44703f4f0acd0bd23f081f089bdb3a5c.ppt
- Количество слайдов: 37
 Regulatory Perspectives on the Thorough QT/QTc Study 2005 FDA/Industry Workshop Washington DC September 16, 2005 Juan (Joanne) Zhang, Ph. D. CDER/FDA *Acknowledgement: Drs. Stella Machado & George Rochester
Regulatory Perspectives on the Thorough QT/QTc Study 2005 FDA/Industry Workshop Washington DC September 16, 2005 Juan (Joanne) Zhang, Ph. D. CDER/FDA *Acknowledgement: Drs. Stella Machado & George Rochester
 Disclaimer • This presentation represents the opinions of the author, and not necessarily those of the U. S. Food and Drug Administration. 2
Disclaimer • This presentation represents the opinions of the author, and not necessarily those of the U. S. Food and Drug Administration. 2
 Outline I. Thorough QT/QTc Study - Introduction II. Design Considerations III. Statistical Test for a Negative ‘Thorough QT/QTc Study’ - Statistical Hypothesis & Test - Intersection-Union Tests IV. False Positive and False Negative Probabilities V. Assay Sensitivity VI. Summary 3
Outline I. Thorough QT/QTc Study - Introduction II. Design Considerations III. Statistical Test for a Negative ‘Thorough QT/QTc Study’ - Statistical Hypothesis & Test - Intersection-Union Tests IV. False Positive and False Negative Probabilities V. Assay Sensitivity VI. Summary 3
 Thorough QT/QTc Study 4
Thorough QT/QTc Study 4
 Introduction • QT interval represents the time between start of ventricular depolarization and end of ventricular repolarization (QT). • QTc is the QT-interval corrected for heart rates 5
Introduction • QT interval represents the time between start of ventricular depolarization and end of ventricular repolarization (QT). • QTc is the QT-interval corrected for heart rates 5
 Introduction (2) • QT/QTc prolongation may cause ventricular arrhythmias including ventricular fibrillation and Torsade de Pointes, which can be fatal even though the degree of this association is not known • Current ICH E 14 Guidance requests all sponsors submitting new drug applications to conduct a thorough QT/QTc study – Generally conducted in early clinical development after some knowledge of the pharmacokinetics of the drug 6
Introduction (2) • QT/QTc prolongation may cause ventricular arrhythmias including ventricular fibrillation and Torsade de Pointes, which can be fatal even though the degree of this association is not known • Current ICH E 14 Guidance requests all sponsors submitting new drug applications to conduct a thorough QT/QTc study – Generally conducted in early clinical development after some knowledge of the pharmacokinetics of the drug 6
 Introduction (3) • ICH E 14 Guidance (Step 4, Adopted May 2005) “The ‘thorough QT/QTc study’ is intended to determine whether the drug has a threshold pharmacologic effect on cardiac repolarization, as detected by QT/QTc prolongation. ” “The study is typically carried out in healthy volunteers (as opposed to individuals at increased risk of arrhythmias) and is used to determine whether or not the effect of a drug on the QT/QTc interval in target patient populations should be studied intensively during later stages of drug development. ” 7
Introduction (3) • ICH E 14 Guidance (Step 4, Adopted May 2005) “The ‘thorough QT/QTc study’ is intended to determine whether the drug has a threshold pharmacologic effect on cardiac repolarization, as detected by QT/QTc prolongation. ” “The study is typically carried out in healthy volunteers (as opposed to individuals at increased risk of arrhythmias) and is used to determine whether or not the effect of a drug on the QT/QTc interval in target patient populations should be studied intensively during later stages of drug development. ” 7
 Negative ‘Thorough QT/QTc Study’ • ICH E 14 Guidance (Step 4, Adopted May 2005) “…a negative ‘thorough QT/QTc study’ is one in which the upper bound of the 95% one-sided confidence interval for the largest time-matched mean effect of the drug on the QTc interval excludes 10 ms. ” 8
Negative ‘Thorough QT/QTc Study’ • ICH E 14 Guidance (Step 4, Adopted May 2005) “…a negative ‘thorough QT/QTc study’ is one in which the upper bound of the 95% one-sided confidence interval for the largest time-matched mean effect of the drug on the QTc interval excludes 10 ms. ” 8
 Non-negative ‘Thorough QT/QTc Study’ • If a ‘thorough QT/QTc study’ is not negative, i. e. , if at least one upper bound of the one-sided 95% confidence interval of the time-matched difference exceeds the threshold of 10 ms, the study is termed “Non-negative”. 9
Non-negative ‘Thorough QT/QTc Study’ • If a ‘thorough QT/QTc study’ is not negative, i. e. , if at least one upper bound of the one-sided 95% confidence interval of the time-matched difference exceeds the threshold of 10 ms, the study is termed “Non-negative”. 9
 Implications of Results from the ‘Thorough QT/QTc Study’ ICH E 14: • IF the ‘thorough QT/QTc study’ is negative – “the collection of baseline and periodic on-therapy ECGs in accordance with the current investigational practices in each therapeutic field is almost always sufficient evaluation during subsequent stages of drug development. ” • IF ‘thorough QT/QTc study’ is non-negative – “additional evaluation in subsequent clinical studies should be performed. ” Hence, expanded ECG safety evaluation during later stages of drug development are needed. 10
Implications of Results from the ‘Thorough QT/QTc Study’ ICH E 14: • IF the ‘thorough QT/QTc study’ is negative – “the collection of baseline and periodic on-therapy ECGs in accordance with the current investigational practices in each therapeutic field is almost always sufficient evaluation during subsequent stages of drug development. ” • IF ‘thorough QT/QTc study’ is non-negative – “additional evaluation in subsequent clinical studies should be performed. ” Hence, expanded ECG safety evaluation during later stages of drug development are needed. 10
 Design Considerations 11
Design Considerations 11
 Design Considerations • To better control variability and potential bias – Randomized, double blinded, placebo and active controlled, single or multiple doses of the drug, crossover or parallel study – Healthy volunteers, it is better to have similar number of males and females in the study (randomization stratified by gender) – Crossover design recommended if possible – For a crossover study, baseline at each period is recommended 12
Design Considerations • To better control variability and potential bias – Randomized, double blinded, placebo and active controlled, single or multiple doses of the drug, crossover or parallel study – Healthy volunteers, it is better to have similar number of males and females in the study (randomization stratified by gender) – Crossover design recommended if possible – For a crossover study, baseline at each period is recommended 12
 Design Considerations (2) • QT data are highly variable, so control of all sources of variability is critical • Replicated QT measurements at each time-point are recommended. Typically ≥ 3 replicates. The mean of these replicates is used. • The baseline QT measurements should be taken as close to the treatment date as possible; typically either – At matching time-points on the day prior to drug treatment; or – At time 0 before dosing, the same day of treatment • QT interval data are adjusted for heart rate (HR) to obtain QTc values which are correlated with HR as little as possible. – Fridericia’s corrected values – Individual corrected values – Bazett’s corrected values 13
Design Considerations (2) • QT data are highly variable, so control of all sources of variability is critical • Replicated QT measurements at each time-point are recommended. Typically ≥ 3 replicates. The mean of these replicates is used. • The baseline QT measurements should be taken as close to the treatment date as possible; typically either – At matching time-points on the day prior to drug treatment; or – At time 0 before dosing, the same day of treatment • QT interval data are adjusted for heart rate (HR) to obtain QTc values which are correlated with HR as little as possible. – Fridericia’s corrected values – Individual corrected values – Bazett’s corrected values 13
 Crossover Design • A crossover QT/QTc study is recommended as opposed to a parallel study if possible since – It requires smaller number of subjects – The precision of estimated treatment differences is greater since subjects act as their own control • Methods and practice applicable in any cross-over trial should be applied in a crossover QT/QTc trial. • Some issues encountered in a crossover-trial need to be considered here; including period effect and carry-over or residual effect. 14
Crossover Design • A crossover QT/QTc study is recommended as opposed to a parallel study if possible since – It requires smaller number of subjects – The precision of estimated treatment differences is greater since subjects act as their own control • Methods and practice applicable in any cross-over trial should be applied in a crossover QT/QTc trial. • Some issues encountered in a crossover-trial need to be considered here; including period effect and carry-over or residual effect. 14
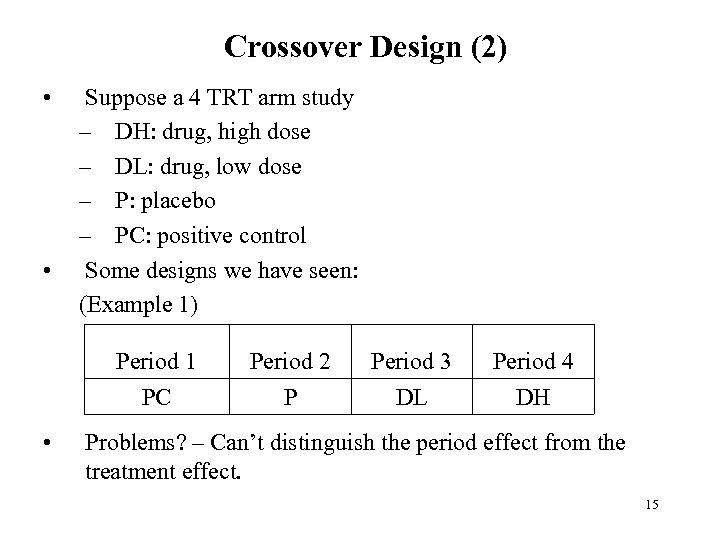 Crossover Design (2) • • Suppose a 4 TRT arm study – DH: drug, high dose – DL: drug, low dose – P: placebo – PC: positive control Some designs we have seen: (Example 1) Period 1 Period 3 Period 4 PC • Period 2 P DL DH Problems? – Can’t distinguish the period effect from the treatment effect. 15
Crossover Design (2) • • Suppose a 4 TRT arm study – DH: drug, high dose – DL: drug, low dose – P: placebo – PC: positive control Some designs we have seen: (Example 1) Period 1 Period 3 Period 4 PC • Period 2 P DL DH Problems? – Can’t distinguish the period effect from the treatment effect. 15
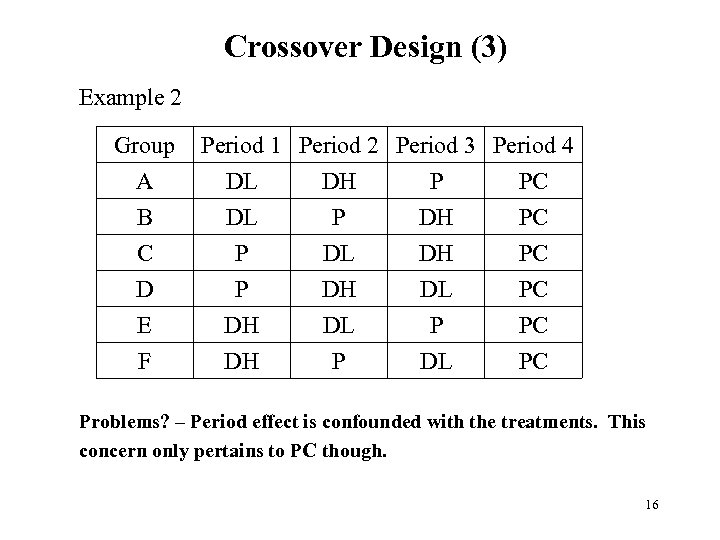 Crossover Design (3) Example 2 Group A B C D E F Period 1 Period 2 Period 3 Period 4 DL DH P PC DL P DH PC P DL DH PC P DH DL PC DH DL P PC DH P DL PC Problems? – Period effect is confounded with the treatments. This concern only pertains to PC though. 16
Crossover Design (3) Example 2 Group A B C D E F Period 1 Period 2 Period 3 Period 4 DL DH P PC DL P DH PC P DL DH PC P DH DL PC DH DL P PC DH P DL PC Problems? – Period effect is confounded with the treatments. This concern only pertains to PC though. 16
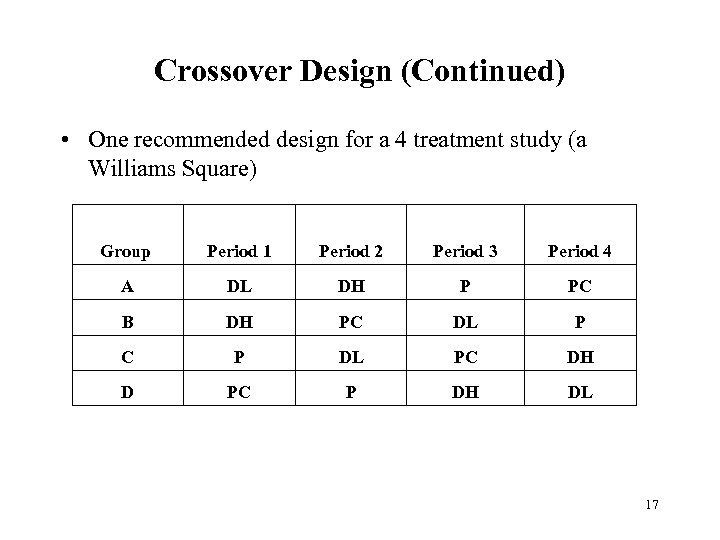 Crossover Design (Continued) • One recommended design for a 4 treatment study (a Williams Square) Group Period 1 Period 2 Period 3 Period 4 A DL DH P PC B DH PC DL P C P DL PC DH D PC P DH DL 17
Crossover Design (Continued) • One recommended design for a 4 treatment study (a Williams Square) Group Period 1 Period 2 Period 3 Period 4 A DL DH P PC B DH PC DL P C P DL PC DH D PC P DH DL 17
 Parallel group studies may have to be used under certain circumstances: • For drugs with long elimination half-lives • For drugs whose carryover effects are prominent 18
Parallel group studies may have to be used under certain circumstances: • For drugs with long elimination half-lives • For drugs whose carryover effects are prominent 18
 Sample Size Required for a Crossover Design and a Parallel Design Notations: • N 1 - # of subjects in a crossover study • N 2 - # of subjects per arm in a parallel study • - observed difference in treatment (adjusted for baseline) for an individual • SD( ) = 1 for a crossover; 2 for a parallel • D – SD for the drug • P – SD for placebo • 12 – Correlation coefficient between the drug and placebo for the same individual (>0) Relation between N 1 and N 2 • In order to make Var(mean of ) the same for both crossover and parallel studies, we have N 2 = ( 2/ 1)2 N 1 • Relation between 1 and 2: 22 = 12 + 2 12 D P • For a 4 arm trial, sample needed for a parallel study can be at least 4 times more than a crossover study 19
Sample Size Required for a Crossover Design and a Parallel Design Notations: • N 1 - # of subjects in a crossover study • N 2 - # of subjects per arm in a parallel study • - observed difference in treatment (adjusted for baseline) for an individual • SD( ) = 1 for a crossover; 2 for a parallel • D – SD for the drug • P – SD for placebo • 12 – Correlation coefficient between the drug and placebo for the same individual (>0) Relation between N 1 and N 2 • In order to make Var(mean of ) the same for both crossover and parallel studies, we have N 2 = ( 2/ 1)2 N 1 • Relation between 1 and 2: 22 = 12 + 2 12 D P • For a 4 arm trial, sample needed for a parallel study can be at least 4 times more than a crossover study 19
 Statistical Test for a Negative ‘Thorough QT/QTc Study’ 20
Statistical Test for a Negative ‘Thorough QT/QTc Study’ 20
 Calculating Time-matched Mean Difference • How to estimate the time-matched population mean effect? Qit: (Baseline adjusted) QT/QTc value for the ith subject at time t after receiving the drug Pit: (Baseline adjusted) QT/QTc value for the ith subject at time t after receiving placebo Time-matched mean difference at time t (suppose equal sample size N in both the drug and placebo groups) Qit/N - Pit/N = (For a crossover) N is the number of subjects 21
Calculating Time-matched Mean Difference • How to estimate the time-matched population mean effect? Qit: (Baseline adjusted) QT/QTc value for the ith subject at time t after receiving the drug Pit: (Baseline adjusted) QT/QTc value for the ith subject at time t after receiving placebo Time-matched mean difference at time t (suppose equal sample size N in both the drug and placebo groups) Qit/N - Pit/N = (For a crossover) N is the number of subjects 21
 Statistical Hypotheses • Hypotheses: H 0: t(D) - t(P) ≥ ms for at least one t H 1: t(D) - t(P) < ms for all t • is the non-inferiority margin (10 ms in the guidance) • t(D) and t(P) are the population means for the drug and placebo at time t, t=1, 2, …, K. K is the total number of time points where QT has been measured. • Claim a negative QT/QTc study if H 0 is rejected • Use = 0. 05 22
Statistical Hypotheses • Hypotheses: H 0: t(D) - t(P) ≥ ms for at least one t H 1: t(D) - t(P) < ms for all t • is the non-inferiority margin (10 ms in the guidance) • t(D) and t(P) are the population means for the drug and placebo at time t, t=1, 2, …, K. K is the total number of time points where QT has been measured. • Claim a negative QT/QTc study if H 0 is rejected • Use = 0. 05 22
 Statistical Test • Let be the observed average time-matched drug placebo difference after baseline adjusted at time t. Let Tt be the test statistic at time t, then Tt = ( - )/SD( ) Reject H 0 if Tt < -t , N-1 for all t, where t , N-1 is the upper tail level critical value for the t distribution with N-1 df, and N is the sample size. The above procedure is to test if all one sided 95% CI upper bounds are < ms at each time point. 23
Statistical Test • Let be the observed average time-matched drug placebo difference after baseline adjusted at time t. Let Tt be the test statistic at time t, then Tt = ( - )/SD( ) Reject H 0 if Tt < -t , N-1 for all t, where t , N-1 is the upper tail level critical value for the t distribution with N-1 df, and N is the sample size. The above procedure is to test if all one sided 95% CI upper bounds are < ms at each time point. 23
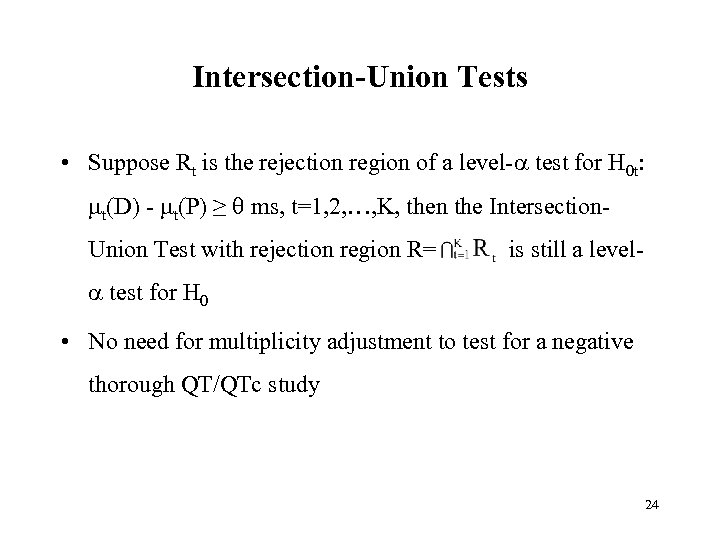 Intersection-Union Tests • Suppose Rt is the rejection region of a level- test for H 0 t: t(D) - t(P) ≥ ms, t=1, 2, …, K, then the Intersection. Union Test with rejection region R= is still a level- test for H 0 • No need for multiplicity adjustment to test for a negative thorough QT/QTc study 24
Intersection-Union Tests • Suppose Rt is the rejection region of a level- test for H 0 t: t(D) - t(P) ≥ ms, t=1, 2, …, K, then the Intersection. Union Test with rejection region R= is still a level- test for H 0 • No need for multiplicity adjustment to test for a negative thorough QT/QTc study 24
 False Positive & False Negative Probabilities 25
False Positive & False Negative Probabilities 25
 Definitions • Type I error: False Negative Probability (FNP) FNP = P(Tt < - t , N-1 for all t | at least one true difference ≥ ) By IUT, FNP can be controlled under (0. 05) level • Type II error: False Positive Probability (FPP) FPP = P(Tt ≥ - t , N-1 for at least one t | true difference < 5 ms at each time point) Why 5 ms chosen: “… drugs that prolong the mean QT/QTc interval by around 5 ms or less do not appear to cause Td. P” – ICH E 14 26
Definitions • Type I error: False Negative Probability (FNP) FNP = P(Tt < - t , N-1 for all t | at least one true difference ≥ ) By IUT, FNP can be controlled under (0. 05) level • Type II error: False Positive Probability (FPP) FPP = P(Tt ≥ - t , N-1 for at least one t | true difference < 5 ms at each time point) Why 5 ms chosen: “… drugs that prolong the mean QT/QTc interval by around 5 ms or less do not appear to cause Td. P” – ICH E 14 26
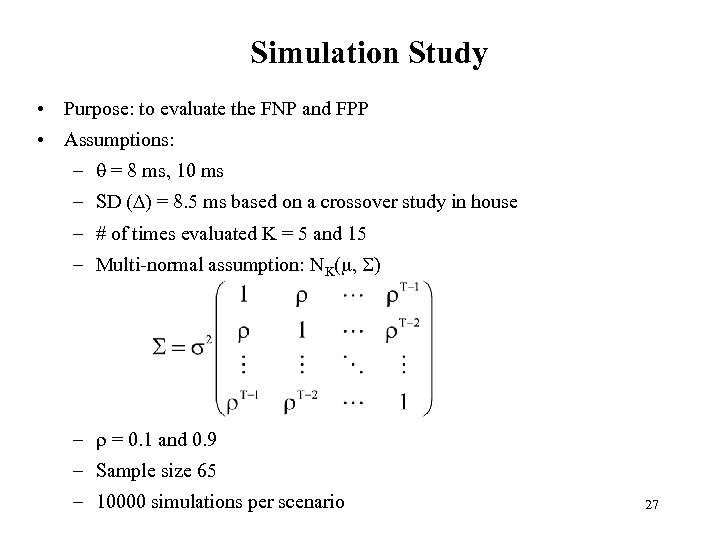 Simulation Study • Purpose: to evaluate the FNP and FPP • Assumptions: – = 8 ms, 10 ms – SD ( ) = 8. 5 ms based on a crossover study in house – # of times evaluated K = 5 and 15 – Multi-normal assumption: NK( , ) – = 0. 1 and 0. 9 – Sample size 65 – 10000 simulations per scenario 27
Simulation Study • Purpose: to evaluate the FNP and FPP • Assumptions: – = 8 ms, 10 ms – SD ( ) = 8. 5 ms based on a crossover study in house – # of times evaluated K = 5 and 15 – Multi-normal assumption: NK( , ) – = 0. 1 and 0. 9 – Sample size 65 – 10000 simulations per scenario 27
 Results: False Negative Probability Table 1 K=5 K=15 (0, 0, 8, 0, 0) (0, 0, 8, 8, 0) (0 -0, 8, 0 -0) (0 -0, 8, 8, 0 -0) 8 0. 1 0. 044 0. 005 0. 051 0. 004 8 0. 9 0. 041 0. 036 0. 047 0. 028 (0, 0, 10, 0, 0) (0, 0, 10, 0) (0 -0, 10, 0 -0) 10 0. 1 0. 037 0. 002 0. 051 0. 002 10 0. 9 0. 052 0. 036 0. 046 0. 031 28
Results: False Negative Probability Table 1 K=5 K=15 (0, 0, 8, 0, 0) (0, 0, 8, 8, 0) (0 -0, 8, 0 -0) (0 -0, 8, 8, 0 -0) 8 0. 1 0. 044 0. 005 0. 051 0. 004 8 0. 9 0. 041 0. 036 0. 047 0. 028 (0, 0, 10, 0, 0) (0, 0, 10, 0) (0 -0, 10, 0 -0) 10 0. 1 0. 037 0. 002 0. 051 0. 002 10 0. 9 0. 052 0. 036 0. 046 0. 031 28
 Results: Power (1 -FPP) Curves ( =(2, 3, 4, 4, 3)’, =0. 1) 29
Results: Power (1 -FPP) Curves ( =(2, 3, 4, 4, 3)’, =0. 1) 29
 Results: Power (1 -FPP) Curves ( =(2, 3, 4, 4, 3)’, =0. 9) 30
Results: Power (1 -FPP) Curves ( =(2, 3, 4, 4, 3)’, =0. 9) 30
 Conclusions This simulation example shows: • FNP is well controlled under the level • FPP (or 1 - Power) is driven by – The true mean difference of the drug and placebo – The Non-inferiority margin – Variability of the data – Sample size 31
Conclusions This simulation example shows: • FNP is well controlled under the level • FPP (or 1 - Power) is driven by – The true mean difference of the drug and placebo – The Non-inferiority margin – Variability of the data – Sample size 31
 Is it true the greater the number of time points, the higher the type II error? (N=65) Table 2 Scenario , K Assumed True Mean Difference r=0. 1 r=0. 9 a 8, 5 (3, 4, 4. 9, 4, 1) 0. 134 0. 109 b 8, 15 (0, 0, 0, 1, 1, 3, 4, 4. 9, 4, 1, 1, 1, 0, 0, 0) 0. 132 0. 103 c 8, 5 (1, 1, 1, 4. 9, 1) 0. 101 0. 106 d 8, 15 (0, 0, 0, 1, 1, 1, 4. 9, 1, 1, 1, 0, 0, 0) 0. 112 0. 097 e 10, 5 (3, 4, 4. 9, 4, 1) 0. 001 f 10, 15 (0, 0, 0, 1, 1, 1, 4. 9, 1, 1, 1, 0, 0, 0) 0. 001 g 10, 5 (1, 1, 1, 4. 9, 1) 0. 001 h 10, 15 (0, 0, 0, 1, 1, 1, 4. 9, 1, 1, 1, 0, 0, 0) 0. 001 32
Is it true the greater the number of time points, the higher the type II error? (N=65) Table 2 Scenario , K Assumed True Mean Difference r=0. 1 r=0. 9 a 8, 5 (3, 4, 4. 9, 4, 1) 0. 134 0. 109 b 8, 15 (0, 0, 0, 1, 1, 3, 4, 4. 9, 4, 1, 1, 1, 0, 0, 0) 0. 132 0. 103 c 8, 5 (1, 1, 1, 4. 9, 1) 0. 101 0. 106 d 8, 15 (0, 0, 0, 1, 1, 1, 4. 9, 1, 1, 1, 0, 0, 0) 0. 112 0. 097 e 10, 5 (3, 4, 4. 9, 4, 1) 0. 001 f 10, 15 (0, 0, 0, 1, 1, 1, 4. 9, 1, 1, 1, 0, 0, 0) 0. 001 g 10, 5 (1, 1, 1, 4. 9, 1) 0. 001 h 10, 15 (0, 0, 0, 1, 1, 1, 4. 9, 1, 1, 1, 0, 0, 0) 0. 001 32
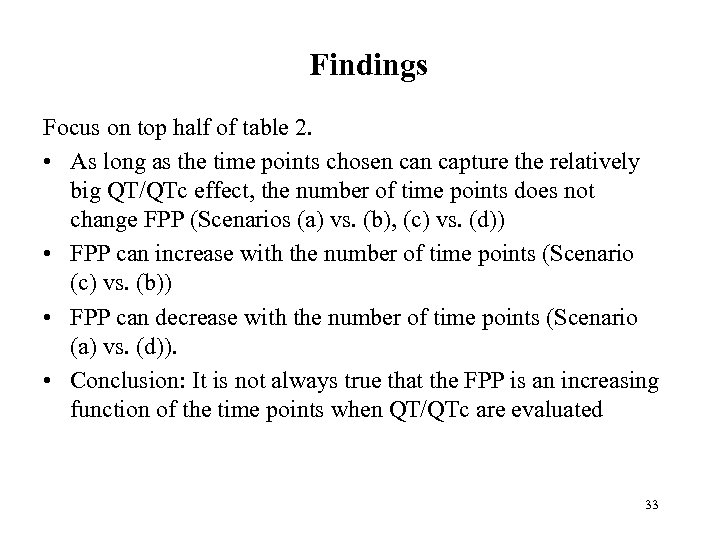 Findings Focus on top half of table 2. • As long as the time points chosen capture the relatively big QT/QTc effect, the number of time points does not change FPP (Scenarios (a) vs. (b), (c) vs. (d)) • FPP can increase with the number of time points (Scenario (c) vs. (b)) • FPP can decrease with the number of time points (Scenario (a) vs. (d)). • Conclusion: It is not always true that the FPP is an increasing function of the time points when QT/QTc are evaluated 33
Findings Focus on top half of table 2. • As long as the time points chosen capture the relatively big QT/QTc effect, the number of time points does not change FPP (Scenarios (a) vs. (b), (c) vs. (d)) • FPP can increase with the number of time points (Scenario (c) vs. (b)) • FPP can decrease with the number of time points (Scenario (a) vs. (d)). • Conclusion: It is not always true that the FPP is an increasing function of the time points when QT/QTc are evaluated 33
 Assay Sensitivity 34
Assay Sensitivity 34
 Assay Sensitivity ICH E 14: “The positive control should have an effect on the mean QT/QTc interval of about 5 ms” The positive control “should be well-characterized and consistently produce an effect on the QT/QTc interval that is around the threshold of regulatory concern (5 ms, section 2. 2. ). ” 35
Assay Sensitivity ICH E 14: “The positive control should have an effect on the mean QT/QTc interval of about 5 ms” The positive control “should be well-characterized and consistently produce an effect on the QT/QTc interval that is around the threshold of regulatory concern (5 ms, section 2. 2. ). ” 35
 Statistical Procedures to Assess Assay Sensitivity • H 0: t(PC) - t(P) < c ms for all t H 1: t(PC) - t(P) ≥ c ms for at least one t • How to choose c? Under discussion. • Challenge: Multiple endpoint issues (can’t apply IUT here) 36
Statistical Procedures to Assess Assay Sensitivity • H 0: t(PC) - t(P) < c ms for all t H 1: t(PC) - t(P) ≥ c ms for at least one t • How to choose c? Under discussion. • Challenge: Multiple endpoint issues (can’t apply IUT here) 36
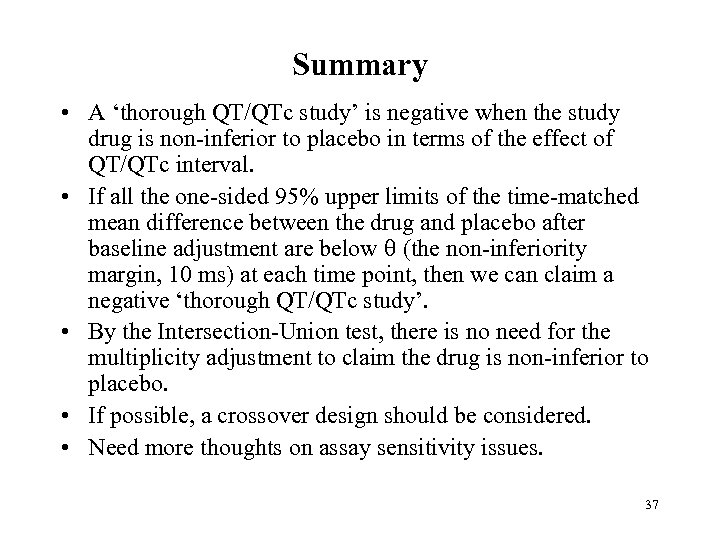 Summary • A ‘thorough QT/QTc study’ is negative when the study drug is non-inferior to placebo in terms of the effect of QT/QTc interval. • If all the one-sided 95% upper limits of the time-matched mean difference between the drug and placebo after baseline adjustment are below (the non-inferiority margin, 10 ms) at each time point, then we can claim a negative ‘thorough QT/QTc study’. • By the Intersection-Union test, there is no need for the multiplicity adjustment to claim the drug is non-inferior to placebo. • If possible, a crossover design should be considered. • Need more thoughts on assay sensitivity issues. 37
Summary • A ‘thorough QT/QTc study’ is negative when the study drug is non-inferior to placebo in terms of the effect of QT/QTc interval. • If all the one-sided 95% upper limits of the time-matched mean difference between the drug and placebo after baseline adjustment are below (the non-inferiority margin, 10 ms) at each time point, then we can claim a negative ‘thorough QT/QTc study’. • By the Intersection-Union test, there is no need for the multiplicity adjustment to claim the drug is non-inferior to placebo. • If possible, a crossover design should be considered. • Need more thoughts on assay sensitivity issues. 37


