28a2c85348bd945fb46fd05bd8ab8cb7.ppt
- Количество слайдов: 69

Reprocessing of instruments for office based practices including Day surgeries, dental practices and aged care facilities Lynne Noring

National standards and State Policy • Australia/ New Zealand 4815 2006 Office based health care facilities –Reprocessing of reusable medical and surgical instruments and equipment, and maintenance of associated environment. • NSW infection Control policy

Cleaning and handling of used equipment

Standard precautions • Treating all used items as a • • • potential source of infection Wash hands and cover any skin lesions with occlusive dressings Wear appropriate personal protection such as utility gloves specially designed repellent masks/eye protection/face shields and fluid resistant aprons or gowns. Thermal disinfect the cleaning brushes either at the beginning or end of each day.

why is the pre-requisite to sterilisation the cleaning process. • Reduce the microbial • • • contaminates. Remove tissue debris, blood and other organic material so the sterilant can make contact with every part of the item being sterilised. To prevent the deterioration of instruments and equipment. If an item is not clean prior to being placed into the steriliser it will not be sterile.

There are 2 types of soil you will come in contact with when cleaning reusable equipment. • Visible contaminates including blood, bone. tissue and inorganic soils such as dirt or dust. • Non-visible contaminates Micro – organisms, bacteria, and virus.

Water quality for cleaning • Water supply should be of • • good quality. Water suitable for drinking is suitable for cleaning. Water with high mineral content is unsuitable due to mineral deposits shortening the equipment life and interfering with the effect of the cleaning agents.

Collection procedures • Procedures for the • collection of used reusable items from treatment areas shall be formulated by each office – based health care facility. Collection equipment shall be puncture and leak resistant, and made of a material that is able to be cleaned.

Reprocessing Area • All used items are to be • cleaned in a designated area to prevent the possible contamination of items processed. On receipt in the reprocessing area, items should be sorted to type and corresponding cleaning method. Example manual, mechanical and cannualted.

Sorting for reprocessing • All equipment that is • • unwrapped is considered to be contaminated whether used or not. A check for completeness and defects shall be made during the sorting process. All facilities should have written procedures for handling specialized equipment.

The equipment the facility should ideally include. • Separate hand washing • • • facilities Adequate bench space with smooth surfaces without crevices. Good lighting Efficient ventilation Adequate storage space Bins for disposal of waste Non-slip flooring Cleaning sinks and washer /disinfector Ultrasonic cleaner Drying facilities.

The four steps to the cleaning process • Pre-rinse –cold water • Wash- hot water and a • • cleaning agent Rinse – 1 or more rinses with hot water 80 to 90 ˚c with a drying agent added, in accordance with manufacturers recommendations. Drying – shall be by means of a drying cabinet or lint free cloths or disposable lint free cloths.

Cleaning agents • Shall be used to remove residual soil and organic matter from instruments and equipment. Only those intended by the manufacturer for use in cleaning medical devices shall be used.

Safe use of cleaning agents • Staff should be trained in • application, handling and safe use of cleaning agents for instrument cleaning. Product data bulletins and material safety data sheets for all cleaning agents shall be obtained, read and implemented.

Cleaning agents should be, • • Biodegradable Non-corrosive Non-toxic Non-abrasive Low foaming Free rinsing Preferably liquid mild alkali formation with a ph range 8. 0 to 10. 8 However some instruments and equipment maybe made of materials where the use of neutral detergents are more appropriate.

Labelling of cleaning agents should include, • • • The name of the product The manufacturer The purpose of the product The directions for dilution and use The batch number The expiry date Advice not to be mixed with other chemicals Safety and first aid Specific storage Hazards nature

Abrasive cleaners • Steel wool • Stainless steel brushes • Powders and pastes

Manual cleaning equipment • Sinks • Assorted brushes with • soft and hard bristles. lumen brushes with a variety of diameters and lengths. Soft lint free cloths

Mechanical cleaning methods • Ultrasonic washers • Indexing/tunnel • • washer disinfectors Stand alone washer/disinfectors Cannulised sonic washers

Thermal disinfection • Thermal disinfection is • not a sterilisation process. Items used for critical procedures must be sterilised. Thermal disinfection uses heat and water at temperatures that destroy pathogenic vegetative organisms.
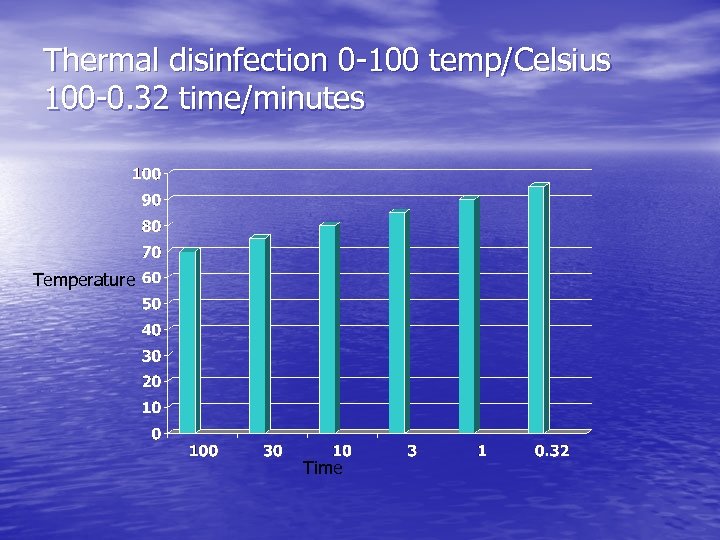
Thermal disinfection 0 -100 temp/Celsius 100 -0. 32 time/minutes Temperature Time

Methods of mechanical cleaning • Washer cycles include, cold pre • • • rinse Hot wash with cleaning agent One or more rinses with hot water at a suitable temperature for thermal disinfection to be achieved. Drain, leaving the contents at a temperature for quick drying. Drying. Washer disinfectors need to cleaned and maintained regularly to prevent colonizing and formation of biofilms which could contaminate the instruments processed within.

Ultrasonic cleaners • The tank must be filled with cold or • • tepid water add a measured amount of recommended detergent. Operate the machine to degas the solution. Tested to see if the transducers are functioning correctly and the results recorded. Rinse the instrument of visible contaminates before immersing the instruments. The instruments should be placed in a suitable basket in an open position before being submerged into the water tank close the lid and commence the cycle. After the specific time, remove the instruments and rinse in clean running hot water.

Method of manual cleaning • Flush the item with running water. • Fill the container or bowl with • • • warm water and neutral or in some cases enzymatic cleaning solution. Dismantle all removable parts or open items before placing into the cleaning solution. Hold the items low in the container and wash all surfaces, brush lumens and valves. Remove stubborn stains by soaking in an approved stain removing solution if the instrument manufacturers recommendation permits.

Method of rinsing and drying for manual cleaning. • Rinse the item thoroughly • • in hot running water. Dry in a drying cabinet or with lint free cloths or disposable lint free cloths. – items should never be dried in ambient air. Then visually inspect the cleanliness of all items.
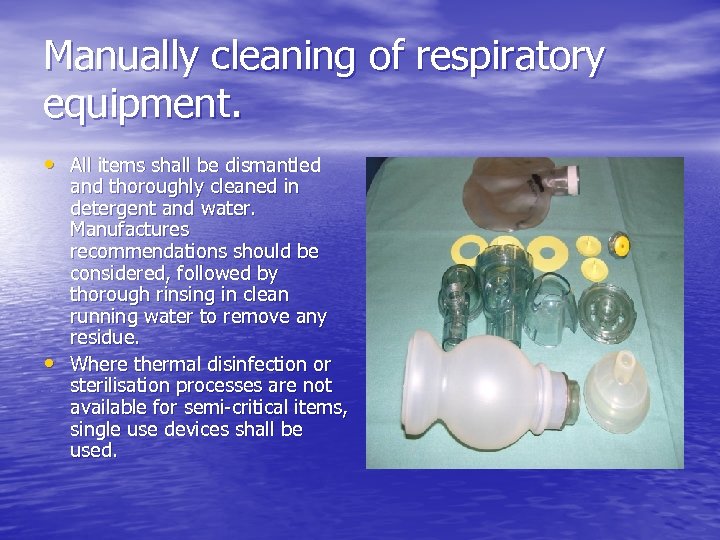
Manually cleaning of respiratory equipment. • All items shall be dismantled • and thoroughly cleaned in detergent and water. Manufactures recommendations should be considered, followed by thorough rinsing in clean running water to remove any residue. Where thermal disinfection or sterilisation processes are not available for semi-critical items, single use devices shall be used.

Chemical disinfection • Chemical disinfection can only • • be used when thermal disinfection is unsuitable. Any chemical disinfectants that are used must be registered with the Therapeutic Goods Administration in Australia Instruments should not be stored in chemical disinfectants before or after any form of processing.
Materials that may inhibit or restrict chemical disinfection. • Hard water • Fibres or lint from • • cleaning materials Food, fluid and fats Body fluids and excrements Organic materials Physical soils
Instrument Grade Disinfectants • High level instrument grade disinfectant is the minimum level to be used on semicritical instruments which contact unbroken mucous membranes that are normally not sterile.

Instrument grade disinfectants • Intermediate or low level • • disinfectants may be used for non-critical instruments which are normally restricted to contact with unbroken skin. There may be instances where a hospital grade disinfection maybe used in a office based health care facility. Formal policies and procedures need to be developed for the use of hospital grade disinfectants.

General housekeeping • There needs to be written ü ü ü policies and procedures for the routine cleaning of all the sterilising and ancillary equipment including; Method Frequency Manufacturers instructions Cleaning agents and materials. All waste should be removed from the reprocessing area via designated disposals exits. AS 4031, AS/NZS 4261, NZS 4304 should also be consulted.

Education and training • Office - based health care facilities responsible for instrument reprocessing are also responsible for ensuring the appropriate education and training of staff is kept up to date on a regular basis.
Interchange of instruments and equipment. • Instruments should not be • • interchanged between necropsy, human and animal use. Instruments used on animals should not be reprocessed in sterilisers and associated equipment and environments used for reprocessing instruments used for humans. Infection transmission hazards exist where these requirements are not met.

Instruments on loan • On receipt into the health care • • facility, instruments on loan from loan companies or other health care facilities or individual clinicians , shall undergo a complete routine cleaning and processing prior to sterilisation. Perceived lack of time shall not be permit the cleaning process to be bypassed. There should be a contracted arrangement between the health care facility and the loan supplier to define the responsibilities of the parties.

Sterilisation of unwrapped instruments. • To avoid danger of contamination, use • • • unwrapped instruments for critical procedures immediately after sterilisation. However this is an unacceptable practice and should only take place when an item is dropped another one is not available and the item must have undergone a full cleaning process prior to being placed in the flash sterilising unit. Lack of time or equipment is not an acceptable excuse for this process to take place. The lack of a drying process causes instruments to corrode, and jointed instruments to become stiff.
Lubrication • If lubricants are required for ü ü basic instruments they need to be, Water miscible Compatible to the sterilising agent. Lubrication is not a way of overcoming inadequate cleaning practices. Routine lubrication of instruments following the cleaning process often results in extremely heavy contamination.

Insulated instruments • Need to be tested to • ensure the integrity of the insulation material This can be done by trained SSD staff member or a biomedical engineer depending on the frequency of the equipment use.
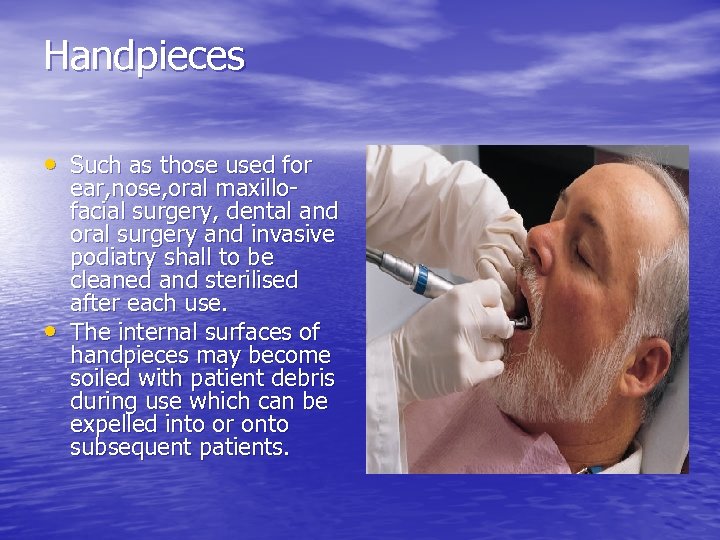
Handpieces • Such as those used for • ear, nose, oral maxillofacial surgery, dental and oral surgery and invasive podiatry shall to be cleaned and sterilised after each use. The internal surfaces of handpieces may become soiled with patient debris during use which can be expelled into or onto subsequent patients.

Processing handpieces. • Documented cleaning procedure must be followed. • At the end of the procedure, handpieces need to be operated and • • run to discharge water and air for a minimum of 30 seconds. This is to flush out any gross debris that may have entered the turbine and air or water lines. Handpieces maybe hand clean and lubricated following manufacturers recommendations or hand cleaned and then processed using an automatic flush-through and lubricated system. Handpieces need to be also packaged and steam sterilised and remain in a sterile package before each use. It is not an acceptable practice wiping or soaking handpieces in a chemical disinfectant. Handpieces pose a challenge to the downward displacement sterilisation process, due to limitations to the air removal process and steam penetration.
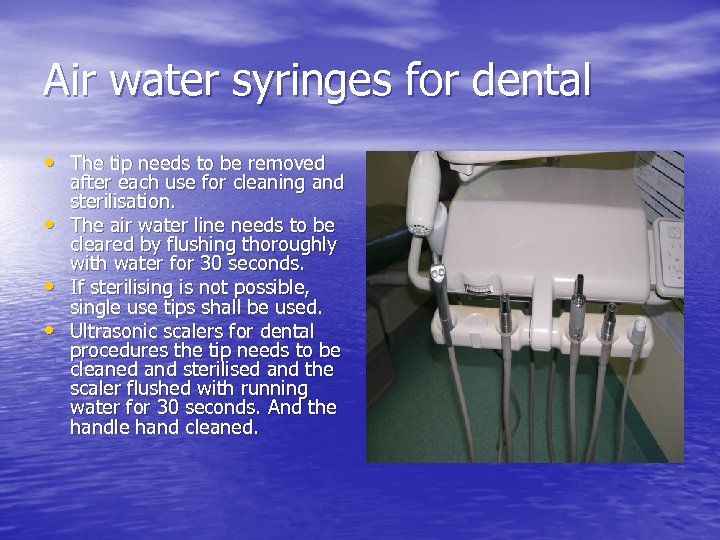
Air water syringes for dental • The tip needs to be removed • • • after each use for cleaning and sterilisation. The air water line needs to be cleared by flushing thoroughly with water for 30 seconds. If sterilising is not possible, single use tips shall be used. Ultrasonic scalers for dental procedures the tip needs to be cleaned and sterilised and the scaler flushed with running water for 30 seconds. And the handle hand cleaned.
Diagnostic ultrasound transducers. • Intracavity probes such as • (transvaginal transrectal or transoesophageal ) require cleaning in accordance to the manufacturers recommendations and either sterilisation if possible or high level disinfectant using only instrument grade disinfectant should be used. Sheaths/ sleeves or protective barriers must not be used as a substitute for cleaning, disinfection or sterilisation procedures.

Use of textiles • Where an office- based health • care facility has a laundry which is dedicated to processing drapes, gowns and other textiles that need to be sterile and area needs to be set a side for inspection, folding and assembly of these textiles. If this is not possible consideration should be given to outsourcing textiles or the use of single use. Inappropriate textiles and laundering practice pose an infection risk.

Packaging and wrapping prior to sterilisation • The purpose of packaging is to • • • provide an effective bacteria barrier against potential contamination and to maintain sterility. Materials should permit the removal of air from the pack and allow penetration of the sterilising agent and removal of the sterilising agent and water vapour. Materials need to be compatible to the sterilising method selected. Sharp instruments should be packaged in such a way the tips cannot pierce the packaging material.

Pack size • The principle determining the • size, mass and contents of packs is that the contents are dry and sterile immediately on completion of the sterilisation cycle. This is usually established when the performance qualifications are conducted on your sterilisers, using thermocouples and biological indicators.

Labelling packs • Labelling should always be done prior • • ü ü ü to sterilisation so the packaging material integrity is not compromised. All Packaged items for sterilisation needs labels for identification of the contents The following is the only methods that should be used for labelling packages for sterilisation, Soft tipped alcohol-based felt marking pens. Pre-printed tapes Pre-printed bags Stamping systems Pre-printed labels Computerized generated labels.

Sealing of packs and bags • The purpose of sealing is • to maintain pack integrity , to ensure the package will remain sealed during the sterilisation process. Sealing is usually achieved by the use of heat seals, self sealing pouches or sterilising indicator tape.

Methods which compromise pack integrity. • String • Non-adhesive tape • Staples • Elastic bands • Paperclips • Ball point pens or pencils

Sterilisation equipment • Methods of sterilisation in • • office -based health care facilities are steam under pressure and dry heat. Steam kills micro-organisms by coagulation of the cell protein. Dry heat kills micro –organisms by oxidation this causes the continuous loss of moisture by the micro-organism in the heated environment.

Steam sterilisation • Steam possess three 1. 2. 3. important qualities that make it an effective sterilant. Latent heat Moisture Penetration

Types of Steam 1. 2. 3. Saturated steam Wet saturated steam Superheated steam Saturated steam is the only steam suitable for steam sterilisation. The maximum allowable steam wetness is 3% which is equivalent to 97% dry saturated steam.

Small steam sterilisers. • In Australia and New • Zealand small steam sterilisers are known as bench top or portable. AS 2182 specifies the manufacturers requirements for this type of steriliser.

Types of sterilising cycles and their intended use. • Type N = the sterilisation of unwrapped, solid items. • Type B = The sterilisation of all wrapped (single or double) or unwrapped • Ø Ø Ø items, including porous and cannulated items that do not exceed the specifications of hollow load type A from the AS 4815 2006 Type S = the sterilisation of items as specified by the manufacturer of the steriliser. The available cycles needs to be capable of sterilising unwrapped solid items and at least one other of the following types; Porous items Small porous items Hollow load type A Hollow load type B Single layer wrapped items Double wrapped items.

Tubing • Tubing poses a challenge to the sterilisation process due to the inability of effective cleaning. There is also a risk of air entrapment during sterilisation. Therefore it is wise to use sterile single use tubing.

Dry heat sterilisers • The portable dry heat type steriliser is • • • different from the steam because air removal is not part of the process. Items need to be sealed within impermeable containers. And can withstand a temperature of 160˚c for a minimum holding time of 120 mins plus penetration time. Dry heat steriliser (hot air Type) are specified in AS 2487 appliances that do not meet the requirements of this standard should not be used under any circumstances. This type of steriliser is not designed to sterilised liquids therefore this should not be attempted.

Peracetic acid liquid chemical sterilisation • Peracetic acid (PAA) is generally used in • • operating theatres or endoscopy units. When used correctly it provides them with a sterile product rather than a chemically disinfected product. PAA is an effective biocide and for sterilisation 35% peracetic acid is mixed with 10 litres of warm water rendering a final concentration of 0. 2%.

PAA as a Sterilant • • • Concentration 0. 2% Temperature range 50 – 56 c Holding Time 12 minutes The cycle time is between 20 -35 minutes and is dependant on the initial water temperature and water pressure. Items need to be thoroughly cleaned before being loaded into purpose built trays that sit into the chamber. As it is a wet process the items are immersed for 12 minutes followed four rinses with filtered portable water. At the end of the cycle filtered air is purged into the chamber to remove excess water. The unit remains sealed until released by the operator. The PAA system is designed for ‘’just in time’’ processing as items cannot be stored following the process.

(LTP) Low Temperature Hydrogen peroxide plasma sterilisation. • LTP is a relatively new process • when compared to other sterilisation methods. The plasma is created in ambient temperature. The substance used for the sterilisation process is hydrogen peroxide (58%). This is in liquid form originally and is vaporised in the chamber then converted to plasma by the addition of an energy field which in this case is created by the turning on of a radio frequency within the chamber during deep vacuum.

LTP how it kills micro-organisms. • LTP destroys micro-organisms by creating a plasma cloud. This cloud contains free radicals known to have a biochemical interaction with cell membranes, enzymes or nucleic acids to disrupt the life functions of micro-organisms.

Loading the sterilisers • Correct loading is essential for • • successful sterilisation. Because the sterilant must contact the surface for sterilisation to be achieved loading trays shall be loosely loaded to capacity. only a single layer of packs shall be loaded on each tray. Packs must remain in the confines of the chamber and not touch the walls, floor or door.

Unloading sterilisers • The load must be removed • • immediately on completion and visual inspection shall be made to ascertain that the load is dry, packaging is intact and that the sterilising indicators colour change has taken place. Directly after the sterilisation process items are very vulnerable to contamination by moisture or improper handling. After sterilisation the loading trays shall be kept away from high activity areas.

Unloading procedures • Procedures for unloading each type of steriliser shall be developed and documented and ongoing compliance with such procedures need to be monitored.

Performance qualification • After commissioning • performance qualification is preformed annually or when significant changes are made to any aspect of reprocessing activities e. g. changes to packaging material, loading configurations and sterilisation process parameters. Also after major repairs to the steriliser.

Performance testing • Leak rate vacuum test • Air removal and steam • • penetration test. Air detector test (where applicable) Note sterilisers that use downward displacement as a method of air removal cannot be effectively performance tested prior to use.
Monitoring • Chemical indicators • biological indicators • enzymatic indicators • Process challenge • • devices. Data loggers Thermocouples and digital readout thermometers.

Monitoring of packaging following sterilisation. • Integrity of the outer wrap • Integrity of seals • Correct labelling including; Ø Date of manufacture Ø Batch number Ø Steriliser cycle or run number Ø Steriliser number Ø Contents of pack and operators • identification Correct colour change of chemical indicator.

Criteria for release of processed items • To release the processed • items, there should be evidence that the process has compiled with all specific requirements, therefore achieving the sterility assurance level or the level of disinfection required. The person responsible for authorizing the release must have full knowledge of all aspects of the validated process and be satisfied that monitoring and control of the entire process has met specifications.

Storage of sterile stock. • Storage areas for sterile stock • • should be controlled to prevent contamination and shall be dedicated for that purpose only. The storage environment should be free of dust, insects and vermin. Storage containers, trolleys and cupboards need to be kept clean and dry and in good condition cardboard boxes should not be used as they are porous and cannot be cleaned and will harbour organisms.

Summary • • • Staff health –infection control Cleaning , disinfection and chemical disinfection Inspect, prepare and packaging Loading for sterilisation Steam Sterilisation Unloading of sterile items Monitoring Release of sterile stock Storage of sterile stock.
Thank you
28a2c85348bd945fb46fd05bd8ab8cb7.ppt