9f4237af375146273322eadd0e45e497.ppt
- Количество слайдов: 24
 Renal Denervation in Patients with Uncontrolled Hypertension: Results of the SYMPLICITY HTN 3 Trial Deepak L. Bhatt, M. D. , M. P. H. , David E. Kandzari, M. D. , William W. O’Neill, M. D. , Ralph D'Agostino, Ph. D. , John M. Flack, M. D. , M. P. H. , Barry T. Katzen, M. D. , Martin B. Leon, M. D. , Minglei Liu, Ph. D. , Laura Mauri, M. D. , M. Sc. , Manuela Negoita, M. D. , Sidney A. Cohen, M. D. , Ph. D. , Suzanne Oparil, M. D. , Krishna Rocha-Singh, M. D. , Raymond R. Townsend, M. D. , George L. Bakris, M. D. , for the SYMPLICITY HTN-3 Investigators
Renal Denervation in Patients with Uncontrolled Hypertension: Results of the SYMPLICITY HTN 3 Trial Deepak L. Bhatt, M. D. , M. P. H. , David E. Kandzari, M. D. , William W. O’Neill, M. D. , Ralph D'Agostino, Ph. D. , John M. Flack, M. D. , M. P. H. , Barry T. Katzen, M. D. , Martin B. Leon, M. D. , Minglei Liu, Ph. D. , Laura Mauri, M. D. , M. Sc. , Manuela Negoita, M. D. , Sidney A. Cohen, M. D. , Ph. D. , Suzanne Oparil, M. D. , Krishna Rocha-Singh, M. D. , Raymond R. Townsend, M. D. , George L. Bakris, M. D. , for the SYMPLICITY HTN-3 Investigators
 Disclosures for Dr. Bhatt Advisory Board: Elsevier Practice Update Cardiology, Medscape Cardiology, Regado Biosciences; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care; Chair: American Heart Association Get With The Guidelines Steering Committee; Data Monitoring Committees: Duke Clinical Research Institute; Harvard Clinical Research Institute; Mayo Clinic; Population Health Research Institute (including Enlig. HTNment); Honoraria: American College of Cardiology (Editor, Clinical Trials, Cardiosource), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees), Harvard Clinical Research Institute (clinical trial steering committee), HMP Communications (Editor in Chief, Journal of Invasive Cardiology); Population Health Research Institute (clinical trial steering committee), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Web. MD (CME steering committees); Research Grants: Amarin, Astra. Zeneca, Bristol-Myers Squibb, Eisai, Ethicon, Medtronic (co-PI of SYMPLICITY HTN-3, steering committee member of SYMPLICITY HTN-4), Roche, Sanofi Aventis, The Medicines Company; Unfunded Research: Flow. Co, PLx Pharma, Takeda. This presentation discusses off-label and investigational uses of various devices. The SYMPLICITY HTN-3 trial was funded by Medtronic, Inc.
Disclosures for Dr. Bhatt Advisory Board: Elsevier Practice Update Cardiology, Medscape Cardiology, Regado Biosciences; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care; Chair: American Heart Association Get With The Guidelines Steering Committee; Data Monitoring Committees: Duke Clinical Research Institute; Harvard Clinical Research Institute; Mayo Clinic; Population Health Research Institute (including Enlig. HTNment); Honoraria: American College of Cardiology (Editor, Clinical Trials, Cardiosource), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees), Harvard Clinical Research Institute (clinical trial steering committee), HMP Communications (Editor in Chief, Journal of Invasive Cardiology); Population Health Research Institute (clinical trial steering committee), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Web. MD (CME steering committees); Research Grants: Amarin, Astra. Zeneca, Bristol-Myers Squibb, Eisai, Ethicon, Medtronic (co-PI of SYMPLICITY HTN-3, steering committee member of SYMPLICITY HTN-4), Roche, Sanofi Aventis, The Medicines Company; Unfunded Research: Flow. Co, PLx Pharma, Takeda. This presentation discusses off-label and investigational uses of various devices. The SYMPLICITY HTN-3 trial was funded by Medtronic, Inc.
 Background • Due to aging of the population and greater trends towards obesity, hypertension is growing in prevalence worldwide. • Approximately 10% of patients with diagnosed hypertension have “resistant” hypertension. • The sympathetic nervous system appears to play an important role in resistant hypertension. • Prior non-blinded studies have suggested that catheterbased renal artery denervation reduces blood pressure in resistant hypertension. Bhatt DL, Kandzari DE, O’Neill WW, et al. . . Bakris GL. N Engl J Med 2014
Background • Due to aging of the population and greater trends towards obesity, hypertension is growing in prevalence worldwide. • Approximately 10% of patients with diagnosed hypertension have “resistant” hypertension. • The sympathetic nervous system appears to play an important role in resistant hypertension. • Prior non-blinded studies have suggested that catheterbased renal artery denervation reduces blood pressure in resistant hypertension. Bhatt DL, Kandzari DE, O’Neill WW, et al. . . Bakris GL. N Engl J Med 2014
 Trial Objectives • SYMPLICITY HTN-3 is the first prospective, multi-center, randomized, blinded, sham controlled study to evaluate both the safety and efficacy of percutaneous renal artery denervation in patients with severe treatment-resistant hypertension. • The trial included 535 patients enrolled by 88 participating US centers. Bhatt DL, Kandzari DE, O’Neill WW, et al. . . Bakris GL. N Engl J Med 2014
Trial Objectives • SYMPLICITY HTN-3 is the first prospective, multi-center, randomized, blinded, sham controlled study to evaluate both the safety and efficacy of percutaneous renal artery denervation in patients with severe treatment-resistant hypertension. • The trial included 535 patients enrolled by 88 participating US centers. Bhatt DL, Kandzari DE, O’Neill WW, et al. . . Bakris GL. N Engl J Med 2014
 Participating Sites Providence Metro. Health Cleveland, OH St. Joseph Mercy Oakland Pontiac, MI U of Michigan St. Joseph Mercy Ann Arbor, MI Southfield, MI Morristown Memorial UH Case Cleveland, OH Morristown, NJ Harper Virginia Commonweath U Detroit, MI Richmond, VA Ypsilanti, MI Brigham & Women’s Women’ Lahey Clinic Burlington, MA Fletcher Allen South Burlington, VT U of Virginia U of Mass Charlottesville, VA Providence Spokane, WA Abbott Northwestern Worcester, MA VA Boston West Roxbury, MA U of Connecticut Farmington, CT Montefiore Bronx, NY SUNY U of Pitt Minneapolis, MN Boston, MA Brooklyn, NY Pittsburg, PA Rhode Island Providence, RI Stony Brook U Aurora St. Lukes Stony Brook, NY Milwaukee, WI St. Mary’s Mary’ St. Francis Rochester, MN Roslyn, NY Wheaton Franciscan Mount Sinai Racine, WI Stanford New York, NY Mercy Medical Palo Alto, CA West Des Moines, IA U of Colorado St. Joseph Orange, CA Scripps Northwestern Memorial Oakbrook Terrace, IL St. Louis, MO Chicago, IL U of Texas Sharp Kaiser Permanente Los Angeles, CA Plano, TX Neptune, NJ Duke Durham, NC Vanderbilt Nashville, TN Carolinas Medical Charlotte, NC Chatanooga, TN Medical USC Charleston, SC Mayo Clinic Dallas, TX Jacksonville, FL Scott & White Shands at UF Temple, TX Gainesville, FL Heart Hospital Austin, TX Emory Methodist Philadelphia, PA Lankenau Wynnewood, PA Deborah Heart & Lung Brown Mills, NJ Geisinger Danville, PA Lancaster General Lancaster, PA U of Maryland Atlanta, GA Houston, TX U of Penn Thomas Jefferson U Dallas VA Dallas, TX New York, NY Jersey Shore Wake Forest Winston-Salem, NC Memorial Baylor Heart Hospital Baylor Cedars-Sinai Lexington, KY Baptist Memorial Columbia University Columbus, OH Germantown, TN Dallas, TX San Diego, CA U of Kentucky Barnes Jewish New York, NY Riverside Methodist Cincinnati, OH Kansas City, MO Langone Columbus, OH Christ Hospital St. Luke’s Luke’ MIdwest Heart La Jolla, CA Los Angeles, CA Ohio State Springfield, IL Chicago, IL New York, NY Cleveland, OH St. John’s John’ Aurora, CO U of Chicago Medical Ctr Weill Cleveland Clinic Baltimore, MD Piedmont Washington Hospital Center Atlanta, GA Washington, DC Forrest General Hattiesburg, MS Memorial Hermann Houston, TX Princeton Baptist Medical Howard University Tampa General Birmingham, AL Ochsner New Orleans, LA U of Alabama Birmingham, AL Tampa, FL Washington, DC U of Miami, FL Baptist Hospital Miami, FL George Washington University Washington, DC UNC Memorial Chapel Hill, NC
Participating Sites Providence Metro. Health Cleveland, OH St. Joseph Mercy Oakland Pontiac, MI U of Michigan St. Joseph Mercy Ann Arbor, MI Southfield, MI Morristown Memorial UH Case Cleveland, OH Morristown, NJ Harper Virginia Commonweath U Detroit, MI Richmond, VA Ypsilanti, MI Brigham & Women’s Women’ Lahey Clinic Burlington, MA Fletcher Allen South Burlington, VT U of Virginia U of Mass Charlottesville, VA Providence Spokane, WA Abbott Northwestern Worcester, MA VA Boston West Roxbury, MA U of Connecticut Farmington, CT Montefiore Bronx, NY SUNY U of Pitt Minneapolis, MN Boston, MA Brooklyn, NY Pittsburg, PA Rhode Island Providence, RI Stony Brook U Aurora St. Lukes Stony Brook, NY Milwaukee, WI St. Mary’s Mary’ St. Francis Rochester, MN Roslyn, NY Wheaton Franciscan Mount Sinai Racine, WI Stanford New York, NY Mercy Medical Palo Alto, CA West Des Moines, IA U of Colorado St. Joseph Orange, CA Scripps Northwestern Memorial Oakbrook Terrace, IL St. Louis, MO Chicago, IL U of Texas Sharp Kaiser Permanente Los Angeles, CA Plano, TX Neptune, NJ Duke Durham, NC Vanderbilt Nashville, TN Carolinas Medical Charlotte, NC Chatanooga, TN Medical USC Charleston, SC Mayo Clinic Dallas, TX Jacksonville, FL Scott & White Shands at UF Temple, TX Gainesville, FL Heart Hospital Austin, TX Emory Methodist Philadelphia, PA Lankenau Wynnewood, PA Deborah Heart & Lung Brown Mills, NJ Geisinger Danville, PA Lancaster General Lancaster, PA U of Maryland Atlanta, GA Houston, TX U of Penn Thomas Jefferson U Dallas VA Dallas, TX New York, NY Jersey Shore Wake Forest Winston-Salem, NC Memorial Baylor Heart Hospital Baylor Cedars-Sinai Lexington, KY Baptist Memorial Columbia University Columbus, OH Germantown, TN Dallas, TX San Diego, CA U of Kentucky Barnes Jewish New York, NY Riverside Methodist Cincinnati, OH Kansas City, MO Langone Columbus, OH Christ Hospital St. Luke’s Luke’ MIdwest Heart La Jolla, CA Los Angeles, CA Ohio State Springfield, IL Chicago, IL New York, NY Cleveland, OH St. John’s John’ Aurora, CO U of Chicago Medical Ctr Weill Cleveland Clinic Baltimore, MD Piedmont Washington Hospital Center Atlanta, GA Washington, DC Forrest General Hattiesburg, MS Memorial Hermann Houston, TX Princeton Baptist Medical Howard University Tampa General Birmingham, AL Ochsner New Orleans, LA U of Alabama Birmingham, AL Tampa, FL Washington, DC U of Miami, FL Baptist Hospital Miami, FL George Washington University Washington, DC UNC Memorial Chapel Hill, NC
 SYMPLICITY HTN-3 Committees Steering Committee Co-PIs: Deepak L. Bhatt, MD, MPH, and George L. Bakris, MD Chair: William O’Neill, MD Members: Sidney A. Cohen, MD, Ph. D; Ralph D'Agostino, Ph. D; Murray Esler, MBBS, Ph. D; John Flack, MD, MPH; David Kandzari, MD; Barry Katzen, MD; Martin Leon, MD; Laura Mauri, MD, MSc; Manuela Negoita, MD; Suzanne Oparil, MD; Krishna Rocha-Singh, MD; Ray Townsend, MD; Paul Sobotka, MD Independent Data & Safety Monitoring Board Chair: Bernard J. Gersh, MB, Ch. B, D. Phil Members: John A. Ambrose, MD; Phyllis August, MD, MPH; Glenn M. Chertow, MD; Stuart Pocock, BSc, MSc, Ph. D Non-voting member: Joseph Massaro, Ph. D Independent Statistical Analysis Validation Harvard Clinical Research Institute Independent Clinical Events Committee Chair: Clive Rosendorff, MD, Ph. D, DSc Med Members: Ladan Golestaneh, MD; Steven Marx, MD; Michele H. Mokrzycki, MD; Joel Neugarten, MD Non-voting members: Sorin Brener, MD; Roxana Mehran, MD Renal Artery Duplex Core Laboratory Michael Jaff, DO, Vas. Core Angiographic Core Laboratory Jeffrey J. Popma, MD Beth Israel Deaconess Medical Center MRA Core Laboratory Milind Y. Desai, MD, C 5 Research Blood Core Laboratory Tania Cochran, Sr. Project Manager ACM Global Laboratory Sponsor Medtronic, Inc.
SYMPLICITY HTN-3 Committees Steering Committee Co-PIs: Deepak L. Bhatt, MD, MPH, and George L. Bakris, MD Chair: William O’Neill, MD Members: Sidney A. Cohen, MD, Ph. D; Ralph D'Agostino, Ph. D; Murray Esler, MBBS, Ph. D; John Flack, MD, MPH; David Kandzari, MD; Barry Katzen, MD; Martin Leon, MD; Laura Mauri, MD, MSc; Manuela Negoita, MD; Suzanne Oparil, MD; Krishna Rocha-Singh, MD; Ray Townsend, MD; Paul Sobotka, MD Independent Data & Safety Monitoring Board Chair: Bernard J. Gersh, MB, Ch. B, D. Phil Members: John A. Ambrose, MD; Phyllis August, MD, MPH; Glenn M. Chertow, MD; Stuart Pocock, BSc, MSc, Ph. D Non-voting member: Joseph Massaro, Ph. D Independent Statistical Analysis Validation Harvard Clinical Research Institute Independent Clinical Events Committee Chair: Clive Rosendorff, MD, Ph. D, DSc Med Members: Ladan Golestaneh, MD; Steven Marx, MD; Michele H. Mokrzycki, MD; Joel Neugarten, MD Non-voting members: Sorin Brener, MD; Roxana Mehran, MD Renal Artery Duplex Core Laboratory Michael Jaff, DO, Vas. Core Angiographic Core Laboratory Jeffrey J. Popma, MD Beth Israel Deaconess Medical Center MRA Core Laboratory Milind Y. Desai, MD, C 5 Research Blood Core Laboratory Tania Cochran, Sr. Project Manager ACM Global Laboratory Sponsor Medtronic, Inc.
 Key Inclusion Criteria • Age ≥ 18 and ≤ 80 years at time of randomization • Stable medication regimen including full tolerated doses of 3 or more antihypertensive medications of different classes, including a diuretic (with no changes for a minimum of 2 weeks prior to screening) and no expected changes for at least 6 months • Office SBP ≥ 160 mm Hg based on an average of 3 blood pressure readings measured at both an initial and a confirmatory screening visit • Written informed consent Bhatt DL, Kandzari DE, O’Neill WW, et al. . . Bakris GL. N Engl J Med 2014
Key Inclusion Criteria • Age ≥ 18 and ≤ 80 years at time of randomization • Stable medication regimen including full tolerated doses of 3 or more antihypertensive medications of different classes, including a diuretic (with no changes for a minimum of 2 weeks prior to screening) and no expected changes for at least 6 months • Office SBP ≥ 160 mm Hg based on an average of 3 blood pressure readings measured at both an initial and a confirmatory screening visit • Written informed consent Bhatt DL, Kandzari DE, O’Neill WW, et al. . . Bakris GL. N Engl J Med 2014
 Key Exclusion Criteria • ABPM 24 hour average SBP <135 mm Hg • e. GFR of <45 m. L/min/1. 73 m 2 • Main renal arteries <4 mm diameter or <20 mm treatable length • Multiple renal arteries where the main renal artery is estimated to supply <75% of the kidney • Renal artery stenosis >50% or aneurysm in either renal artery • History of prior renal artery intervention Bhatt DL, Kandzari DE, O’Neill WW, et al. . . Bakris GL. N Engl J Med 2014
Key Exclusion Criteria • ABPM 24 hour average SBP <135 mm Hg • e. GFR of <45 m. L/min/1. 73 m 2 • Main renal arteries <4 mm diameter or <20 mm treatable length • Multiple renal arteries where the main renal artery is estimated to supply <75% of the kidney • Renal artery stenosis >50% or aneurysm in either renal artery • History of prior renal artery intervention Bhatt DL, Kandzari DE, O’Neill WW, et al. . . Bakris GL. N Engl J Med 2014
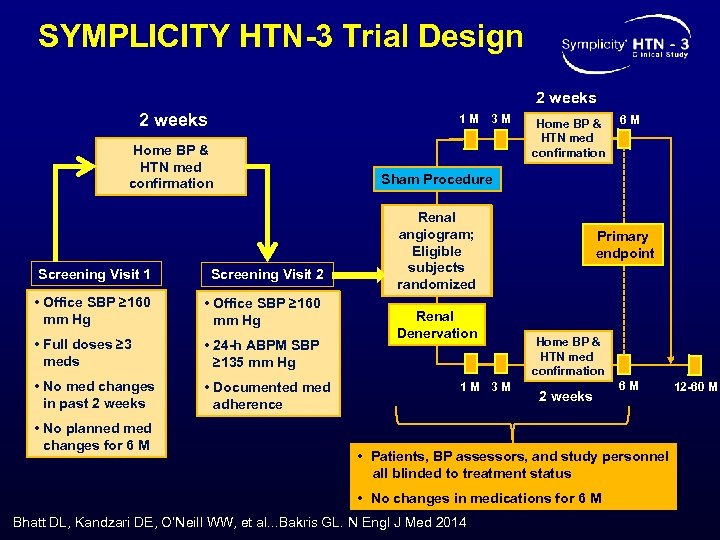 SYMPLICITY HTN-3 Trial Design 2 weeks 1 M Home BP & HTN med confirmation Screening Visit 1 Screening Visit 2 • Office SBP ≥ 160 mm Hg • Full doses ≥ 3 meds • 24 -h ABPM SBP ≥ 135 mm Hg • No med changes in past 2 weeks • Documented med adherence • No planned med changes for 6 M 3 M Home BP & HTN med confirmation 6 M Sham Procedure Renal angiogram; Eligible subjects randomized Renal Denervation 1 M 3 M Primary endpoint Home BP & HTN med confirmation 2 weeks 6 M • Patients, BP assessors, and study personnel all blinded to treatment status • No changes in medications for 6 M Bhatt DL, Kandzari DE, O’Neill WW, et al. . . Bakris GL. N Engl J Med 2014 12 -60 M
SYMPLICITY HTN-3 Trial Design 2 weeks 1 M Home BP & HTN med confirmation Screening Visit 1 Screening Visit 2 • Office SBP ≥ 160 mm Hg • Full doses ≥ 3 meds • 24 -h ABPM SBP ≥ 135 mm Hg • No med changes in past 2 weeks • Documented med adherence • No planned med changes for 6 M 3 M Home BP & HTN med confirmation 6 M Sham Procedure Renal angiogram; Eligible subjects randomized Renal Denervation 1 M 3 M Primary endpoint Home BP & HTN med confirmation 2 weeks 6 M • Patients, BP assessors, and study personnel all blinded to treatment status • No changes in medications for 6 M Bhatt DL, Kandzari DE, O’Neill WW, et al. . . Bakris GL. N Engl J Med 2014 12 -60 M
 Primary Safety Endpoint • The rate of Major Adverse Events (MAE) in the treatment group compared with an Objective Performance Criterion (OPC) • OPC = 9. 8% (derived from historical data) • MAE was defined as all-cause mortality, end-stage renal disease, embolic event resulting in end-organ damage, renal artery or other vascular complication, hypertensive crisis through 30 days, or new renal artery stenosis within six months Bhatt DL, Kandzari DE, O’Neill WW, et al. . . Bakris GL. N Engl J Med 2014
Primary Safety Endpoint • The rate of Major Adverse Events (MAE) in the treatment group compared with an Objective Performance Criterion (OPC) • OPC = 9. 8% (derived from historical data) • MAE was defined as all-cause mortality, end-stage renal disease, embolic event resulting in end-organ damage, renal artery or other vascular complication, hypertensive crisis through 30 days, or new renal artery stenosis within six months Bhatt DL, Kandzari DE, O’Neill WW, et al. . . Bakris GL. N Engl J Med 2014
 Efficacy Endpoints Primary Effectiveness Endpoint: • Comparison of office SBP change from baseline to 6 months in RDN arm compared with change from baseline to 6 months in control arm Endpoint = (SBPRDN 6 month – SBPRDN Baseline) – (SBPCTL 6 month – SBPCTL Baseline) • Superiority margin of 5 mm Hg Powered Secondary Effectiveness Endpoint: • Comparison of mean 24 -hour ambulatory (ABPM) SBP change from baseline to 6 months in RDN arm compared with change from baseline to 6 months in control arm • Superiority margin of 2 mm Hg Bhatt DL, Kandzari DE, O’Neill WW, et al. . . Bakris GL. N Engl J Med 2014
Efficacy Endpoints Primary Effectiveness Endpoint: • Comparison of office SBP change from baseline to 6 months in RDN arm compared with change from baseline to 6 months in control arm Endpoint = (SBPRDN 6 month – SBPRDN Baseline) – (SBPCTL 6 month – SBPCTL Baseline) • Superiority margin of 5 mm Hg Powered Secondary Effectiveness Endpoint: • Comparison of mean 24 -hour ambulatory (ABPM) SBP change from baseline to 6 months in RDN arm compared with change from baseline to 6 months in control arm • Superiority margin of 2 mm Hg Bhatt DL, Kandzari DE, O’Neill WW, et al. . . Bakris GL. N Engl J Med 2014
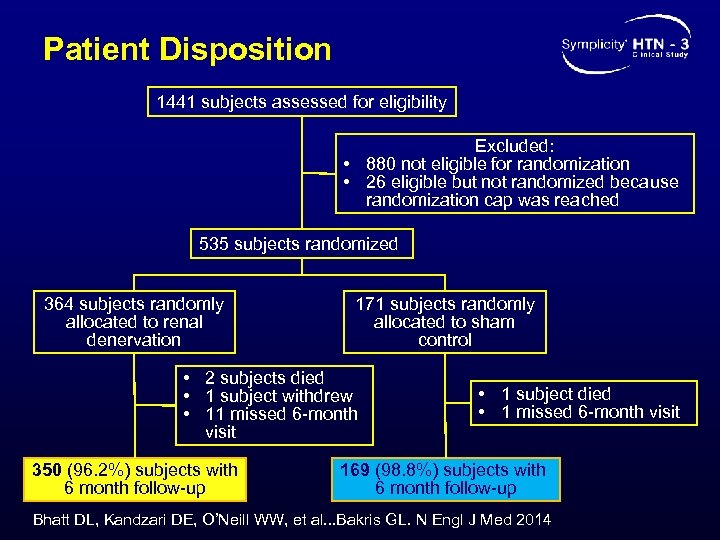 Patient Disposition 1441 subjects assessed for eligibility Excluded: • 880 not eligible for randomization • 26 eligible but not randomized because randomization cap was reached 535 subjects randomized 364 subjects randomly allocated to renal denervation 171 subjects randomly allocated to sham control • 2 subjects died • 1 subject withdrew • 11 missed 6 -month visit 350 (96. 2%) subjects with 6 month follow-up • 1 subject died • 1 missed 6 -month visit 169 (98. 8%) subjects with 6 month follow-up Bhatt DL, Kandzari DE, O’Neill WW, et al. . . Bakris GL. N Engl J Med 2014
Patient Disposition 1441 subjects assessed for eligibility Excluded: • 880 not eligible for randomization • 26 eligible but not randomized because randomization cap was reached 535 subjects randomized 364 subjects randomly allocated to renal denervation 171 subjects randomly allocated to sham control • 2 subjects died • 1 subject withdrew • 11 missed 6 -month visit 350 (96. 2%) subjects with 6 month follow-up • 1 subject died • 1 missed 6 -month visit 169 (98. 8%) subjects with 6 month follow-up Bhatt DL, Kandzari DE, O’Neill WW, et al. . . Bakris GL. N Engl J Med 2014
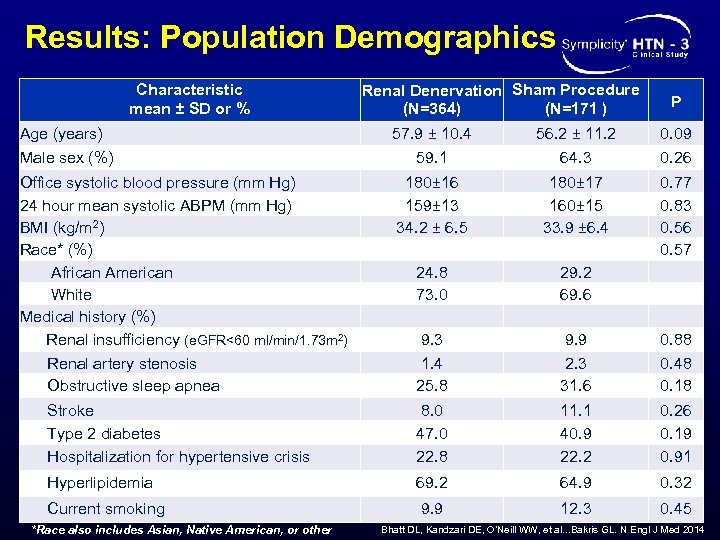 Results: Population Demographics Characteristic mean ± SD or % Renal Denervation Sham Procedure (N=364) (N=171 ) P Age (years) Male sex (%) 57. 9 ± 10. 4 59. 1 56. 2 ± 11. 2 64. 3 0. 09 0. 26 Office systolic blood pressure (mm Hg) 24 hour mean systolic ABPM (mm Hg) BMI (kg/m 2) Race* (%) African American White Medical history (%) Renal insufficiency (e. GFR<60 ml/min/1. 73 m 2) Renal artery stenosis Obstructive sleep apnea 180± 16 159± 13 34. 2 ± 6. 5 24. 8 73. 0 9. 3 1. 4 25. 8 180± 17 160± 15 33. 9 ± 6. 4 29. 2 69. 6 9. 9 2. 3 31. 6 0. 77 0. 83 0. 56 0. 57 0. 88 0. 48 0. 18 Stroke Type 2 diabetes Hospitalization for hypertensive crisis 8. 0 47. 0 22. 8 11. 1 40. 9 22. 2 0. 26 0. 19 0. 91 Hyperlipidemia 69. 2 64. 9 0. 32 Current smoking 9. 9 12. 3 0. 45 *Race also includes Asian, Native American, or other Bhatt DL, Kandzari DE, O’Neill WW, et al. . . Bakris GL. N Engl J Med 2014
Results: Population Demographics Characteristic mean ± SD or % Renal Denervation Sham Procedure (N=364) (N=171 ) P Age (years) Male sex (%) 57. 9 ± 10. 4 59. 1 56. 2 ± 11. 2 64. 3 0. 09 0. 26 Office systolic blood pressure (mm Hg) 24 hour mean systolic ABPM (mm Hg) BMI (kg/m 2) Race* (%) African American White Medical history (%) Renal insufficiency (e. GFR<60 ml/min/1. 73 m 2) Renal artery stenosis Obstructive sleep apnea 180± 16 159± 13 34. 2 ± 6. 5 24. 8 73. 0 9. 3 1. 4 25. 8 180± 17 160± 15 33. 9 ± 6. 4 29. 2 69. 6 9. 9 2. 3 31. 6 0. 77 0. 83 0. 56 0. 57 0. 88 0. 48 0. 18 Stroke Type 2 diabetes Hospitalization for hypertensive crisis 8. 0 47. 0 22. 8 11. 1 40. 9 22. 2 0. 26 0. 19 0. 91 Hyperlipidemia 69. 2 64. 9 0. 32 Current smoking 9. 9 12. 3 0. 45 *Race also includes Asian, Native American, or other Bhatt DL, Kandzari DE, O’Neill WW, et al. . . Bakris GL. N Engl J Med 2014
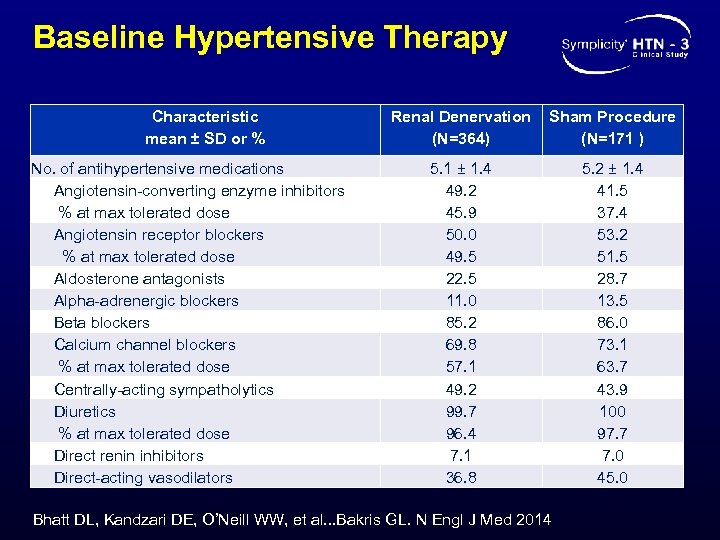 Baseline Hypertensive Therapy Characteristic mean ± SD or % No. of antihypertensive medications Angiotensin-converting enzyme inhibitors % at max tolerated dose Angiotensin receptor blockers % at max tolerated dose Aldosterone antagonists Alpha-adrenergic blockers Beta blockers Calcium channel blockers % at max tolerated dose Centrally-acting sympatholytics Diuretics % at max tolerated dose Direct renin inhibitors Direct-acting vasodilators Renal Denervation (N=364) Sham Procedure (N=171 ) 5. 1 ± 1. 4 49. 2 45. 9 50. 0 49. 5 22. 5 11. 0 85. 2 69. 8 57. 1 49. 2 99. 7 96. 4 7. 1 36. 8 5. 2 ± 1. 4 41. 5 37. 4 53. 2 51. 5 28. 7 13. 5 86. 0 73. 1 63. 7 43. 9 100 97. 7 7. 0 45. 0 Bhatt DL, Kandzari DE, O’Neill WW, et al. . . Bakris GL. N Engl J Med 2014
Baseline Hypertensive Therapy Characteristic mean ± SD or % No. of antihypertensive medications Angiotensin-converting enzyme inhibitors % at max tolerated dose Angiotensin receptor blockers % at max tolerated dose Aldosterone antagonists Alpha-adrenergic blockers Beta blockers Calcium channel blockers % at max tolerated dose Centrally-acting sympatholytics Diuretics % at max tolerated dose Direct renin inhibitors Direct-acting vasodilators Renal Denervation (N=364) Sham Procedure (N=171 ) 5. 1 ± 1. 4 49. 2 45. 9 50. 0 49. 5 22. 5 11. 0 85. 2 69. 8 57. 1 49. 2 99. 7 96. 4 7. 1 36. 8 5. 2 ± 1. 4 41. 5 37. 4 53. 2 51. 5 28. 7 13. 5 86. 0 73. 1 63. 7 43. 9 100 97. 7 7. 0 45. 0 Bhatt DL, Kandzari DE, O’Neill WW, et al. . . Bakris GL. N Engl J Med 2014
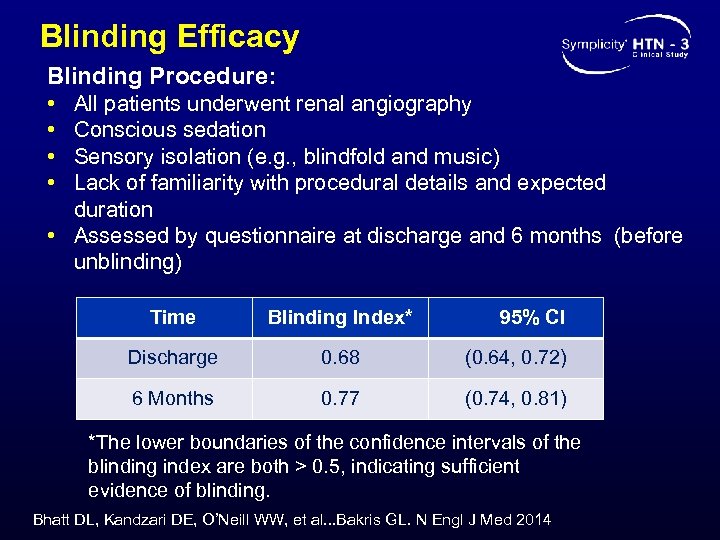 Blinding Efficacy Blinding Procedure: • • All patients underwent renal angiography Conscious sedation Sensory isolation (e. g. , blindfold and music) Lack of familiarity with procedural details and expected duration • Assessed by questionnaire at discharge and 6 months (before unblinding) Time Blinding Index* 95% CI Discharge 0. 68 (0. 64, 0. 72) 6 Months 0. 77 (0. 74, 0. 81) *The lower boundaries of the confidence intervals of the blinding index are both > 0. 5, indicating sufficient evidence of blinding. Bhatt DL, Kandzari DE, O’Neill WW, et al. . . Bakris GL. N Engl J Med 2014
Blinding Efficacy Blinding Procedure: • • All patients underwent renal angiography Conscious sedation Sensory isolation (e. g. , blindfold and music) Lack of familiarity with procedural details and expected duration • Assessed by questionnaire at discharge and 6 months (before unblinding) Time Blinding Index* 95% CI Discharge 0. 68 (0. 64, 0. 72) 6 Months 0. 77 (0. 74, 0. 81) *The lower boundaries of the confidence intervals of the blinding index are both > 0. 5, indicating sufficient evidence of blinding. Bhatt DL, Kandzari DE, O’Neill WW, et al. . . Bakris GL. N Engl J Med 2014
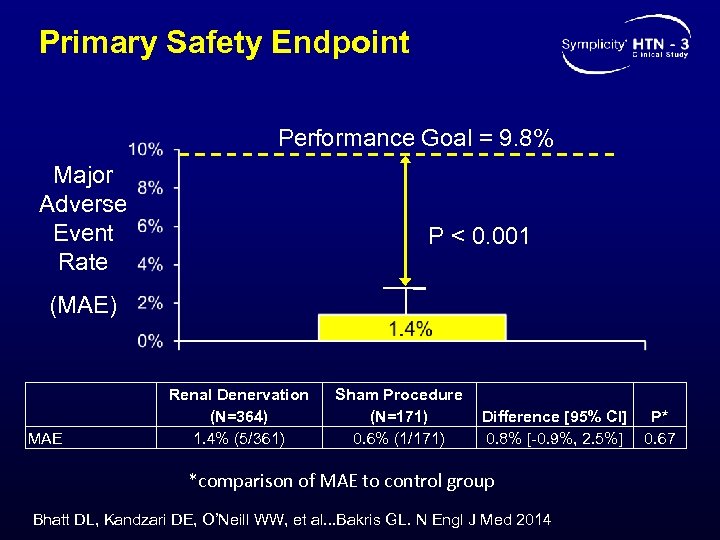 Primary Safety Endpoint Performance Goal = 9. 8% Major Adverse Event Rate P < 0. 001 (MAE) MAE Renal Denervation (N=364) 1. 4% (5/361) Sham Procedure (N=171) 0. 6% (1/171) Difference [95% CI] 0. 8% [-0. 9%, 2. 5%] *comparison of MAE to control group Bhatt DL, Kandzari DE, O’Neill WW, et al. . . Bakris GL. N Engl J Med 2014 P* 0. 67
Primary Safety Endpoint Performance Goal = 9. 8% Major Adverse Event Rate P < 0. 001 (MAE) MAE Renal Denervation (N=364) 1. 4% (5/361) Sham Procedure (N=171) 0. 6% (1/171) Difference [95% CI] 0. 8% [-0. 9%, 2. 5%] *comparison of MAE to control group Bhatt DL, Kandzari DE, O’Neill WW, et al. . . Bakris GL. N Engl J Med 2014 P* 0. 67
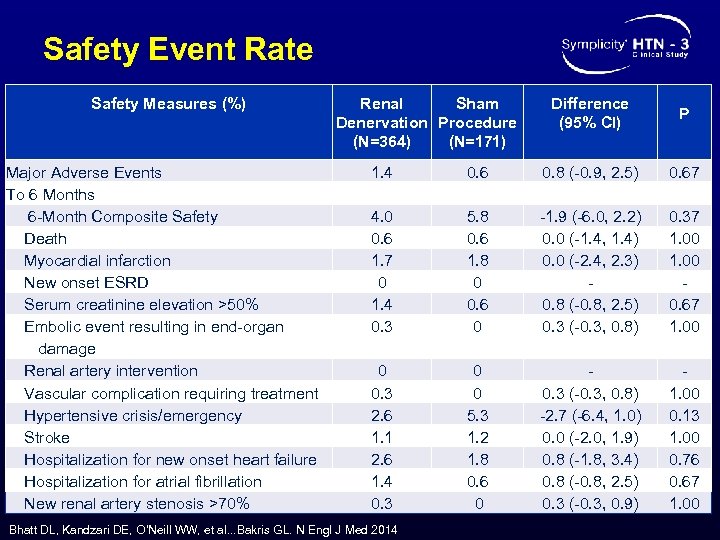 Safety Event Rate Safety Measures (%) Major Adverse Events To 6 Months 6 -Month Composite Safety Death Myocardial infarction New onset ESRD Serum creatinine elevation >50% Embolic event resulting in end-organ damage Renal artery intervention Vascular complication requiring treatment Hypertensive crisis/emergency Stroke Hospitalization for new onset heart failure Hospitalization for atrial fibrillation New renal artery stenosis >70% Renal Sham Denervation Procedure (N=364) (N=171) Difference (95% CI) P 1. 4 4. 0 0. 6 1. 7 0 1. 4 0. 3 0. 6 5. 8 0. 6 1. 8 0 0. 6 0 0. 8 (-0. 9, 2. 5) -1. 9 (-6. 0, 2. 2) 0. 0 (-1. 4, 1. 4) 0. 0 (-2. 4, 2. 3) 0. 8 (-0. 8, 2. 5) 0. 3 (-0. 3, 0. 8) 0. 67 0 0. 3 2. 6 1. 1 2. 6 1. 4 0. 3 0 0 5. 3 1. 2 1. 8 0. 6 0 0. 3 (-0. 3, 0. 8) -2. 7 (-6. 4, 1. 0) 0. 0 (-2. 0, 1. 9) 0. 8 (-1. 8, 3. 4) 0. 8 (-0. 8, 2. 5) 0. 3 (-0. 3, 0. 9) 1. 00 0. 13 1. 00 0. 76 0. 67 1. 00 Bhatt DL, Kandzari DE, O’Neill WW, et al. . . Bakris GL. N Engl J Med 2014 0. 37 1. 00 0. 67 1. 00
Safety Event Rate Safety Measures (%) Major Adverse Events To 6 Months 6 -Month Composite Safety Death Myocardial infarction New onset ESRD Serum creatinine elevation >50% Embolic event resulting in end-organ damage Renal artery intervention Vascular complication requiring treatment Hypertensive crisis/emergency Stroke Hospitalization for new onset heart failure Hospitalization for atrial fibrillation New renal artery stenosis >70% Renal Sham Denervation Procedure (N=364) (N=171) Difference (95% CI) P 1. 4 4. 0 0. 6 1. 7 0 1. 4 0. 3 0. 6 5. 8 0. 6 1. 8 0 0. 6 0 0. 8 (-0. 9, 2. 5) -1. 9 (-6. 0, 2. 2) 0. 0 (-1. 4, 1. 4) 0. 0 (-2. 4, 2. 3) 0. 8 (-0. 8, 2. 5) 0. 3 (-0. 3, 0. 8) 0. 67 0 0. 3 2. 6 1. 1 2. 6 1. 4 0. 3 0 0 5. 3 1. 2 1. 8 0. 6 0 0. 3 (-0. 3, 0. 8) -2. 7 (-6. 4, 1. 0) 0. 0 (-2. 0, 1. 9) 0. 8 (-1. 8, 3. 4) 0. 8 (-0. 8, 2. 5) 0. 3 (-0. 3, 0. 9) 1. 00 0. 13 1. 00 0. 76 0. 67 1. 00 Bhatt DL, Kandzari DE, O’Neill WW, et al. . . Bakris GL. N Engl J Med 2014 0. 37 1. 00 0. 67 1. 00
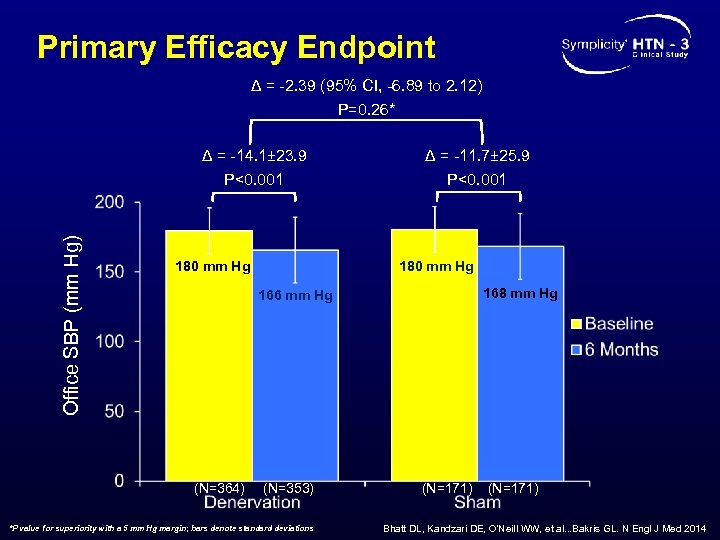 Primary Efficacy Endpoint Δ = -2. 39 (95% CI, -6. 89 to 2. 12) P=0. 26* Office SBP (mm Hg) Δ = -14. 1± 23. 9 P<0. 001 180 mm Hg Δ = -11. 7± 25. 9 P<0. 001 180 mm Hg 168 mm Hg 166 mm Hg (N=364) (N=353) *P value for superiority with a 5 mm Hg margin; bars denote standard deviations (N=171) Bhatt DL, Kandzari DE, O’Neill WW, et al. . . Bakris GL. N Engl J Med 2014
Primary Efficacy Endpoint Δ = -2. 39 (95% CI, -6. 89 to 2. 12) P=0. 26* Office SBP (mm Hg) Δ = -14. 1± 23. 9 P<0. 001 180 mm Hg Δ = -11. 7± 25. 9 P<0. 001 180 mm Hg 168 mm Hg 166 mm Hg (N=364) (N=353) *P value for superiority with a 5 mm Hg margin; bars denote standard deviations (N=171) Bhatt DL, Kandzari DE, O’Neill WW, et al. . . Bakris GL. N Engl J Med 2014
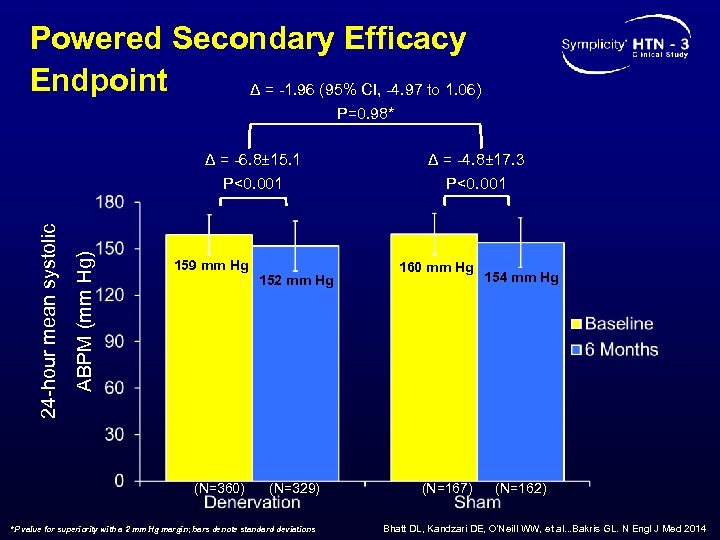 Powered Secondary Efficacy Endpoint Δ = -1. 96 (95% CI, -4. 97 to 1. 06) P=0. 98* ABPM (mm Hg) 24 -hour mean systolic Δ = -6. 8± 15. 1 P<0. 001 159 mm Hg (N=360) 152 mm Hg (N=329) *P value for superiority with a 2 mm Hg margin; bars denote standard deviations Δ = -4. 8± 17. 3 P<0. 001 160 mm Hg (N=167) 154 mm Hg (N=162) Bhatt DL, Kandzari DE, O’Neill WW, et al. . . Bakris GL. N Engl J Med 2014
Powered Secondary Efficacy Endpoint Δ = -1. 96 (95% CI, -4. 97 to 1. 06) P=0. 98* ABPM (mm Hg) 24 -hour mean systolic Δ = -6. 8± 15. 1 P<0. 001 159 mm Hg (N=360) 152 mm Hg (N=329) *P value for superiority with a 2 mm Hg margin; bars denote standard deviations Δ = -4. 8± 17. 3 P<0. 001 160 mm Hg (N=167) 154 mm Hg (N=162) Bhatt DL, Kandzari DE, O’Neill WW, et al. . . Bakris GL. N Engl J Med 2014
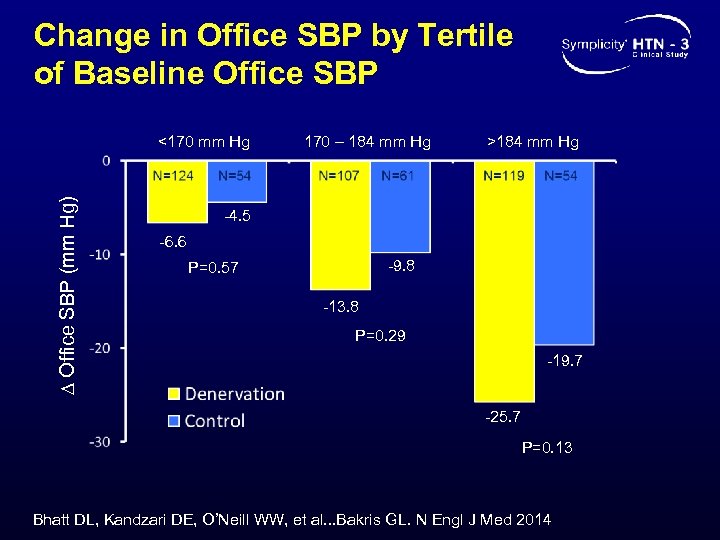 Change in Office SBP by Tertile of Baseline Office SBP ∆ Office SBP (mm Hg) <170 mm Hg 170 – 184 mm Hg >184 mm Hg -4. 5 -6. 6 -9. 8 P=0. 57 -13. 8 P=0. 29 -19. 7 -25. 7 P=0. 13 Bhatt DL, Kandzari DE, O’Neill WW, et al. . . Bakris GL. N Engl J Med 2014
Change in Office SBP by Tertile of Baseline Office SBP ∆ Office SBP (mm Hg) <170 mm Hg 170 – 184 mm Hg >184 mm Hg -4. 5 -6. 6 -9. 8 P=0. 57 -13. 8 P=0. 29 -19. 7 -25. 7 P=0. 13 Bhatt DL, Kandzari DE, O’Neill WW, et al. . . Bakris GL. N Engl J Med 2014
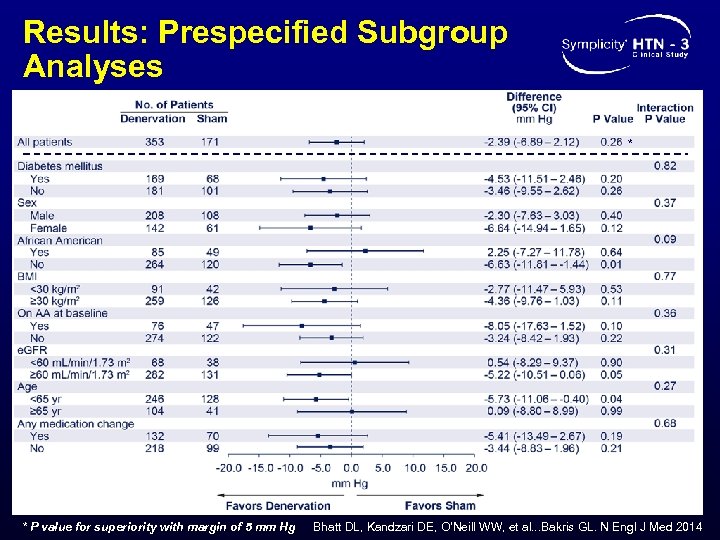 Results: Prespecified Subgroup Analyses * * P value for superiority with margin of 5 mm Hg Bhatt DL, Kandzari DE, O’Neill WW, et al. . . Bakris GL. N Engl J Med 2014
Results: Prespecified Subgroup Analyses * * P value for superiority with margin of 5 mm Hg Bhatt DL, Kandzari DE, O’Neill WW, et al. . . Bakris GL. N Engl J Med 2014
 Potential Limitations • Drug adherence not measured by blood levels, but adherence was measured by patient diaries at baseline and 6 months. • Medication changes did occur, but results unchanged even when these patients were censored. • Duration of primary endpoint may have been too short, but prior studies had found benefit by 6 months. • Operator learning curve is always a possibility, but we found no relationship with procedural volume in the trial. • Biological confirmation of denervation did not occur, as there is no accepted measure, but appropriate energy delivery was confirmed. Bhatt DL, Kandzari DE, O’Neill WW, et al. . . Bakris GL. N Engl J Med 2014
Potential Limitations • Drug adherence not measured by blood levels, but adherence was measured by patient diaries at baseline and 6 months. • Medication changes did occur, but results unchanged even when these patients were censored. • Duration of primary endpoint may have been too short, but prior studies had found benefit by 6 months. • Operator learning curve is always a possibility, but we found no relationship with procedural volume in the trial. • Biological confirmation of denervation did not occur, as there is no accepted measure, but appropriate energy delivery was confirmed. Bhatt DL, Kandzari DE, O’Neill WW, et al. . . Bakris GL. N Engl J Med 2014
 Conclusions • In a prospective, multicenter, randomized, blinded, sham controlled trial of patients with uncontrolled resistant hypertension, percutaneous renal denervation was safe but not associated with significant additional reductions in office or ambulatory blood pressure. • These results underscore the importance of blinding and sham controls in evaluations of new devices. • Further study in rigorously designed clinical trials will be necessary to confirm previously reported benefits of renal denervation in patients with resistant hypertension or to validate alternate methods of renal denervation. Bhatt DL, Kandzari DE, O’Neill WW, et al. . . Bakris GL. N Engl J Med 2014
Conclusions • In a prospective, multicenter, randomized, blinded, sham controlled trial of patients with uncontrolled resistant hypertension, percutaneous renal denervation was safe but not associated with significant additional reductions in office or ambulatory blood pressure. • These results underscore the importance of blinding and sham controls in evaluations of new devices. • Further study in rigorously designed clinical trials will be necessary to confirm previously reported benefits of renal denervation in patients with resistant hypertension or to validate alternate methods of renal denervation. Bhatt DL, Kandzari DE, O’Neill WW, et al. . . Bakris GL. N Engl J Med 2014
 For Full Details, Please Go to WWW. NEJM. ORG Bhatt DL, Kandzari DE, O’Neill WW, et al. . . Bakris GL. N Engl J Med 2014
For Full Details, Please Go to WWW. NEJM. ORG Bhatt DL, Kandzari DE, O’Neill WW, et al. . . Bakris GL. N Engl J Med 2014


