11dffec128e1d9ad2b433f17192919ac.ppt
- Количество слайдов: 87

Reinhard Baildon, M. D. Executive Director Clinical Development Pfizer Global Research & Development 1

Voriconazole i Introduction i In vitro and in vivo Data i Clinical Pharmacology i Efficacy i Safety i Conclusion 2

3

Voriconazole Development Program i First in human: 1991 i IND for oral/IV: 08/95, 04/96 i NDA submitted 11/00 i Extensive, frequent discussion with Division i Collaboration with NIAID Mycoses Study Group (MSG) and European Organisation for Research and Treatment of Cancer (EORTC) i External Data Review Committees (DRCs) for rigorous, blinded efficacy assessments 4
Sponsor Section Craig Brater, MD John Camm, MD George Drusano, MD Frederick Fraunfelder, MD Willis Maddrey, MD Thomas Patterson, MD Guy Paulus, MD, Ph. D John Rex, MD Robert Rubin, MD Jeremy Ruskin, MD Eugene Schiff, MD Thomas Walsh, MD Paul Watkins, MD Andrew Whelton, MD University of Indiana St. George’s Hospital Albany Medical College Casey Eye Institute University of Texas, Dallas University of Texas, San Antonio Consultant University of Texas, Houston Harvard University Massachusetts General Hospital University of Miami National Cancer Institute University of North Carolina Consultant 5

Voriconazole Superior outcome and survival benefit in primary therapy of acute invasive aspergillosis i Efficacy in patients with Scedosporium and Fusarium infections i Efficacy in Candida infections i Appropriate option for empirical therapy i Better tolerated than amphotericin B formulations i Acceptable overall safety profile i Manageable drug-drug interactions i 6

Voriconazole Clinical Program Invasive Aspergillosis i Global Comparative Aspergillosis Study (307/602) i Non-Comparative Aspergillosis Study (304) i Historical Control Study (1003) Emerging Pathogens i Scedosporium Infections i Fusarium Infections Candida Infections i Esophageal Candidiasis Study (305) i Pooled Efficacy Data Empirical Therapy Study (603/MSG 42) 7
Voriconazole N N HO Me F N N N F F Voriconazole N N HO N N F F Fluconazole 8

Voriconazole i Introduction i In vitro and in vivo Data i Clinical Pharmacology i Efficacy i Safety i Conclusion 9
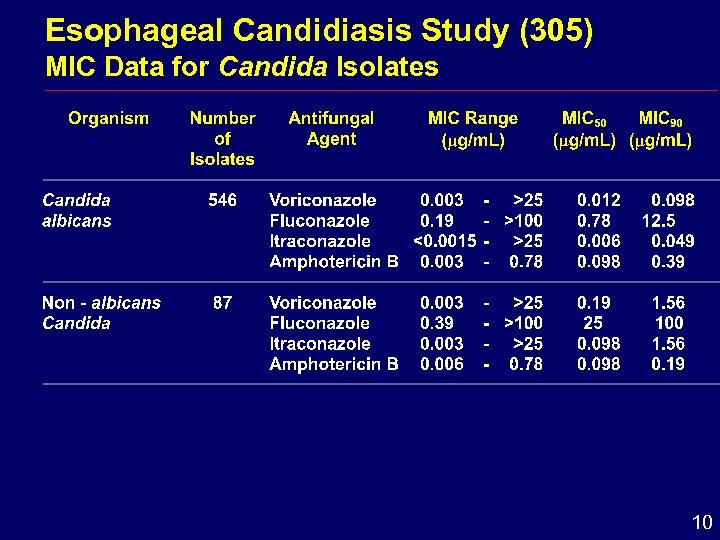
Esophageal Candidiasis Study (305) MIC Data for Candida Isolates 10
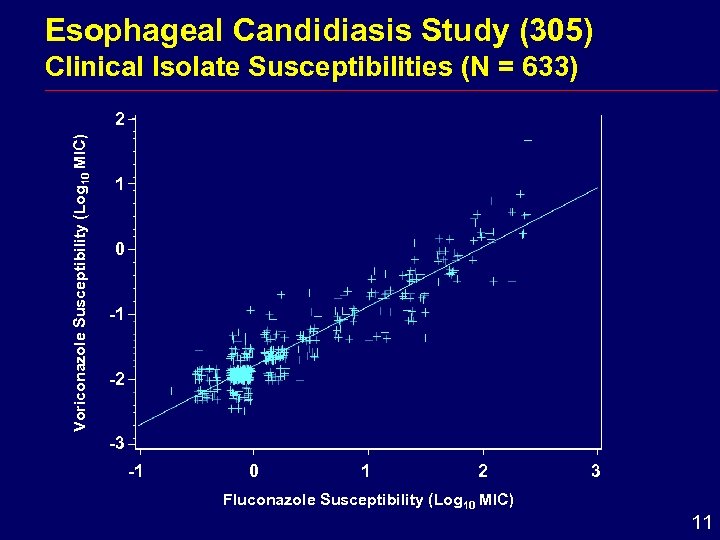
Esophageal Candidiasis Study (305) Clinical Isolate Susceptibilities (N = 633) Voriconazole Susceptibility (Log 10 MIC) 2 1 0 -1 -2 -3 -1 0 1 2 Fluconazole Susceptibility (Log 10 MIC) 3 11
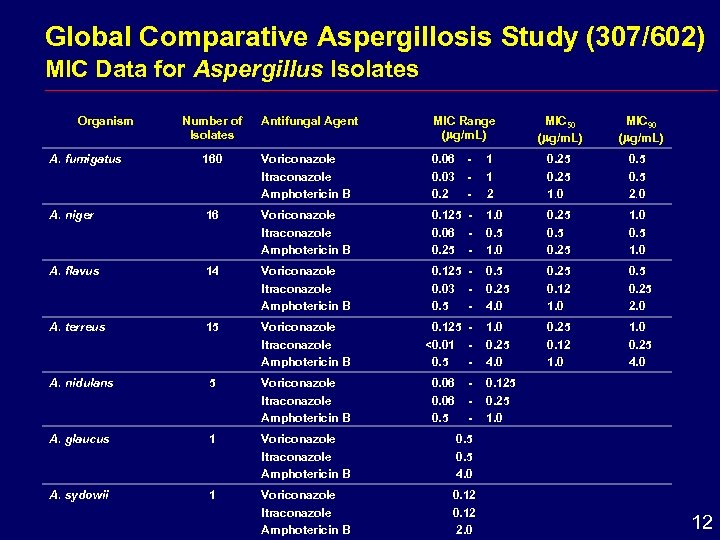
Global Comparative Aspergillosis Study (307/602) MIC Data for Aspergillus Isolates Organism Number of Isolates Antifungal Agent MIC Range ( g/m. L) MIC 50 ( g/m. L) MIC 90 ( g/m. L) A. fumigatus 160 Voriconazole Itraconazole Amphotericin B 0. 06 0. 03 0. 2 - 1 1 2 0. 25 1. 0 0. 5 2. 0 A. niger 16 Voriconazole Itraconazole Amphotericin B 0. 125 0. 06 0. 25 - 1. 0 0. 5 1. 0 0. 25 1. 0 0. 5 1. 0 A. flavus 14 Voriconazole Itraconazole Amphotericin B 0. 125 0. 03 0. 5 - 0. 5 0. 25 4. 0 0. 25 0. 12 1. 0 0. 5 0. 25 2. 0 A. terreus 15 Voriconazole Itraconazole Amphotericin B 0. 125 <0. 01 0. 5 - 1. 0 0. 25 4. 0 0. 25 0. 12 1. 0 0. 25 4. 0 A. nidulans 5 Voriconazole Itraconazole Amphotericin B A. glaucus 1 Voriconazole Itraconazole Amphotericin B 0. 5 4. 0 A. sydowii 1 Voriconazole Itraconazole Amphotericin B 0. 12 2. 0 0. 06 0. 5 - 0. 125 0. 25 1. 0 12

Voriconazole In Vivo Model Immunocompromised guinea pigs (cyclophosphamide and dexamethasone) i Dunkin Hartley guinea pigs i > 90% reduction in neutrophils i Direct IV inoculation i Efficacy measured i. Survival i. Cure i. Tissue burden 13
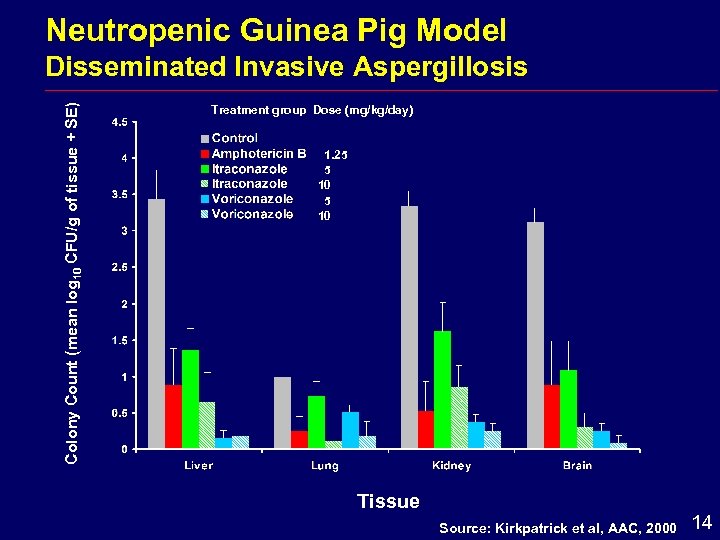
Neutropenic Guinea Pig Model Colony Count (mean log 10 CFU/g of tissue + SE) Disseminated Invasive Aspergillosis Treatment group Dose (mg/kg/day) 1. 25 5 10 Tissue Source: Kirkpatrick et al, AAC, 2000 14

Voriconazole i In vitro potency against yeasts 60 -fold higher than for fluconazole i Cidality against Aspergillus and other moulds i In vitro potency translates into in vivo efficacy in severely immunocompromised animals 15

Voriconazole i Introduction i In vitro and in vivo Data i Clinical Pharmacology i Efficacy i Safety i Conclusion 16

Voriconazole Clinical Pharmacology i Absorption and distribution i Metabolism and excretion i Non-linear pharmacokinetics i Loading dose regimen i Factors influencing pharmacokinetic variability i Drug-drug interactions 17

Voriconazole i Oral bioavailability of 96% i Volume of distribution of 4. 6 L/kg i Plasma protein binding 58% 18
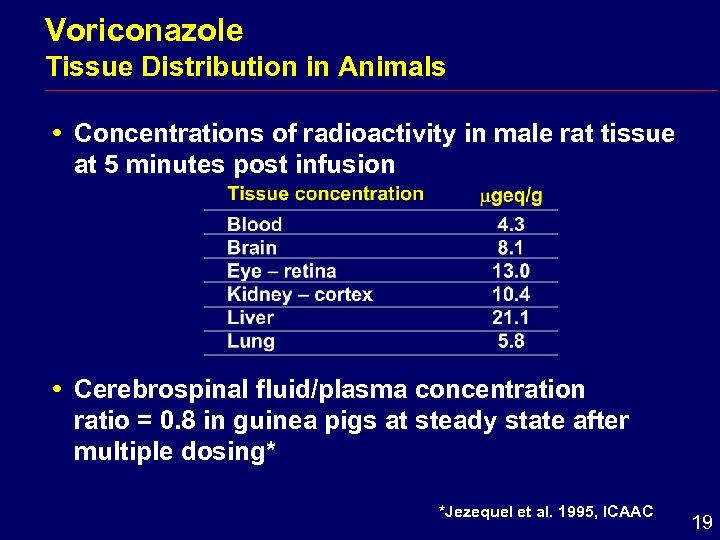
Voriconazole Tissue Distribution in Animals i Concentrations of radioactivity in male rat tissue at 5 minutes post infusion i Cerebrospinal fluid/plasma concentration ratio = 0. 8 in guinea pigs at steady state after multiple dosing* *Jezequel et al. 1995, ICAAC 19

Voriconazole Metabolism and Excretion i Metabolized primarily by the hepatic cytochrome P 450 isoenzymes CYP 2 C 19, CYP 2 C 9 and CYP 3 A 4 i CYP 2 C 19 exhibits genetic polymorphism i Extensive metabolism to a major circulating Noxide metabolite (72% at 1 hour) and several minor metabolites i Metabolite present in toxicology species, does not contribute to efficacy i Less than 2% of a dose excreted unchanged in the urine 20
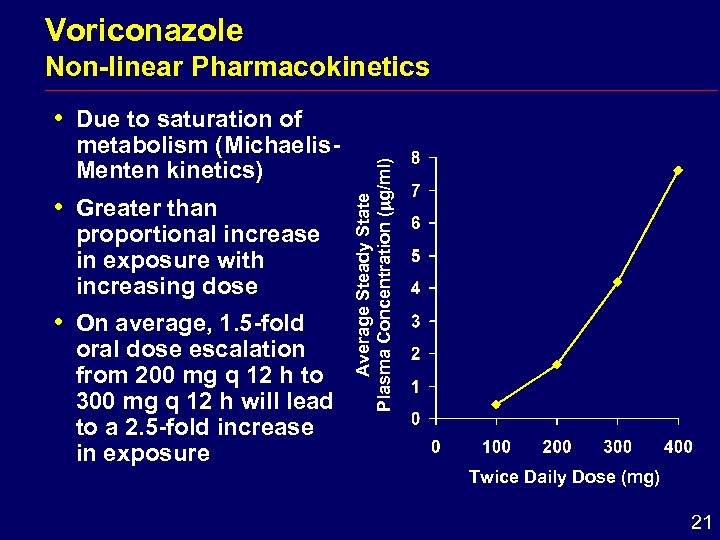
Voriconazole i Due to saturation of metabolism (Michaelis. Menten kinetics) i Greater than proportional increase in exposure with increasing dose i On average, 1. 5 -fold oral dose escalation from 200 mg q 12 h to 300 mg q 12 h will lead to a 2. 5 -fold increase in exposure Average Steady State Plasma Concentration ( g/ml) Non-linear Pharmacokinetics Twice Daily Dose (mg) 21
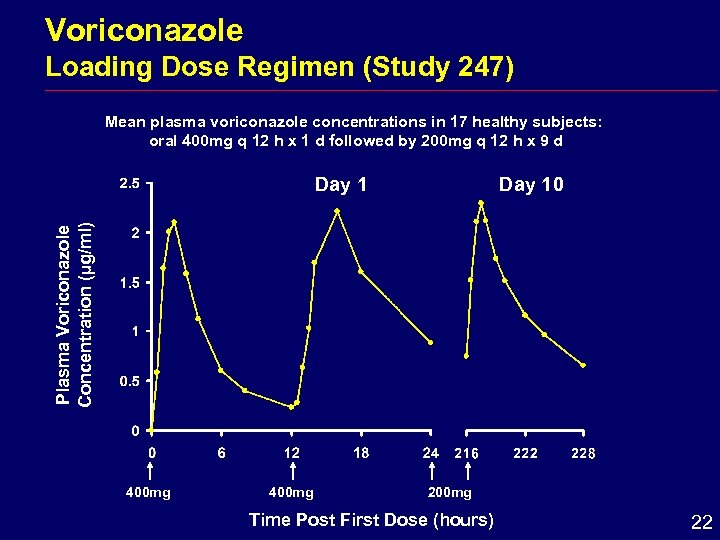
Voriconazole Loading Dose Regimen (Study 247) Mean plasma voriconazole concentrations in 17 healthy subjects: oral 400 mg q 12 h x 1 d followed by 200 mg q 12 h x 9 d Day 10 Plasma Voriconazole Concentration (µg/ml) Day 1 400 mg 200 mg Time Post First Dose (hours) 22
Voriconazole Factors Influencing Pharmacokinetic Variability i CYP 2 C 19 genotype i Race i Gender and age in adults i Children (2 - < 12 years) i Body weight i Hepatic impairment i Renal impairment i Concomitant medications 23
Voriconazole Factors Influencing Pharmacokinetic Variability i CYP 2 C 19 genotype i Race No dose adjustment i Gender and age in adults i Children (2 - < 12 years) i Body weight i Hepatic impairment i Renal impairment i Concomitant medications 24
Voriconazole Factors Influencing Pharmacokinetic Variability i CYP 2 C 19 genotype i Race i Gender and age in adults i Children (2 - < 12 years) i Body weight i Hepatic impairment i Renal impairment i Concomitant medications No dose adjustment 25
Voriconazole Factors Influencing Pharmacokinetic Variability i CYP 2 C 19 genotype i Race i Gender and age in adults i Children (2 - < 12 years): maintenance dose of 4 mg/kg IV q 12 h i Body weight i Hepatic impairment i Renal impairment i Concomitant medications 26
Voriconazole Factors Influencing Pharmacokinetic Variability i CYP 2 C 19 genotype i Race i Gender and age in adults i Children (2 - < 12 years) i Body weight: under 40 kg halve oral maintenance dose i Hepatic impairment i Renal impairment i Concomitant medications 27
Voriconazole Factors Influencing Pharmacokinetic Variability i CYP 2 C 19 genotype i Race i Gender and age in adults i Children (2 - < 12 years) i Body weight i Hepatic impairment: halve maintenance dose i Renal impairment i Concomitant medications 28
Voriconazole Factors Influencing Pharmacokinetic Variability i CYP 2 C 19 genotype i Race i Gender and age in adults i Children (2 - < 12 years) i Body weight i Hepatic impairment i Renal impairment: use oral in patients with serum creatinine > 2. 5 mg/d. L i Concomitant medications 29
Voriconazole Factors Influencing Pharmacokinetic Variability i CYP 2 C 19 genotype i Race i Gender and age in adults i Children (2 - < 12 years) i Body weight i Hepatic impairment i Renal impairment i Concomitant medications: drug-drug interactions 30

Voriconazole Drug-drug Interactions Explored in 19 studies including 365 volunteers i Effect of nine other drugs on voriconazole i Effect of voriconazole on 11 other drugs Recommendations i Contraindications i Dose adjustments of voriconazole or concomitant medications i Monitor concentrations or effects of concomitant medications i No adjustments needed 31

Voriconazole Drug-drug Interactions: Contraindications The following drugs are contraindicated: i Rifampin*, barbiturates (long-acting), carbamazepine (decreased voriconazole exposure) i Sirolimus*, terfenadine, astemizole, cisapride, pimozide, quinidine, ergot alkaloids (voriconazole increases exposure to these medications) * Studied in volunteers, other interactions predicted 32
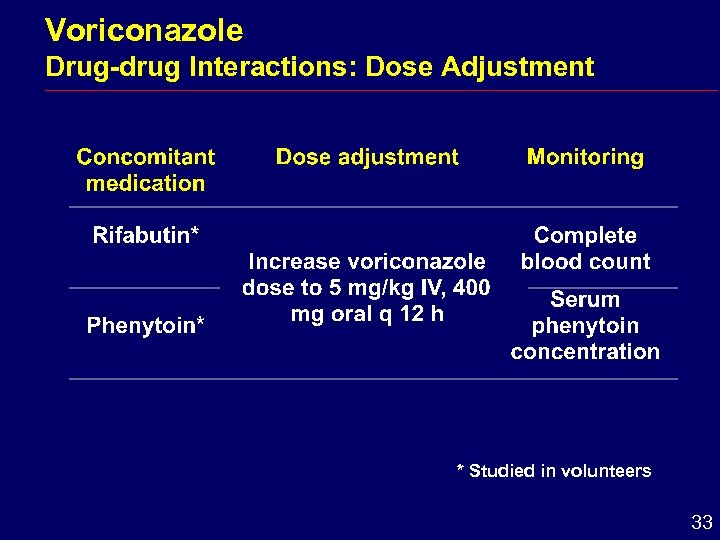
Voriconazole Drug-drug Interactions: Dose Adjustment * Studied in volunteers 33
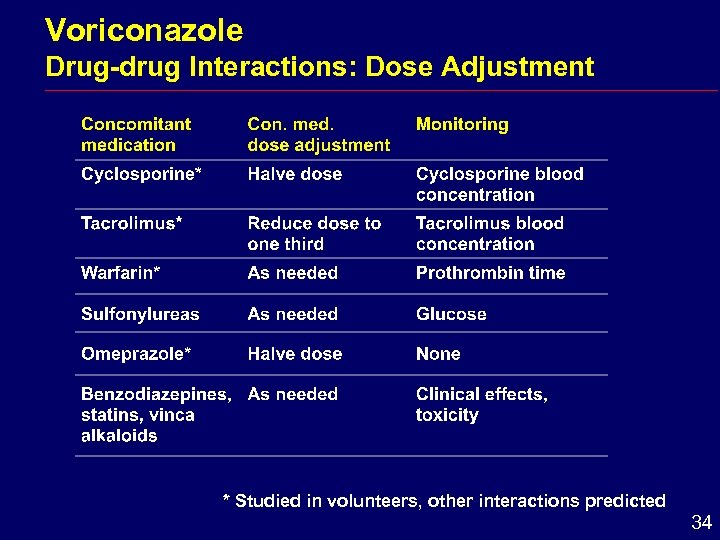
Voriconazole Drug-drug Interactions: Dose Adjustment * Studied in volunteers, other interactions predicted 34

Voriconazole Drug-drug Interactions i No dose adjustment required when voriconazole is administered with: i Macrolide antibiotics i Indinavir i Cimetidine i Ranitidine i Digoxin i Mycophenolate i Prednisolone All studied in volunteers 35

Voriconazole Summary of Pharmacokinetics i Rapid and consistent absorption with high oral bioavailability (96%) i Large volume of distribution (4. 6 L/kg) i Non-linear elimination i Hepatic metabolism by CYP 2 C 19, 2 C 9 and 3 A 4 isoenzymes i Increased exposure in cirrhosis i Metabolic drug interactions well-characterized 36

Voriconazole Superior outcome and survival benefit in primary therapy of acute invasive aspergillosis i Efficacy in patients with Scedosporium and Fusarium infections i Efficacy in Candida infections i Appropriate option for empirical therapy i Better tolerated than amphotericin B formulations i Acceptable overall safety profile i Manageable drug-drug interactions i 37
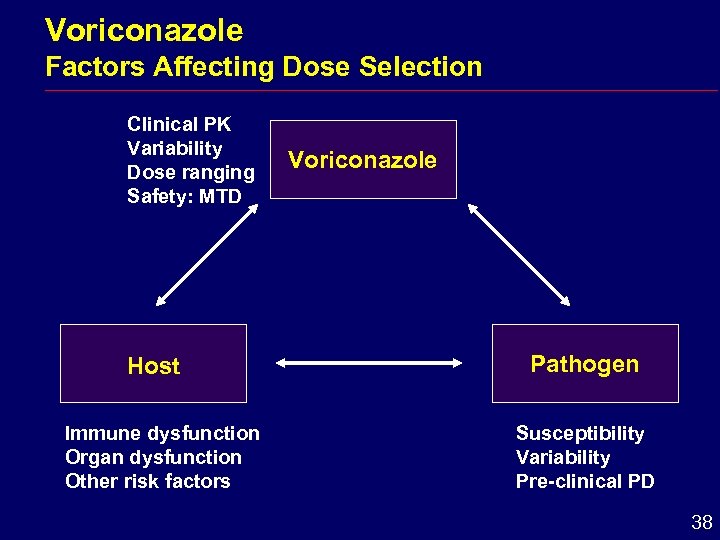
Voriconazole Factors Affecting Dose Selection Clinical PK Variability Dose ranging Safety: MTD Host Immune dysfunction Organ dysfunction Other risk factors Voriconazole Pathogen Susceptibility Variability Pre-clinical PD 38

Voriconazole Dose Selection i Target maximum tolerated dose i Aim for plasma concentrations above MIC for common pathogens 39
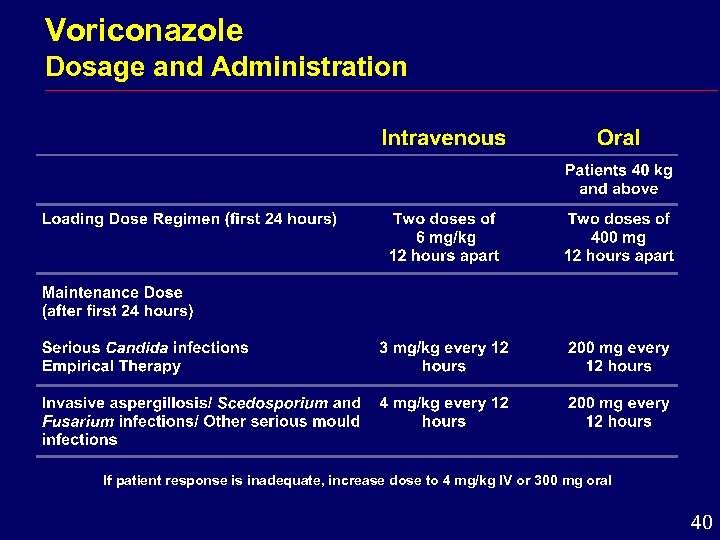
Voriconazole Dosage and Administration If patient response is inadequate, increase dose to 4 mg/kg IV or 300 mg oral 40

41

Voriconazole i Introduction i In vitro and in vivo data i Clinical Pharmacology i Efficacy i Safety i Conclusion 42
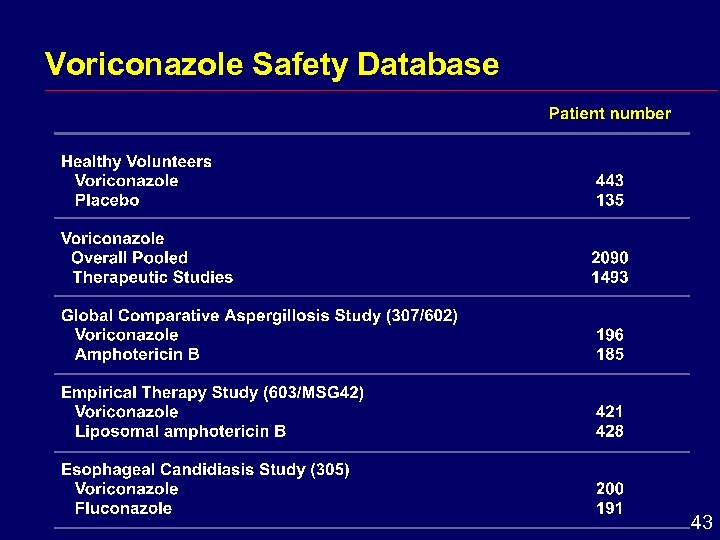
Voriconazole Safety Database 43
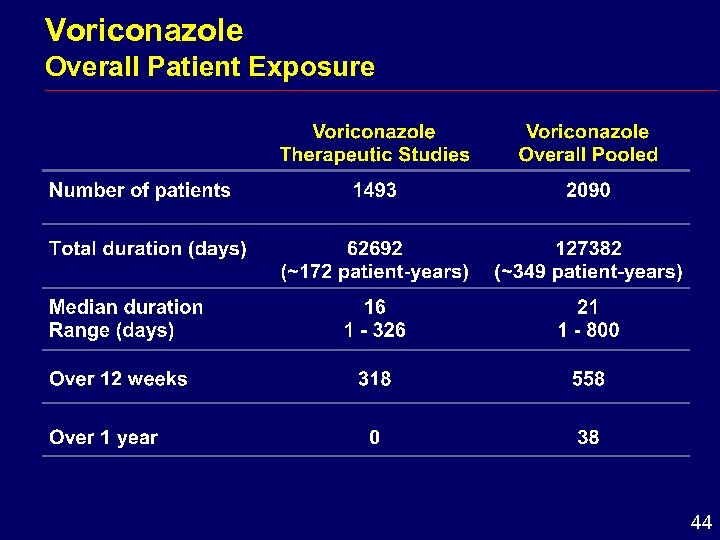
Voriconazole Overall Patient Exposure 44
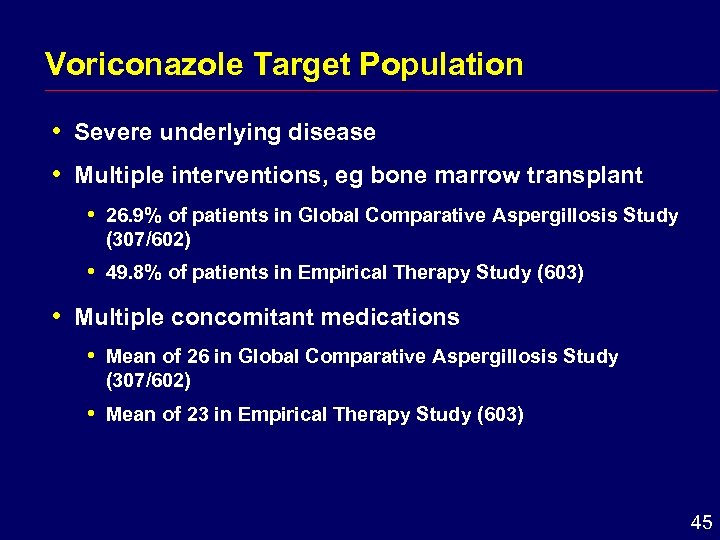
Voriconazole Target Population i Severe underlying disease i Multiple interventions, eg bone marrow transplant i i i 26. 9% of patients in Global Comparative Aspergillosis Study (307/602) 49. 8% of patients in Empirical Therapy Study (603) Multiple concomitant medications i Mean of 26 in Global Comparative Aspergillosis Study (307/602) i Mean of 23 in Empirical Therapy Study (603) 45

Overview of Safety Presentation i Deaths and discontinuations i Adverse i Special events safety topics i Emerging clinical, animal, published data i Thorough investigation 46
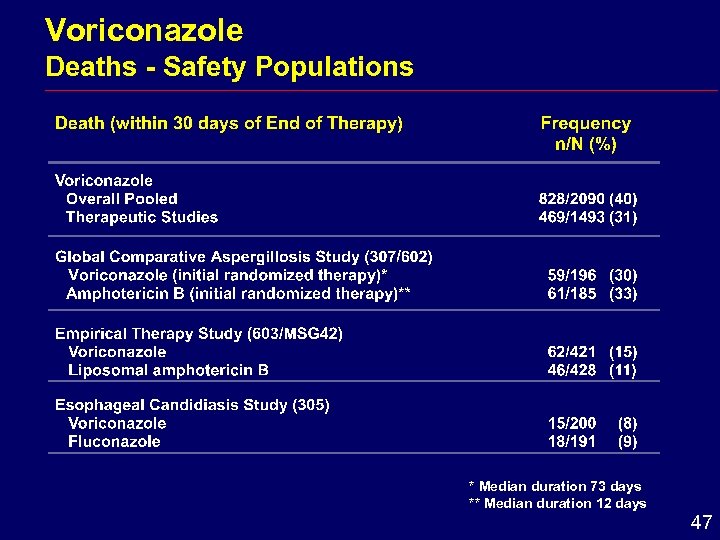
Voriconazole Deaths - Safety Populations * Median duration 73 days ** Median duration 12 days 47
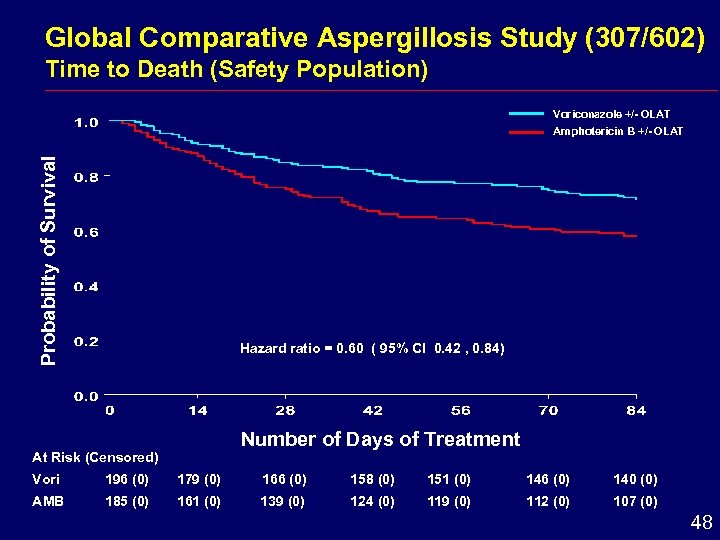
Global Comparative Aspergillosis Study (307/602) Time to Death (Safety Population) Voriconazole +/- OLAT Probability of Survival Amphotericin B +/- OLAT Hazard ratio = 0. 60 ( 95% CI 0. 42 , 0. 84) Number of Days of Treatment At Risk (Censored) Vori 196 (0) 179 (0) 166 (0) 158 (0) 151 (0) 146 (0) 140 (0) AMB 185 (0) 161 (0) 139 (0) 124 (0) 119 (0) 112 (0) 107 (0) 48
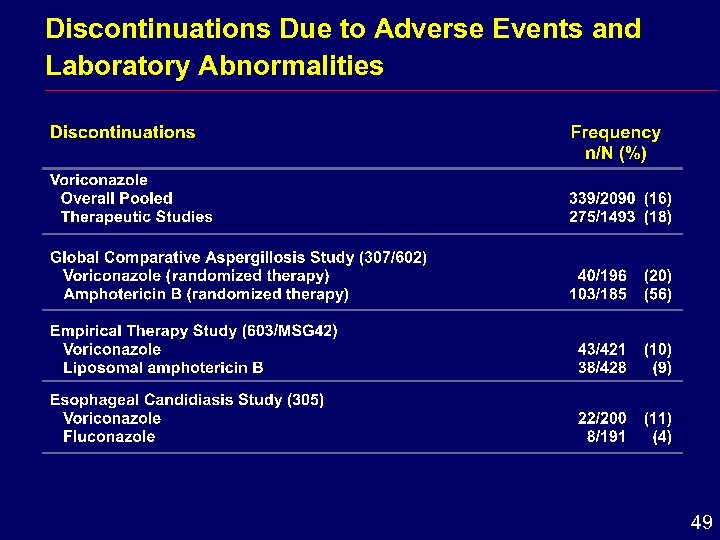
Discontinuations Due to Adverse Events and Laboratory Abnormalities 49
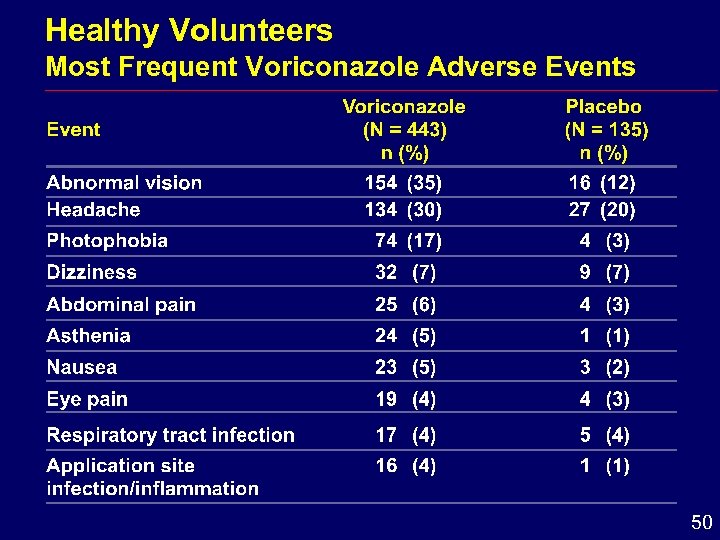
Healthy Volunteers Most Frequent Voriconazole Adverse Events 50
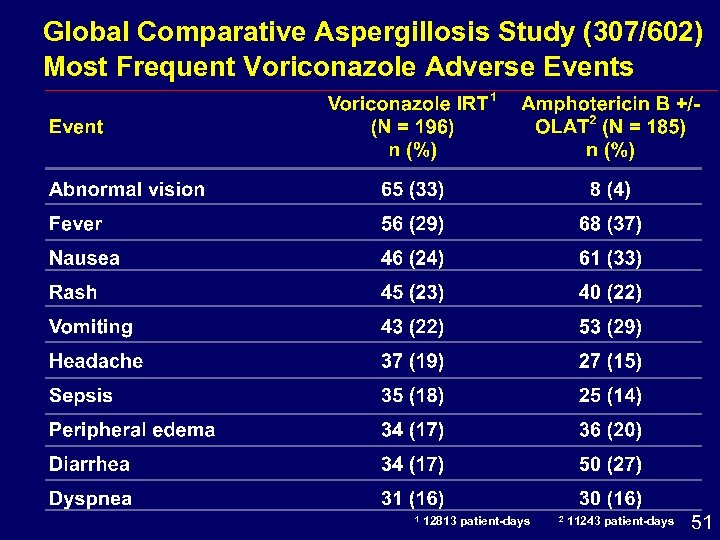
Global Comparative Aspergillosis Study (307/602) Most Frequent Voriconazole Adverse Events 1 12813 patient-days 2 11243 patient-days 51
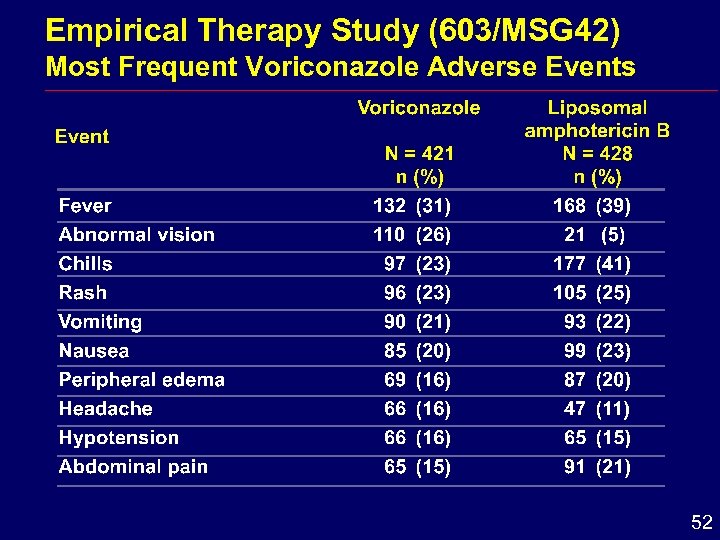
Empirical Therapy Study (603/MSG 42) Most Frequent Voriconazole Adverse Events 52
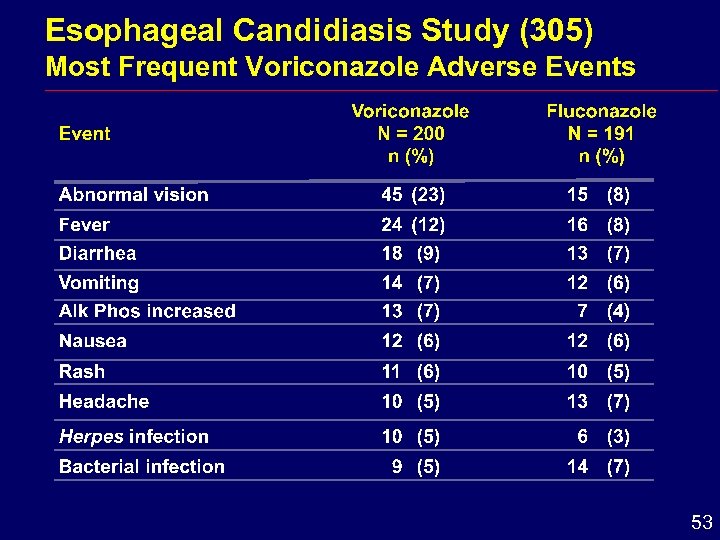
Esophageal Candidiasis Study (305) Most Frequent Voriconazole Adverse Events 53

Voriconazole Special Safety Topics i Visual disturbances i Hepatic i Skin adverse events reactions i Other topics i Cardiac adverse events i Anaphylactoid i Renal reactions function i Sepsis i Hallucinations 54
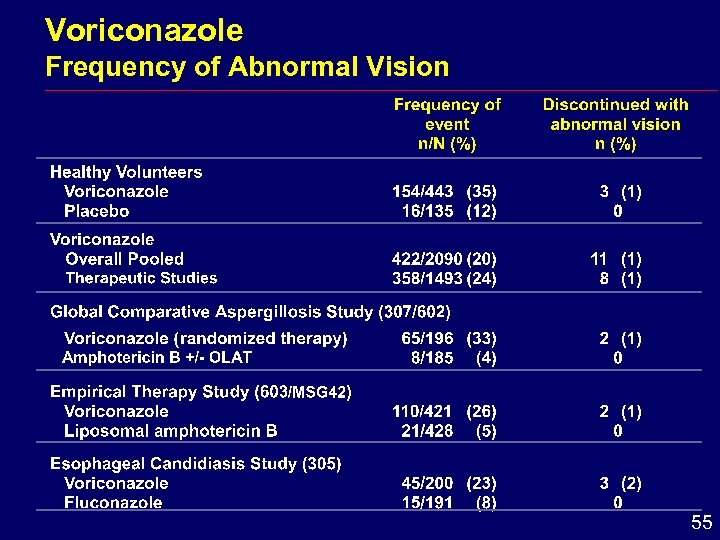
Voriconazole Frequency of Abnormal Vision 55
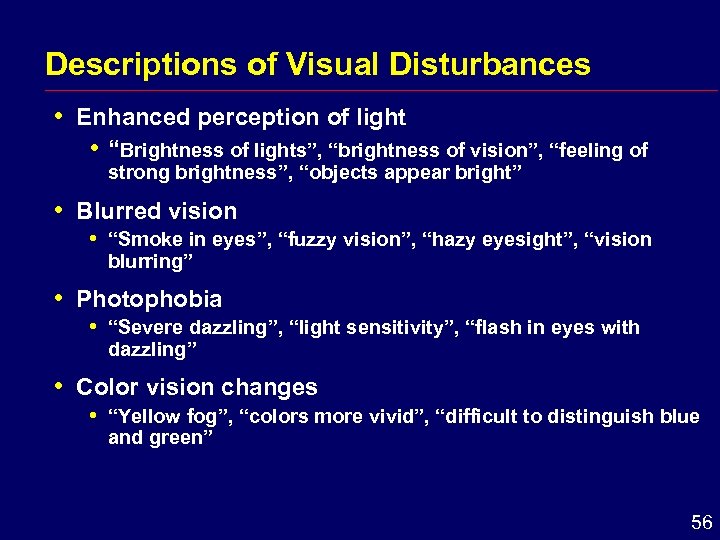
Descriptions of Visual Disturbances i Enhanced perception of light i “Brightness of lights”, “brightness of vision”, “feeling of strong brightness”, “objects appear bright” i Blurred vision i i Photophobia i i “Smoke in eyes”, “fuzzy vision”, “hazy eyesight”, “vision blurring” “Severe dazzling”, “light sensitivity”, “flash in eyes with dazzling” Color vision changes i “Yellow fog”, “colors more vivid”, “difficult to distinguish blue and green” 56
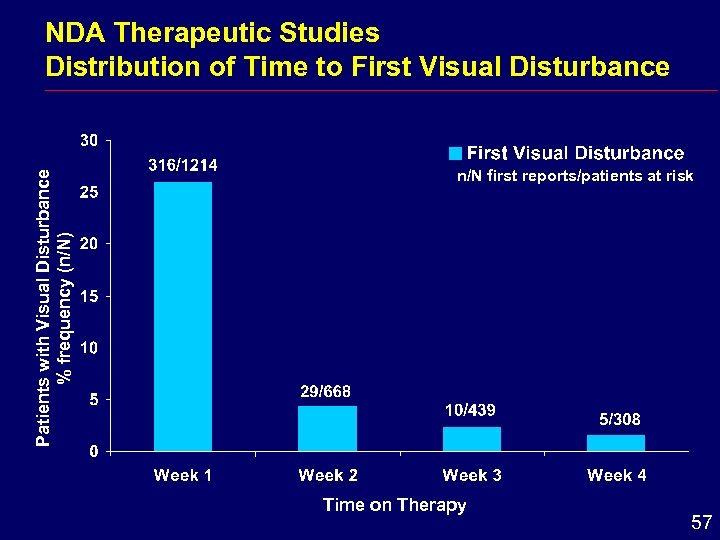
Patients with Visual Disturbance % frequency (n/N) NDA Therapeutic Studies Distribution of Time to First Visual Disturbance n/N first reports/patients at risk Time on Therapy 57
Multiple Dose Visual Function Study (1004) i Double-blind, randomized, placebo-controlled, parallel group study (N = 18/group), treatment duration 28. 5 days Oral voriconazole i Usual loading dose regimen, followed by 300 mg q 12 h i i ERG at screening and on Days 1, 8, 29 and 43 i Tests on Days 3, 7, 28 and 42 Farnsworth-Munsell 100 Hue test i Humphrey Visual field test i Slit lamp test i Visual acuity test i External eye examinations i Funduscopy (indirect and direct) i 58
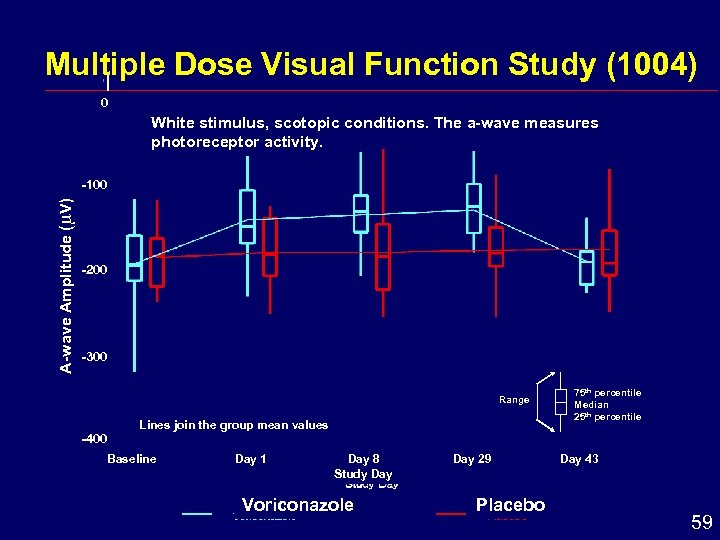
Multiple Dose Visual Function Study (1004) 0 White stimulus, scotopic conditions. The a-wave measures photoreceptor activity. A-wave Amplitude ( V) -100 -200 -300 Range -400 Lines join the group mean values Baseline Day 1 Day 8 Study Day Voriconazole Day 29 Placebo 75 th percentile Median 25 th percentile Day 43 59
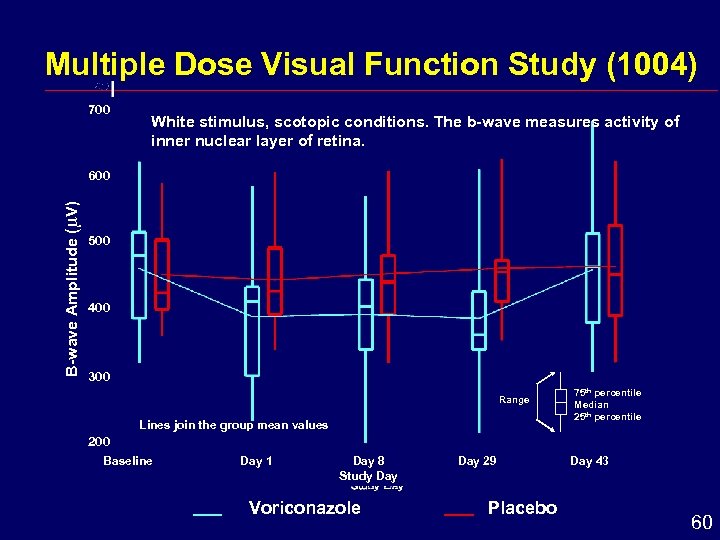
Multiple Dose Visual Function Study (1004) 700 White stimulus, scotopic conditions. The b-wave measures activity of inner nuclear layer of retina. B-wave Amplitude ( V) 600 500 400 300 Range Lines join the group mean values 75 th percentile Median 25 th percentile 200 Baseline Day 1 Day 8 Study Day Voriconazole Day 29 Placebo Day 43 60
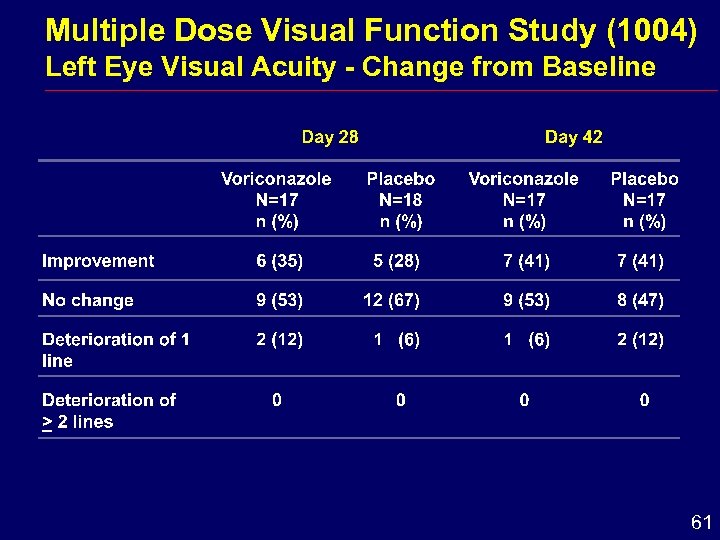
Multiple Dose Visual Function Study (1004) Left Eye Visual Acuity - Change from Baseline 61
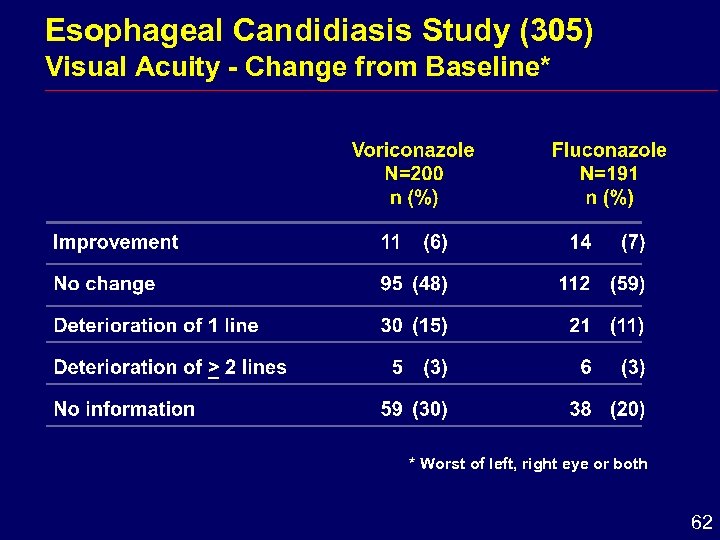
Esophageal Candidiasis Study (305) Visual Acuity - Change from Baseline* * Worst of left, right eye or both 62
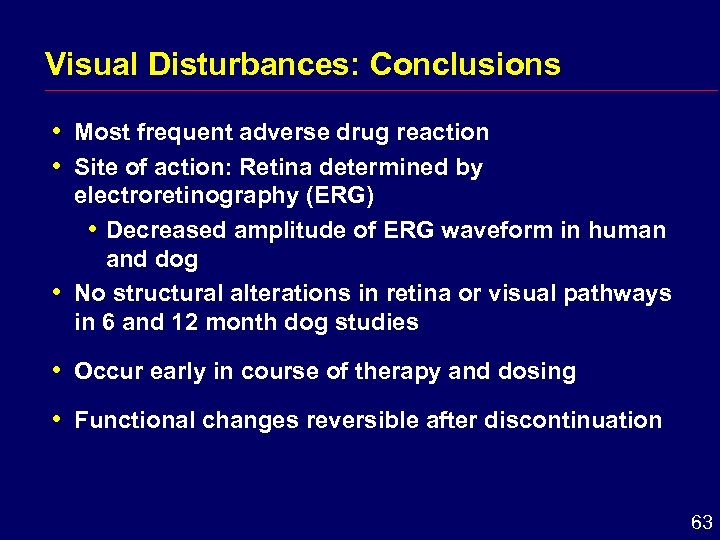
Visual Disturbances: Conclusions i i i Most frequent adverse drug reaction Site of action: Retina determined by electroretinography (ERG) i Decreased amplitude of ERG waveform in human and dog No structural alterations in retina or visual pathways in 6 and 12 month dog studies i Occur early in course of therapy and dosing i Functional changes reversible after discontinuation 63

Voriconazole Special Safety Topics i Visual disturbances i Hepatic i Skin adverse events reactions i Other topics i Cardiac adverse events i Anaphylactoid i Renal reactions function i Sepsis i Hallucinations 64
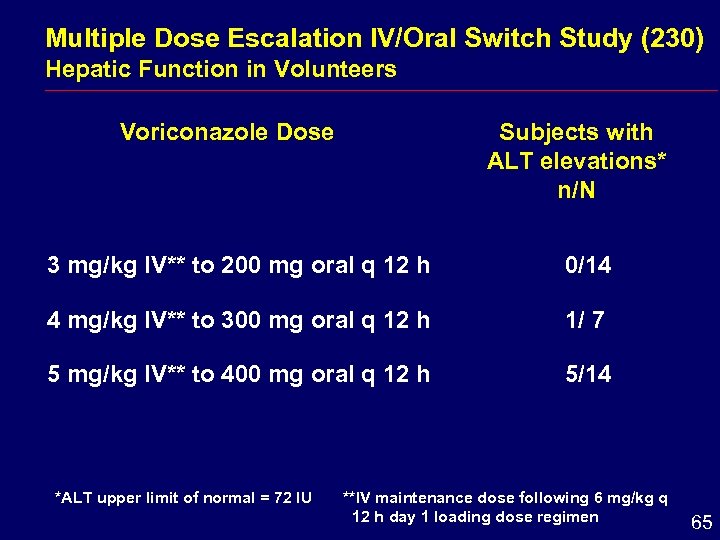
Multiple Dose Escalation IV/Oral Switch Study (230) Hepatic Function in Volunteers Voriconazole Dose Subjects with ALT elevations* n/N 3 mg/kg IV** to 200 mg oral q 12 h 0/14 4 mg/kg IV** to 300 mg oral q 12 h 1/ 7 5 mg/kg IV** to 400 mg oral q 12 h 5/14 *ALT upper limit of normal = 72 IU **IV maintenance dose following 6 mg/kg q 12 h day 1 loading dose regimen 65
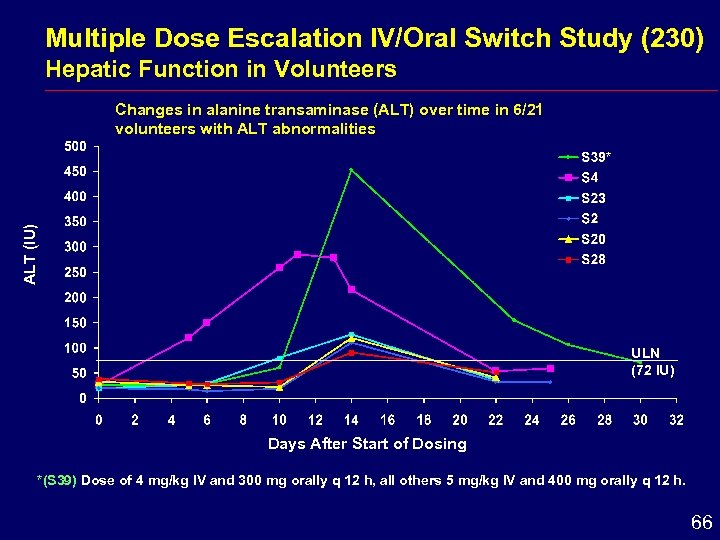
Multiple Dose Escalation IV/Oral Switch Study (230) Hepatic Function in Volunteers ALT (IU) Changes in alanine transaminase (ALT) over time in 6/21 volunteers with ALT abnormalities ULN (72 IU) Days After Start of Dosing *(S 39) Dose of 4 mg/kg IV and 300 mg orally q 12 h, all others 5 mg/kg IV and 400 mg orally q 12 h. 66
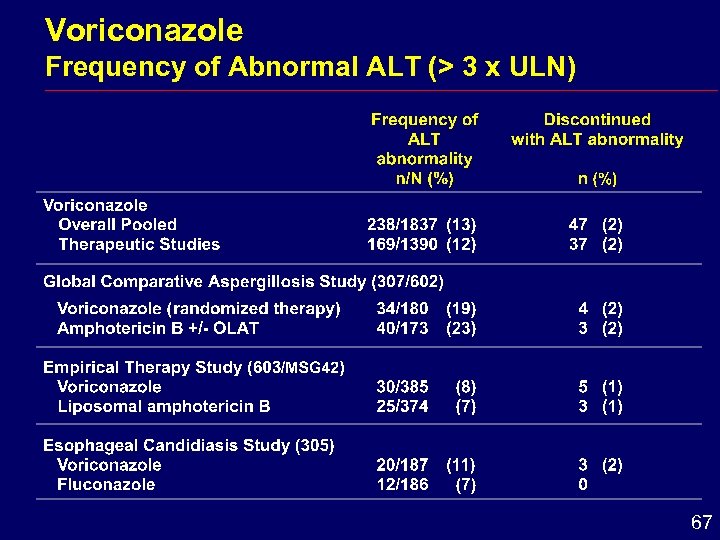
Voriconazole Frequency of Abnormal ALT (> 3 x ULN) 67
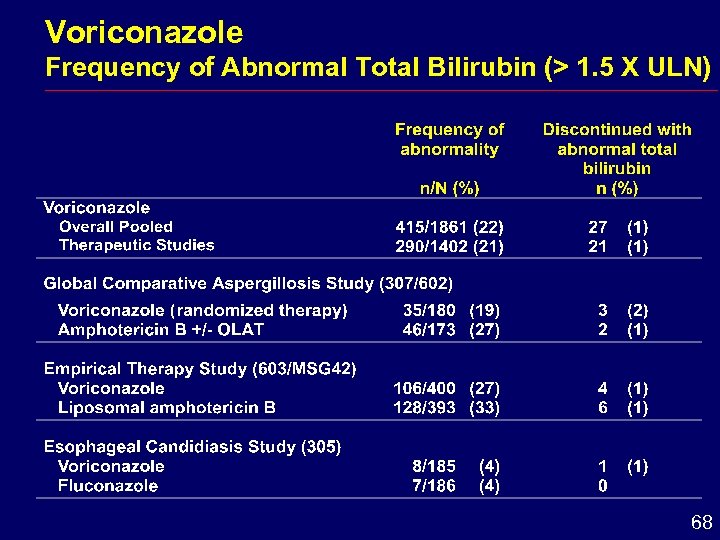
Voriconazole Frequency of Abnormal Total Bilirubin (> 1. 5 X ULN) 68
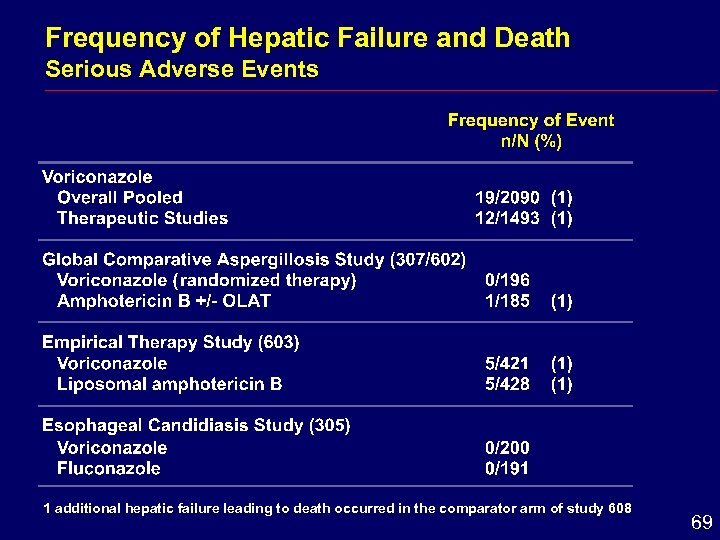
Frequency of Hepatic Failure and Death Serious Adverse Events 1 additional hepatic failure leading to death occurred in the comparator arm of study 608 69
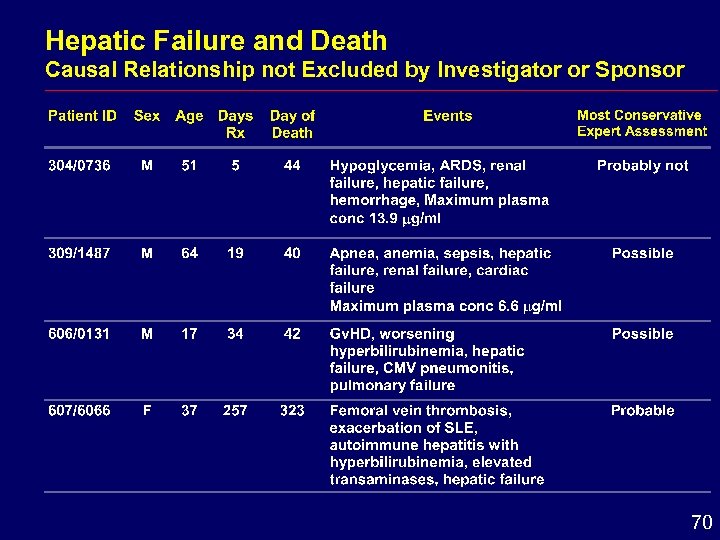
Hepatic Failure and Death Causal Relationship not Excluded by Investigator or Sponsor 70

Hepatic Conclusions i Enzyme elevations reversible after dose withdrawal i Comparative studies hepatic adverse effects i Similar frequency for voriconazole and amphotericin B formulations i Greater frequency for voriconazole than for fluconazole i Monitoring of hepatic function is recommended 71

Voriconazole Special Safety Topics i Visual disturbances i Hepatic i Skin adverse events reactions i Other topics i Cardiac adverse events i Anaphylactoid i Renal reactions function i Sepsis i Hallucinations 72

Descriptions of Skin Adverse Events Rash “rash on trunk and arms”, “facial erythema”, “exanthema”, “generalized erythema”, “head, neck and shoulder erythema”, “neck redness”, “dermatitis”, “allergic skin reaction” Photosensitivity Reaction “photosensitivity skin reaction”, “photosensitization of face”, “photosensitivity rash”, “skin rash in sun exposed regions”, “sunburn” 73
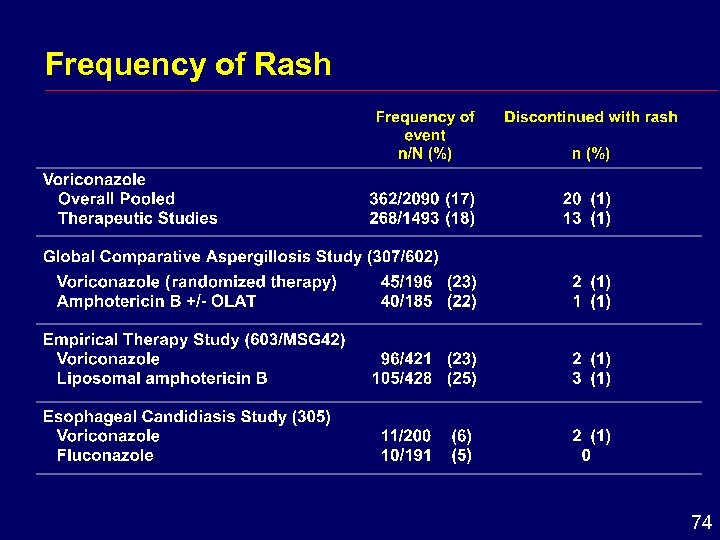
Frequency of Rash 74
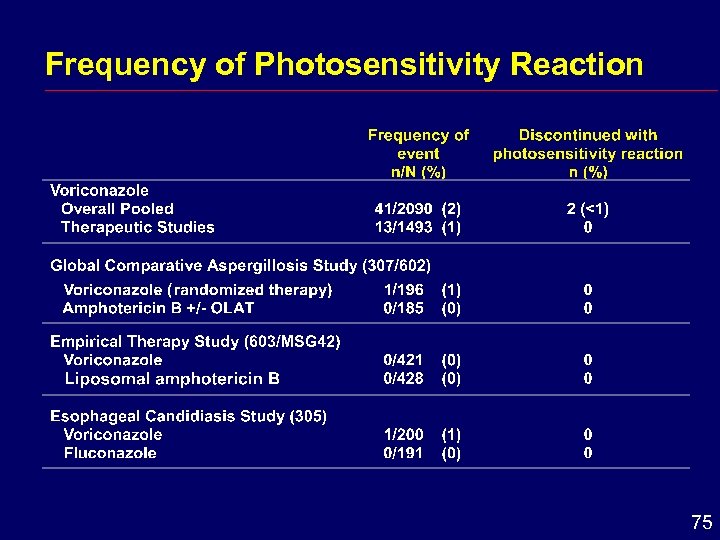
Frequency of Photosensitivity Reaction 75

Skin Conclusions i In comparative studies, skin adverse effects occurred with a similar frequency in voriconazole, amphotericin B- and fluconazole-treated patients i Photosensitivity potential cannot be excluded 76

Voriconazole Special Safety Topics i Visual disturbances i Hepatic i Skin adverse events reactions i Other topics i Cardiac adverse events i Anaphylactoid i Renal reactions function i Sepsis i Hallucinations 77

Other Safety Issues i Cardiac adverse events i i One cardiac death Thorough in vitro, Phase 1 and clinical investigations i Anaphylactoid reactions i Renal function i i Sepsis and host resistance i i Proposed to monitor creatinine No association identified Hallucinations i i Role for voriconazole not excluded No impact on therapy 78
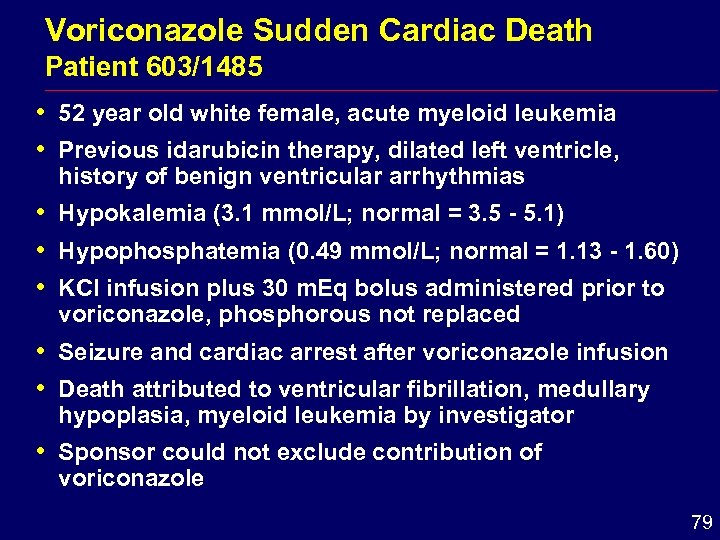
Voriconazole Sudden Cardiac Death Patient 603/1485 i 52 year old white female, acute myeloid leukemia i Previous idarubicin therapy, dilated left ventricle, history of benign ventricular arrhythmias i Hypokalemia (3. 1 mmol/L; normal = 3. 5 - 5. 1) i Hypophosphatemia (0. 49 mmol/L; normal = 1. 13 - 1. 60) i KCl infusion plus 30 m. Eq bolus administered prior to voriconazole, phosphorous not replaced i Seizure and cardiac arrest after voriconazole infusion i Death attributed to ventricular fibrillation, medullary hypoplasia, myeloid leukemia by investigator i Sponsor could not exclude contribution of voriconazole 79
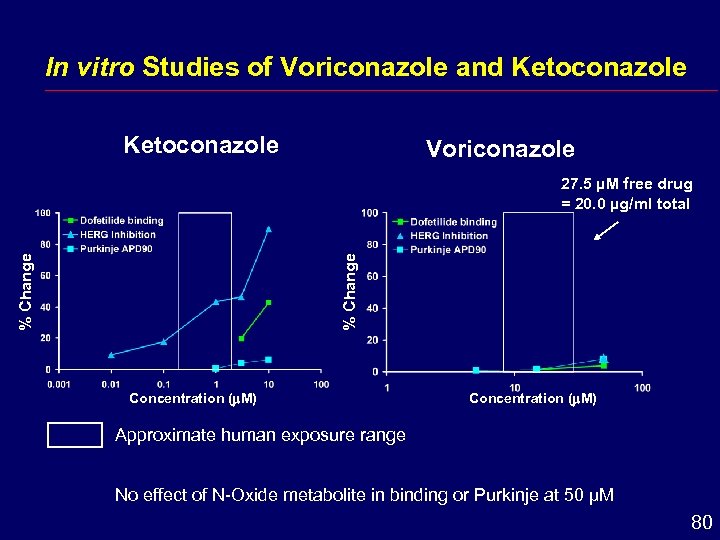
In vitro Studies of Voriconazole and Ketoconazole Voriconazole % Change 27. 5 µM free drug = 20. 0 µg/ml total Concentration ( M) Approximate human exposure range No effect of N-Oxide metabolite in binding or Purkinje at 50 µM 80
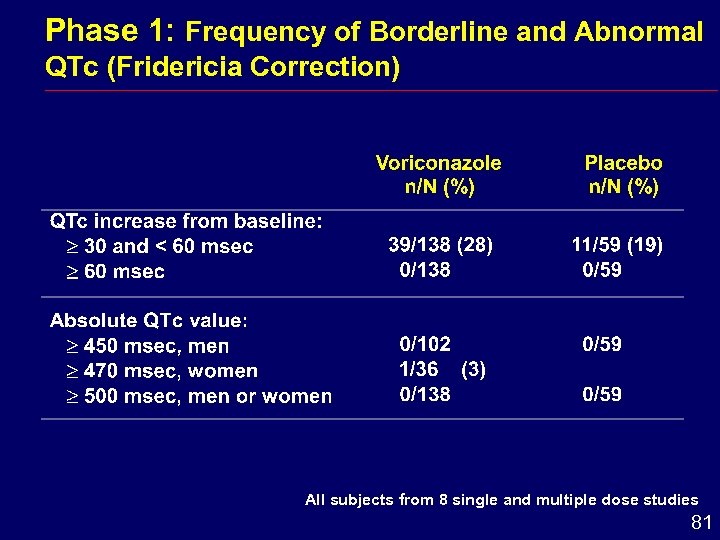
Phase 1: Frequency of Borderline and Abnormal QTc (Fridericia Correction) All subjects from 8 single and multiple dose studies 81
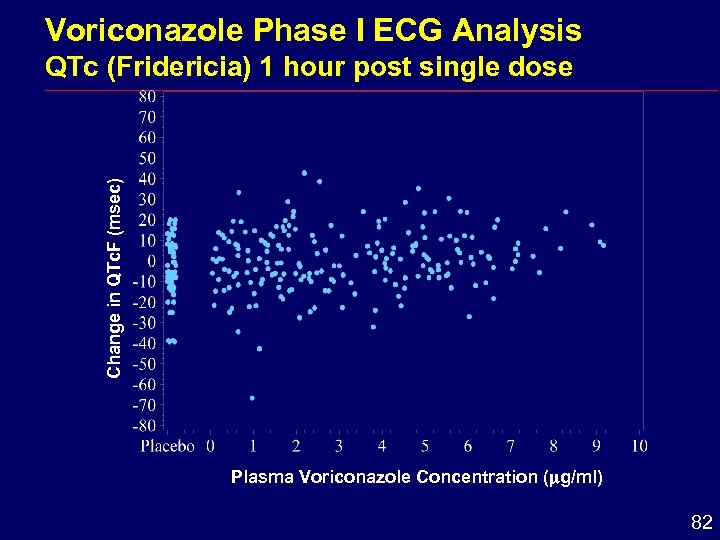
Voriconazole Phase I ECG Analysis Change in QTc. F (msec) QTc (Fridericia) 1 hour post single dose Plasma Voriconazole Concentration ( g/ml) 82
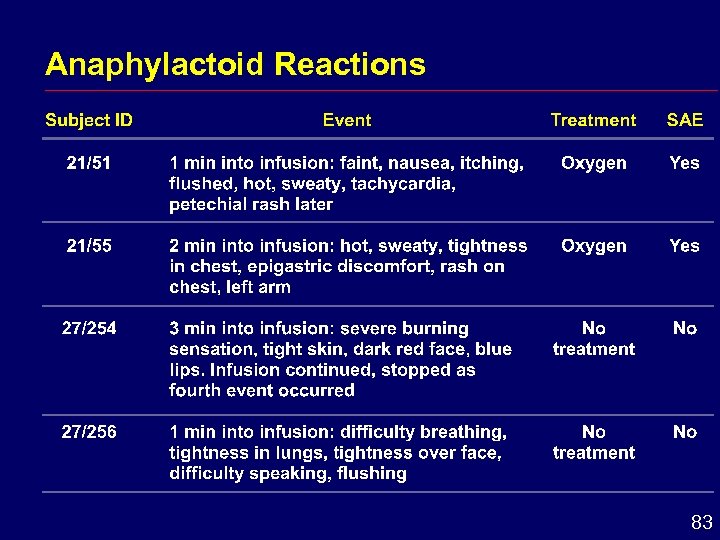
Anaphylactoid Reactions 83
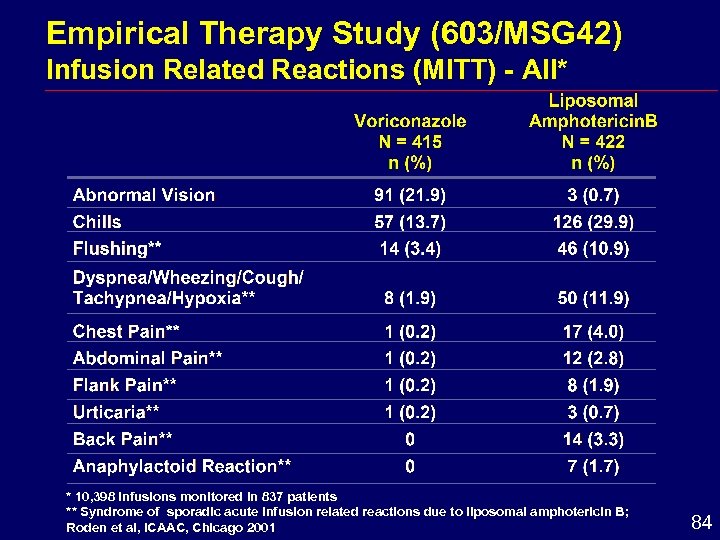
Empirical Therapy Study (603/MSG 42) Infusion Related Reactions (MITT) - All* * 10, 398 infusions monitored in 837 patients ** Syndrome of sporadic acute infusion related reactions due to liposomal amphotericin B; Roden et al, ICAAC, Chicago 2001 84

Other Safety Issues i Cardiac adverse events i i One cardiac death Thorough in vitro, Phase 1 and clinical investigations i Anaphylactoid reactions i Renal function i i Sepsis and host resistance i i Proposed to monitor creatinine No association identified Hallucinations i i Role for voriconazole not excluded No impact on therapy 85
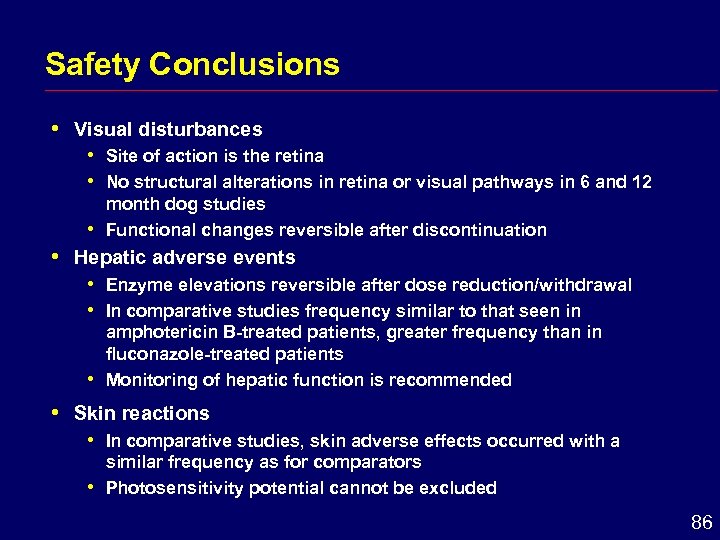
Safety Conclusions i Visual disturbances i i Hepatic adverse events i i Site of action is the retina No structural alterations in retina or visual pathways in 6 and 12 month dog studies Functional changes reversible after discontinuation Enzyme elevations reversible after dose reduction/withdrawal In comparative studies frequency similar to that seen in amphotericin B-treated patients, greater frequency than in fluconazole-treated patients Monitoring of hepatic function is recommended Skin reactions i i In comparative studies, skin adverse effects occurred with a similar frequency as for comparators Photosensitivity potential cannot be excluded 86

Voriconazole i Superior outcome and survival benefit in primary therapy of acute invasive aspergillosis i Efficacy in patients with Scedosporium and Fusarium infections i Efficacy in Candida infections i Appropriate option for empirical therapy i Better tolerated than amphotericin B formulations i Acceptable overall safety profile i Manageable drug-drug interactions 87
11dffec128e1d9ad2b433f17192919ac.ppt