5ad591e4b938fc229b6893319d05d5cb.ppt
- Количество слайдов: 30

Regulations of China Medical Device Sunjingsheng Beijing institute of medical device testing 2013. 11 1
---Brief Introduction of BIMT ØBIMT, with its former name Beijing Medical Device Testing Station , was established in 1983 and it was attached to the former Beijing Medical Equipment Institute. ØBIMT has reformed into a public institution with independent judicial person since 2000, which was an affiliate of BJDA. ØSame time It is a subordinate agency of SFDA.
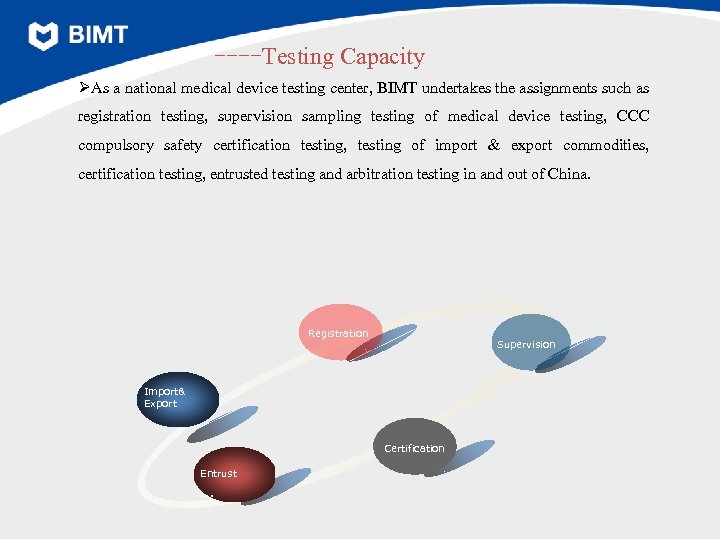
----Testing Capacity ØAs a national medical device testing center, BIMT undertakes the assignments such as registration testing, supervision sampling testing of medical device testing, CCC compulsory safety certification testing, testing of import & export commodities, certification testing, entrusted testing and arbitration testing in and out of China. Registration Supervision Import& Export Certification Entrust …
Content 1. Present situation of CFDA 2. Present medical device regulations system 3. Present medical device standards system 4
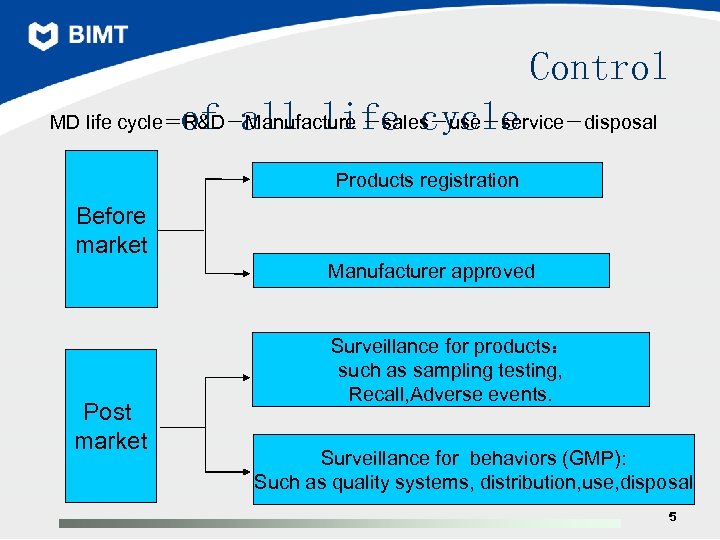
Control of all life cycle MD life cycle=R&D-Manufacture -sales-use-service-disposal Products registration Before market Manufacturer approved Post market Surveillance for products: such as sampling testing, Recall, Adverse events. Surveillance for behaviors (GMP): Such as quality systems, distribution, use, disposal 5
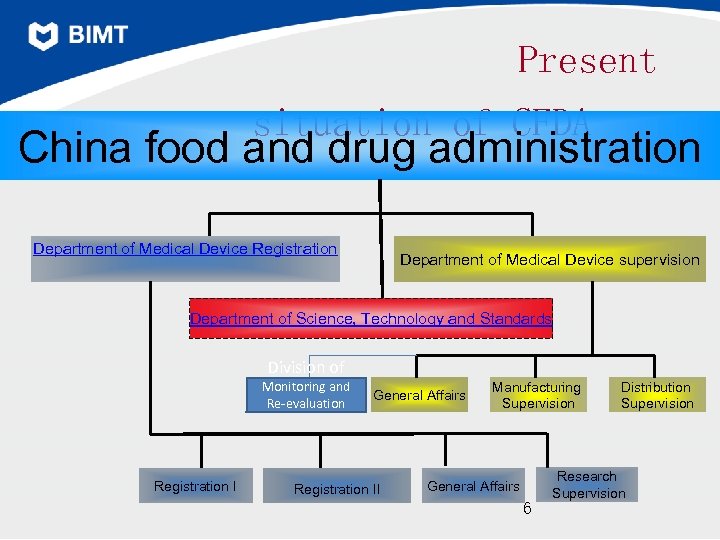
Present situation of CFDA China food and drug administration Department of Medical Device Registration Department of Medical Device supervision Department of Science, Technology and Standards Division of Monitoring and Re-evaluation Registration I General Affairs Registration II Manufacturing Supervision General Affairs 6 Distribution Supervision Research Supervision

Department of Medical Device Registration • To conduct registration for Class III and import medical devices in strict accordance with the conditions and procedures prescribed by law, take the correspondent responsibilities, optimize registration control procedures, organize and implement classification administration, and supervise the implementation of good practices for medical devices. 7

Department of Medical Device Supervision • To track and analyze medical device safety situation and existing problems, and to put forward recommendations on system, mechanism and performance improvement; to supervise the low-level administrative departments in conducting administrative licensing by law, in performing the administrative duty, in detecting and rectifying illegal and improper acts in time; to organize and conduct medical device adverse events monitoring and reevaluation. 8

Department of Science, Technology and Standards To organize and implement major science and technology programs for food and drug supervision, accelerate the construction of food and drug testing system, electronic supervision tracking system, and information system; to draft qualification requirements and testing norms governing food and drug testing institutions, and supervise their implementation; to organize the drafting of standards for drugs, medical devices, cosmetics, and catalogues, pharmaceutical use requirements, standards for immediate packaging materials and containers, and participate in the drafting of food safety standards. 9

Technical Support institutes • Center of Medical Device Evaluation • Center of Medical Device Standards Management • Medical Device Standards Technical Committees • Institute of Medical Device Testing • Center of Drug Adverse Events Monitoring and Reevaluation. 10

Characteristics of structure reform • Strengthen post market supervision • Stress on R&D and standards 11
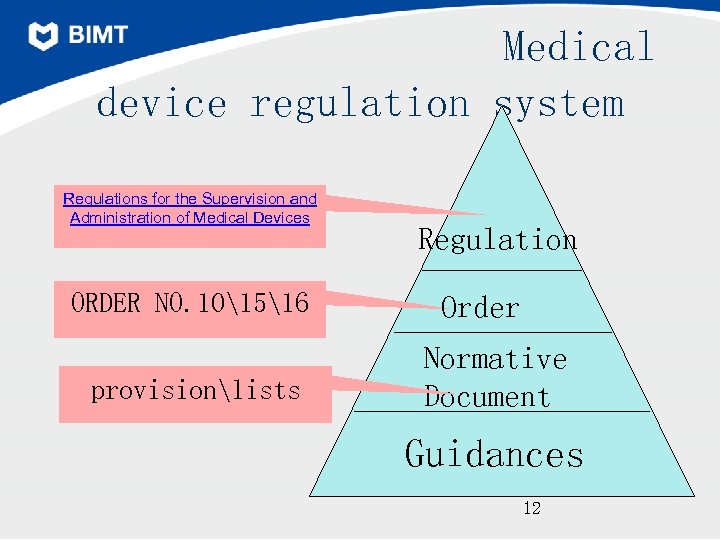
Medical device regulation system Regulations for the Supervision and Administration of Medical Devices ORDER NO. 101516 provisionlists Regulation Order Normative Document Guidances 12

Regulations for the Supervision and Administration of Medical Devices • 条例: Regulations for the Supervision and Administration of Medical Devices State council directive NO. 276 2000. 01. 04 2000. 04. 01 implement Release 13
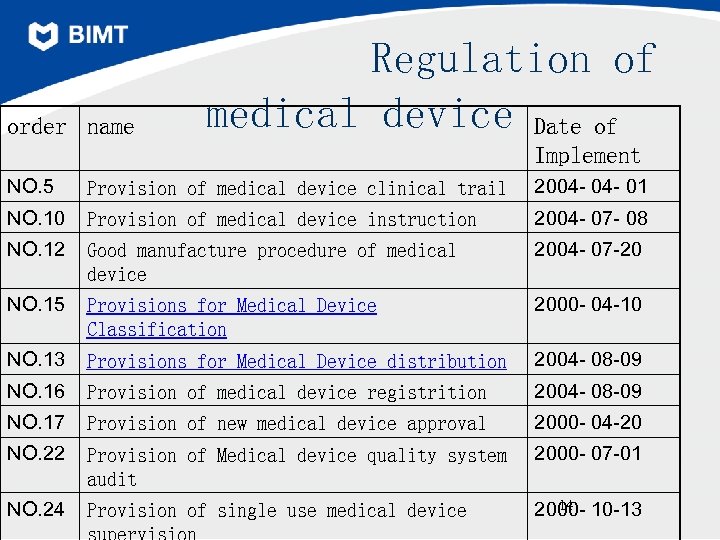
order name Regulation of medical device Date of Implement NO. 5 Provision of medical device clinical trail 2004 - 01 NO. 10 Provision of medical device instruction 2004 - 07 - 08 NO. 12 Good manufacture procedure of medical device 2004 - 07 -20 NO. 15 Provisions for Medical Device Classification 2000 - 04 -10 NO. 13 Provisions for Medical Device distribution 2004 - 08 -09 NO. 16 Provision of medical device registrition 2004 - 08 -09 NO. 17 Provision of new medical device approval 2000 - 04 -20 NO. 22 Provision of Medical device quality system audit 2000 - 07 -01 NO. 24 Provision of single use medical device 14 2000 - 10 -13

Technical guidances 国家食品药品监督管理局发布的医疗器械技术审评指导原则 第二类纤维内窥镜产品注册技术审查指导原则 第二类硬管内窥镜产品注册技术审查指导原则 中频电疗产品注册技术审查指导原则 B型超声诊断设备(第二类)产品注册技术审查指导原则 心电图机产品注册技术审查指导原则 电动手术台指导原则 磁疗产品注册技术审查指导原则 电动病床产品注册技术审查指导原则 3 A类半导体激光治疗机产品注册技术审查指导原则 电子血压计(示波法)产品注册技术审查指导原则 红外乳腺检查仪产品注册技术审查指导原则 注射泵产品注册技术审查指导原则 超声理疗设备产品注册技术审查指导原则 牙科综合治疗机产品注册技术审查指导原则 15

Medical Device Classification • The State shall classify medical devices and administer them based on this classification – Class I Medical Devices are those for which safety and effectiveness can be ensured through routine administration; – Class II Medical Devices are those for which further control is required to ensure their safety and effectiveness – Class III Medical Devices are those which are implanted into the human body, or used for life support or sustenance, or pose potential 16


Order NO. 15 Provisions for Medical Device Classification • Article 3 The Provisions are meant to direct the formulation of The Category of Medical Device Classification as well as to determine the classes of newly registered products. • Article 4 The classification of medical devices should be determined by a combined judgement on three respects: its structural characteristics, form of operation as well as conditions for use. Specifically, their classification can be based on Criteria for Medical Device Classification (see appendix). • 20
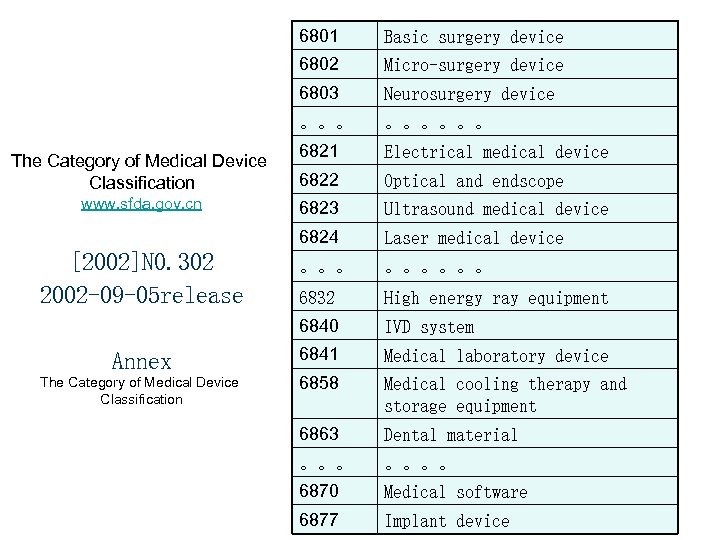
6801 Basic surgery device 6802 Micro-surgery device 6803 Neurosurgery device 。。。。。。 6821 Electrical medical device 6822 Optical and endscope 6823 Ultrasound medical device 6824 Laser medical device 。。。。。。 6832 High energy ray equipment 6840 IVD system Annex 6841 Medical laboratory device The Category of Medical Device Classification 6858 Medical cooling therapy and storage equipment 6863 Dental material 。。。。 6870 Medical software 6877 Implant device The Category of Medical Device Classification www. sfda. gov. cn [2002]NO. 302 2002 -09 -05 release 21
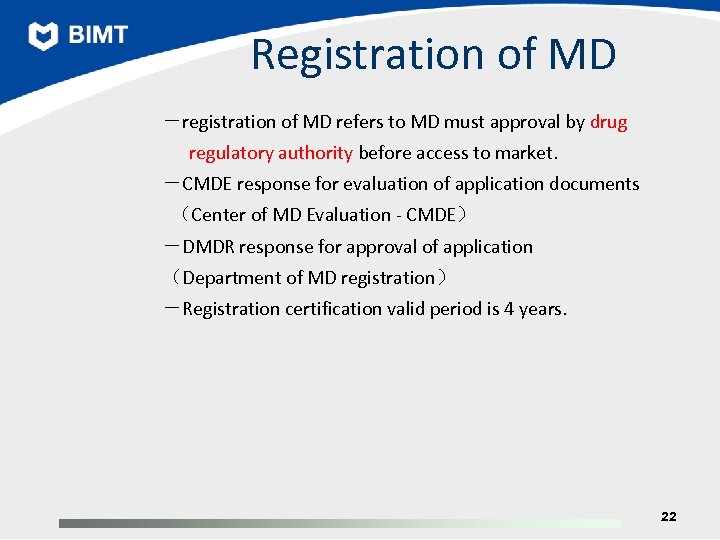
Registration of MD -registration of MD refers to MD must approval by drug regulatory authority before access to market. -CMDE response for evaluation of application documents (Center of MD Evaluation - CMDE) -DMDR response for approval of application (Department of MD registration) -Registration certification valid period is 4 years. 22

• • • Classification registration Article 8 The State shall implement a product registration system for the manufacturing of medical devices. Class I medical devices shall be inspected, approved and granted with a registration certificate by the drug regulatory authority of the government of the municipalities consisting of districts. Class II medical devices shall be inspected, approved and granted with registration certificates by the drug regulatory authorities of provinces, autonomous regions and municipalities directly under the central government. Class III medical devices shall be inspected, approved and granted with registration certificates by the drug regulatory authority directly under the State Council. Importing MD shall be inspected, approved and granted with registration certificates by the drug regulatory authority directly under the State Council. 23
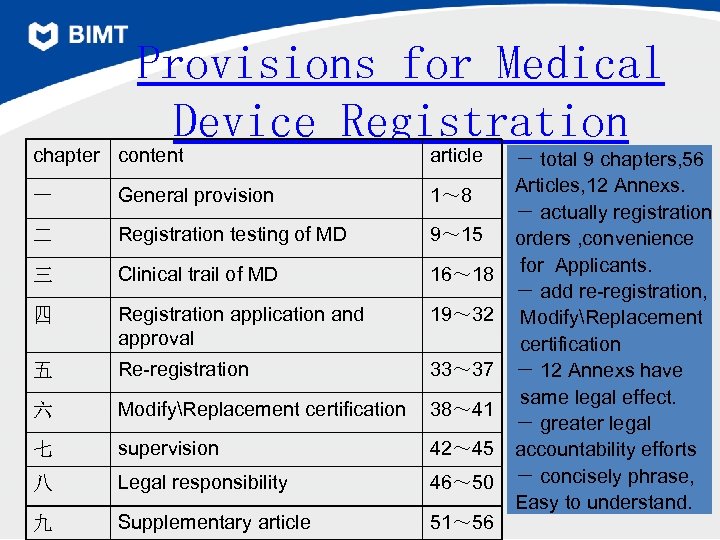
Provisions for Medical Device Registration chapter content article 一 General provision 1~ 8 二 Registration testing of MD 9~ 15 三 Clinical trail of MD 16~ 18 四 Registration application and approval 19~ 32 五 Re-registration 33~ 37 六 ModifyReplacement certification 38~ 41 七 supervision 42~ 45 八 Legal responsibility 46~ 50 九 Supplementary article 51~ 56 - total 9 chapters, 56 Articles, 12 Annexs. - actually registration orders , convenience for Applicants. - add re-registration, ModifyReplacement certification - 12 Annexs have same legal effect. - greater legal accountability efforts - concisely phrase, Easy to understand. 24
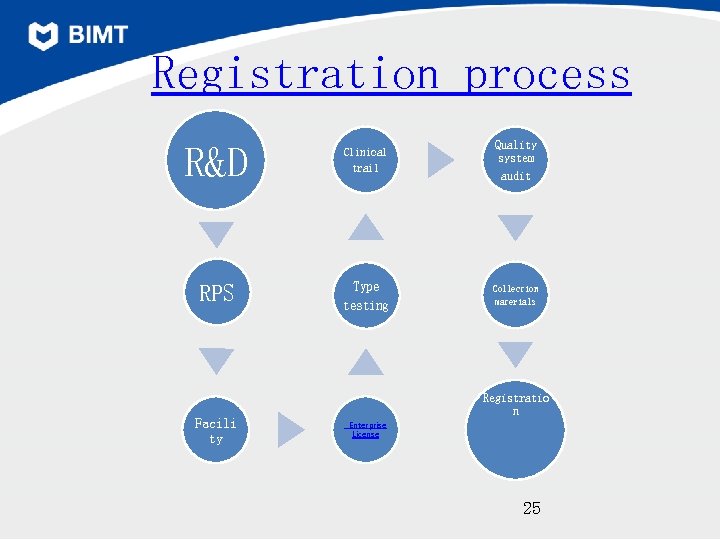
Registration process R&D Clinical trail Quality system audit RPS Type testing Collection materials Facili ty Registratio n Enterprise License 25

Standards of china MD -Category national standard (GB, GB/T) mandatory(GB, YY、YZB)、 industrial standard(YY, YY/T) recommend (GB/T, YY/T) registration product standard(YZB)。 -until DEC. 2012, There are 1050 MD standards,of which national standards 180,of which mandatory standards 90; industrial standards 870,of whichmandatorystandards 320. 26
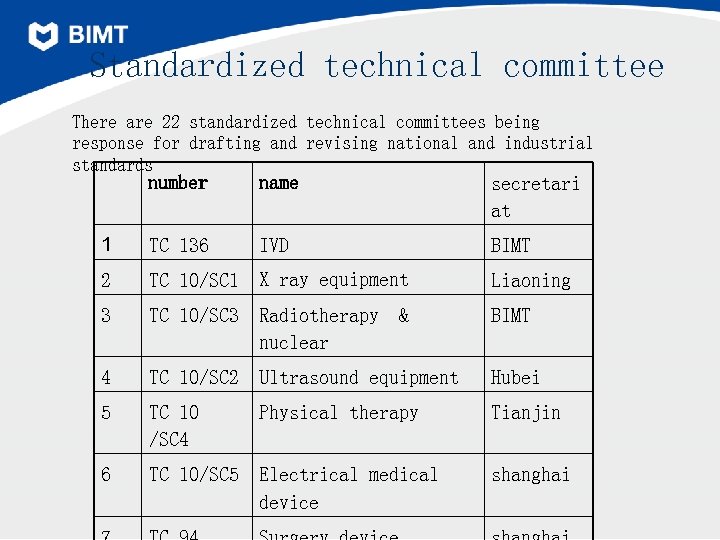
Standardized technical committee There are 22 standardized technical committees being response for drafting and revising national and industrial standards number name secretari at 1 TC 136 IVD BIMT 2 TC 10/SC 1 X ray equipment Liaoning 3 TC 10/SC 3 Radiotherapy nuclear BIMT 4 TC 10/SC 2 Ultrasound equipment Hubei 5 TC 10 /SC 4 Physical therapy Tianjin 6 TC 10/SC 5 Electrical medical device shanghai &

RPS - MD Should have RPS including national standards and industrial standards,but requirements of RPS shall not be lower than requirements of national and industrial standards. - manufacturers are responsible for RPS. - Chinese authority address “RPS is industrial standard , if no corresponding national or industrial standard” 28
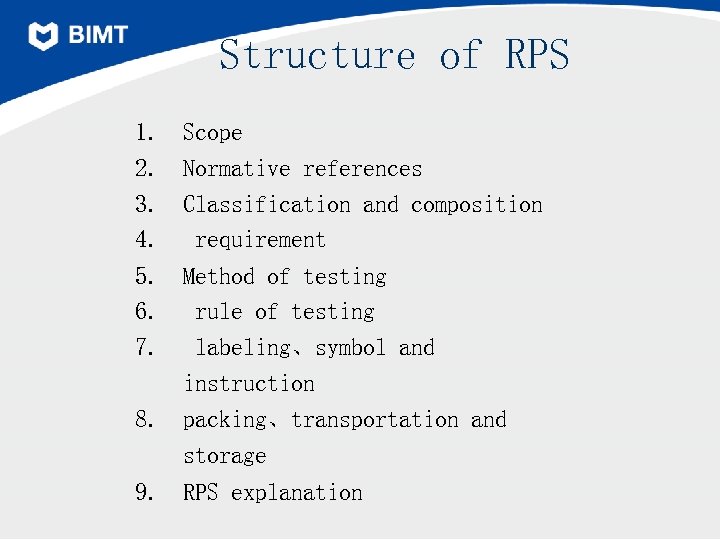
Structure of RPS 1. Scope 2. Normative references 3. Classification and composition 4. requirement 5. Method of testing 6. rule of testing 7. labeling、symbol and instruction 8. packing、transportation and storage 9. RPS explanation

• thanks! 30
5ad591e4b938fc229b6893319d05d5cb.ppt