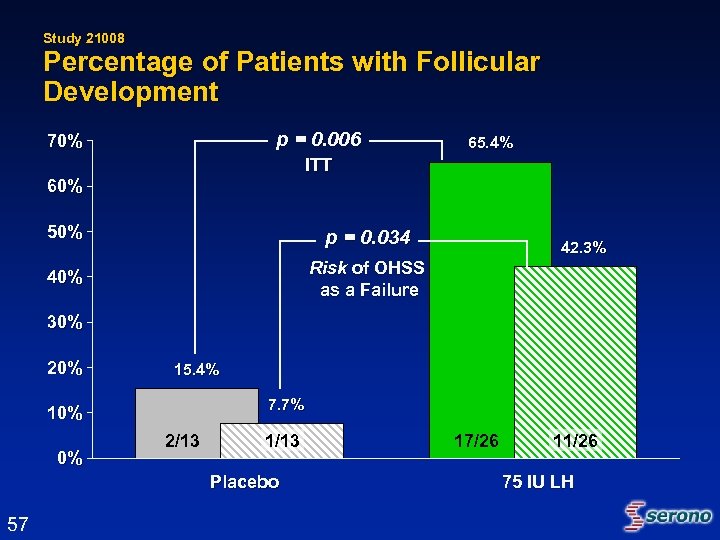
Study 21008 Percentage of Patients with Follicular Development p = 0. 006 70% 65. 4% ITT 60% 50% p = 0. 034 40% Risk of OHSS as a Failure 42. 3% 30% 20% 15. 4% 7. 7% 10% 0% 2/13 1/13 Placebo 57 17/26 11/26 75 IU LH
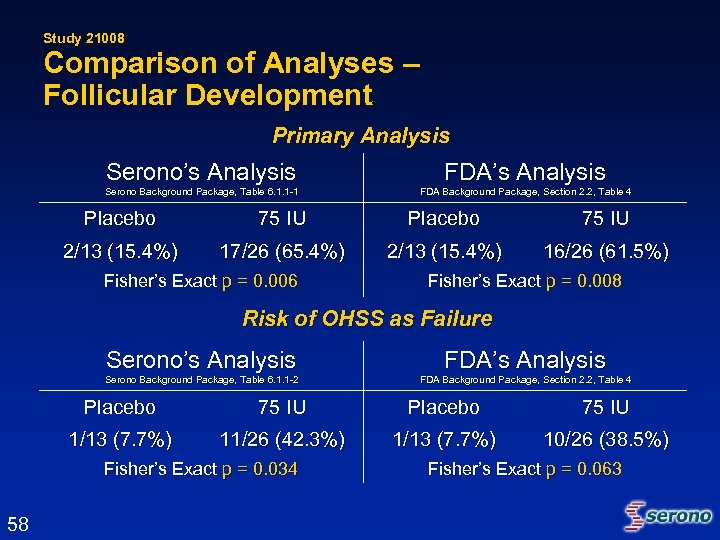
Study 21008 Comparison of Analyses – Follicular Development Primary Analysis Serono’s Analysis FDA’s Analysis Serono Background Package, Table 6. 1. 1 -1 FDA Background Package, Section 2. 2, Table 4 Placebo 75 IU 2/13 (15. 4%) 17/26 (65. 4%) 2/13 (15. 4%) 16/26 (61. 5%) Fisher’s Exact p = 0. 006 Fisher’s Exact p = 0. 008 Risk of OHSS as Failure Serono’s Analysis FDA’s Analysis Serono Background Package, Table 6. 1. 1 -2 FDA Background Package, Section 2. 2, Table 4 Placebo 75 IU 1/13 (7. 7%) 11/26 (42. 3%) 1/13 (7. 7%) 10/26 (38. 5%) Fisher’s Exact p = 0. 034 58 Fisher’s Exact p = 0. 063

Studies 21008 and 21415 Protocol Definition of Success “The primary efficacy endpoint will be follicular development as defined by the following three parameters, all of which must be true: – At least one follicle with a mean diameter 17 mm and – Preovulatory E 2 serum level 109 pg/m. L (400 pmol/L) and – Mid-luteal phase P 4 level 7. 9 ng/m. L (25 nmol/L) Should any patient be cancelled for Risk of OHSS, that patient will be counted as achieving follicular development. Should any patient achieve pregnancy, that patient will be counted as having achieved follicular development. ” 59
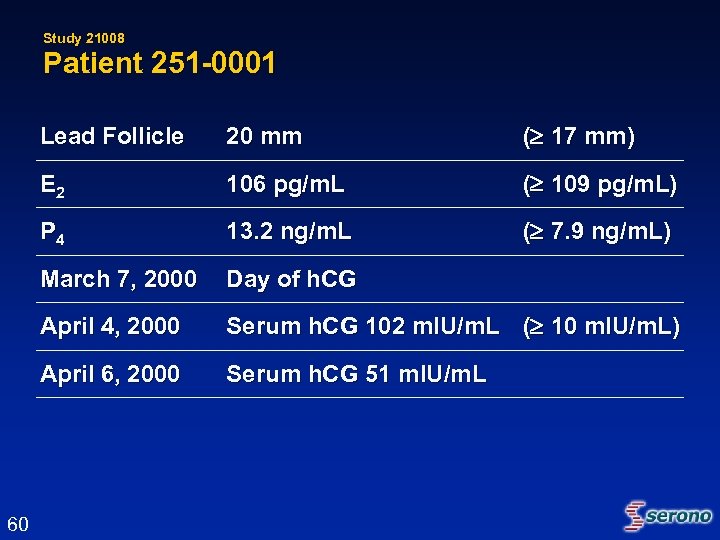
Study 21008 Patient 251 -0001 Lead Follicle ( 17 mm) E 2 106 pg/m. L ( 109 pg/m. L) P 4 13. 2 ng/m. L ( 7. 9 ng/m. L) March 7, 2000 Day of h. CG April 4, 2000 Serum h. CG 102 m. IU/m. L ( 10 m. IU/m. L) April 6, 2000 60 20 mm Serum h. CG 51 m. IU/m. L

Efficacy Overview Extension Study 21415 61
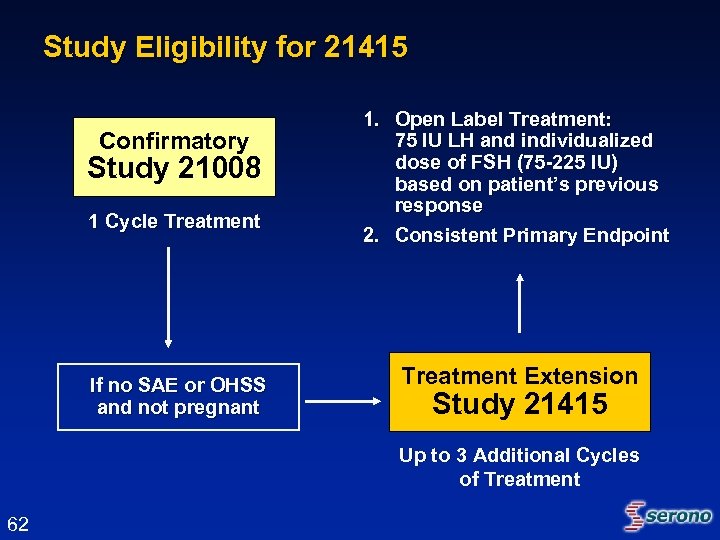
Study Eligibility for 21415 Confirmatory Study 21008 1 Cycle Treatment If no SAE or OHSS and not pregnant 1. Open Label Treatment: 75 IU LH and individualized dose of FSH (75 -225 IU) based on patient’s previous response 2. Consistent Primary Endpoint Treatment Extension Study 21415 Up to 3 Additional Cycles of Treatment 62
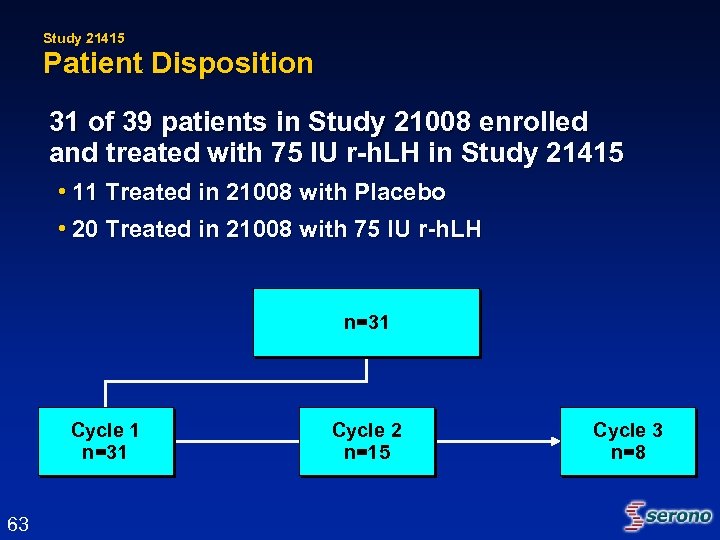
Study 21415 Patient Disposition 31 of 39 patients in Study 21008 enrolled and treated with 75 IU r-h. LH in Study 21415 • 11 Treated in 21008 with Placebo • 20 Treated in 21008 with 75 IU r-h. LH n=31 Cycle 1 n=31 63 Cycle 2 n=15 Cycle 3 n=8
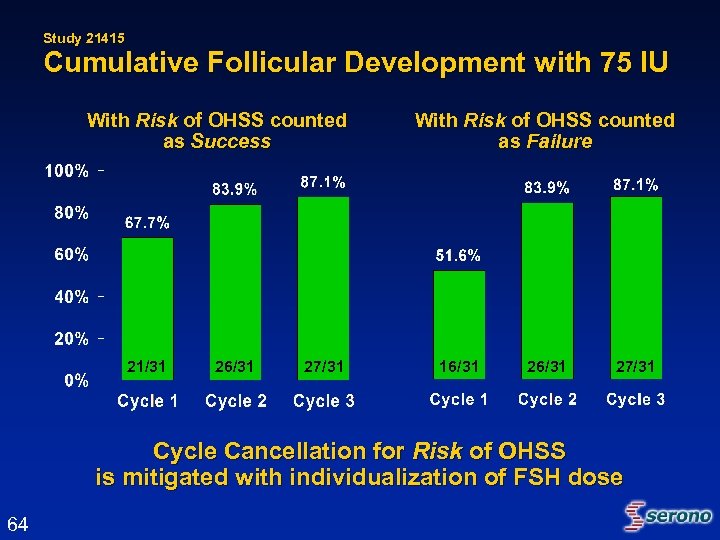
Study 21415 Cumulative Follicular Development with 75 IU With Risk of OHSS counted as Success 21/31 26/31 27/31 With Risk of OHSS counted as Failure 16/31 27/31 Cycle Cancellation for Risk of OHSS is mitigated with individualization of FSH dose 64

Studies 21008 and 21415 Impact of Cycle Cancellation for Risk of OHSS on Response in Subsequent Cycles • Cycle cancellation due to the Risk of OHSS is a normal precaution in clinical practice • Ovarian over-response is a treatment effect and provides guidance for next cycle of treatment • 4 of the 11 patients whose cycles were cancelled due to Risk of OHSS in 1 st cycle of Study 21008 or 21415 achieved pregnancy in Study 21415 with adjustment of FSH dose in subsequent cycle 65
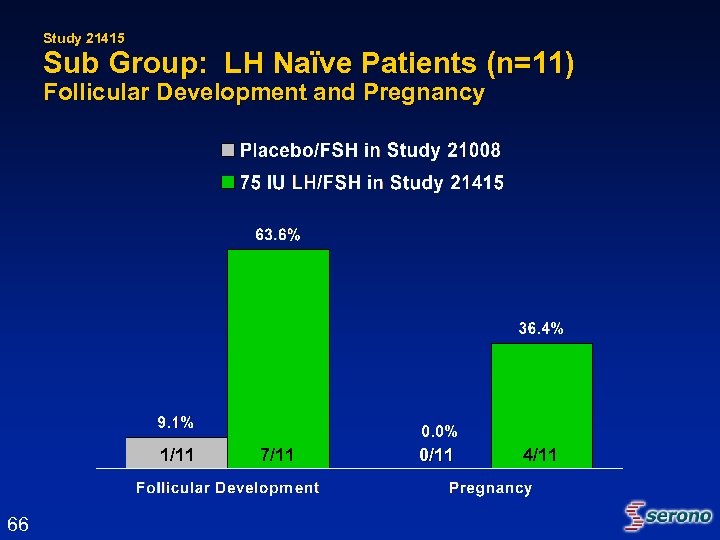
Study 21415 Sub Group: LH Naïve Patients (n=11) Follicular Development and Pregnancy 1/11 66 7/11 0/11 4/11
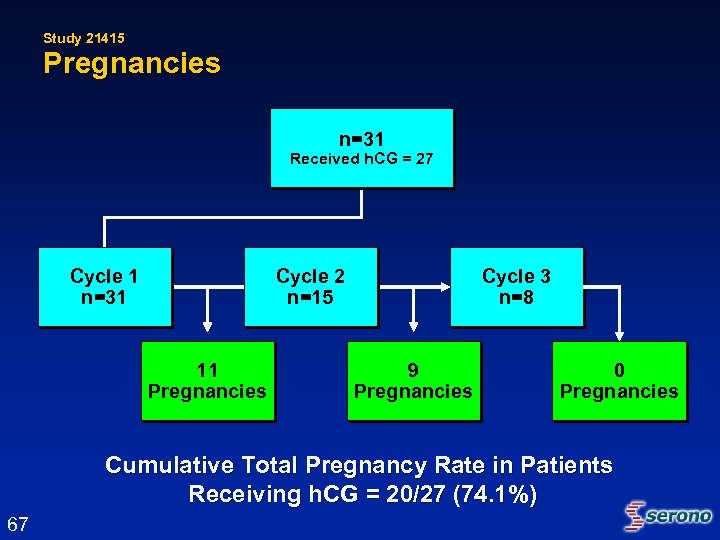
Study 21415 Pregnancies n=31 Received h. CG = 27 Cycle 1 n=31 Cycle 2 n=15 11 Pregnancies Cycle 3 n=8 9 Pregnancies 0 Pregnancies Cumulative Total Pregnancy Rate in Patients Receiving h. CG = 20/27 (74. 1%) 67
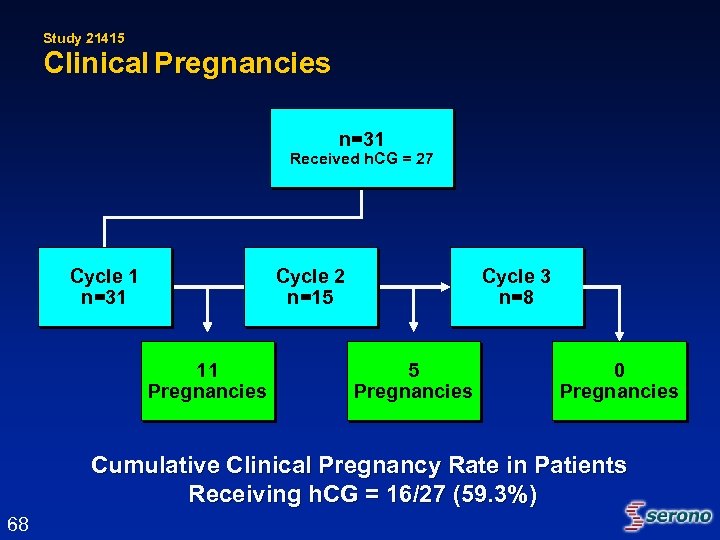
Study 21415 Clinical Pregnancies n=31 Received h. CG = 27 Cycle 1 n=31 Cycle 2 n=15 11 Pregnancies Cycle 3 n=8 5 Pregnancies 0 Pregnancies Cumulative Clinical Pregnancy Rate in Patients Receiving h. CG = 16/27 (59. 3%) 68

Summary of Pregnancy Results and Pregnancy Outcomes 69
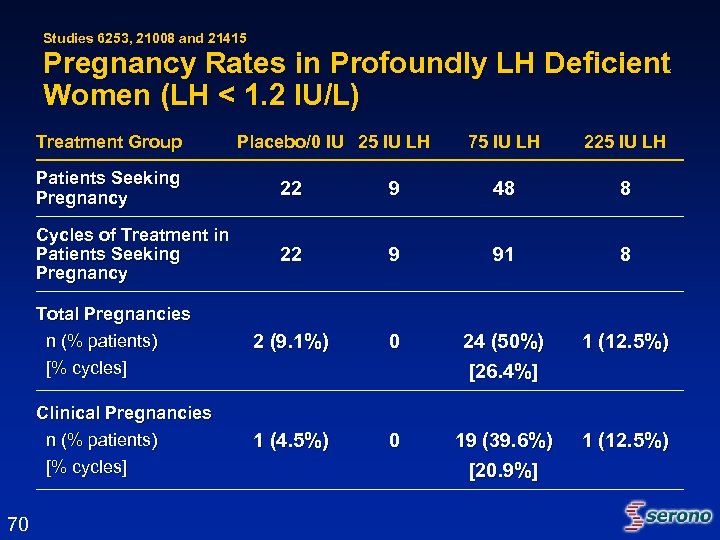
Studies 6253, 21008 and 21415 Pregnancy Rates in Profoundly LH Deficient Women (LH < 1. 2 IU/L) Treatment Group Placebo/0 IU 25 IU LH 75 IU LH 225 IU LH Patients Seeking Pregnancy 9 48 8 Cycles of Treatment in Patients Seeking Pregnancy 22 9 91 8 Total Pregnancies n (% patients) [% cycles] 2 (9. 1%) 0 24 (50%) [26. 4%] 1 (12. 5%) Clinical Pregnancies n (% patients) [% cycles] 70 22 1 (4. 5%) 0 19 (39. 6%) [20. 9%] 1 (12. 5%)
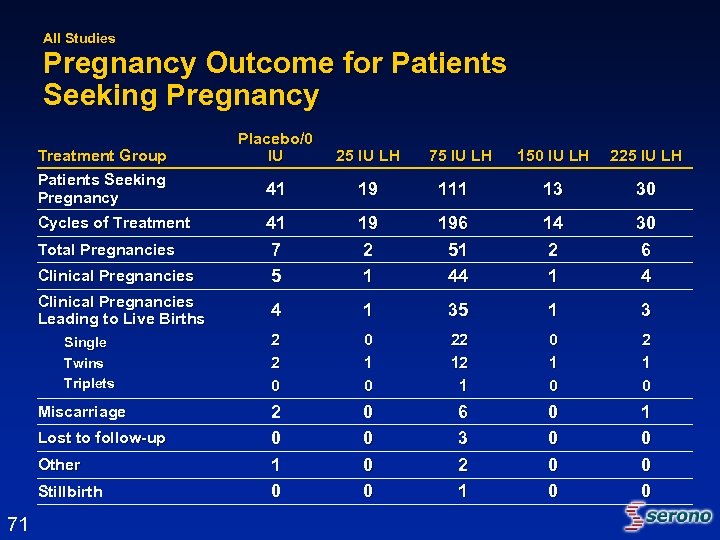
All Studies Pregnancy Outcome for Patients Seeking Pregnancy Treatment Group Patients Seeking Pregnancy Cycles of Treatment Total Pregnancies Clinical Pregnancies Leading to Live Births Single Twins Triplets Miscarriage Lost to follow-up Other Stillbirth 71 Placebo/0 IU 25 IU LH 41 19 41 7 5 75 IU LH 150 IU LH 225 IU LH 111 13 30 19 2 1 196 51 44 14 2 1 30 6 4 4 1 35 1 3 2 2 0 0 1 0 22 12 1 0 2 0 1 0 0 0 6 3 2 1 0 0 0

Efficacy Conclusions 72
Efficacy Conclusions • Study 6253 provides rationale for selection of 75 IU r-h. LH as the appropriate dose for HH patients with profound LH deficiency (LH < 1. 2 IU/L) – No benefit of 25 IU dose of r-h. LH – No additional benefit of 225 IU dose of r-h. LH • Study 21008, the randomized, double-blind, placebo-controlled study, confirmed the efficacy of 75 IU r-h. LH dose in the profoundly LH deficient (LH < 1. 2 IU/L) patient population 73
Efficacy Conclusions (cont’d) • Study 21415 supports the efficacy of 75 IU as used in standard clinical practice with individualized dosing – Cumulative follicular development rate 87. 1% – Cumulative pregnancy rate 74. 1% • Overall, a 50% (24/48) pregnancy rate in profoundly LH-deficient women* (LH < 1. 2 IU/L) on 75 IU dose * Studies 6253, 21008, and 21415 74

Overview of Safety 75

Safety Conclusions • Largest safety database in Female HH Patients (170 patients, 152 received r-h. LH in 283 cycles) • No increase in adverse events when r-h. LH is co-administered with r-h. FSH, compared to r-h. FSH alone • Similar rates of OHSS across all dose groups, including r-h. FSH alone • Safety profile of r-h. LH comparable to currently marketed gonadotropins 76

Overall Conclusions Luveris® Clinical Development Program • Among women with HH, a cut-off value of 1. 2 IU/L differentiates between LH-dependence and LH-independence • Follicular development is an appropriate endpoint in this population and correlates with pregnancy • Canceling a cycle is prudent clinical practice in an over-responding patient with follicular development • Women with profound LH-deficiency clearly benefit from treatment with Luveris® 75 IU • The safety profile of Luveris® is similar to other gonadotropins and is not different from treatment with FSH alone 77

Clinical Perspective and Risk/Benefit Assessment Nanette F. Santoro, MD Professor and Director, Division of Reproductive Endocrinology Department of Obstetrics and Gynecology and Women’s Health Albert Einstein College of Medicine 78

Hypogonadotropic Hypogonadism (HH) Women and Infertility • Absence of pubertal development • Single endocrine factor • Potential to be highly fertile when ovarian responsiveness is restored • LH (in addition to FSH) is needed for optimal follicle growth • Induction of follicular development as a prelude to fertility is therapeutic goal 79

Follicular Maturation FSH • Induces early growth • Controls follicle number LH • Provides estrogen precursors • Needed for latter stages of growth 80
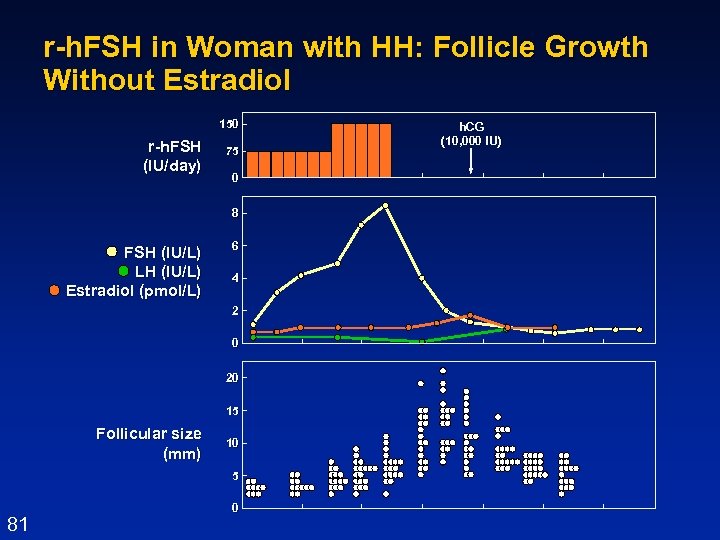
r-h. FSH in Woman with HH: Follicle Growth Without Estradiol 150 r-h. FSH (IU/day) 75 0 8 FSH (IU/L) LH (IU/L) Estradiol (pmol/L) 6 4 2 0 20 15 Follicular size (mm) 10 5 81 0 3/19/2018 h. CG (10, 000 IU)
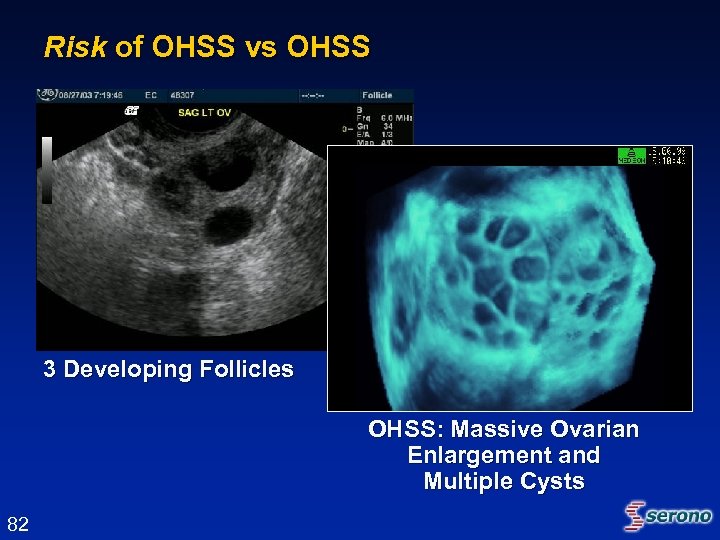
Risk of OHSS vs OHSS 3 Developing Follicles OHSS: Massive Ovarian Enlargement and Multiple Cysts 82

Follicular Development in HH • Give back what’s missing – Gn. RH highly effective if intact pituitary function, but not available – Alternative strategy: exogenous gonadotropins (u-h. MG), but combined fixed ratio and IM administration is a limitation to treatment – Optimal strategy: stand-alone recombinant human LH and FSH allows titration and individualization 83

Recombinant LH • Permissive and obligatory for follicle growth • FSH tailoring is needed in gonadotropin cycles, therefore: r. LH dose should be ‘guaranteed’ to be sufficient! • 75 IU dose is adequate • Maximizes return on investment (cycle) for patient and clinician 84
Risks vs. Benefits Risks • Known complications of gonadotropins in infertility treatment – Ovarian Hyperstimulation Syndrome (OHSS) – Multiple births • Other minimal/transient treatment-related adverse events (minor) • Risks mitigated with proper diagnosis, dosing and observation 85
Risks vs. Benefits • Optimal folliculogenesis and endocrine profile based on individualized treatment • Convenience (subcutaneous, self-administered) • Safety profile comparable to currently marketed gonadotropins • High pregnancy rate in this profoundly LH-deficient patient population 86

Summary • HH is a rare patient group for whom LH is critical during folliculogenesis • Provision of r. LH to r. FSH allows maximum flexibility in treatment of these patients • Benefit/risk profile in favor of approval and making product available to patients and physicians 87

Summary and Conclusions Pamela Williamson Joyce, RAC Vice President, Regulatory Affairs & Quality Assurance – U. S. Serono, Inc. 88

Conclusions • Clear need for LH in treatment of patients with this rare condition • Appropriate patient population – HH women, profoundly LH deficient • Optimal dose of 75 IU safe and effective • Follicular development, as prospectively defined, most appropriate to determine action of LH • Most extensive database in r-h. LH in hypogonadotropic hypogonadal women (n=170) 89

Conclusions • Largest double-blind placebo-controlled clinical trial (21008) in patients with this rare condition • Pivotal trial positive whether cycle cancellation due to Risk of OHSS analyzed as efficacy success or failure • Additional supportive efficacy, safety and pregnancy data (21415) 90

Conclusions • No increase in adverse events compared to placebo; safety similar to that of other gonadotropin products • Luveris® is effective for treatment of infertile women with profound LH deficiency (LH < 1. 2 IU/L) • Positive Benefit/Risk profile 91
a9c6f63e809d9d2ec1ab4ffb1fca901b.ppt