WSU_Renal_Review (2).ppt
- Количество слайдов: 100

RENAL PATHOLOGY Greg Balko, MD May 27, 2009

SET 1

SET 1

Nephrotic Syndrome – Proteinuria > 3. 5 gm/day, due to excessive permeability of glomerular capillary wall. – Leads to hypoalbuminemia, decreased colloid oncotic pressure and edema. – Accompanied by sodium and water retention, hyperlipidemia, vulnerability to infection and thrombotic complications. – Causes of the nephrotic syndrome: • Amyloidosis. • diabetic glomerular disease. • foot process disease. • minimal change glomerulopathy. • focal-segmental glomerulosclerosis. • membranous glomerulopathy. • membranoproliferative glomerulonephritis*.

Nephritic Syndrome (Nephritis) • • Features: – Acute inflammation of glomeruli. – Hematuria (including red cell casts). – Mild to moderate proteinuria. – Oliguria, hypertension and mild edema. Many causes including: – Post-streptococcal glomerulonephritis. – RPGN syndromes. – Membranoproliferative glomerulonephritis. * – Bacterial endocarditis. – Lupus. – Cryoglobulinemia. – Wegener's granulomatosis. – Polyarteritis. – Ig. A nephropathy.
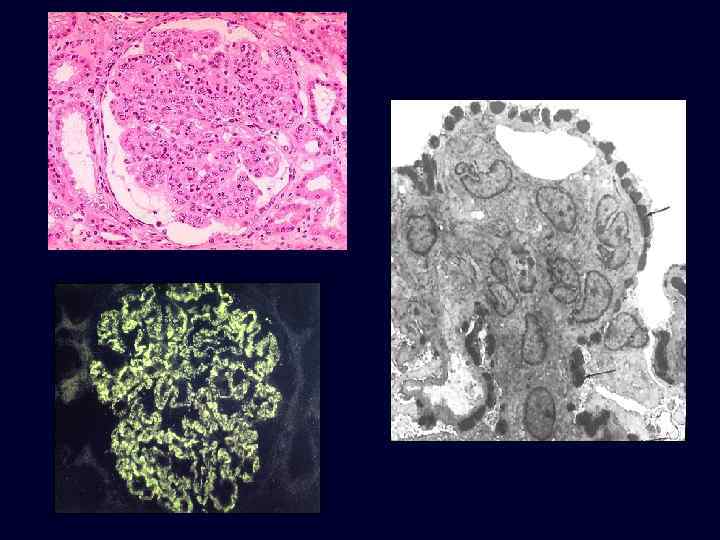
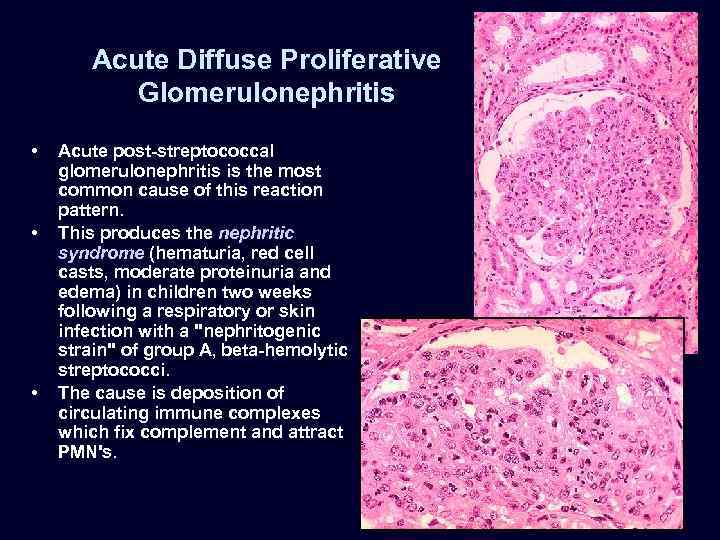
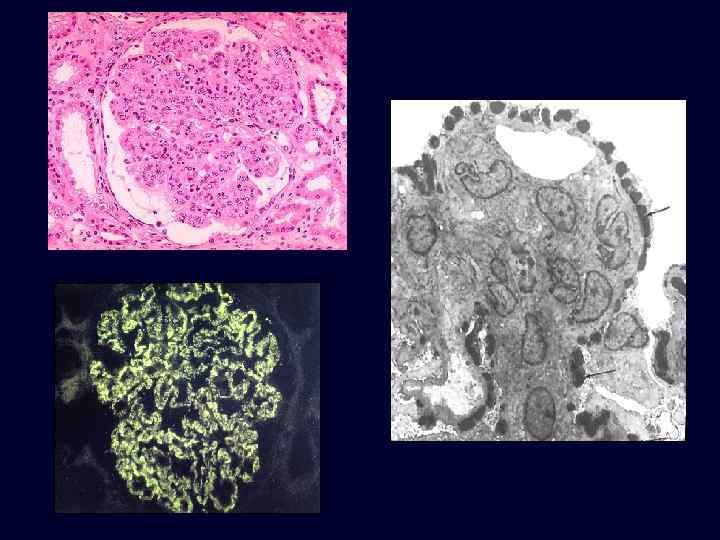
Acute Diffuse Proliferative Glomerulonephritis • • • Acute post-streptococcal glomerulonephritis is the most common cause of this reaction pattern. This produces the nephritic syndrome (hematuria, red cell casts, moderate proteinuria and edema) in children two weeks following a respiratory or skin infection with a "nephritogenic strain" of group A, beta-hemolytic streptococci. The cause is deposition of circulating immune complexes which fix complement and attract PMN's.
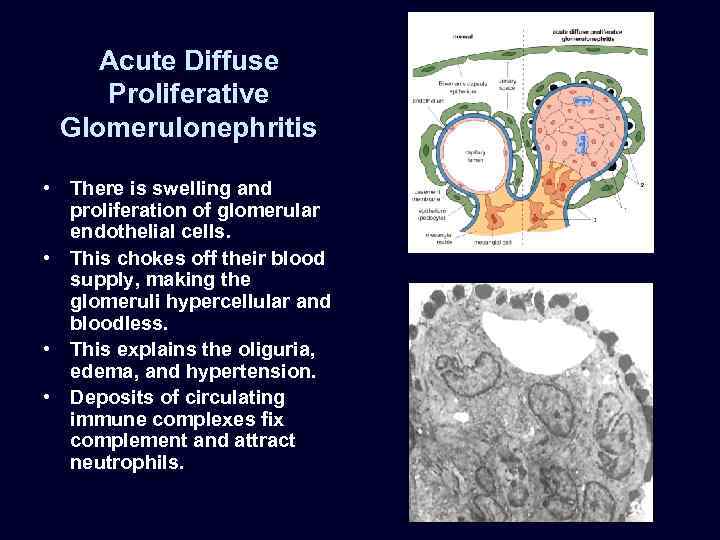
Acute Diffuse Proliferative Glomerulonephritis • There is swelling and proliferation of glomerular endothelial cells. • This chokes off their blood supply, making the glomeruli hypercellular and bloodless. • This explains the oliguria, edema, and hypertension. • Deposits of circulating immune complexes fix complement and attract neutrophils.
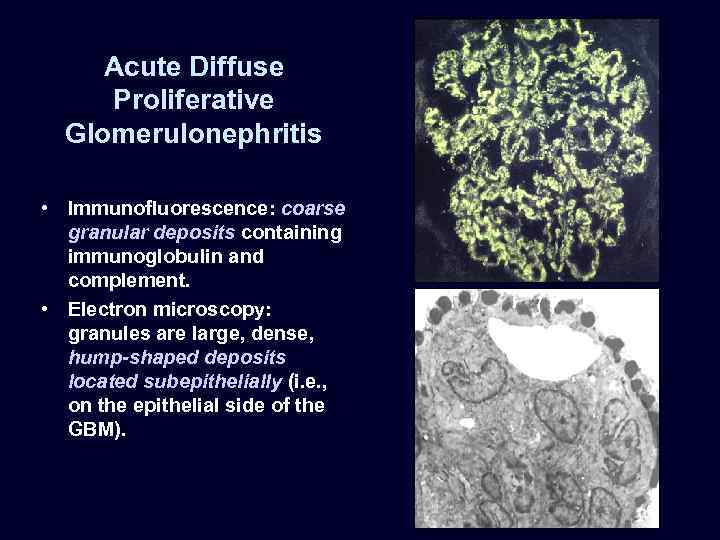
Acute Diffuse Proliferative Glomerulonephritis • Immunofluorescence: coarse granular deposits containing immunoglobulin and complement. • Electron microscopy: granules are large, dense, hump-shaped deposits located subepithelially (i. e. , on the epithelial side of the GBM).

Acute Diffuse Proliferative Glomerulonephritis: Post-streptococcal GN • Children: 95% recover. • Adults: – epidemic form: good prognosis. – sporadic form: 40% develop rapidly progressive disease, chronic renal failure.
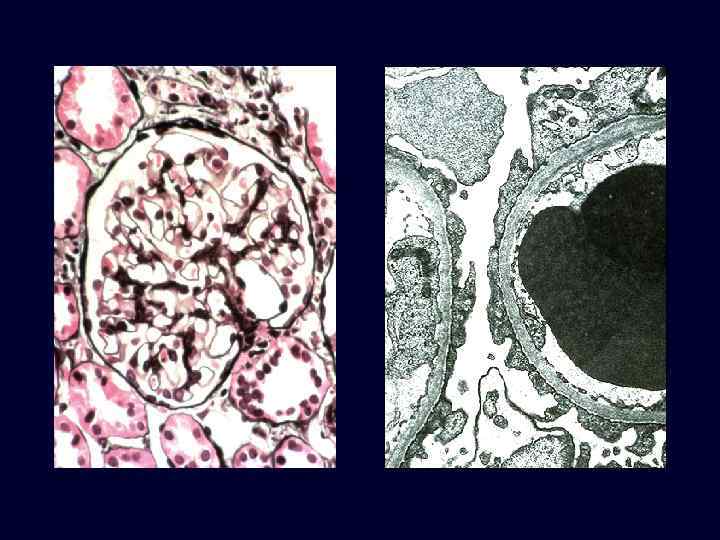
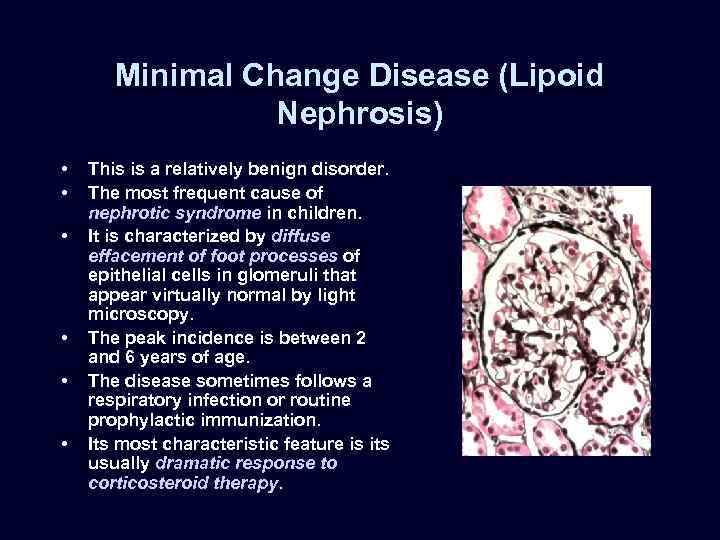
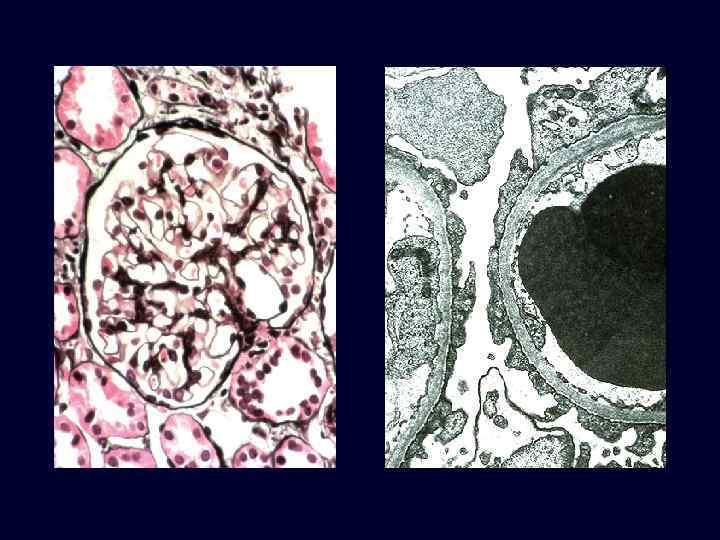
Minimal Change Disease (Lipoid Nephrosis) • • • This is a relatively benign disorder. The most frequent cause of nephrotic syndrome in children. It is characterized by diffuse effacement of foot processes of epithelial cells in glomeruli that appear virtually normal by light microscopy. The peak incidence is between 2 and 6 years of age. The disease sometimes follows a respiratory infection or routine prophylactic immunization. Its most characteristic feature is its usually dramatic response to corticosteroid therapy.
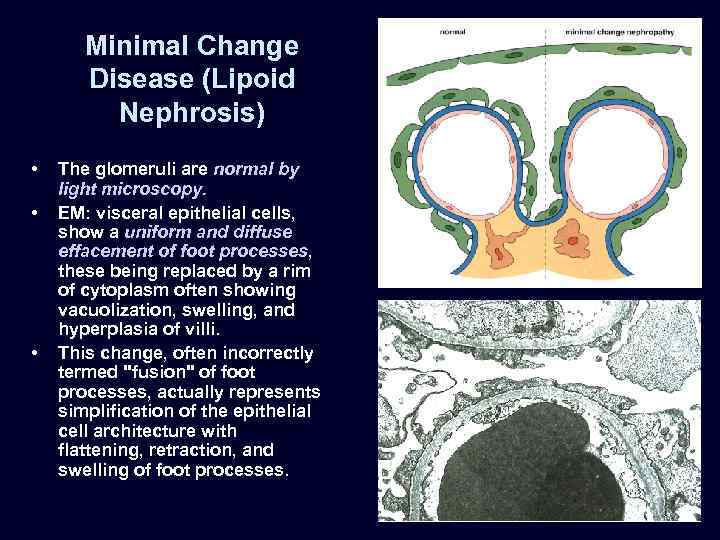
Minimal Change Disease (Lipoid Nephrosis) • • • The glomeruli are normal by light microscopy. EM: visceral epithelial cells, show a uniform and diffuse effacement of foot processes, these being replaced by a rim of cytoplasm often showing vacuolization, swelling, and hyperplasia of villi. This change, often incorrectly termed "fusion" of foot processes, actually represents simplification of the epithelial cell architecture with flattening, retraction, and swelling of foot processes.
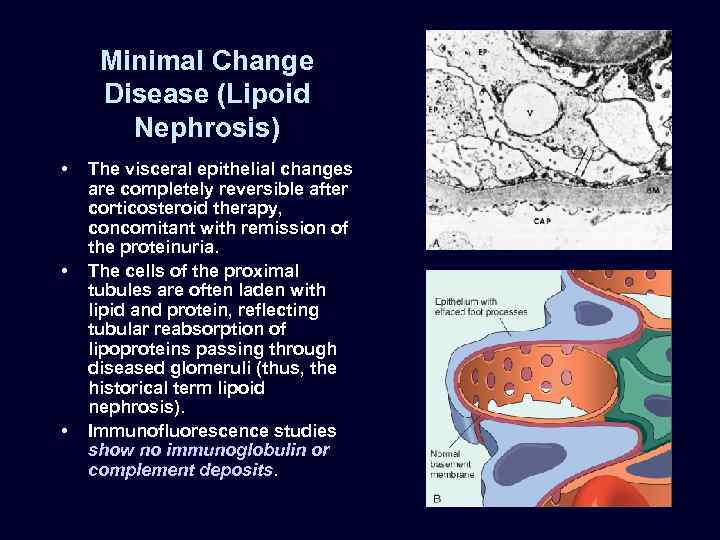
Minimal Change Disease (Lipoid Nephrosis) • • • The visceral epithelial changes are completely reversible after corticosteroid therapy, concomitant with remission of the proteinuria. The cells of the proximal tubules are often laden with lipid and protein, reflecting tubular reabsorption of lipoproteins passing through diseased glomeruli (thus, the historical term lipoid nephrosis). Immunofluorescence studies show no immunoglobulin or complement deposits.
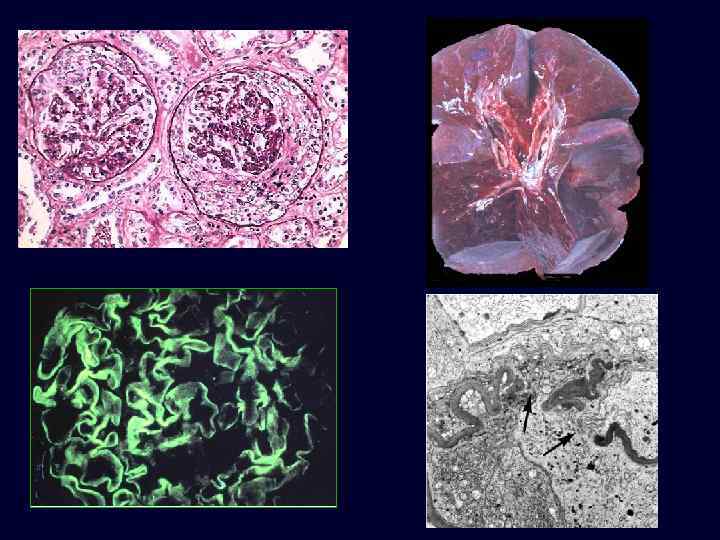
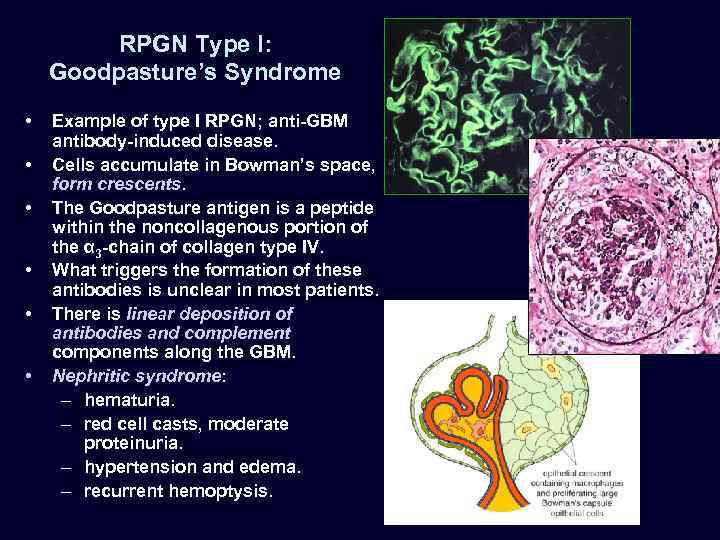
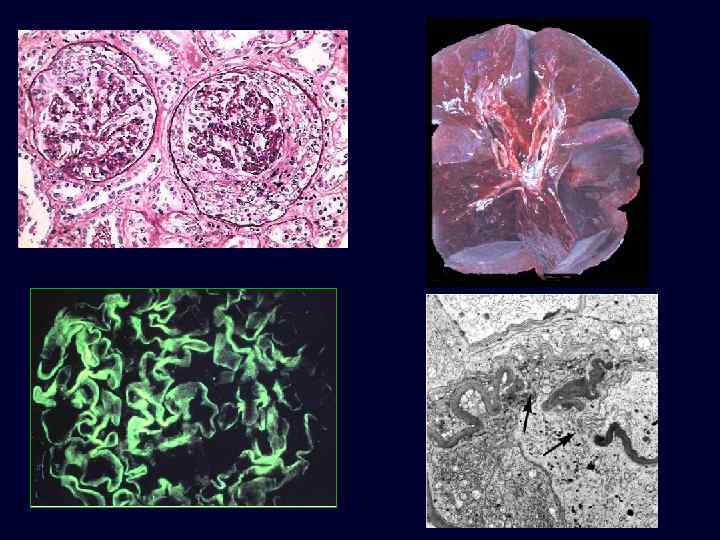
RPGN Type I: Goodpasture’s Syndrome • • • Example of type I RPGN; anti-GBM antibody-induced disease. Cells accumulate in Bowman’s space, form crescents. The Goodpasture antigen is a peptide within the noncollagenous portion of the α 3 -chain of collagen type IV. What triggers the formation of these antibodies is unclear in most patients. There is linear deposition of antibodies and complement components along the GBM. Nephritic syndrome: – hematuria. – red cell casts, moderate proteinuria. – hypertension and edema. – recurrent hemoptysis.
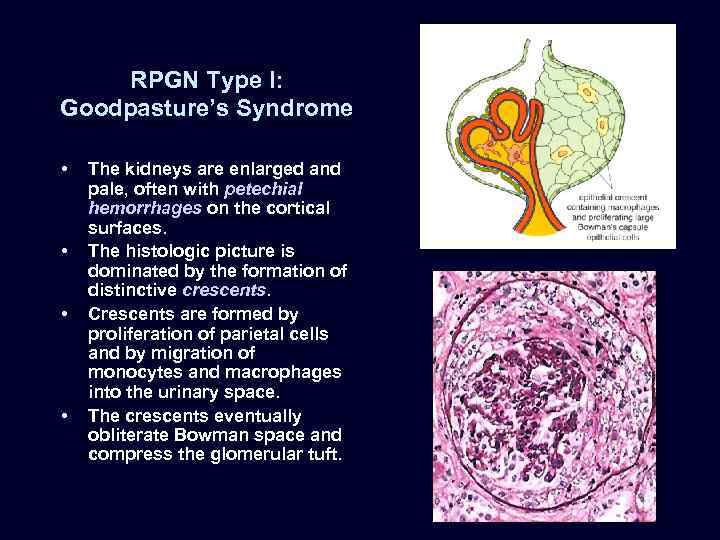
RPGN Type I: Goodpasture’s Syndrome • • The kidneys are enlarged and pale, often with petechial hemorrhages on the cortical surfaces. The histologic picture is dominated by the formation of distinctive crescents. Crescents are formed by proliferation of parietal cells and by migration of monocytes and macrophages into the urinary space. The crescents eventually obliterate Bowman space and compress the glomerular tuft.
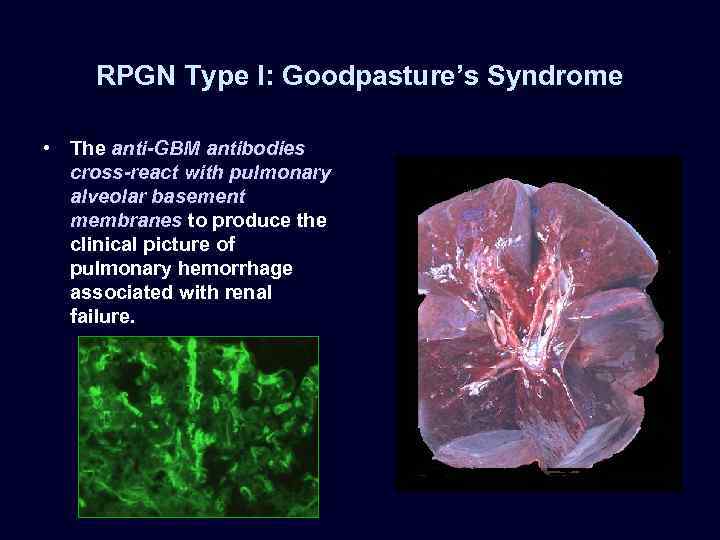
RPGN Type I: Goodpasture’s Syndrome • The anti-GBM antibodies cross-react with pulmonary alveolar basement membranes to produce the clinical picture of pulmonary hemorrhage associated with renal failure.
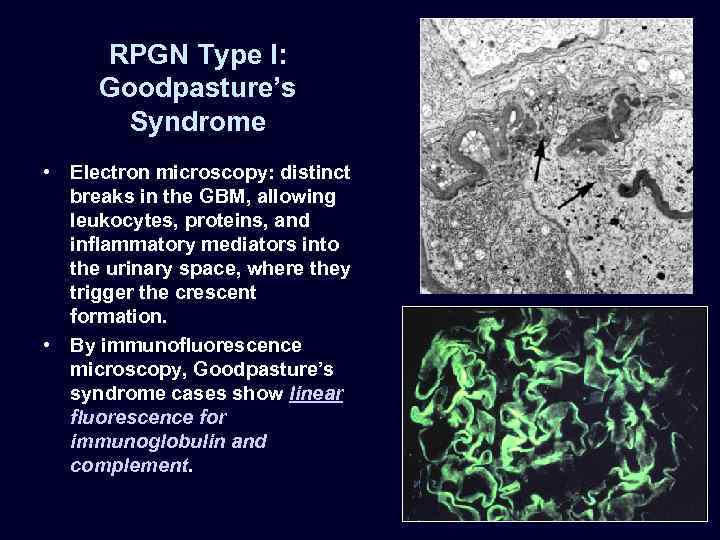
RPGN Type I: Goodpasture’s Syndrome • Electron microscopy: distinct breaks in the GBM, allowing leukocytes, proteins, and inflammatory mediators into the urinary space, where they trigger the crescent formation. • By immunofluorescence microscopy, Goodpasture’s syndrome cases show linear fluorescence for immunoglobulin and complement.
Rapidly Progressive Glomerulonephritis • • • All three types of RPGN may be associated with a welldefined renal or extrarenal disease, but in many cases (approximately 50%), the disorder is idiopathic. Of the patients with this syndrome, about one fifth have anti-GBM antibodyinduced disease without lung involvement; another one fourth have immune complex -mediated disease RPGN; and the remainder are of the pauci-immune type. The common denominator in all types of RPGN is severe glomerular injury.
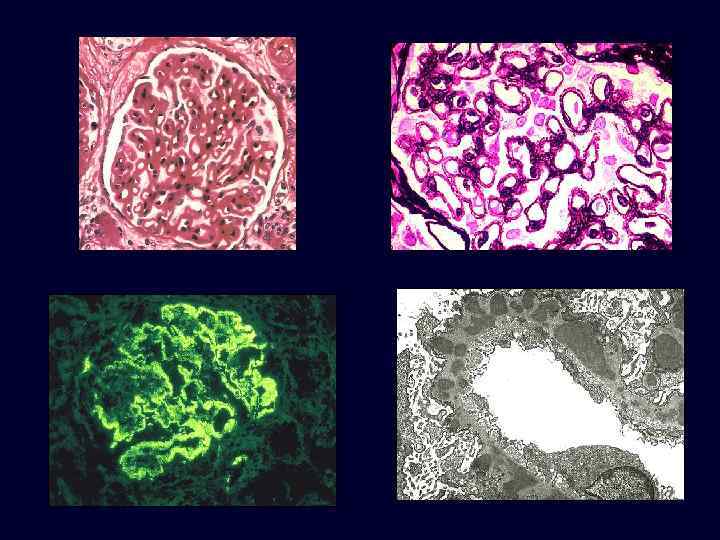
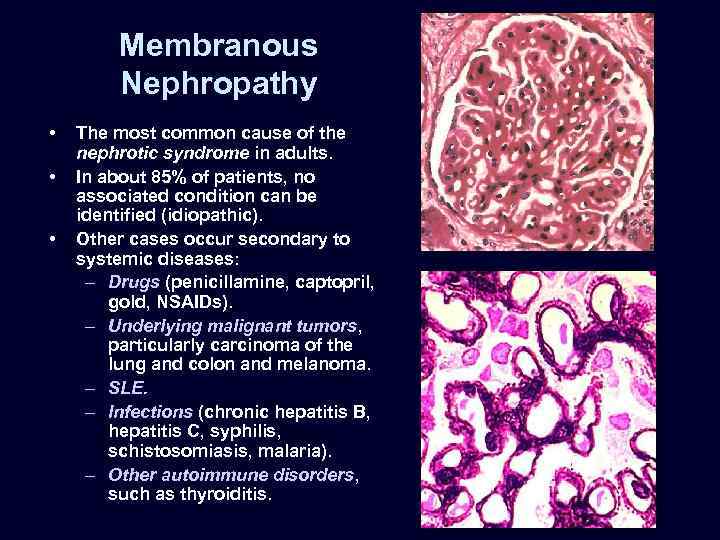
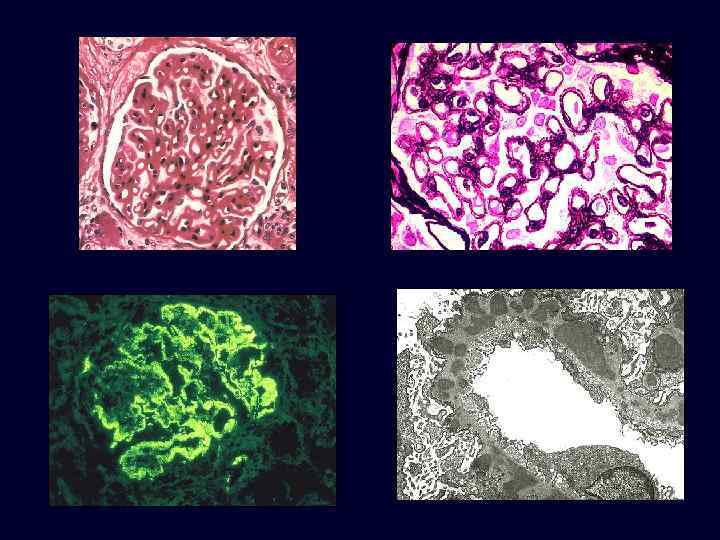
Membranous Nephropathy • • • The most common cause of the nephrotic syndrome in adults. In about 85% of patients, no associated condition can be identified (idiopathic). Other cases occur secondary to systemic diseases: – Drugs (penicillamine, captopril, gold, NSAIDs). – Underlying malignant tumors, particularly carcinoma of the lung and colon and melanoma. – SLE. – Infections (chronic hepatitis B, hepatitis C, syphilis, schistosomiasis, malaria). – Other autoimmune disorders, such as thyroiditis.
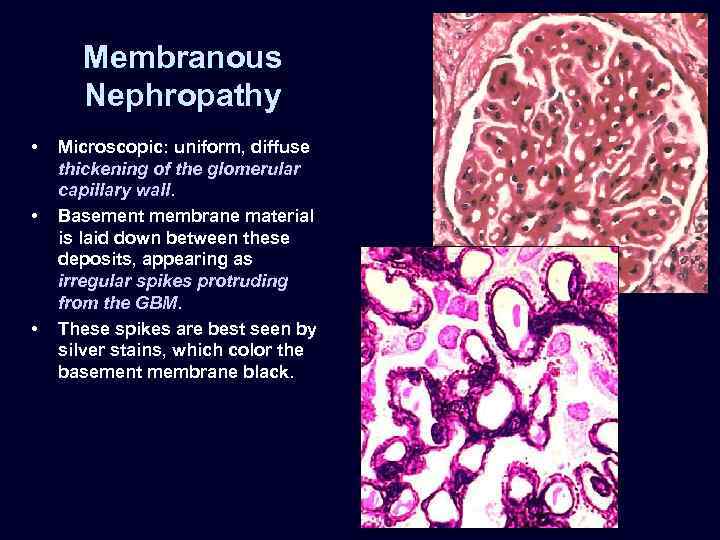
Membranous Nephropathy • • • Microscopic: uniform, diffuse thickening of the glomerular capillary wall. Basement membrane material is laid down between these deposits, appearing as irregular spikes protruding from the GBM. These spikes are best seen by silver stains, which color the basement membrane black.
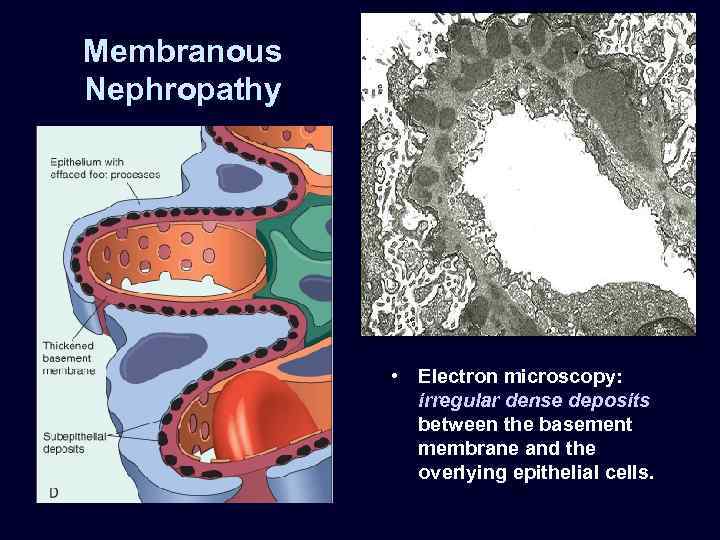
Membranous Nephropathy • Electron microscopy: irregular dense deposits between the basement membrane and the overlying epithelial cells.
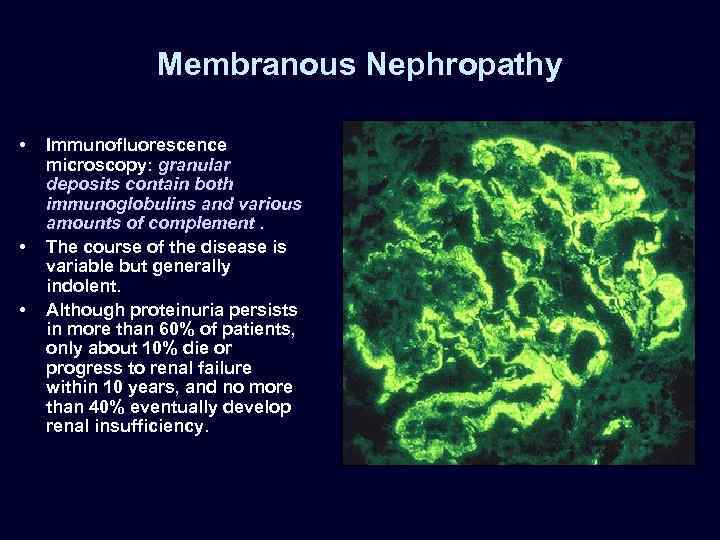
Membranous Nephropathy • • • Immunofluorescence microscopy: granular deposits contain both immunoglobulins and various amounts of complement. The course of the disease is variable but generally indolent. Although proteinuria persists in more than 60% of patients, only about 10% die or progress to renal failure within 10 years, and no more than 40% eventually develop renal insufficiency.

SET 2

SET 2
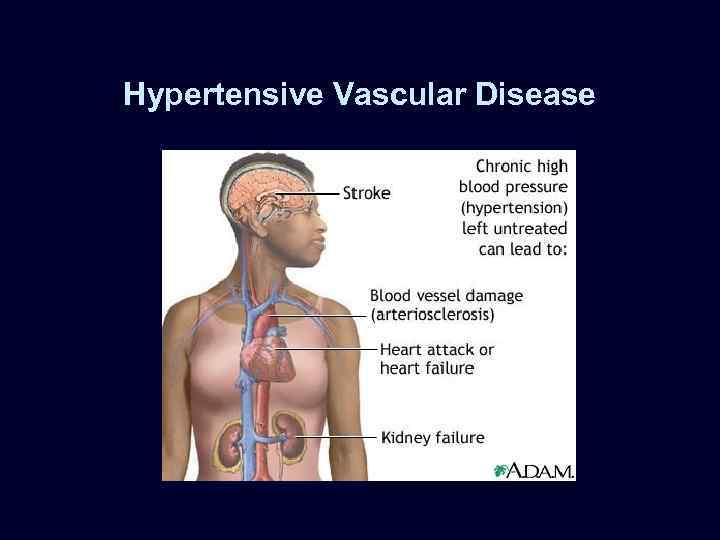
Hypertensive Vascular Disease
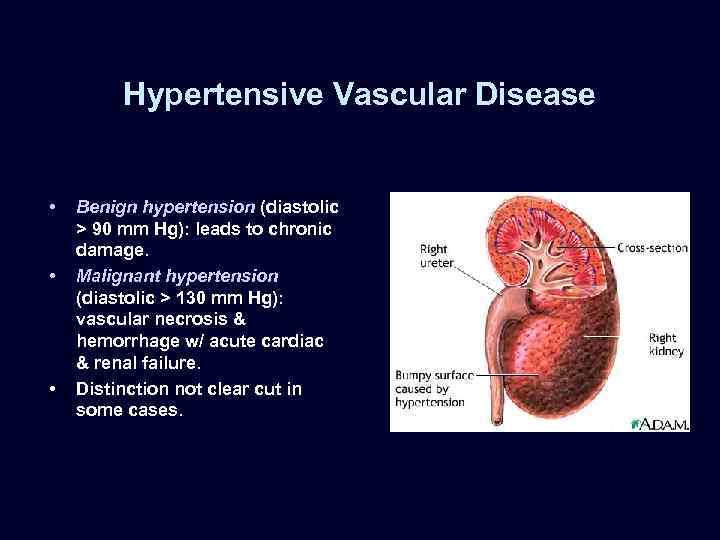
Hypertensive Vascular Disease • • • Benign hypertension (diastolic > 90 mm Hg): leads to chronic damage. Malignant hypertension (diastolic > 130 mm Hg): vascular necrosis & hemorrhage w/ acute cardiac & renal failure. Distinction not clear cut in some cases.
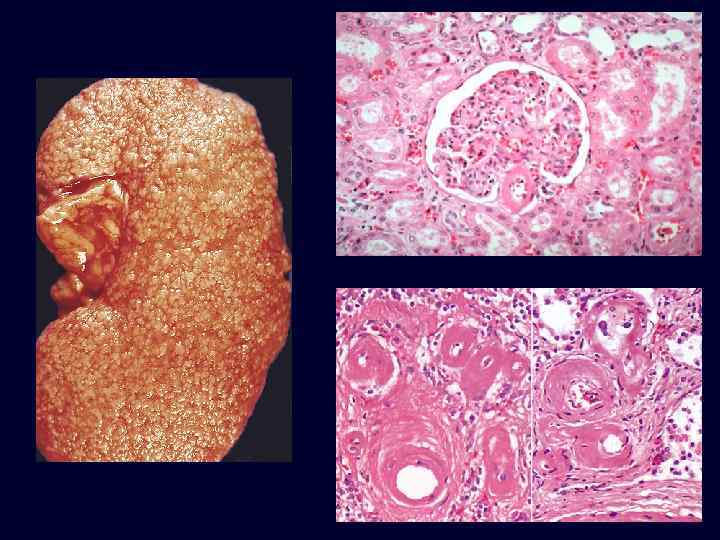
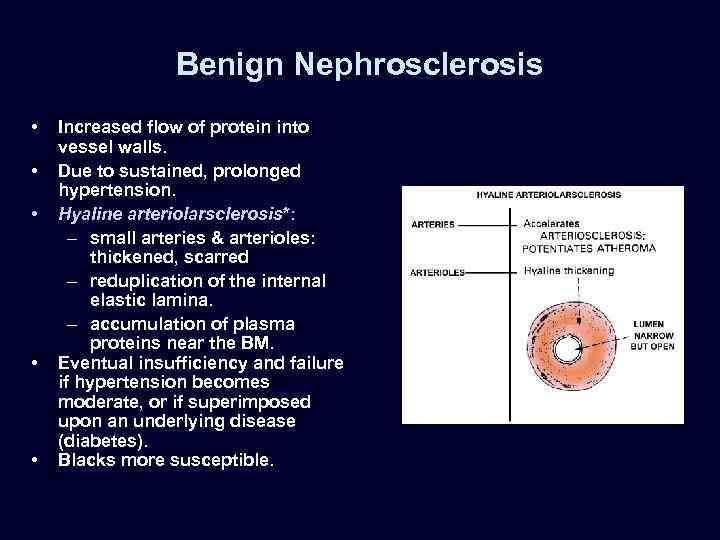
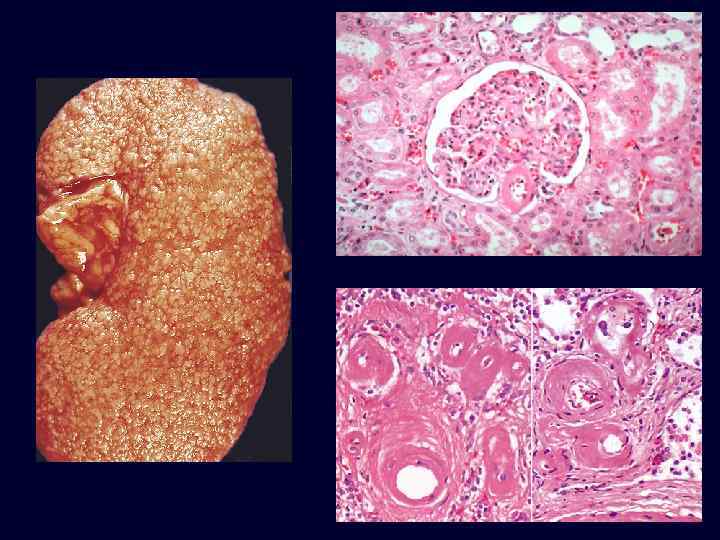
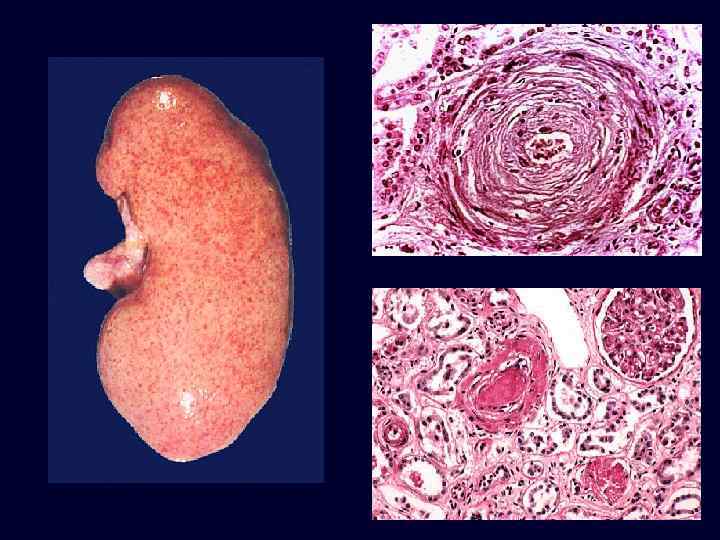
Benign Nephrosclerosis • • • Increased flow of protein into vessel walls. Due to sustained, prolonged hypertension. Hyaline arteriolarsclerosis*: – small arteries & arterioles: thickened, scarred – reduplication of the internal elastic lamina. – accumulation of plasma proteins near the BM. Eventual insufficiency and failure if hypertension becomes moderate, or if superimposed upon an underlying disease (diabetes). Blacks more susceptible.
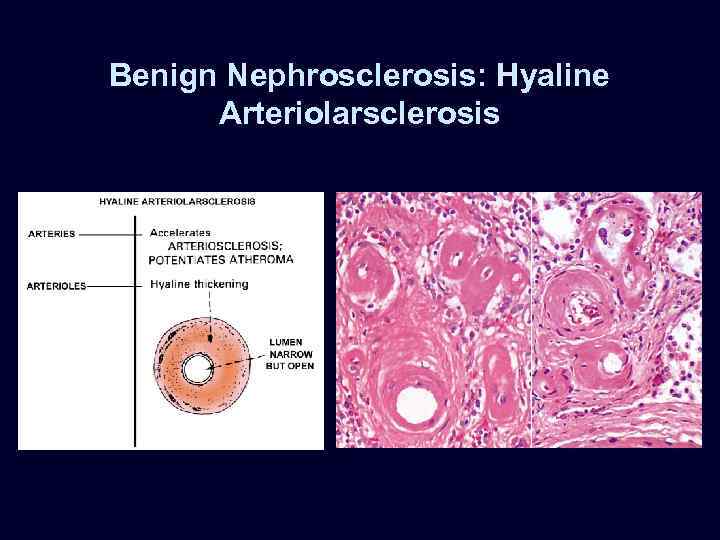
Benign Nephrosclerosis: Hyaline Arteriolarsclerosis
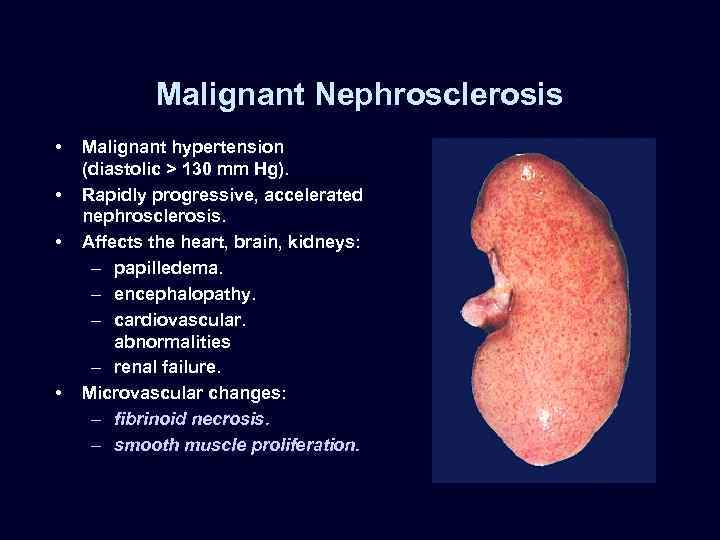
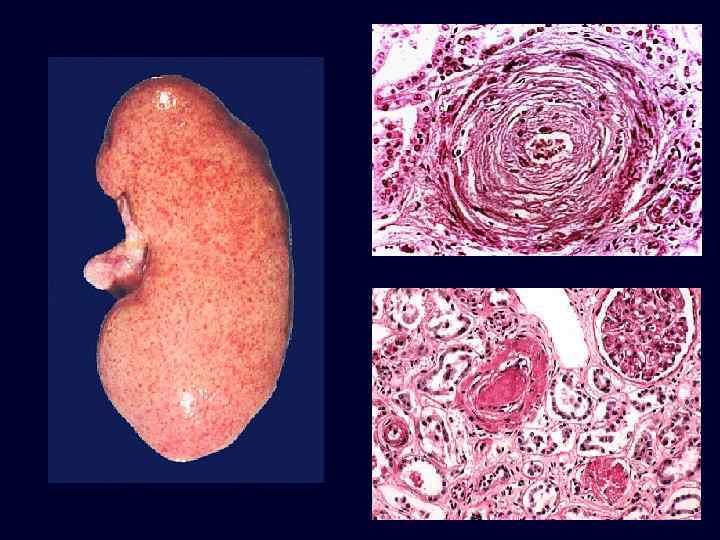
Malignant Nephrosclerosis • • Malignant hypertension (diastolic > 130 mm Hg). Rapidly progressive, accelerated nephrosclerosis. Affects the heart, brain, kidneys: – papilledema. – encephalopathy. – cardiovascular. abnormalities – renal failure. Microvascular changes: – fibrinoid necrosis. – smooth muscle proliferation.
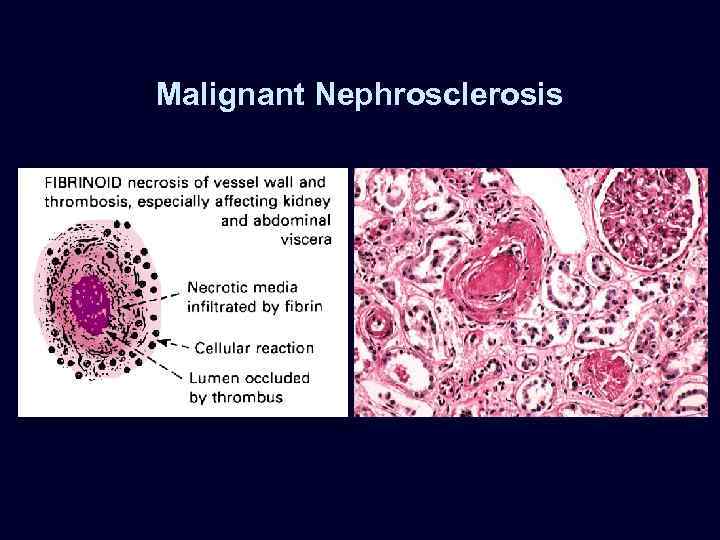
Malignant Nephrosclerosis
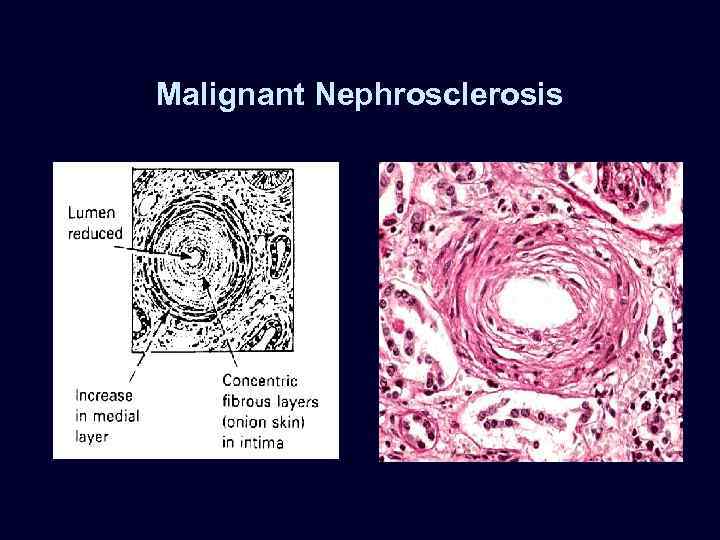
Malignant Nephrosclerosis
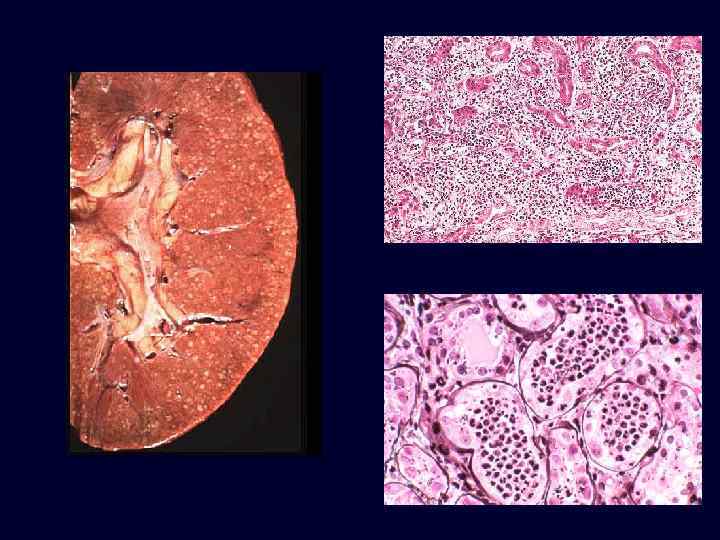
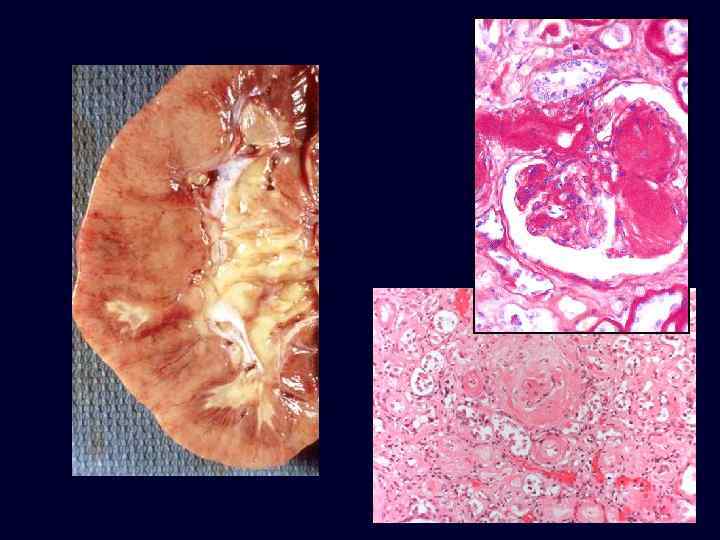
Diabetic Nephropathy • • • The kidneys are prime targets of diabetes. Renal failure is second only to myocardial infarction as a cause of death from this disease. Three lesions are encountered: – glomerular lesions. – renal vascular lesions, mainly arteriolosclerosis. – pyelonephritis, including necrotizing papillitis.
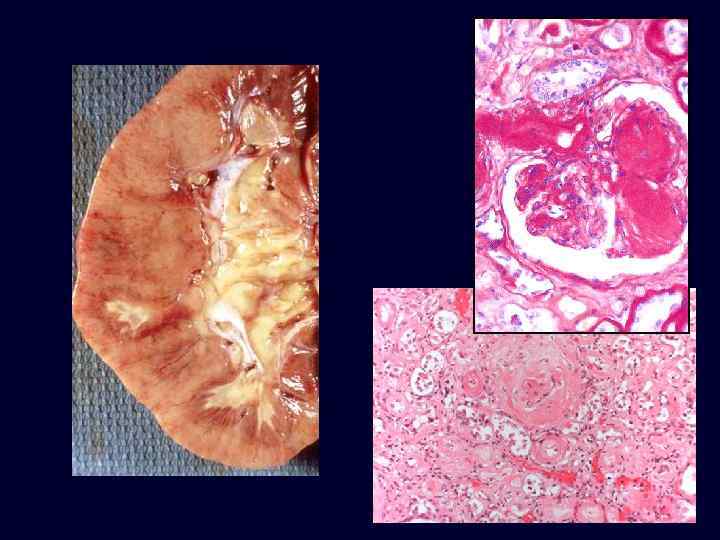

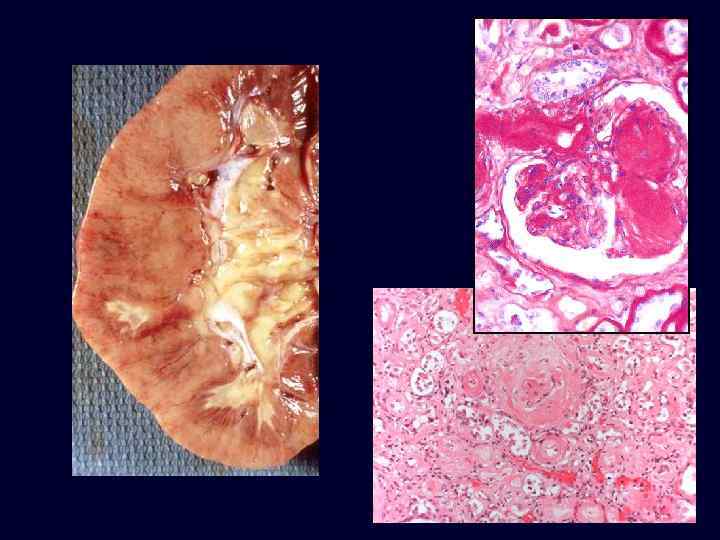
Diabetic Nephropathy • The most important glomerular lesions: – capillary basement membrane thickening. – diffuse mesangial sclerosis. – nodular glomerulosclerosis. • The glomerular capillary basement membranes are thickened throughout their entire length.
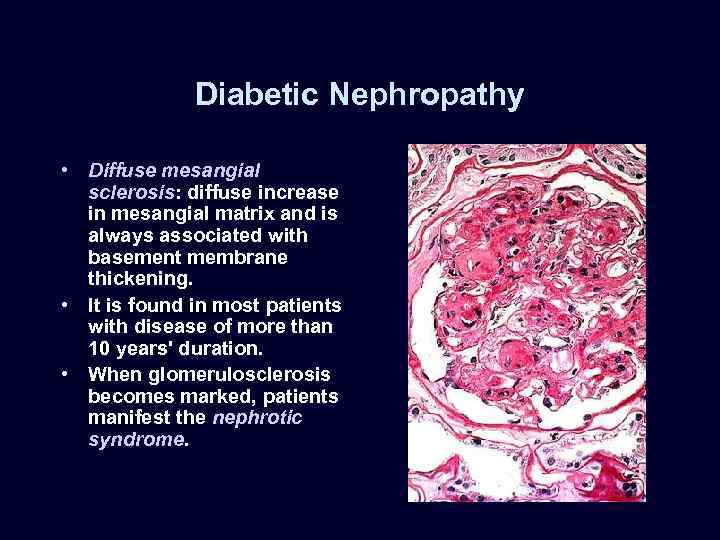
Diabetic Nephropathy • Diffuse mesangial sclerosis: diffuse increase in mesangial matrix and is always associated with basement membrane thickening. • It is found in most patients with disease of more than 10 years' duration. • When glomerulosclerosis becomes marked, patients manifest the nephrotic syndrome.
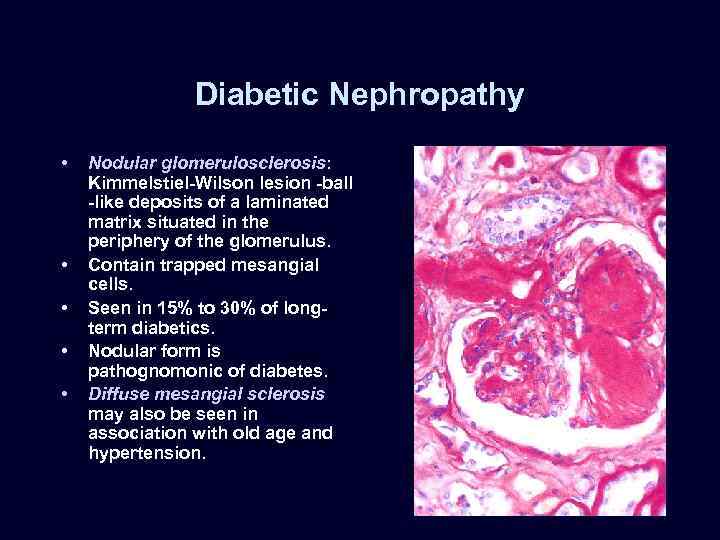
Diabetic Nephropathy • • • Nodular glomerulosclerosis: Kimmelstiel-Wilson lesion -ball -like deposits of a laminated matrix situated in the periphery of the glomerulus. Contain trapped mesangial cells. Seen in 15% to 30% of longterm diabetics. Nodular form is pathognomonic of diabetes. Diffuse mesangial sclerosis may also be seen in association with old age and hypertension.
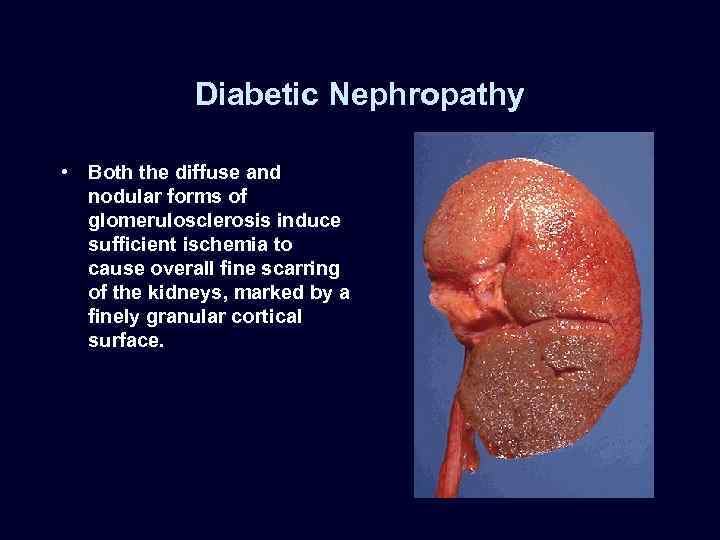
Diabetic Nephropathy • Both the diffuse and nodular forms of glomerulosclerosis induce sufficient ischemia to cause overall fine scarring of the kidneys, marked by a finely granular cortical surface.
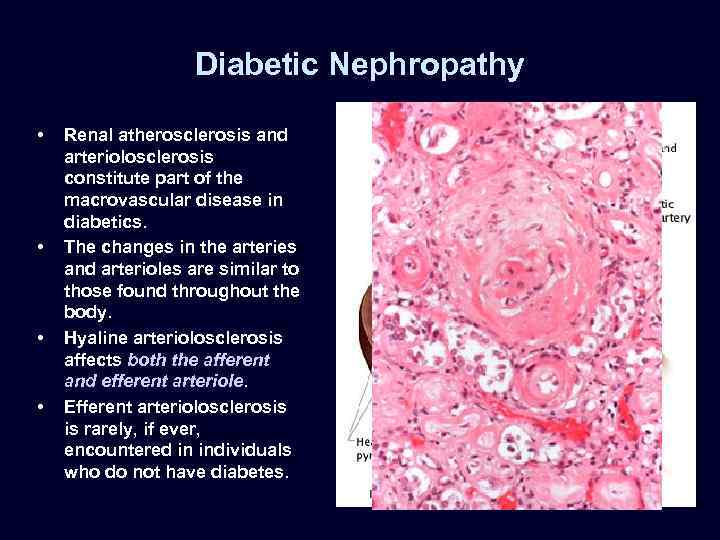
Diabetic Nephropathy • • Renal atherosclerosis and arteriolosclerosis constitute part of the macrovascular disease in diabetics. The changes in the arteries and arterioles are similar to those found throughout the body. Hyaline arteriolosclerosis affects both the afferent and efferent arteriole. Efferent arteriolosclerosis is rarely, if ever, encountered in individuals who do not have diabetes.
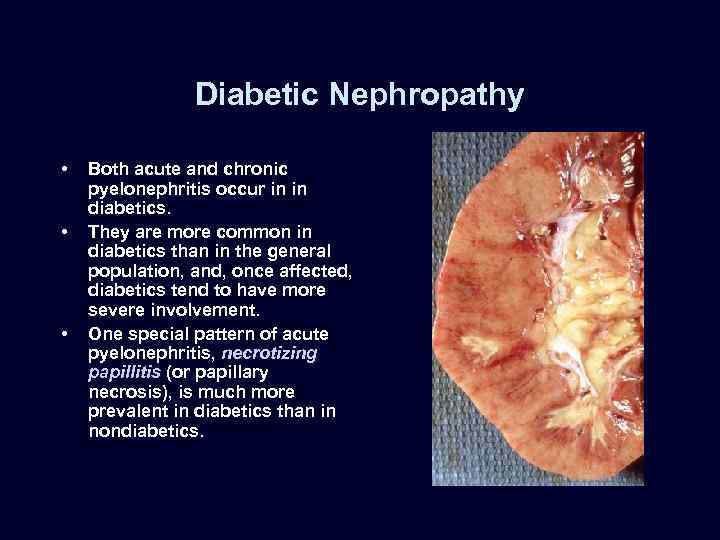
Diabetic Nephropathy • • • Both acute and chronic pyelonephritis occur in in diabetics. They are more common in diabetics than in the general population, and, once affected, diabetics tend to have more severe involvement. One special pattern of acute pyelonephritis, necrotizing papillitis (or papillary necrosis), is much more prevalent in diabetics than in nondiabetics.
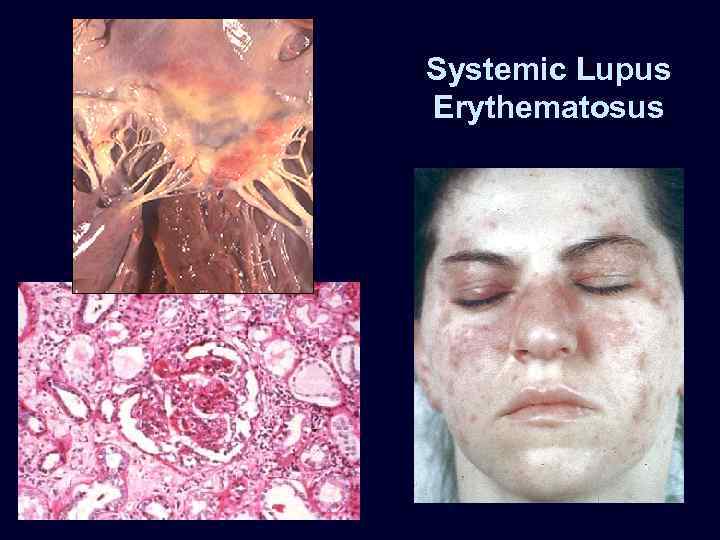
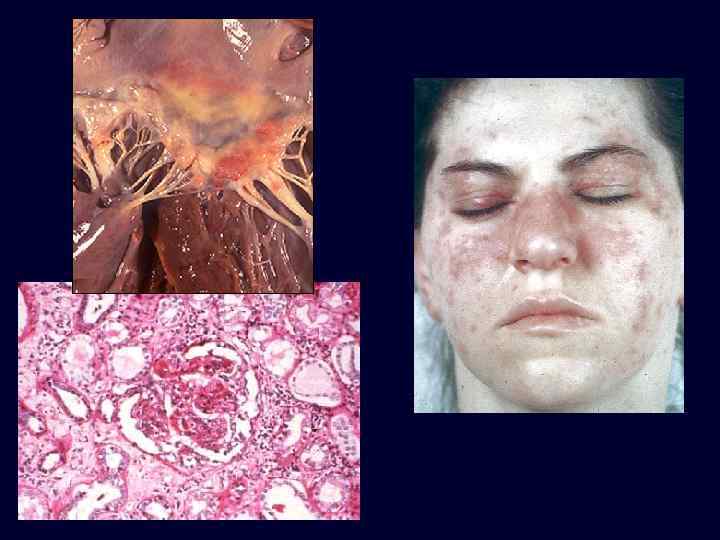
Systemic Lupus Erythematosus
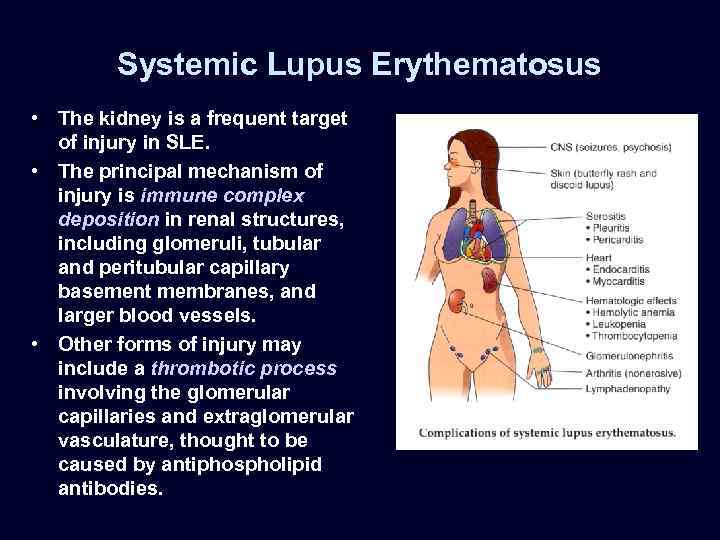
Systemic Lupus Erythematosus • The kidney is a frequent target of injury in SLE. • The principal mechanism of injury is immune complex deposition in renal structures, including glomeruli, tubular and peritubular capillary basement membranes, and larger blood vessels. • Other forms of injury may include a thrombotic process involving the glomerular capillaries and extraglomerular vasculature, thought to be caused by antiphospholipid antibodies.
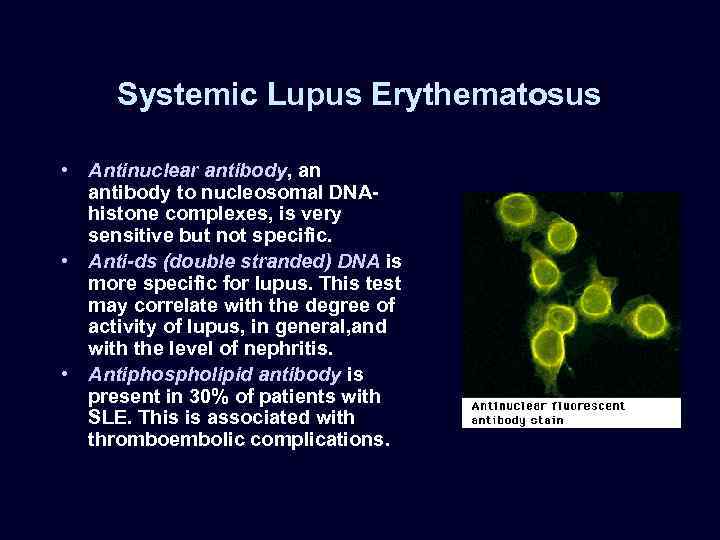
Systemic Lupus Erythematosus • Antinuclear antibody, an antibody to nucleosomal DNAhistone complexes, is very sensitive but not specific. • Anti-ds (double stranded) DNA is more specific for lupus. This test may correlate with the degree of activity of lupus, in general, and with the level of nephritis. • Antiphospholipid antibody is present in 30% of patients with SLE. This is associated with thromboembolic complications.

Systemic Lupus Erythematosus • World Health Organization (WHO) classification of lupus nephritis: – minimal or no detectable abnormalities (class I), rare, seen in renal biopsies from less than 5% of SLE patients. – mesangial lupus glomerulonephritis (class II). – focal proliferative glomerulonephritis (class III). – diffuse proliferative glomerulonephritis (class IV). – membranous glomerulonephritis (class V). • None of these patterns is specific for lupus.

Set 3
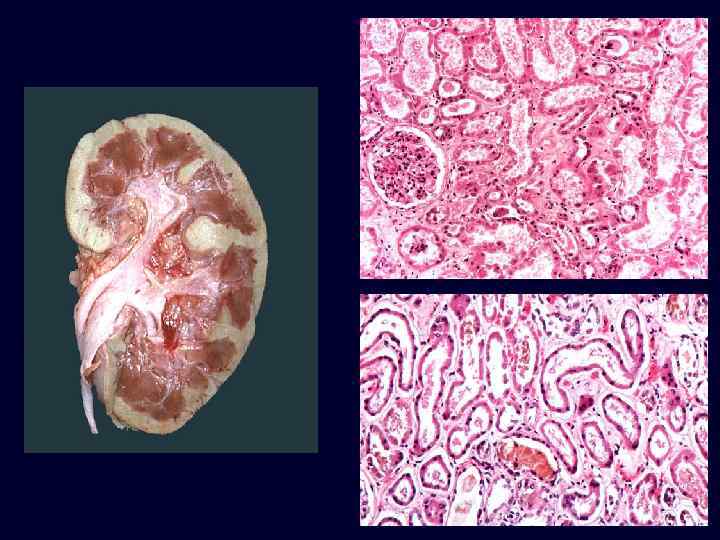
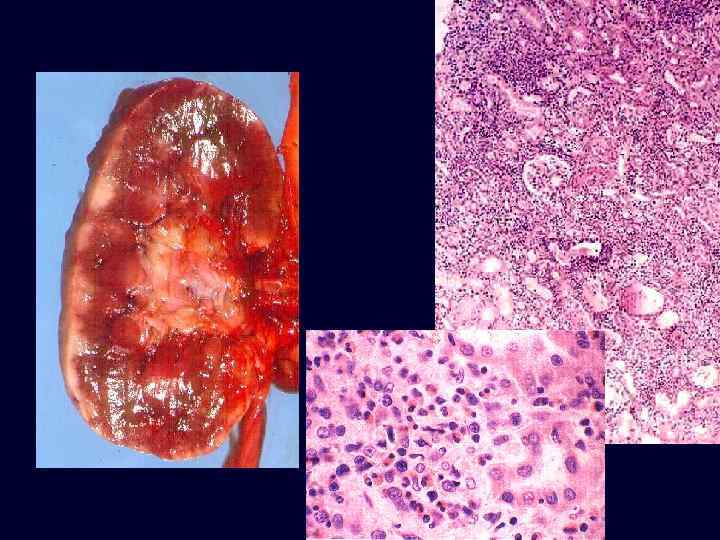
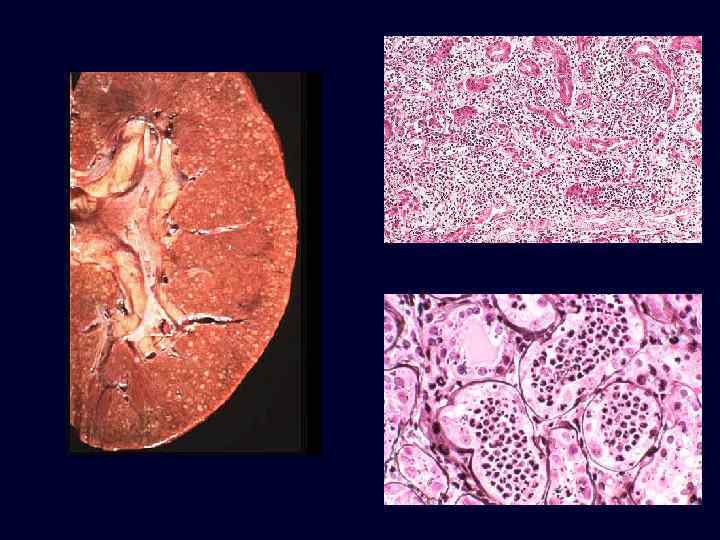
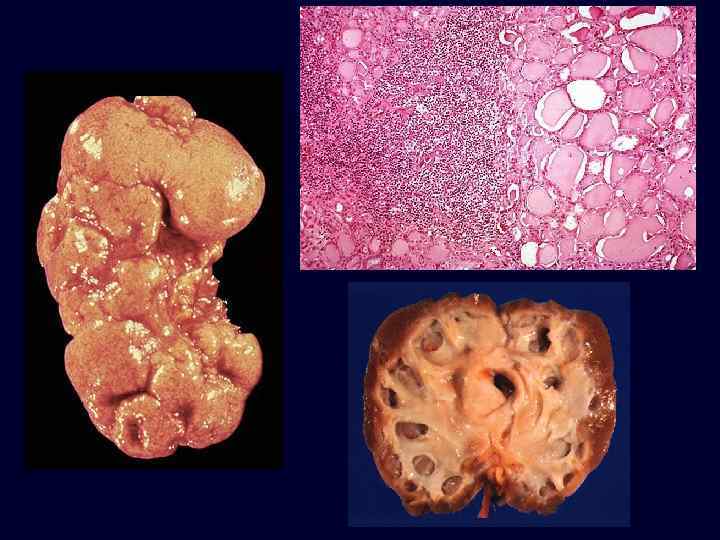

Set 3
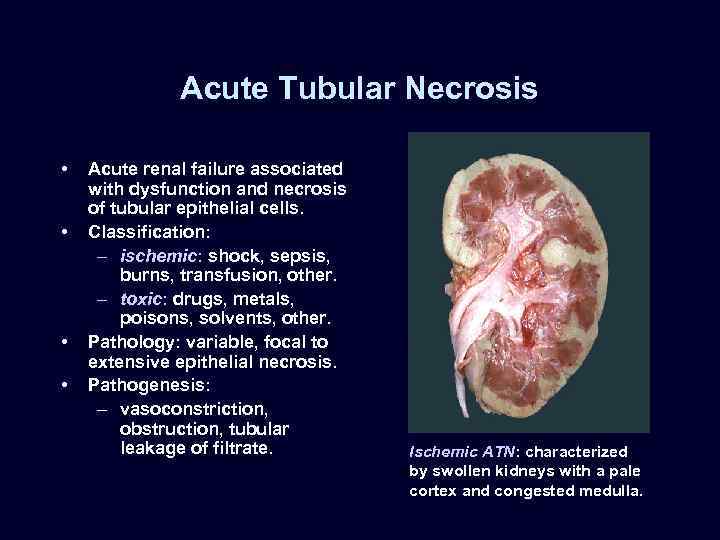
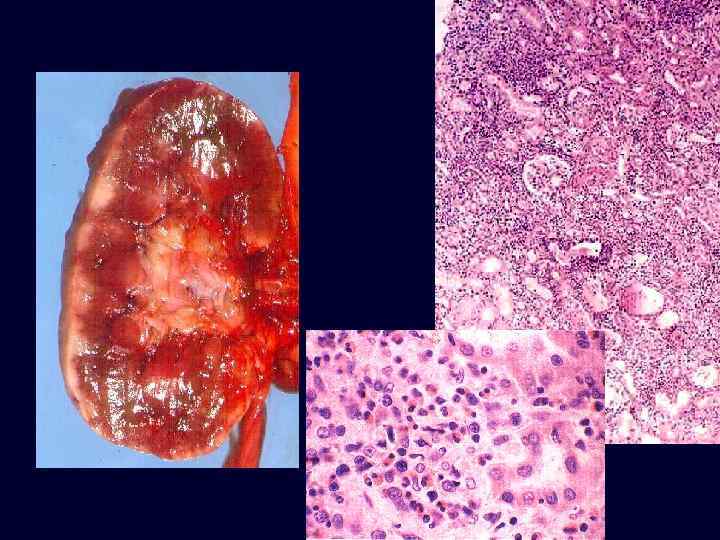
Acute Tubular Necrosis • • Acute renal failure associated with dysfunction and necrosis of tubular epithelial cells. Classification: – ischemic: shock, sepsis, burns, transfusion, other. – toxic: drugs, metals, poisons, solvents, other. Pathology: variable, focal to extensive epithelial necrosis. Pathogenesis: – vasoconstriction, obstruction, tubular leakage of filtrate. Ischemic ATN: characterized by swollen kidneys with a pale cortex and congested medulla.
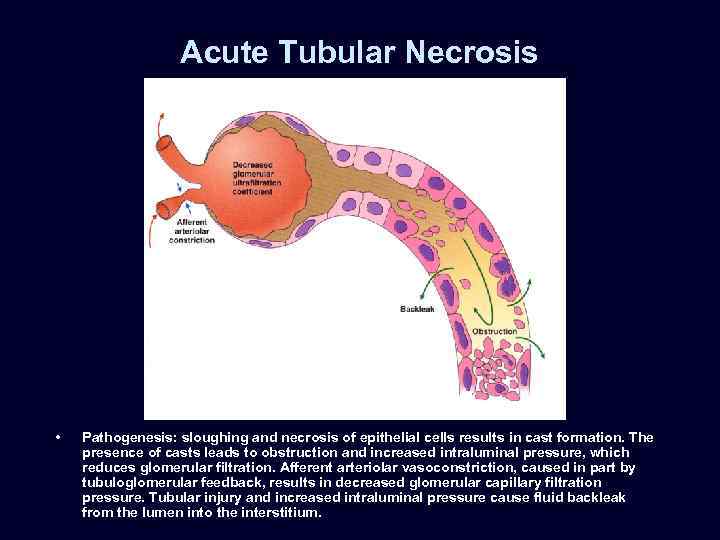
Acute Tubular Necrosis • Pathogenesis: sloughing and necrosis of epithelial cells results in cast formation. The presence of casts leads to obstruction and increased intraluminal pressure, which reduces glomerular filtration. Afferent arteriolar vasoconstriction, caused in part by tubuloglomerular feedback, results in decreased glomerular capillary filtration pressure. Tubular injury and increased intraluminal pressure cause fluid backleak from the lumen into the interstitium.
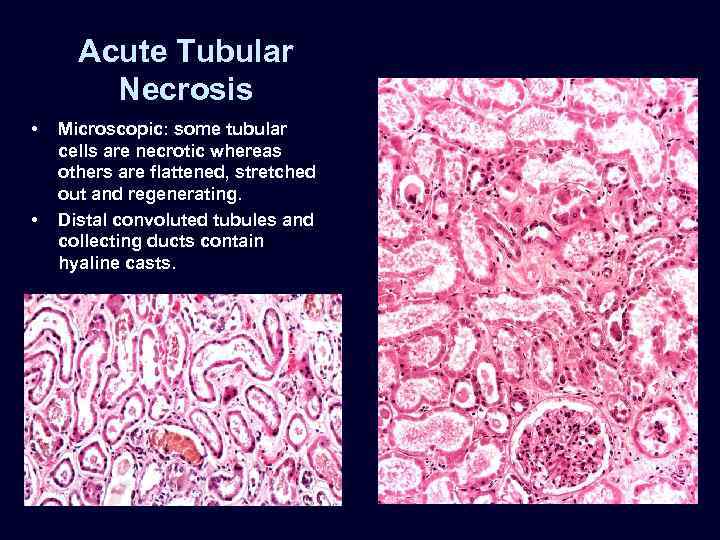
Acute Tubular Necrosis • • Microscopic: some tubular cells are necrotic whereas others are flattened, stretched out and regenerating. Distal convoluted tubules and collecting ducts contain hyaline casts.
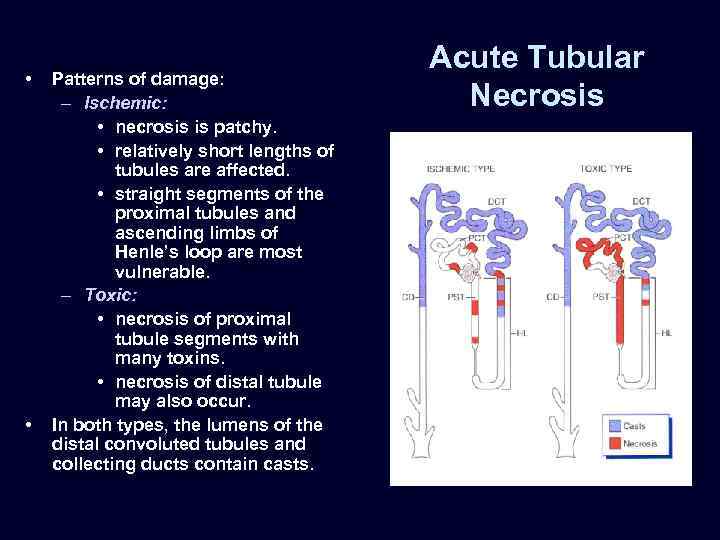
• • Patterns of damage: – Ischemic: • necrosis is patchy. • relatively short lengths of tubules are affected. • straight segments of the proximal tubules and ascending limbs of Henle’s loop are most vulnerable. – Toxic: • necrosis of proximal tubule segments with many toxins. • necrosis of distal tubule may also occur. In both types, the lumens of the distal convoluted tubules and collecting ducts contain casts. Acute Tubular Necrosis

Acute Drug-Induced Interstitial Nephritis • A well-recognized adverse reaction to a constantly increasing number of drugs. • Most frequently occurs with synthetic penicillins (methicillin, ampicillin), other synthetic antibiotics (rifampin), diuretics (thiazides), NSAIDs, and miscellaneous drugs (allopurinol, cimetidine). • Begins about 15 days after exposure and is characterized by fever, eosinophilia, and rash in about 25% of patients, and renal abnormalities (hematuria, mild proteinuria, and leukocyturia often including eosinophils). • A rising serum creatinine level or acute renal failure with oliguria develops in about 50% of cases, particularly in older patients.
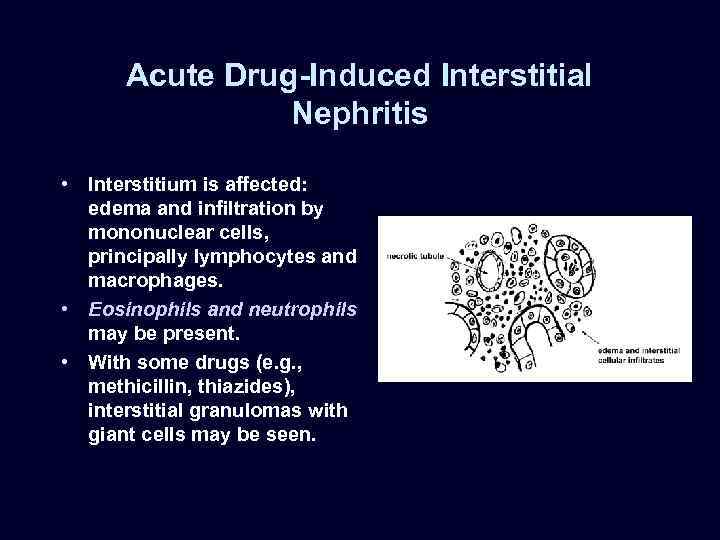
Acute Drug-Induced Interstitial Nephritis • Interstitium is affected: edema and infiltration by mononuclear cells, principally lymphocytes and macrophages. • Eosinophils and neutrophils may be present. • With some drugs (e. g. , methicillin, thiazides), interstitial granulomas with giant cells may be seen.
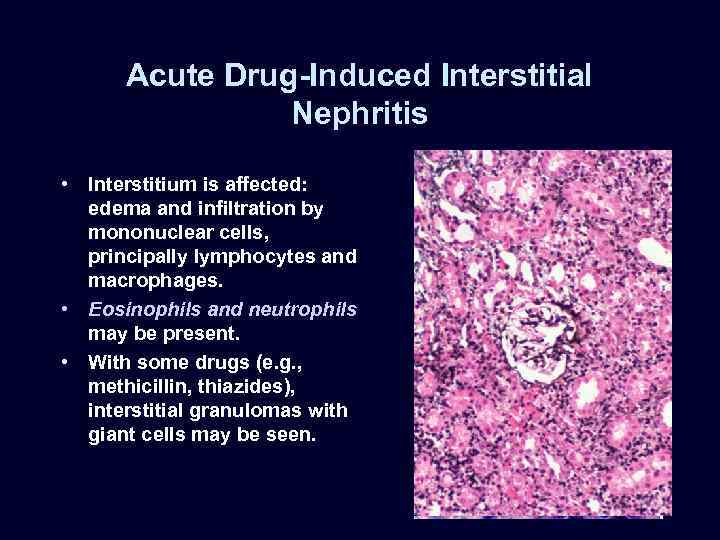
Acute Drug-Induced Interstitial Nephritis • Interstitium is affected: edema and infiltration by mononuclear cells, principally lymphocytes and macrophages. • Eosinophils and neutrophils may be present. • With some drugs (e. g. , methicillin, thiazides), interstitial granulomas with giant cells may be seen.
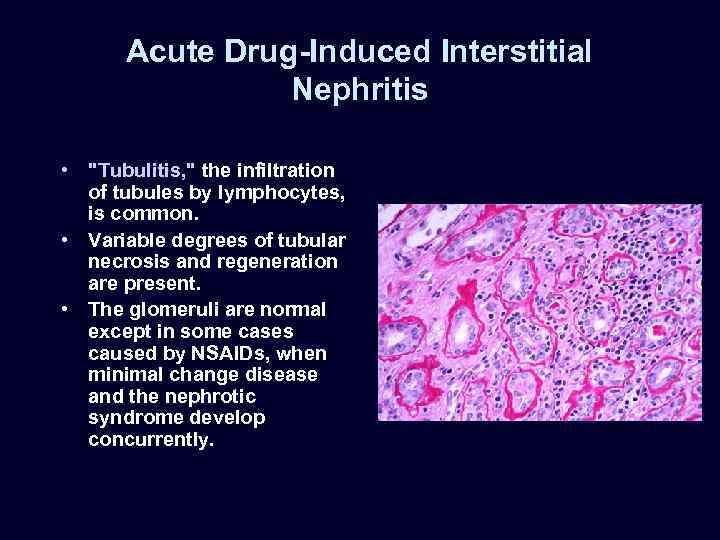
Acute Drug-Induced Interstitial Nephritis • "Tubulitis, " the infiltration of tubules by lymphocytes, is common. • Variable degrees of tubular necrosis and regeneration are present. • The glomeruli are normal except in some cases caused by NSAIDs, when minimal change disease and the nephrotic syndrome develop concurrently.
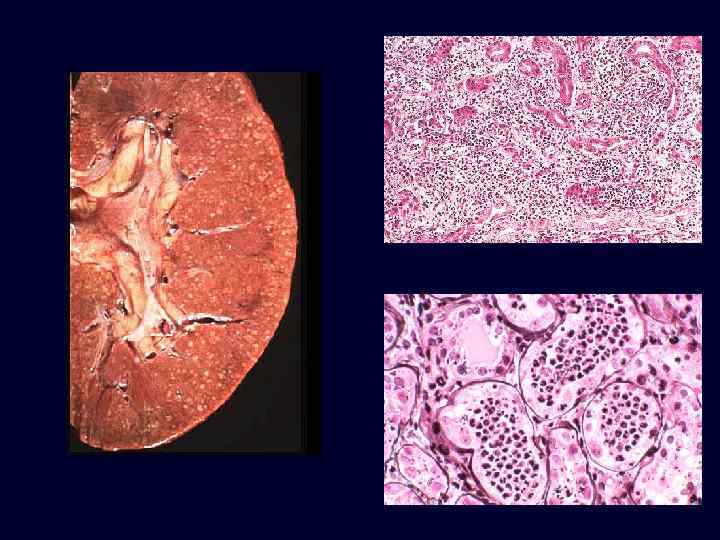
Acute Pyelonephritis • • Causes: – ascending infection vesicoureteral reflux into the renal pelvis and papillae; E. coli, Proteus, Enterobacter. – hematogenous seeding due to septicemia or endocarditis; Staphlococcus and E. coli. Patchy process, pinpoint microabscesses on cortical surface.
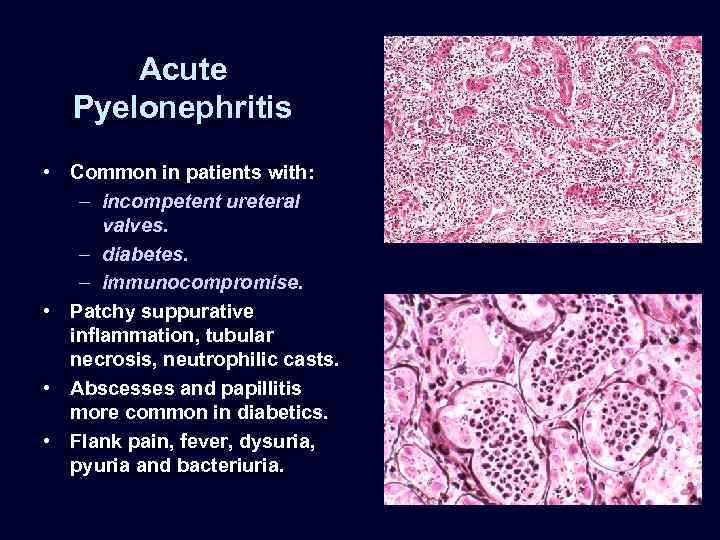
Acute Pyelonephritis • Common in patients with: – incompetent ureteral valves. – diabetes. – immunocompromise. • Patchy suppurative inflammation, tubular necrosis, neutrophilic casts. • Abscesses and papillitis more common in diabetics. • Flank pain, fever, dysuria, pyuria and bacteriuria.
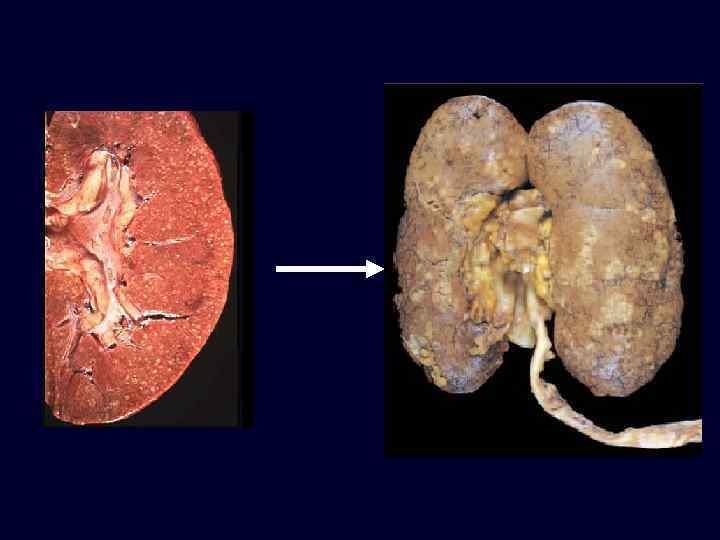
Chronic Pyelonephritis • • • Chronic tubulointerstitial inflammation with renal scarring. Important cause of end-stage renal disease. Two forms: – reflux-associated: common, congenital vesicourtehral reflux or intrarenal reflux. – obstructive: posterior urethral valves, ureteral calculi or abnormalities.
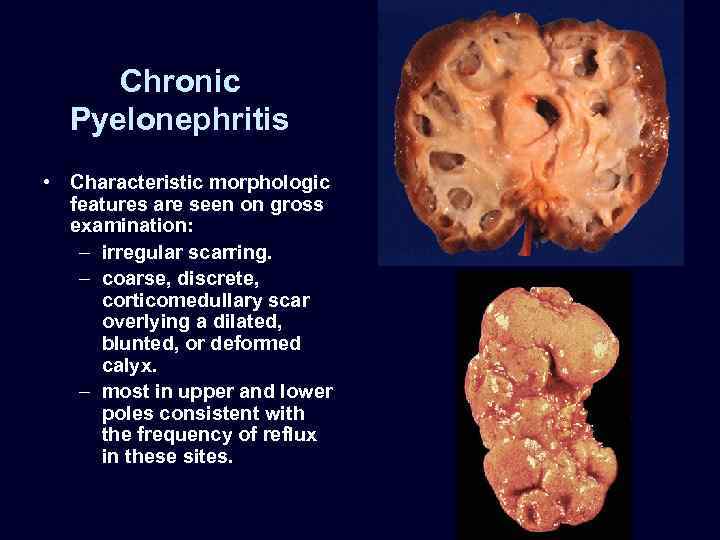
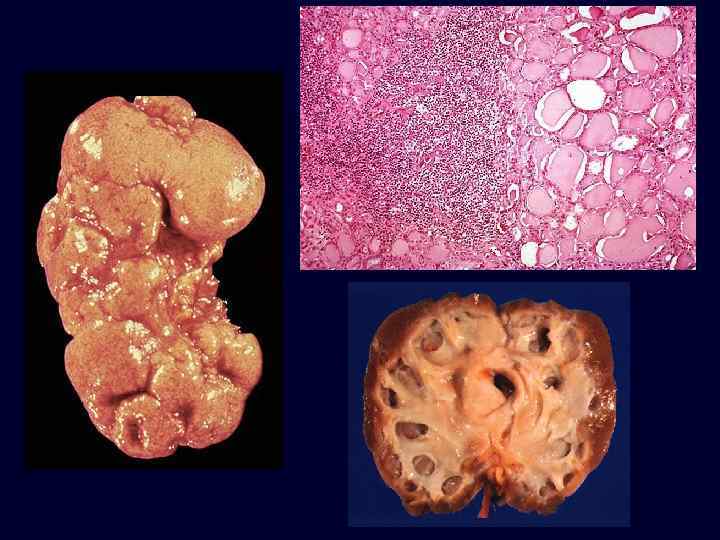
Chronic Pyelonephritis • Characteristic morphologic features are seen on gross examination: – irregular scarring. – coarse, discrete, corticomedullary scar overlying a dilated, blunted, or deformed calyx. – most in upper and lower poles consistent with the frequency of reflux in these sites.
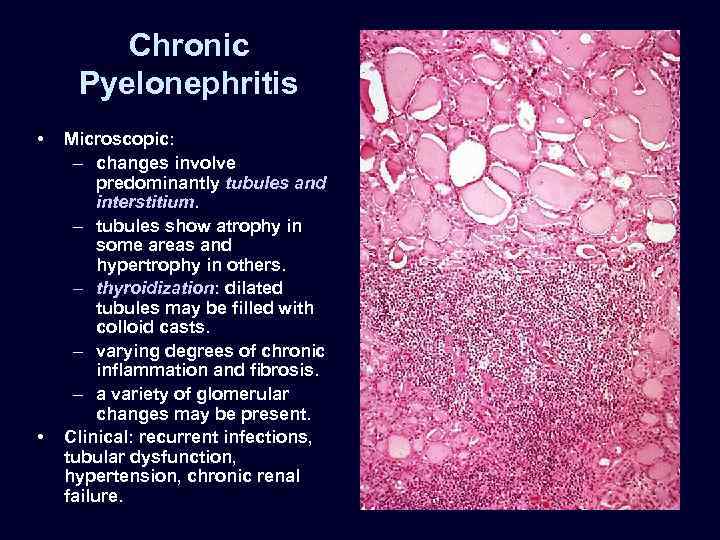
Chronic Pyelonephritis • • Microscopic: – changes involve predominantly tubules and interstitium. – tubules show atrophy in some areas and hypertrophy in others. – thyroidization: dilated tubules may be filled with colloid casts. – varying degrees of chronic inflammation and fibrosis. – a variety of glomerular changes may be present. Clinical: recurrent infections, tubular dysfunction, hypertension, chronic renal failure.

SET 4

SET 4
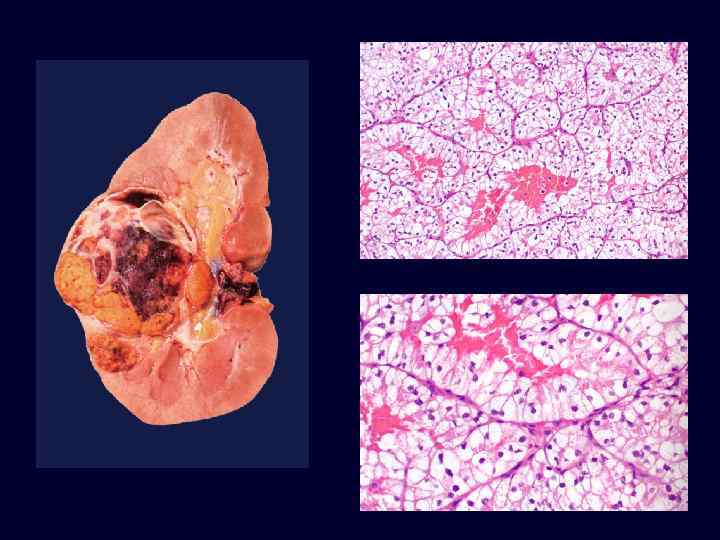
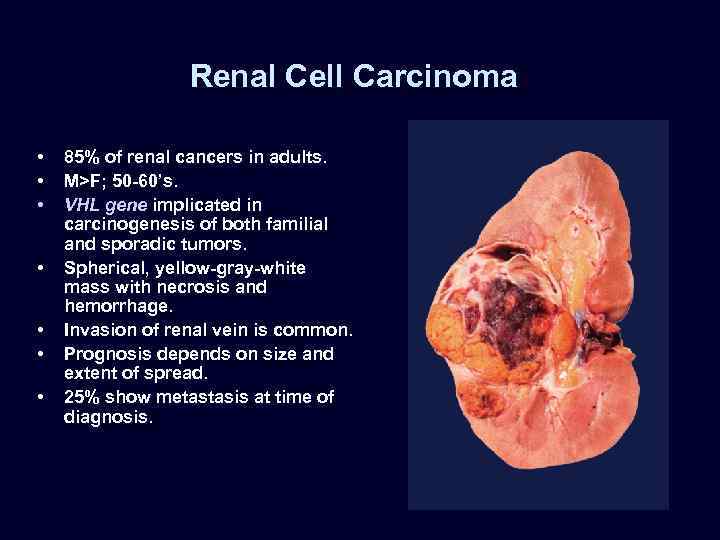
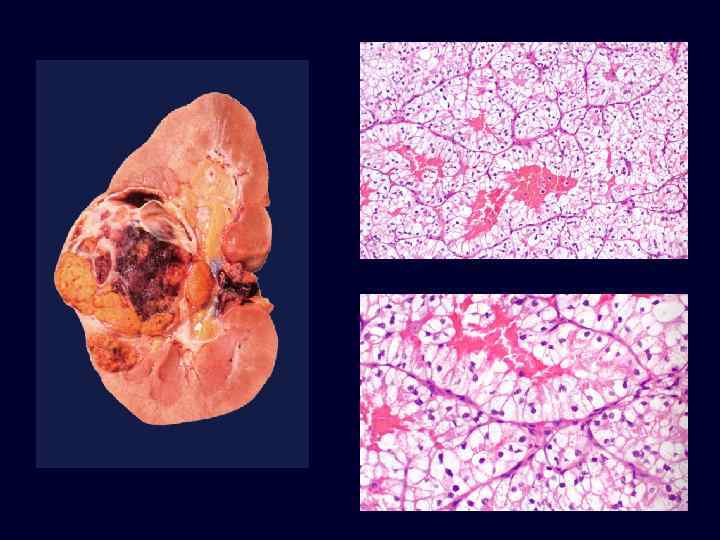
Renal Cell Carcinoma • • 85% of renal cancers in adults. M>F; 50 -60’s. VHL gene implicated in carcinogenesis of both familial and sporadic tumors. Spherical, yellow-gray-white mass with necrosis and hemorrhage. Invasion of renal vein is common. Prognosis depends on size and extent of spread. 25% show metastasis at time of diagnosis.
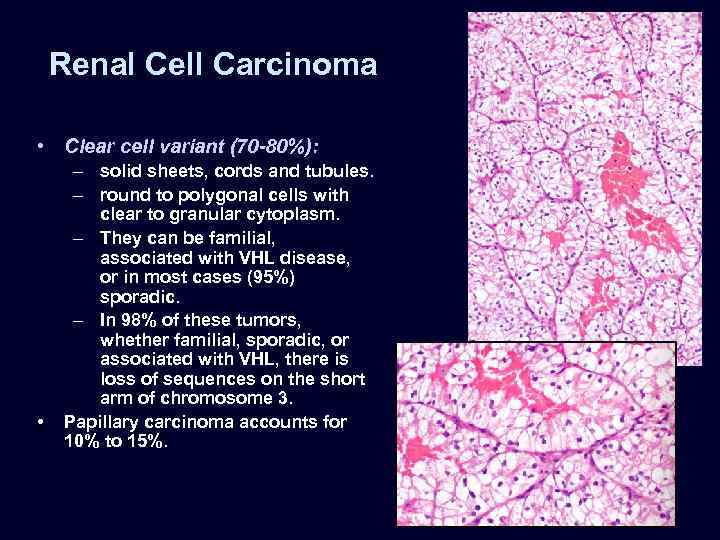
Renal Cell Carcinoma • Clear cell variant (70 -80%): • – solid sheets, cords and tubules. – round to polygonal cells with clear to granular cytoplasm. – They can be familial, associated with VHL disease, or in most cases (95%) sporadic. – In 98% of these tumors, whether familial, sporadic, or associated with VHL, there is loss of sequences on the short arm of chromosome 3. Papillary carcinoma accounts for 10% to 15%.

Renal Cell Carcinoma • Classic triad: hematuria, costovertebral pain, palpable mass. • May produce systemic symptoms: – polycythemia. – hypercalcemia. – hypertension. – hepatic dysfunction. – feminization, masculinization. – Cushing’s syndrome. – eosinophilia, amyloidosis.
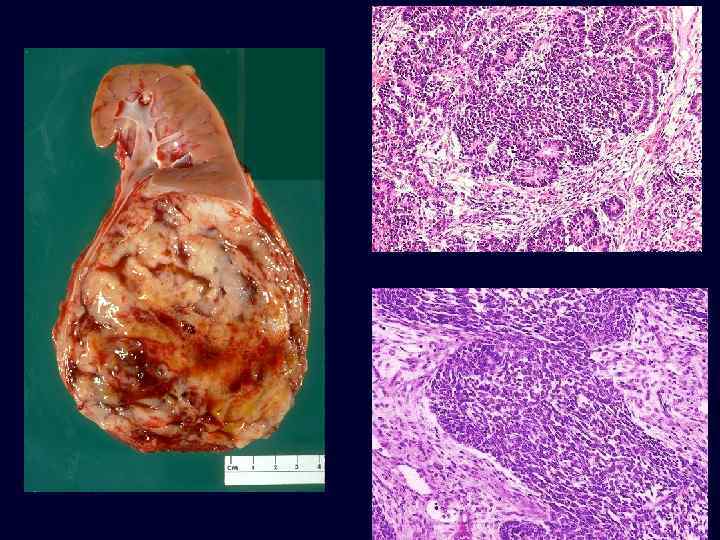
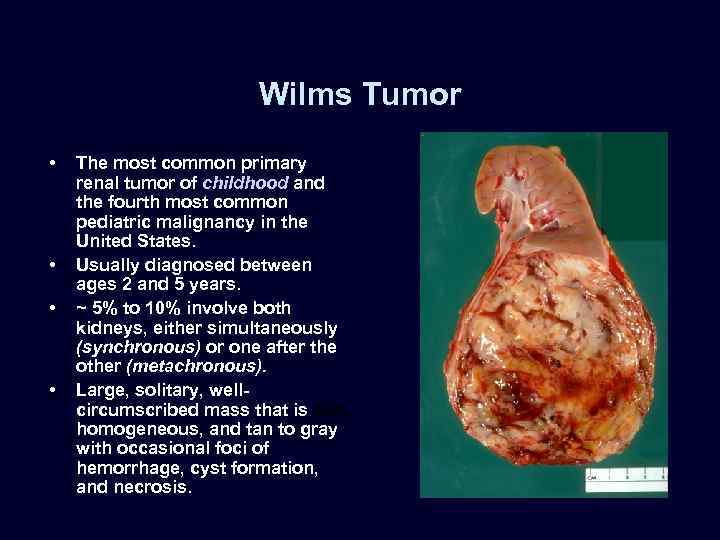
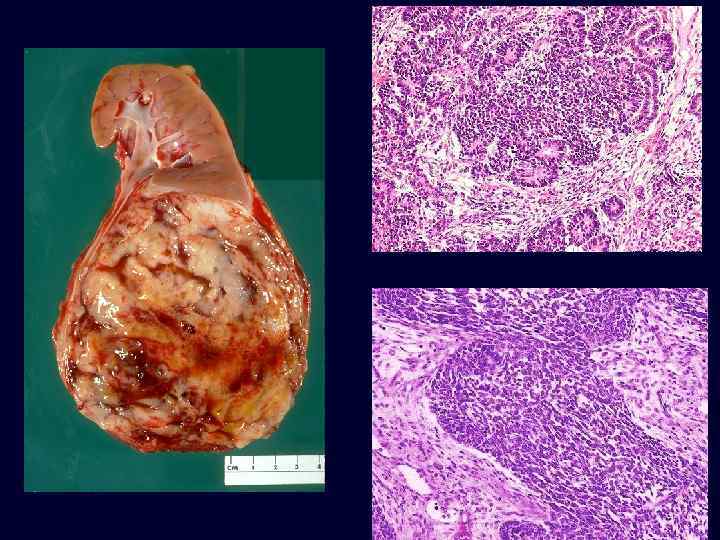
Wilms Tumor • • The most common primary renal tumor of childhood and the fourth most common pediatric malignancy in the United States. Usually diagnosed between ages 2 and 5 years. ~ 5% to 10% involve both kidneys, either simultaneously (synchronous) or one after the other (metachronous). Large, solitary, wellcircumscribed mass that is soft, homogeneous, and tan to gray with occasional foci of hemorrhage, cyst formation, and necrosis.

Wilms Tumor • Tumor risk is increased in association with three recognizable groups of congenital malformations associated with distinct chromosomal loci. – WAGR syndrome: • aniridia, genital anomalies, and mental retardation • 33% chance of developing Wilms tumor. • deletions of 11 p 13, tumor-associated gene, WT 1. – Denys-Drash syndrome: • gonadal dysgenesis (male pseudohermaphroditism) and earlyonset renal failure. • patients are at a much higher risk for Wilms tumor (>90%) • missense mutation of WT 1 gene located at chromosome 11 p 13. – Beckwith-Wiedemann syndrome: • characterized by enlargement of body organs (organomegaly), macroglossia, hemihypertrophy, omphalocele, and abnormal large cells in adrenal cortex (adrenal cytomegaly). • The genetic locus that is involved in these patients is in chromosome 11 distal to the WT 1 locus.
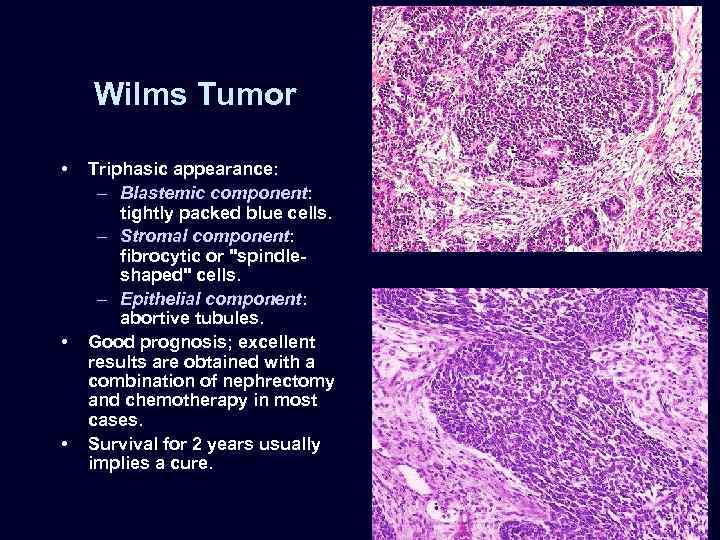
Wilms Tumor • • • Triphasic appearance: – Blastemic component: tightly packed blue cells. – Stromal component: fibrocytic or "spindleshaped" cells. – Epithelial component: abortive tubules. Good prognosis; excellent results are obtained with a combination of nephrectomy and chemotherapy in most cases. Survival for 2 years usually implies a cure.
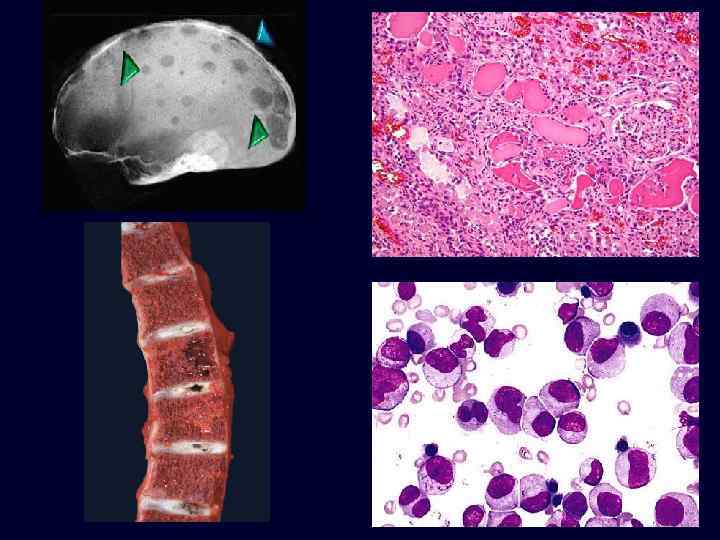
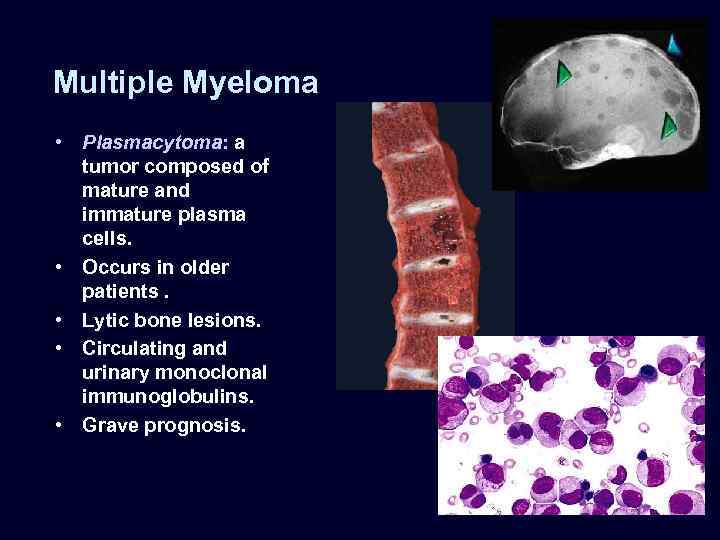
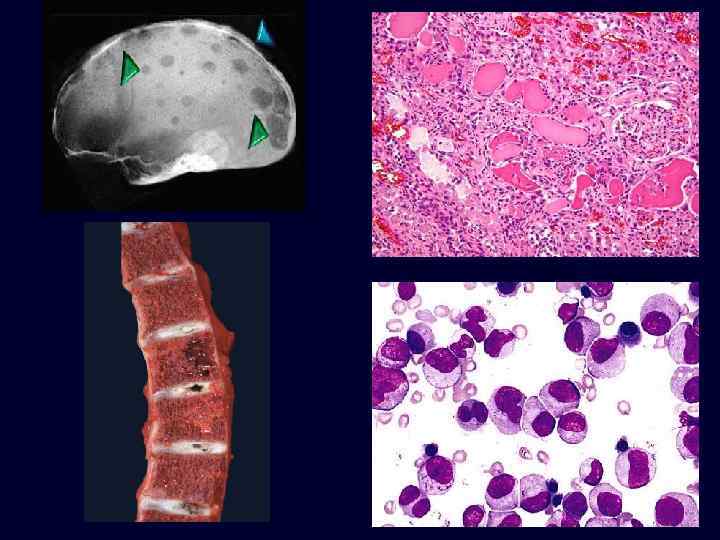
Multiple Myeloma • Plasmacytoma: a tumor composed of mature and immature plasma cells. • Occurs in older patients. • Lytic bone lesions. • Circulating and urinary monoclonal immunoglobulins. • Grave prognosis.
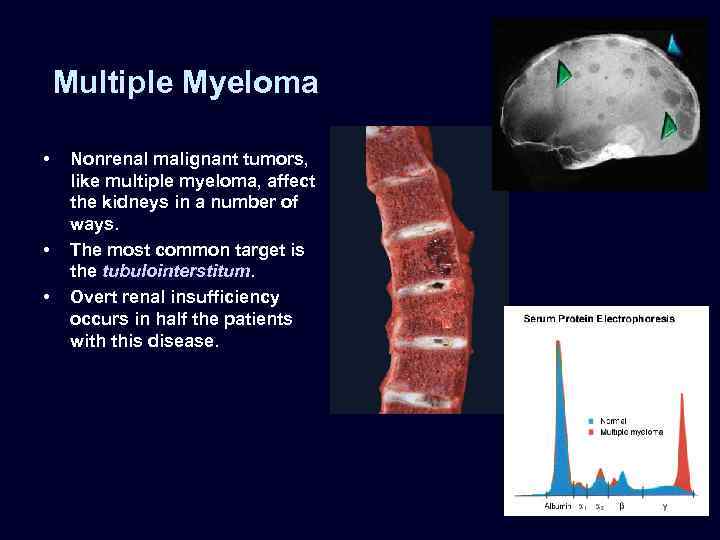
Multiple Myeloma • • • Nonrenal malignant tumors, like multiple myeloma, affect the kidneys in a number of ways. The most common target is the tubulointerstitum. Overt renal insufficiency occurs in half the patients with this disease.
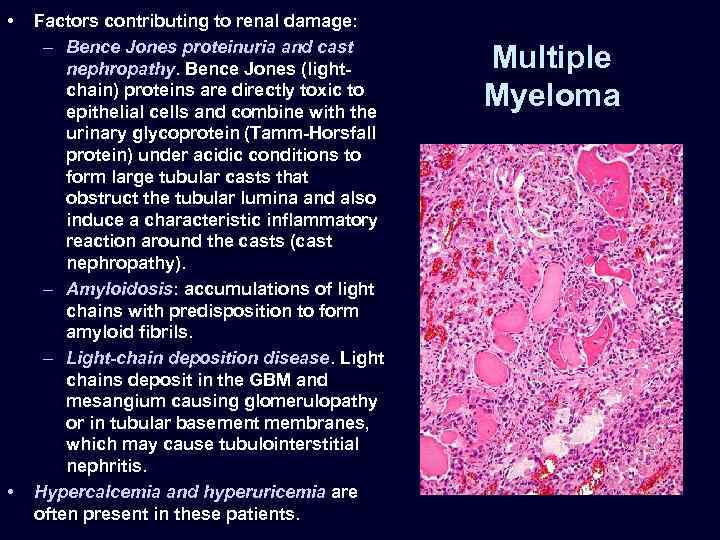
• • Factors contributing to renal damage: – Bence Jones proteinuria and cast nephropathy. Bence Jones (lightchain) proteins are directly toxic to epithelial cells and combine with the urinary glycoprotein (Tamm-Horsfall protein) under acidic conditions to form large tubular casts that obstruct the tubular lumina and also induce a characteristic inflammatory reaction around the casts (cast nephropathy). – Amyloidosis: accumulations of light chains with predisposition to form amyloid fibrils. – Light-chain deposition disease. Light chains deposit in the GBM and mesangium causing glomerulopathy or in tubular basement membranes, which may cause tubulointerstitial nephritis. Hypercalcemia and hyperuricemia are often present in these patients. Multiple Myeloma
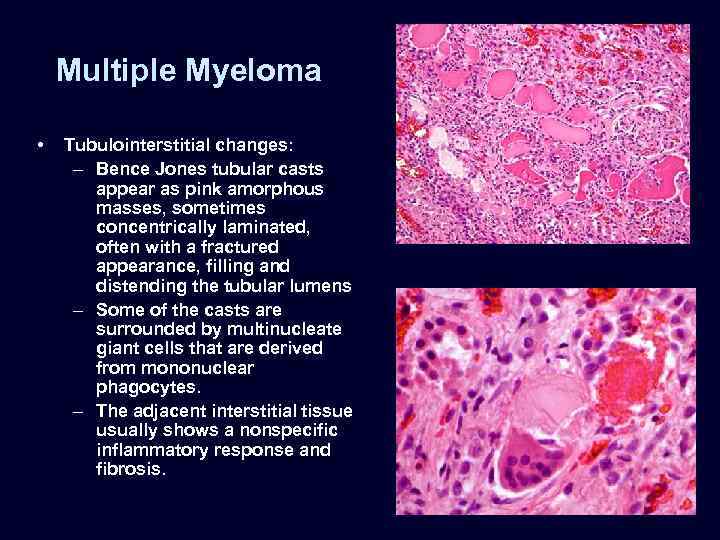
Multiple Myeloma • Tubulointerstitial changes: – Bence Jones tubular casts appear as pink amorphous masses, sometimes concentrically laminated, often with a fractured appearance, filling and distending the tubular lumens – Some of the casts are surrounded by multinucleate giant cells that are derived from mononuclear phagocytes. – The adjacent interstitial tissue usually shows a nonspecific inflammatory response and fibrosis.
WSU_Renal_Review (2).ppt