27b0319227aafd36de8bdcf2b3dc3be0.ppt
- Количество слайдов: 33
 Representation of Markush structures — from molecules towards patents Szabolcs Csepregi August 2010, ACS National meeting, Boston Solutions for Cheminformatics
Representation of Markush structures — from molecules towards patents Szabolcs Csepregi August 2010, ACS National meeting, Boston Solutions for Cheminformatics
 Contents • Chem. Axon • What are Markush structures? • How to get them? • What can be done with them? – Enumeration – Storage, search • Challenges in chemical representation • Under development August 2010, ACS National meeting, Boston
Contents • Chem. Axon • What are Markush structures? • How to get them? • What can be done with them? – Enumeration – Storage, search • Challenges in chemical representation • Under development August 2010, ACS National meeting, Boston
 Chem. Axon • Cheminformatics toolkits and applications • HQ: Budapest, Hungary • Founded: 1998 • Main customers: pharma, biotech, publishing • 3 rd party applications and web sites. (e. g. Integrity, Reaxis, PDB ligand search, ELN-s, registration systems, etc) August 2010, ACS National meeting, Boston
Chem. Axon • Cheminformatics toolkits and applications • HQ: Budapest, Hungary • Founded: 1998 • Main customers: pharma, biotech, publishing • 3 rd party applications and web sites. (e. g. Integrity, Reaxis, PDB ligand search, ELN-s, registration systems, etc) August 2010, ACS National meeting, Boston
 Chem. Axon Main products: – – Structure drawing & visualization (Marvin family) Chemical DB tools (JChem family) Property predictions (Calculator plugins) Drug discovery tools (Reactor, JKlustor, etc. ) Development strategy: customer-driven August 2010, ACS National meeting, Boston
Chem. Axon Main products: – – Structure drawing & visualization (Marvin family) Chemical DB tools (JChem family) Property predictions (Calculator plugins) Drug discovery tools (Reactor, JKlustor, etc. ) Development strategy: customer-driven August 2010, ACS National meeting, Boston
 What are Markush structures and how to get them? August 2010, ACS National meeting, Boston
What are Markush structures and how to get them? August 2010, ACS National meeting, Boston
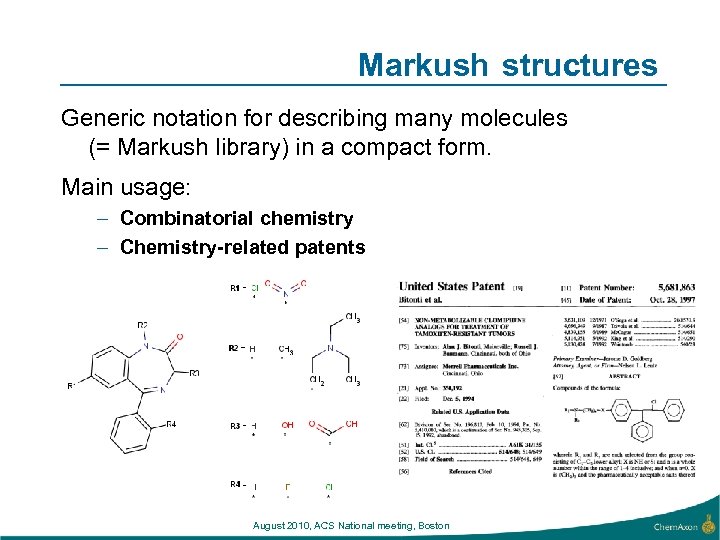 Markush structures Generic notation for describing many molecules (= Markush library) in a compact form. Main usage: – Combinatorial chemistry – Chemistry-related patents August 2010, ACS National meeting, Boston
Markush structures Generic notation for describing many molecules (= Markush library) in a compact form. Main usage: – Combinatorial chemistry – Chemistry-related patents August 2010, ACS National meeting, Boston
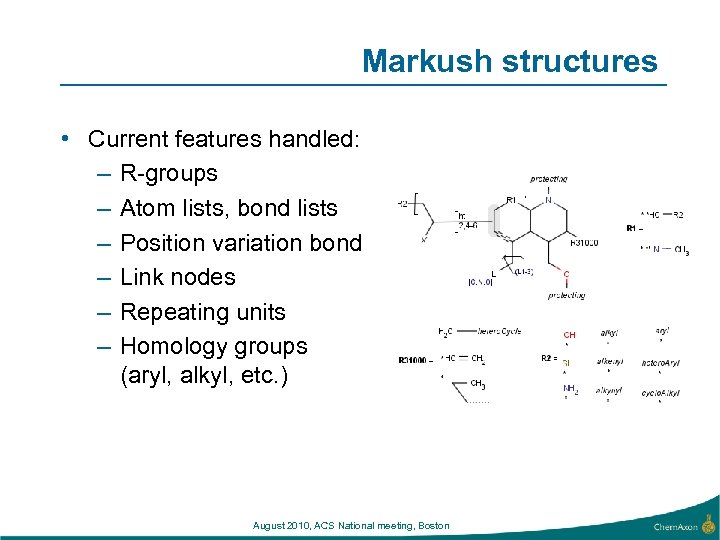 Markush structures • Current features handled: – R-groups – Atom lists, bond lists – Position variation bond – Link nodes – Repeating units – Homology groups (aryl, alkyl, etc. ) August 2010, ACS National meeting, Boston
Markush structures • Current features handled: – R-groups – Atom lists, bond lists – Position variation bond – Link nodes – Repeating units – Homology groups (aryl, alkyl, etc. ) August 2010, ACS National meeting, Boston
 Chem. Axon Markush project Goals: – Extend structural search capabilities to combinatorial Markush structures – Markush enumeration Complications: – Practical examples may be very complex, methods using explicit enumeration may be impossible – Extension of current molecular formats (generic features) Timeline – – Pilot study started in 2005 Q 4, First prototype shown at UGM, 2006 June Released in JChem 5. 0, 2008 Markush DARC format support 5. 3. 0 2010 August 2010, ACS National meeting, Boston
Chem. Axon Markush project Goals: – Extend structural search capabilities to combinatorial Markush structures – Markush enumeration Complications: – Practical examples may be very complex, methods using explicit enumeration may be impossible – Extension of current molecular formats (generic features) Timeline – – Pilot study started in 2005 Q 4, First prototype shown at UGM, 2006 June Released in JChem 5. 0, 2008 Markush DARC format support 5. 3. 0 2010 August 2010, ACS National meeting, Boston
 How to get Markush structures? • Drawing – Marvin Sketch August 2010, ACS National meeting, Boston
How to get Markush structures? • Drawing – Marvin Sketch August 2010, ACS National meeting, Boston
 How to get Markush structures? • Patent literature – Markush DARC format (*. vmn) • Compatible with Thomson Reuters MMS patent Markush database (Test set available. ) August 2010, ACS National meeting, Boston
How to get Markush structures? • Patent literature – Markush DARC format (*. vmn) • Compatible with Thomson Reuters MMS patent Markush database (Test set available. ) August 2010, ACS National meeting, Boston
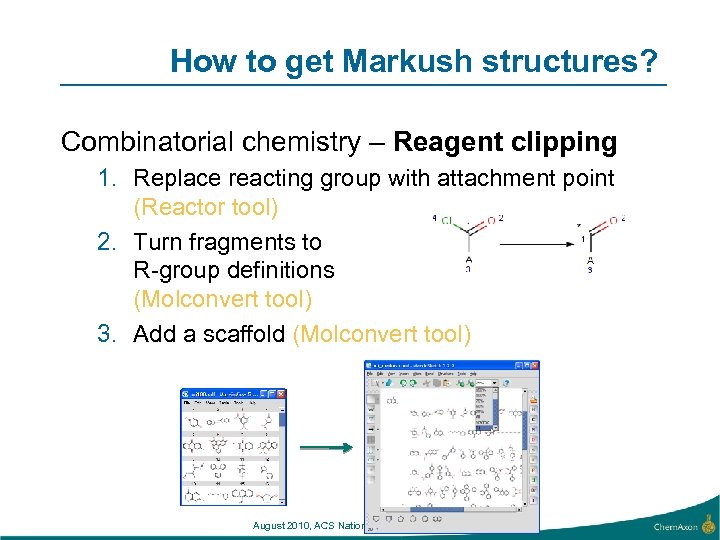 How to get Markush structures? Combinatorial chemistry – Reagent clipping 1. Replace reacting group with attachment point (Reactor tool) 2. Turn fragments to R-group definitions (Molconvert tool) 3. Add a scaffold (Molconvert tool) August 2010, ACS National meeting, Boston
How to get Markush structures? Combinatorial chemistry – Reagent clipping 1. Replace reacting group with attachment point (Reactor tool) 2. Turn fragments to R-group definitions (Molconvert tool) 3. Add a scaffold (Molconvert tool) August 2010, ACS National meeting, Boston
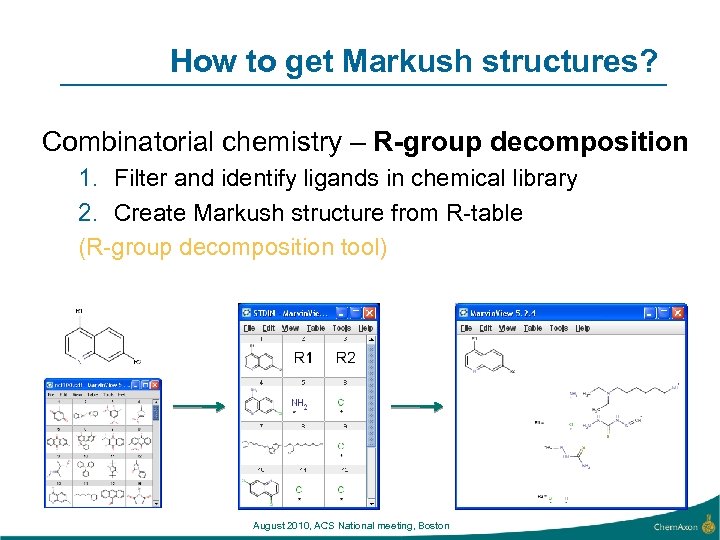 How to get Markush structures? Combinatorial chemistry – R-group decomposition 1. Filter and identify ligands in chemical library 2. Create Markush structure from R-table (R-group decomposition tool) August 2010, ACS National meeting, Boston
How to get Markush structures? Combinatorial chemistry – R-group decomposition 1. Filter and identify ligands in chemical library 2. Create Markush structure from R-table (R-group decomposition tool) August 2010, ACS National meeting, Boston
 What to do with them? August 2010, ACS National meeting, Boston
What to do with them? August 2010, ACS National meeting, Boston
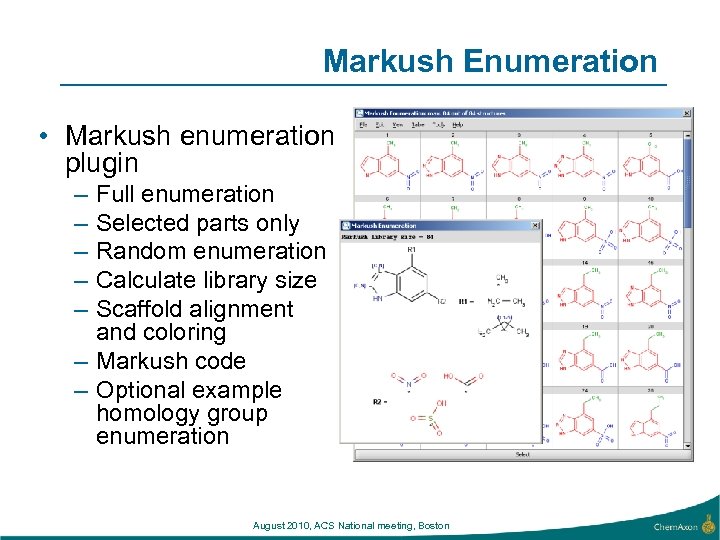 Markush Enumeration • Markush enumeration plugin – – – Full enumeration Selected parts only Random enumeration Calculate library size Scaffold alignment and coloring – Markush code – Optional example homology group enumeration August 2010, ACS National meeting, Boston
Markush Enumeration • Markush enumeration plugin – – – Full enumeration Selected parts only Random enumeration Calculate library size Scaffold alignment and coloring – Markush code – Optional example homology group enumeration August 2010, ACS National meeting, Boston
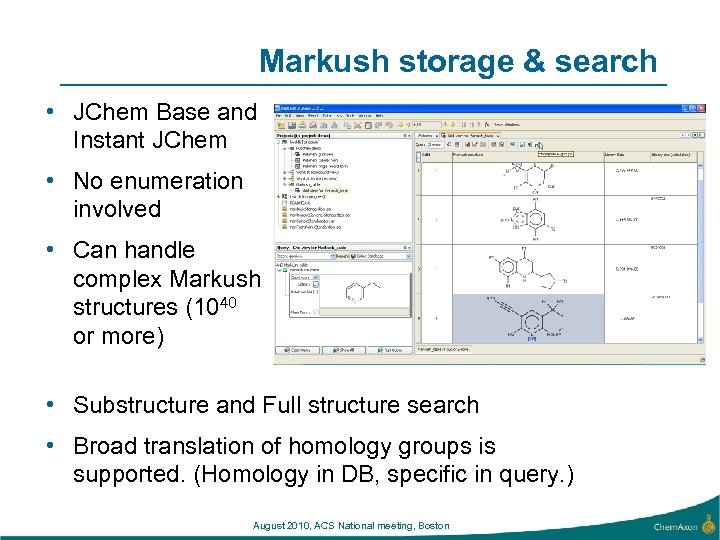 Markush storage & search • JChem Base and Instant JChem • No enumeration involved • Can handle complex Markush structures (1040 or more) • Substructure and Full structure search • Broad translation of homology groups is supported. (Homology in DB, specific in query. ) August 2010, ACS National meeting, Boston
Markush storage & search • JChem Base and Instant JChem • No enumeration involved • Can handle complex Markush structures (1040 or more) • Substructure and Full structure search • Broad translation of homology groups is supported. (Homology in DB, specific in query. ) August 2010, ACS National meeting, Boston
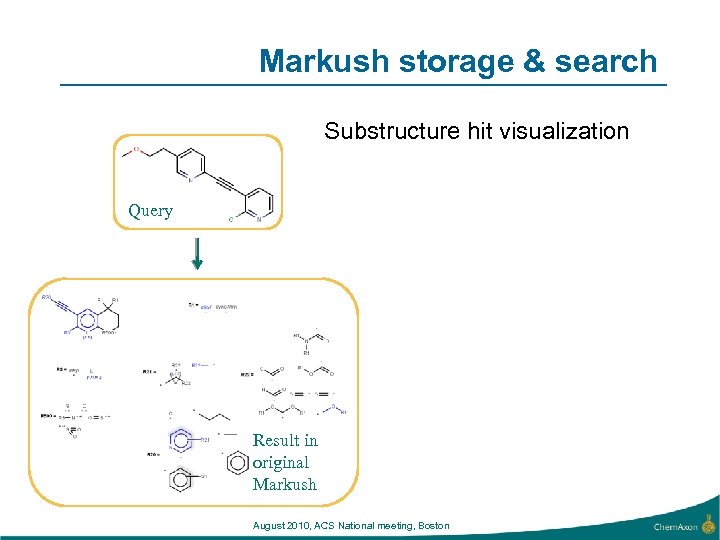 Markush storage & search Substructure hit visualization Query Result in original Markush August 2010, ACS National meeting, Boston
Markush storage & search Substructure hit visualization Query Result in original Markush August 2010, ACS National meeting, Boston
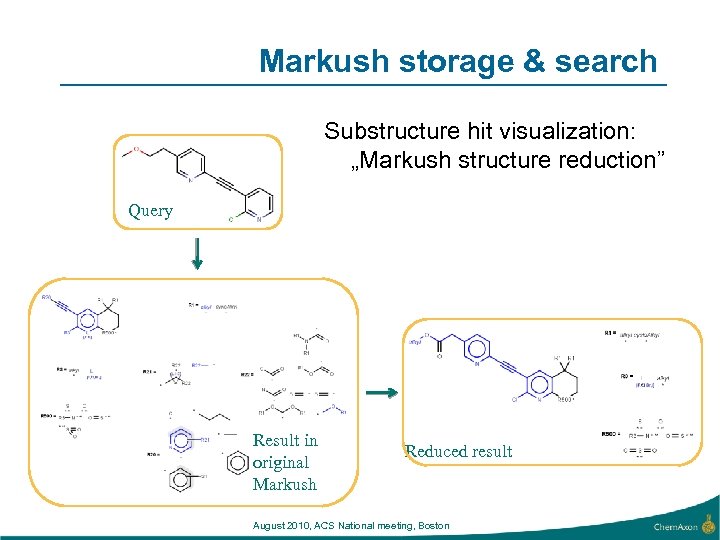 Markush storage & search Substructure hit visualization: „Markush structure reduction” Query Result in original Markush Reduced result August 2010, ACS National meeting, Boston
Markush storage & search Substructure hit visualization: „Markush structure reduction” Query Result in original Markush Reduced result August 2010, ACS National meeting, Boston
 Main use cases • Patent search hits refining / visualization, • White space analysis, • Patent busting, • Markush structure curation, • In-house storage of small Markush DB, • etc. . . August 2010, ACS National meeting, Boston
Main use cases • Patent search hits refining / visualization, • White space analysis, • Patent busting, • Markush structure curation, • In-house storage of small Markush DB, • etc. . . August 2010, ACS National meeting, Boston
 MMS evaluation Instant JChem project August 2010, ACS National meeting, Boston
MMS evaluation Instant JChem project August 2010, ACS National meeting, Boston
 Challenges in chemical representation (solved) August 2010, ACS National meeting, Boston
Challenges in chemical representation (solved) August 2010, ACS National meeting, Boston
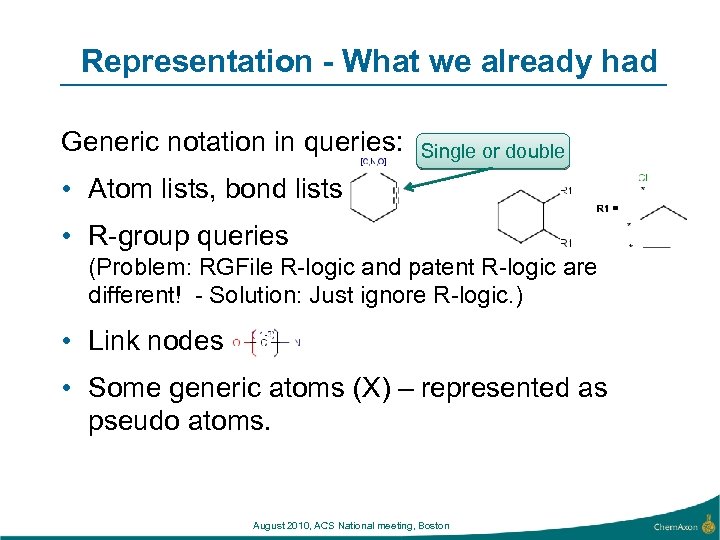 Representation - What we already had Generic notation in queries: Single or double • Atom lists, bond lists • R-group queries (Problem: RGFile R-logic and patent R-logic are different! - Solution: Just ignore R-logic. ) • Link nodes • Some generic atoms (X) – represented as pseudo atoms. August 2010, ACS National meeting, Boston
Representation - What we already had Generic notation in queries: Single or double • Atom lists, bond lists • R-group queries (Problem: RGFile R-logic and patent R-logic are different! - Solution: Just ignore R-logic. ) • Link nodes • Some generic atoms (X) – represented as pseudo atoms. August 2010, ACS National meeting, Boston
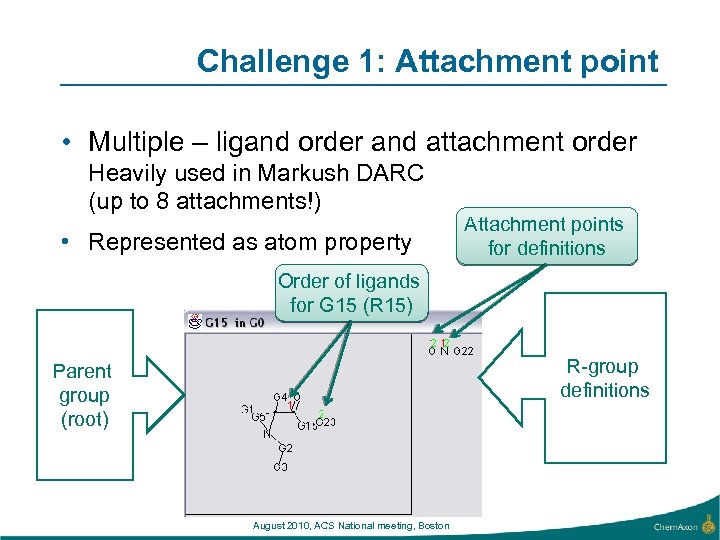 Challenge 1: Attachment point • Multiple – ligand order and attachment order Heavily used in Markush DARC (up to 8 attachments!) • Represented as atom property Attachment points for definitions Order of ligands for G 15 (R 15) R-group definitions Parent group (root) August 2010, ACS National meeting, Boston
Challenge 1: Attachment point • Multiple – ligand order and attachment order Heavily used in Markush DARC (up to 8 attachments!) • Represented as atom property Attachment points for definitions Order of ligands for G 15 (R 15) R-group definitions Parent group (root) August 2010, ACS National meeting, Boston
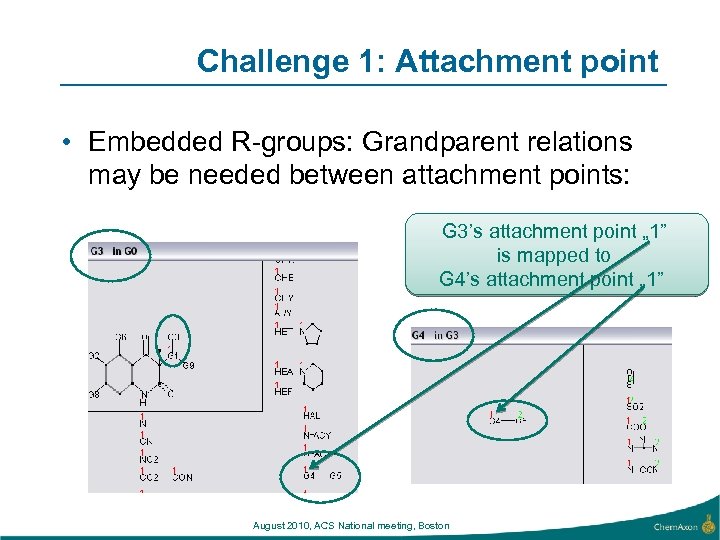 Challenge 1: Attachment point • Embedded R-groups: Grandparent relations may be needed between attachment points: G 3’s attachment point „ 1” is mapped to G 4’s attachment point „ 1” August 2010, ACS National meeting, Boston
Challenge 1: Attachment point • Embedded R-groups: Grandparent relations may be needed between attachment points: G 3’s attachment point „ 1” is mapped to G 4’s attachment point „ 1” August 2010, ACS National meeting, Boston
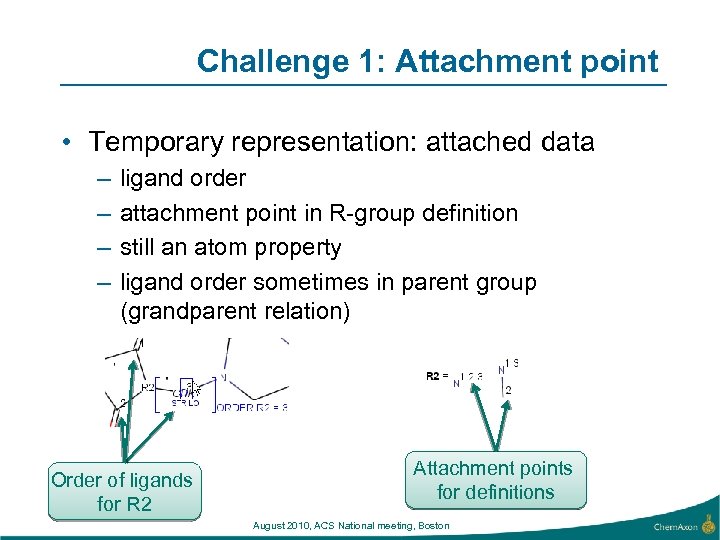 Challenge 1: Attachment point • Temporary representation: attached data – – ligand order attachment point in R-group definition still an atom property ligand order sometimes in parent group (grandparent relation) Order of ligands for R 2 Attachment points for definitions August 2010, ACS National meeting, Boston
Challenge 1: Attachment point • Temporary representation: attached data – – ligand order attachment point in R-group definition still an atom property ligand order sometimes in parent group (grandparent relation) Order of ligands for R 2 Attachment points for definitions August 2010, ACS National meeting, Boston
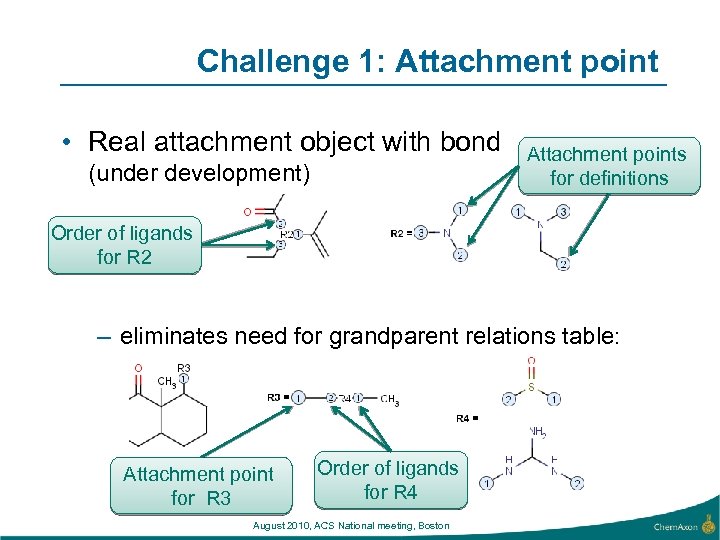 Challenge 1: Attachment point • Real attachment object with bond (under development) Attachment points for definitions Order of ligands for R 2 – eliminates need for grandparent relations table: Attachment point for R 3 Order of ligands for R 4 August 2010, ACS National meeting, Boston
Challenge 1: Attachment point • Real attachment object with bond (under development) Attachment points for definitions Order of ligands for R 2 – eliminates need for grandparent relations table: Attachment point for R 3 Order of ligands for R 4 August 2010, ACS National meeting, Boston
 Challenge 2: Abbreviations • Superatom S-groups were originally in Marvin (~700 built-in shortcuts) – Expand / Contract – Search code already handled them in specific structures. • M. DARC had 21 shortcuts + 31 peptides. • Attachment point next to abbreviations – Needed to be visible „outside” and handled correctly „inside”. – New attachment point solves this also: August 2010, ACS National meeting, Boston
Challenge 2: Abbreviations • Superatom S-groups were originally in Marvin (~700 built-in shortcuts) – Expand / Contract – Search code already handled them in specific structures. • M. DARC had 21 shortcuts + 31 peptides. • Attachment point next to abbreviations – Needed to be visible „outside” and handled correctly „inside”. – New attachment point solves this also: August 2010, ACS National meeting, Boston
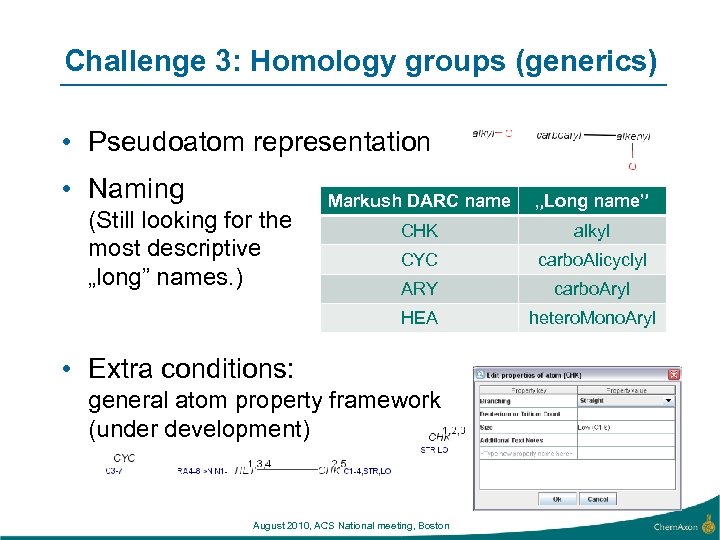 Challenge 3: Homology groups (generics) • Pseudoatom representation • Naming „Long name” CHK alkyl CYC carbo. Alicyclyl ARY carbo. Aryl HEA (Still looking for the most descriptive „long” names. ) Markush DARC name hetero. Mono. Aryl • Extra conditions: general atom property framework (under development) August 2010, ACS National meeting, Boston
Challenge 3: Homology groups (generics) • Pseudoatom representation • Naming „Long name” CHK alkyl CYC carbo. Alicyclyl ARY carbo. Aryl HEA (Still looking for the most descriptive „long” names. ) Markush DARC name hetero. Mono. Aryl • Extra conditions: general atom property framework (under development) August 2010, ACS National meeting, Boston
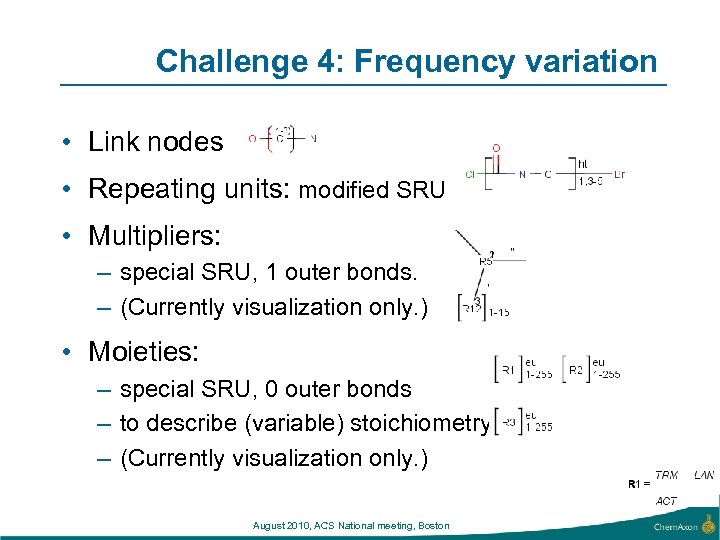 Challenge 4: Frequency variation • Link nodes • Repeating units: modified SRU • Multipliers: – special SRU, 1 outer bonds. – (Currently visualization only. ) • Moieties: – special SRU, 0 outer bonds – to describe (variable) stoichiometry – (Currently visualization only. ) August 2010, ACS National meeting, Boston
Challenge 4: Frequency variation • Link nodes • Repeating units: modified SRU • Multipliers: – special SRU, 1 outer bonds. – (Currently visualization only. ) • Moieties: – special SRU, 0 outer bonds – to describe (variable) stoichiometry – (Currently visualization only. ) August 2010, ACS National meeting, Boston
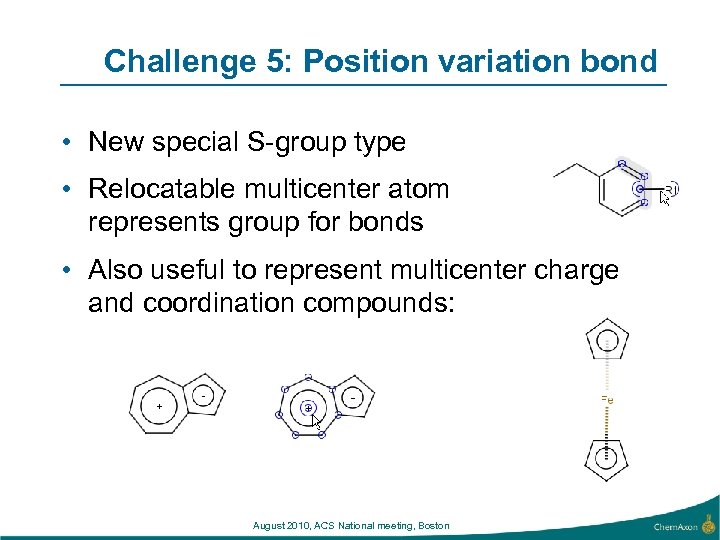 Challenge 5: Position variation bond • New special S-group type • Relocatable multicenter atom represents group for bonds • Also useful to represent multicenter charge and coordination compounds: August 2010, ACS National meeting, Boston
Challenge 5: Position variation bond • New special S-group type • Relocatable multicenter atom represents group for bonds • Also useful to represent multicenter charge and coordination compounds: August 2010, ACS National meeting, Boston
 What (else) keep us busy August 2010, ACS National meeting, Boston
What (else) keep us busy August 2010, ACS National meeting, Boston
 Under development • Further improvements in Markush DARC support: – Ring segment groups (XX form a ring) – New, more robust representation for attachment points – Homology properties (low alkyl, fused aryl, C 1 -3, N 2 -5, etc) • Ranking of results • New ways to navigate/zoom Markush structures • Maximum common substructure search • Biased enumeration and covering Markush – based on examples in patent. • Improve search speed to handle larger Markush sets. • Other Markush formats – Markush In. Ch. I standard committee • Overlap analysis of Markush structures • Conditions for Markush variables August 2010, ACS National meeting, Boston
Under development • Further improvements in Markush DARC support: – Ring segment groups (XX form a ring) – New, more robust representation for attachment points – Homology properties (low alkyl, fused aryl, C 1 -3, N 2 -5, etc) • Ranking of results • New ways to navigate/zoom Markush structures • Maximum common substructure search • Biased enumeration and covering Markush – based on examples in patent. • Improve search speed to handle larger Markush sets. • Other Markush formats – Markush In. Ch. I standard committee • Overlap analysis of Markush structures • Conditions for Markush variables August 2010, ACS National meeting, Boston
 Summary • Markush structure storage, search and enumeration at Chem. Axon now patent coverage • Compatible patent data is available from Thomson Reuters • Well thought out chemical representation • Continuous development, improvements in the pipeline August 2010, ACS National meeting, Boston
Summary • Markush structure storage, search and enumeration at Chem. Axon now patent coverage • Compatible patent data is available from Thomson Reuters • Well thought out chemical representation • Continuous development, improvements in the pipeline August 2010, ACS National meeting, Boston
 Acknowledgements • Development team: Nóra Máté, Róbert Wágner, Szilárd Dóránt, Tamás Csizmazia, Tim Dudgeon, Erika Bíró, Ali Baharev, Ferenc Csizmadia, et al. • Tim Miller, Steve Hajkowski, Gez Cross and Linda Clark at Thomson Reuters for useful discussions, help and example Markush DARC files • Many early adopters and colleagues within the field for suggestions and feedback August 2010, ACS National meeting, Boston
Acknowledgements • Development team: Nóra Máté, Róbert Wágner, Szilárd Dóránt, Tamás Csizmazia, Tim Dudgeon, Erika Bíró, Ali Baharev, Ferenc Csizmadia, et al. • Tim Miller, Steve Hajkowski, Gez Cross and Linda Clark at Thomson Reuters for useful discussions, help and example Markush DARC files • Many early adopters and colleagues within the field for suggestions and feedback August 2010, ACS National meeting, Boston


