d63a68ebb4120cd8a8a550b00deeba6c.ppt
- Количество слайдов: 28

Renal Nerve Ablation: Will It Become the treatment Of Choice for Resistant Hypertension? Jie Wang MD Ph. D Mark Gelfand, MS Howard Levin, MD Disclosure: JW was a consultant and shareholder of Ardian; MG/HL were Co-founders of Ardian

Three Different Diseases, One Common Pathway § Heart Failure, Hypertension and Chronic Renal Failure have many different initial causes § Irrespective of initial cause, all follow a common pathway on their progression to end-stage disease § Physiologic changes caused by the kidney are responsible for this progression including: § Abnormal hormone secretion (renin, aldosterone, Norepi) § Renal, systemic and pulmonary vasoconstriction § Salt and water retention § The Common Pathway: Renal Nerve Hyperactivity

Renal Sympathetic Efferent Nerve Activity: Kidney as Recipient of Sympathetic Signals Renal Efferent Nerves Renal Ischemia ↑ Adenosine production BNP resistance ↑ Renin Release RAAS activation ↑ Sodium Retention ↓ Renal Blood Flow

Renal Sympathetic Afferent Nerves: Kidney as Origin of Central Sympathetic Drive Vasoconstriction Atherosclerosis Renal Afferent Nerves Hypertrophy Arrhythmia Oxygen Consumption Heart Failure Insulin Resistance ↑ Renin Release RAAS activation ↑ Sodium Retention ↓ Renal Blood Flow
Previous Data Confirm Positive Effects of Renal Nerve Blocking § Renal injury/ dysfunction identified as mechanism of increased sympathetic nervous system activity leading to HTN (Campese, 2002) § Blocking of renal nerve activity controls HTN in animals with chronic renal insufficiency (Campese, 1995) § Surgical renal denervation done to eliminate intractable pain in patients with polycystic kidney disease also eliminates HTN (Valente, 2001) § Denervation (by nephrectomy) eliminates HTN in humans on dialysis with severe HTN refractory to multi-drug therapy (Converse, 1992) § Blocking of renal nerve activity increases diuresis/ naturesis in rats with CHF (Dibona) § Blocking of renal nerve activity limits ventricular remodeling in rats post-MI (Tozawa, 2002)

Totally Novel Therapy, Original Plan § Intelligent, programmable fully implanted infusion pump with the catheter tip placed in the proximity of the renal nerve § Programmable battery operated electronic device similar in size and complexity to a pacemaker § Integrated electronic feedbacks to guide therapy § Percutaneous refilling of the reservoir once per month

How Does It Work? -Original Thought § Catheter is implanted in the periarterial space outside of the renal artery (Gerota’s fascia) § This technique is similar to percutaneous drainage of perinephric abscess § As needed, a bolus of a nerve blocking drug is infused and temporarily blocks renal nerve activity § Drug can be an approved drug such as Marcaine, local anesthetic commonly used for pain management

Our Animal Studies Confirmed The Clinical Utility Of This Concept § Obtained data from the 11 dogs with micro-embolization model of Acute HF § 8 dogs had Renal Nerve Block (RNB) created by injecting 10 ml of Marcaine (bupivicaine) inside Gerota’s fascia § 3 dogs served as controls § Urine output (Urine output/every 15 min) significantly increased compared to controls § Both naturesis and diuresis were observed confirming physiologic basis for effect § Same results found in other 6 dogs with microembolization model of chronic CHF
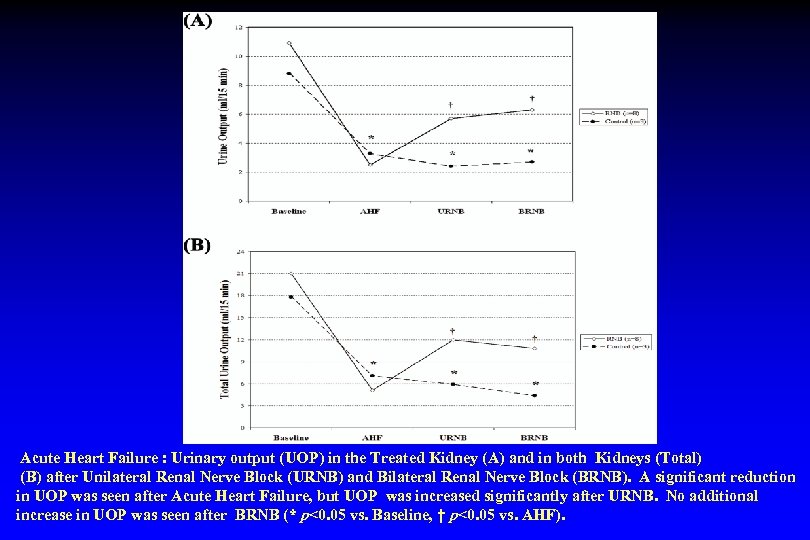
Acute Heart Failure : Urinary output (UOP) in the Treated Kidney (A) and in both Kidneys (Total) (B) after Unilateral Renal Nerve Block (URNB) and Bilateral Renal Nerve Block (BRNB). A significant reduction in UOP was seen after Acute Heart Failure, but UOP was increased significantly after URNB. No additional increase in UOP was seen after BRNB (* p<0. 05 vs. Baseline, † p<0. 05 vs. AHF).
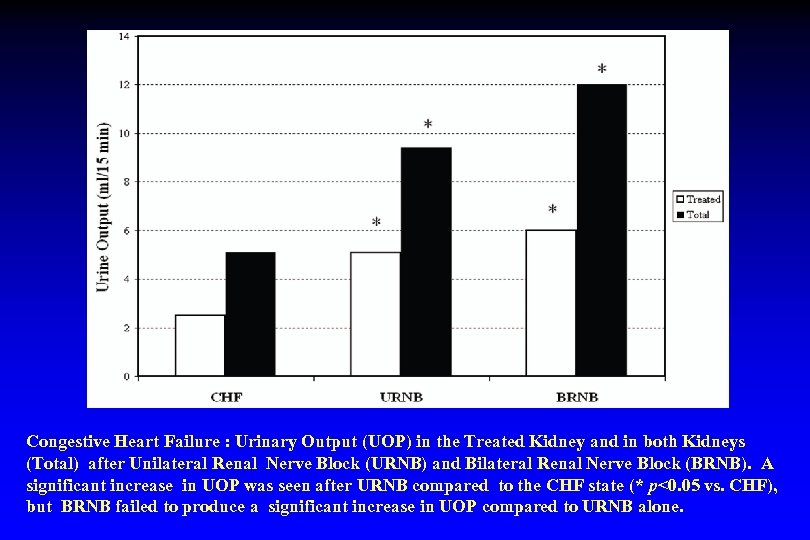
Congestive Heart Failure : Urinary Output (UOP) in the Treated Kidney and in both Kidneys (Total) after Unilateral Renal Nerve Block (URNB) and Bilateral Renal Nerve Block (BRNB). A significant increase in UOP was seen after URNB compared to the CHF state (* p<0. 05 vs. CHF), but BRNB failed to produce a significant increase in UOP compared to URNB alone.
Proposed Two Step Approach To Prove Clinical Utility in Humans Step 1 § Acute study of renal nerve block using CT guided technique to demonstrate either: § Diuresis and neurohormonal effects in patients with HF and fluid overload § Lowering of blood pressure and neurohormonal effects in patients with hypertension

Acute Human Feasibility Study in 5 -10 Patients § Class III-IV CHF patients refractory to diuretics or patients with BP > 140/90 on medication § Injection of drug to block RN using CT guided technique § Comparison of urine volume, urine and serum electrolytes, hemodynamic and neurohormonal effects from 6 hours pre- to 12 hours postinjection

Intervention Performed In 5 Patient § Successful test of RN blocking technique § Performed under CAT Scan Guidance § Needle placed at the hilum of the kidney § Single injection of Marcaine resulted as predicted in: § Nerve block for 12 hours § Reduction in blood pressure

Proposed Two Step Approach To Prove Clinical Utility in Humans Step 2 § Chronic (1 -3 month) study of renal nerve block using minimally invasively implanted port to demonstrate either: § Diuresis and neurohormonal effects in patients with HF and fluid overload § Lowering of blood pressure and neurohormonal effects in patients with hypertension

What the Therapy/Device Should be? § Device Based Therapy § Interventional/Trans-catheter § Similar to Current Medical Practice

Renal Nerve Anatomy Allows a Catheter-Based Approach • Standard interventional technique • 4 -6 two-minute treatments per artery • Proprietary RF Generator − Automated − Low-power − Built-in safety algorithms In the United States: Caution: Investigational Device. Limited by U. S. law to investigational use. 16
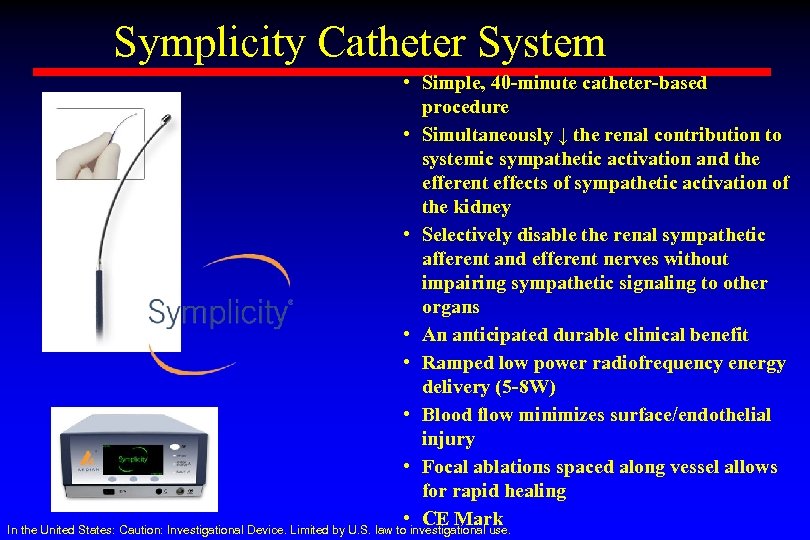
Symplicity Catheter System • Simple, 40 -minute catheter-based procedure • Simultaneously ↓ the renal contribution to systemic sympathetic activation and the efferent effects of sympathetic activation of the kidney • Selectively disable the renal sympathetic afferent and efferent nerves without impairing sympathetic signaling to other organs • An anticipated durable clinical benefit • Ramped low power radiofrequency energy delivery (5 -8 W) • Blood flow minimizes surface/endothelial injury • Focal ablations spaced along vessel allows for rapid healing • CE Mark In the United States: Caution: Investigational Device. Limited by U. S. law to investigational use.

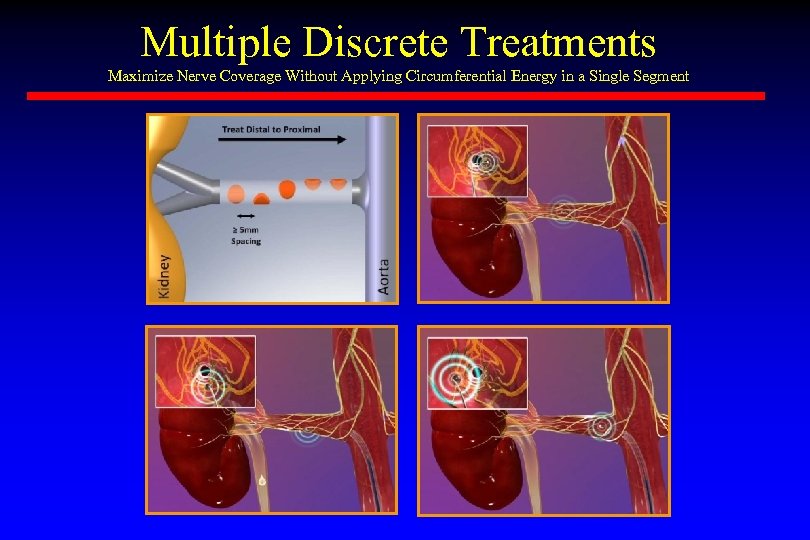
Multiple Discrete Treatments Maximize Nerve Coverage Without Applying Circumferential Energy in a Single Segment

Study Aims Krum et al. Lancet. 2009; 373(9671): 1275 -1281.

Inclusion/Exclusion Criteria Key Inclusion Criteria • Office SBP ≥ 160 mm. Hg despite 3+ anti-hypertensive medications (including diuretic), or confirmed intolerance to medications • e. GFR (MDRD formula) of ≥ 45 m. L/min/1. 73 m 2 Key Exclusion Criteria • Known secondary cause of hypertension • Type I diabetes mellitus • Currently taking clonidine, moxonidine, or rilmenidine • Renovascular abnormalities: significant renal artery stenosis, prior renal stenting or angioplasty, dual renal arteries Krum et al. Lancet. 2009; 373(9671): 1275 -1281.
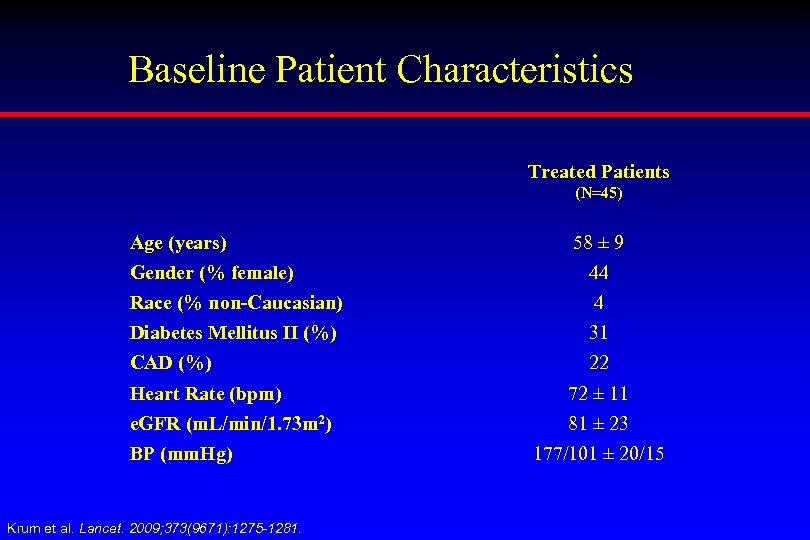
Baseline Patient Characteristics Treated Patients (N=45) Age (years) 58 ± 9 Gender (% female) 44 Race (% non-Caucasian) 4 Diabetes Mellitus II (%) 31 CAD (%) 22 Heart Rate (bpm) 72 ± 11 e. GFR (m. L/min/1. 73 m 2) 81 ± 23 BP (mm. Hg) Krum et al. Lancet. 2009; 373(9671): 1275 -1281. 177/101 ± 20/15

Results: Procedure Characteristics & Safety • Procedure time: median 38 (IQR 34 -48) minutes • Treatment delivered without complication in 43/45: – 1 renal artery dissection during catheter delivery (before RF energy application) – 1 femoral pseudoaneurysm, manually reduced without further sequelae
Vascular and Renal Safety • No chronic vascular complications – 18 patients with angiograms at 14 -30 days post-procedure – 38 of 38 patients with CTA/MRA 6 months post-procedure • No chronic renal (∆ e. GFR) complications – 3 Month: 0. 4 m. L/min/1. 73 m 2 (95% CI: -4. 2 to 4. 9; N=31; p=0. 87) – 12 Month: -3. 1 m. L/min/1. 73 m 2 (95% CI: -9. 0 to 2. 8; N=28; p=0. 31) • No orthostatic or electrolyte disturbances

Blood Pressure Response Change in Blood Pressure (mm. Hg) 10 0 Systolic Diastolic -18 -11 -23 -10 -23 -11 1 month (n=70) 3 months (n=64) 6 months (n=56) -25 -12 -27 -13 -10 -20 -30 -40 89% Responder Rate 9 months 12 months (n=40) (n=34) Repeated measures ANOVA: P<0. 001 for SBP & DBP P<0. 00001 vs. baseline for each SBP & DBP

The Potential Market § Developed Countries: Drug-Resistant Hypertension § China: Hypertension Patient, 160 millions § Optimized Drug Therapies: 6. 1%, 3. 23 millions
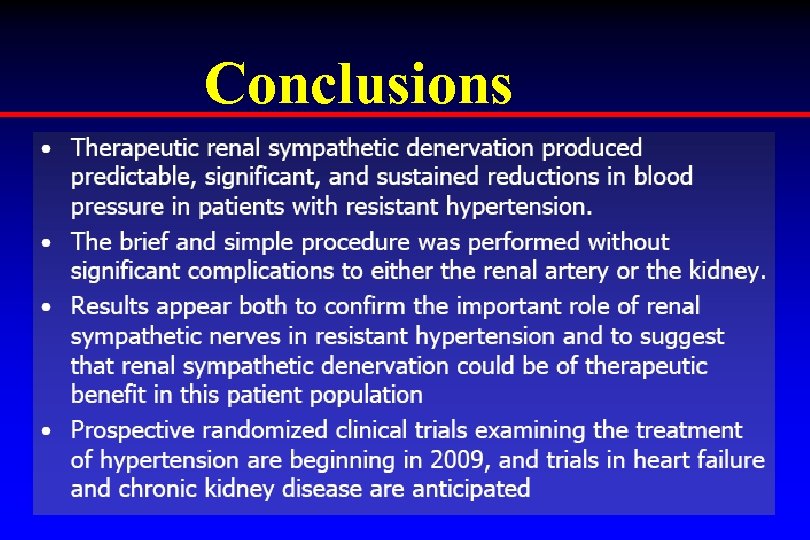
Conclusions
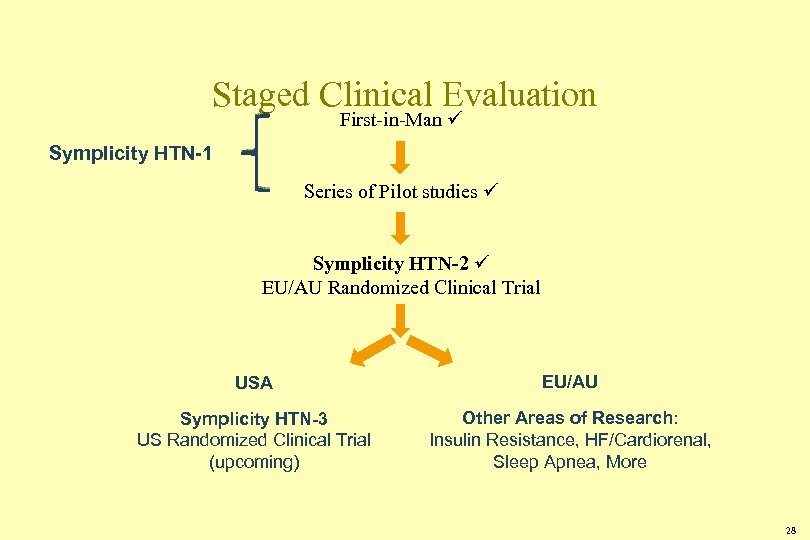
Staged Clinical Evaluation First-in-Man Symplicity HTN-1 Series of Pilot studies Symplicity HTN-2 EU/AU Randomized Clinical Trial USA EU/AU Symplicity HTN-3 US Randomized Clinical Trial (upcoming) Other Areas of Research: Insulin Resistance, HF/Cardiorenal, Sleep Apnea, More 28
d63a68ebb4120cd8a8a550b00deeba6c.ppt