9bb431cb7830801b644a04a19c5a350e.ppt
- Количество слайдов: 27
 REGULATION IN THE PROGRESS, CHALLENGES AND OPPORTUNITIES OF MEDICAL DEVICE IN COLOMBIA. Dr. Blanca Elvira Cajigas General Director Ottawa, Canada 7 Septiembre de 2013 PM 06 -CAT-G 17 V 4 01/07/2012
REGULATION IN THE PROGRESS, CHALLENGES AND OPPORTUNITIES OF MEDICAL DEVICE IN COLOMBIA. Dr. Blanca Elvira Cajigas General Director Ottawa, Canada 7 Septiembre de 2013 PM 06 -CAT-G 17 V 4 01/07/2012
 CONTENTS I. REGULATION PROGRESS IN MEDICAL DEVICES IN COLOMBIA. II. CHALLENGES AND OPPORTUNITIES FOR STRENGTHENING THE REGULATION OF MEDICAL DEVICES IN THE REGIONAL AREA PM 06 -CAT-G 17 V 4 01/07/2012
CONTENTS I. REGULATION PROGRESS IN MEDICAL DEVICES IN COLOMBIA. II. CHALLENGES AND OPPORTUNITIES FOR STRENGTHENING THE REGULATION OF MEDICAL DEVICES IN THE REGIONAL AREA PM 06 -CAT-G 17 V 4 01/07/2012
 I. REGULATION PROGRESS IN MEDICAL DEVICES IN COLOMBIA. PM 06 -CAT-G 17 V 4 01/07/2012
I. REGULATION PROGRESS IN MEDICAL DEVICES IN COLOMBIA. PM 06 -CAT-G 17 V 4 01/07/2012
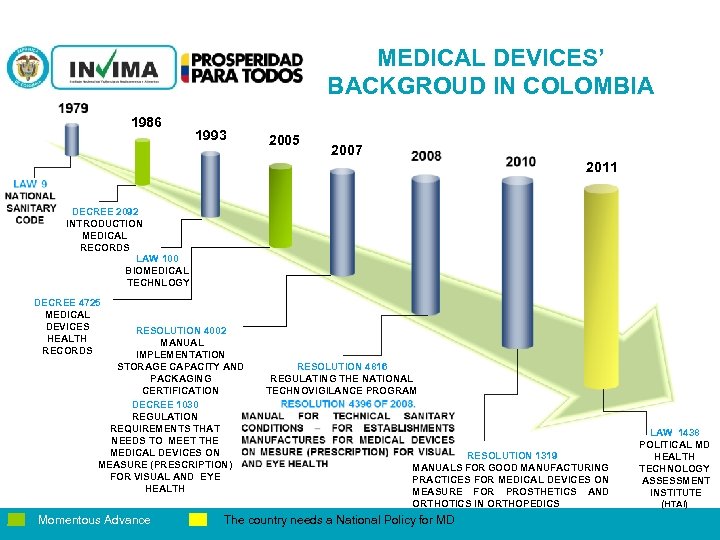 MEDICAL DEVICES’ BACKGROUD IN COLOMBIA 1986 1993 2005 2007 2011 DECREE 2092 INTRODUCTION MEDICAL RECORDS LAW 100 BIOMEDICAL TECHNLOGY DECREE 4725 MEDICAL DEVICES HEALTH RECORDS RESOLUTION 4002 MANUAL IMPLEMENTATION STORAGE CAPACITY AND PACKAGING CERTIFICATION DECREE 1030 REGULATION REQUIREMENTS THAT NEEDS TO MEET THE MEDICAL DEVICES ON MEASURE (PRESCRIPTION) FOR VISUAL AND EYE HEALTH Momentous Advance RESOLUTION 4816 REGULATING THE NATIONAL TECHNOVIGILANCE PROGRAM RESOLUTION 1319 MANUALS FOR GOOD MANUFACTURING PRACTICES FOR MEDICAL DEVICES ON MEASURE FOR PROSTHETICS AND ORTHOTICS IN ORTHOPEDICS PM 06 -CAT-G 17 V 4 01/07/2012 The country needs a National Policy for MD LAW 1438 POLITICAL MD HEALTH TECHNOLOGY ASSESSMENT INSTITUTE (HTAI)
MEDICAL DEVICES’ BACKGROUD IN COLOMBIA 1986 1993 2005 2007 2011 DECREE 2092 INTRODUCTION MEDICAL RECORDS LAW 100 BIOMEDICAL TECHNLOGY DECREE 4725 MEDICAL DEVICES HEALTH RECORDS RESOLUTION 4002 MANUAL IMPLEMENTATION STORAGE CAPACITY AND PACKAGING CERTIFICATION DECREE 1030 REGULATION REQUIREMENTS THAT NEEDS TO MEET THE MEDICAL DEVICES ON MEASURE (PRESCRIPTION) FOR VISUAL AND EYE HEALTH Momentous Advance RESOLUTION 4816 REGULATING THE NATIONAL TECHNOVIGILANCE PROGRAM RESOLUTION 1319 MANUALS FOR GOOD MANUFACTURING PRACTICES FOR MEDICAL DEVICES ON MEASURE FOR PROSTHETICS AND ORTHOTICS IN ORTHOPEDICS PM 06 -CAT-G 17 V 4 01/07/2012 The country needs a National Policy for MD LAW 1438 POLITICAL MD HEALTH TECHNOLOGY ASSESSMENT INSTITUTE (HTAI)
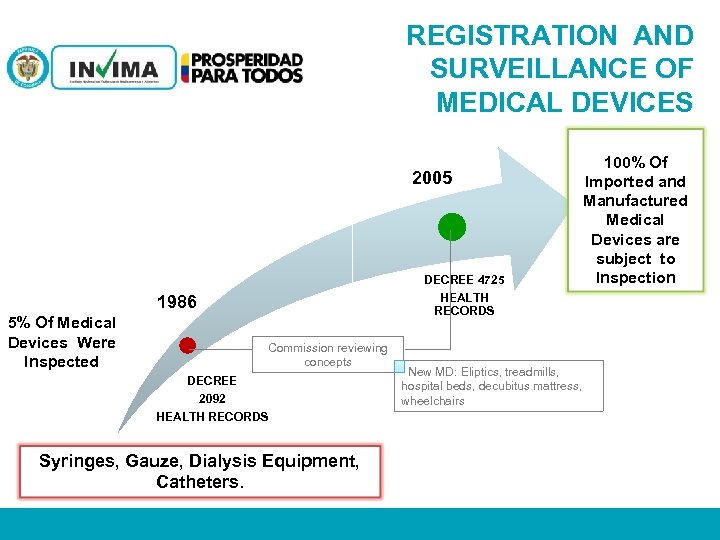 REGISTRATION AND SURVEILLANCE OF MEDICAL DEVICES 2005 DECREE 4725 HEALTH RECORDS 1986 5% Of Medical Devices Were Inspected 100% Of Imported and Manufactured Medical Devices are subject to Inspection Commission reviewing concepts DECREE 2092 HEALTH RECORDS New MD: Eliptics, treadmills, hospital beds, decubitus mattress, wheelchairs Syringes, Gauze, Dialysis Equipment, Catheters. PM 06 -CAT-G 17 V 4 01/07/2012
REGISTRATION AND SURVEILLANCE OF MEDICAL DEVICES 2005 DECREE 4725 HEALTH RECORDS 1986 5% Of Medical Devices Were Inspected 100% Of Imported and Manufactured Medical Devices are subject to Inspection Commission reviewing concepts DECREE 2092 HEALTH RECORDS New MD: Eliptics, treadmills, hospital beds, decubitus mattress, wheelchairs Syringes, Gauze, Dialysis Equipment, Catheters. PM 06 -CAT-G 17 V 4 01/07/2012
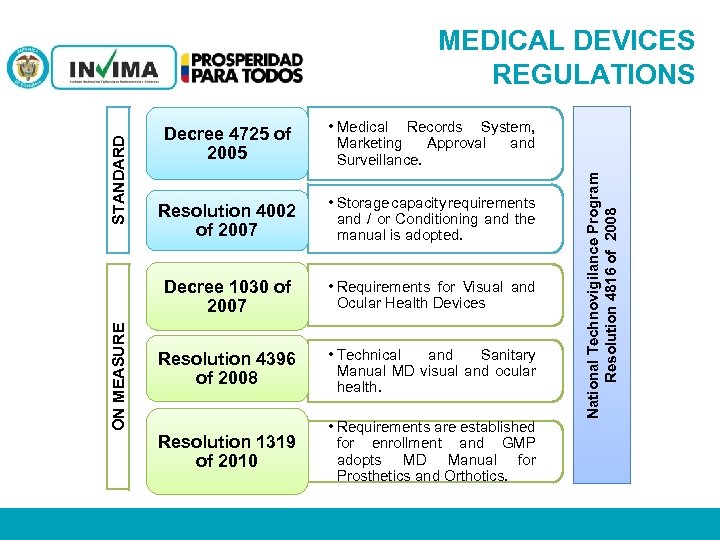 MEDICAL DEVICES REGULATIONS Resolution 4002 of 2007 • Storage capacity requirements and / or Conditioning and the manual is adopted. • Requirements for Visual and Ocular Health Devices Resolution 4396 of 2008 • Technical and Sanitary Manual MD visual and ocular health. Resolution 1319 of 2010 • Requirements are established for enrollment and GMP adopts MD Manual for Prosthetics and Orthotics. PM 06 -CAT-G 17 V 4 01/07/2012 National Technovigilance Program Resolution 4816 of 2008 STANDARD • Medical Records System, Marketing Approval and Surveillance. Decree 1030 of 2007 ON MEASURE Decree 4725 of 2005
MEDICAL DEVICES REGULATIONS Resolution 4002 of 2007 • Storage capacity requirements and / or Conditioning and the manual is adopted. • Requirements for Visual and Ocular Health Devices Resolution 4396 of 2008 • Technical and Sanitary Manual MD visual and ocular health. Resolution 1319 of 2010 • Requirements are established for enrollment and GMP adopts MD Manual for Prosthetics and Orthotics. PM 06 -CAT-G 17 V 4 01/07/2012 National Technovigilance Program Resolution 4816 of 2008 STANDARD • Medical Records System, Marketing Approval and Surveillance. Decree 1030 of 2007 ON MEASURE Decree 4725 of 2005
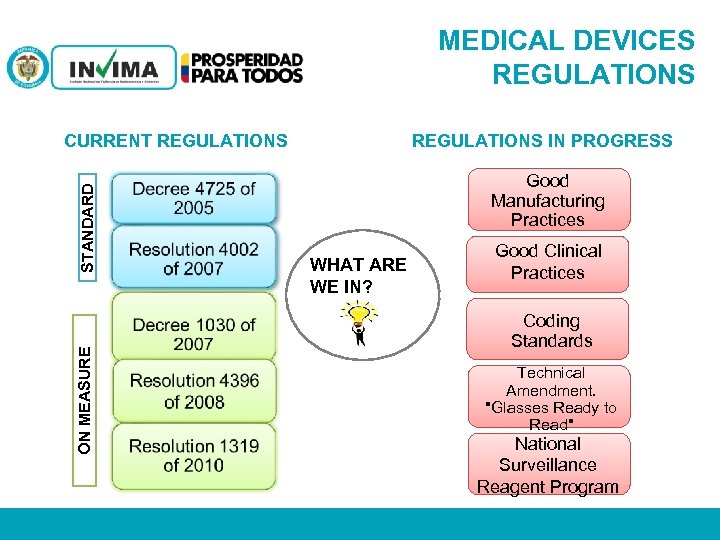 MEDICAL DEVICES REGULATIONS IN PROGRESS Good Manufacturing Practices WHAT ARE WE IN? Good Clinical Practices Coding Standards ON MEASURE STANDARD CURRENT REGULATIONS Technical Amendment. "Glasses Ready to Read" National Surveillance Reagent Program PM 06 -CAT-G 17 V 4 01/07/2012
MEDICAL DEVICES REGULATIONS IN PROGRESS Good Manufacturing Practices WHAT ARE WE IN? Good Clinical Practices Coding Standards ON MEASURE STANDARD CURRENT REGULATIONS Technical Amendment. "Glasses Ready to Read" National Surveillance Reagent Program PM 06 -CAT-G 17 V 4 01/07/2012
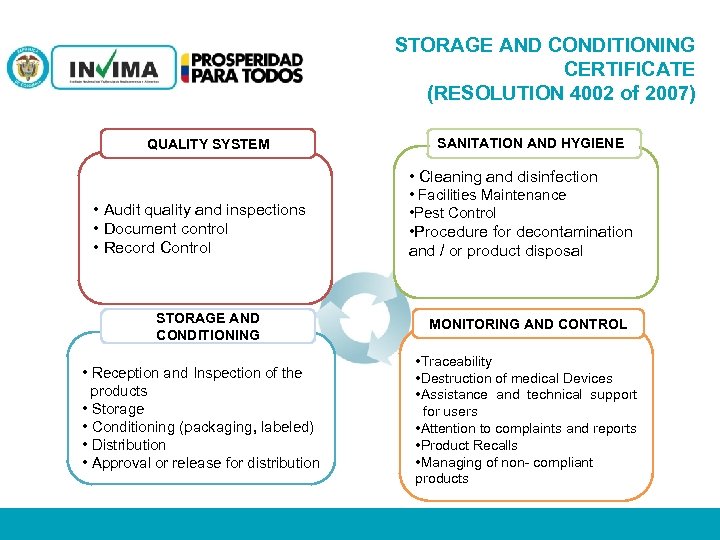 STORAGE AND CONDITIONING CERTIFICATE (RESOLUTION 4002 of 2007) SANITATION AND HYGIENE QUALITY SYSTEM • Cleaning and disinfection • Audit quality and inspections • Document control • Record Control STORAGE AND CONDITIONING • Facilities Maintenance • Pest Control • Procedure for decontamination and / or product disposal SANEAMIENTO E HIGIENE MONITORING AND CONTROL • Reception and Inspection of the products • Storage • Conditioning (packaging, labeled) • Distribution • Approval or release for distribution • Traceability • Destruction of medical Devices • Assistance and technical support for users • Attention to complaints and reports • Product Recalls • Managing of non- compliant products PM 06 -CAT-G 17 V 4 01/07/2012
STORAGE AND CONDITIONING CERTIFICATE (RESOLUTION 4002 of 2007) SANITATION AND HYGIENE QUALITY SYSTEM • Cleaning and disinfection • Audit quality and inspections • Document control • Record Control STORAGE AND CONDITIONING • Facilities Maintenance • Pest Control • Procedure for decontamination and / or product disposal SANEAMIENTO E HIGIENE MONITORING AND CONTROL • Reception and Inspection of the products • Storage • Conditioning (packaging, labeled) • Distribution • Approval or release for distribution • Traceability • Destruction of medical Devices • Assistance and technical support for users • Attention to complaints and reports • Product Recalls • Managing of non- compliant products PM 06 -CAT-G 17 V 4 01/07/2012
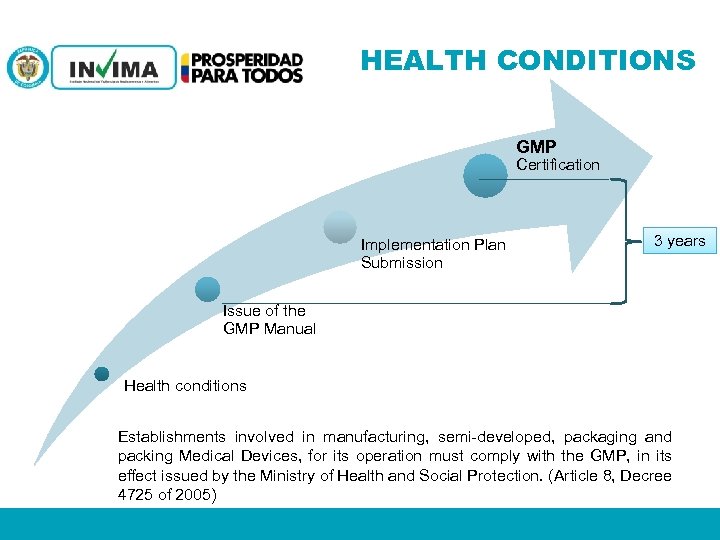 HEALTH CONDITIONS GMP Certification Implementation Plan Submission 3 years Issue of the GMP Manual Health conditions Establishments involved in manufacturing, semi-developed, packaging and packing Medical Devices, for its operation must comply with the GMP, in its effect issued by the Ministry of Health and Social Protection. (Article 8, Decree 4725 of 2005) PM 06 -CAT-G 17 V 4 01/07/2012
HEALTH CONDITIONS GMP Certification Implementation Plan Submission 3 years Issue of the GMP Manual Health conditions Establishments involved in manufacturing, semi-developed, packaging and packing Medical Devices, for its operation must comply with the GMP, in its effect issued by the Ministry of Health and Social Protection. (Article 8, Decree 4725 of 2005) PM 06 -CAT-G 17 V 4 01/07/2012
 Good Manufacturing Practices - GMP Manual of Good Manufacturing Practices for Medical Devices. Ministry of Health and Social Protection - INVIMA. Bearing in mind the International Standards ISO 13485 and ISO 14971. Directive 93/42/EEC, and International Countries Concerning FDA, ANMAT. Proposed Resolution Designed Annex 1. Manual of Good Practices Designed Annex 2. Inspection and Certification Guide PM 06 -CAT-G 17 V 4 01/07/2012 WHAT ARE WE IN?
Good Manufacturing Practices - GMP Manual of Good Manufacturing Practices for Medical Devices. Ministry of Health and Social Protection - INVIMA. Bearing in mind the International Standards ISO 13485 and ISO 14971. Directive 93/42/EEC, and International Countries Concerning FDA, ANMAT. Proposed Resolution Designed Annex 1. Manual of Good Practices Designed Annex 2. Inspection and Certification Guide PM 06 -CAT-G 17 V 4 01/07/2012 WHAT ARE WE IN?
 EXTERNAL ORTHOPEDIC PROSTHETIC AND ORTHOTICS (RESOLUTION 1319 OF 2010) Manual of Good Manufacturing Practices for the development and adjustment of prescription medical devices for orthopedics prosthetic and orthotics, Ministry of Health and Social Protection - INVIMA Competencies – Health Secretaries Profile and requirements for the Technical Department The time of registration for manufacturers (Adding 3 months) PM 06 -CAT-G 17 V 4 01/07/2012 WHAT ARE WE IN?
EXTERNAL ORTHOPEDIC PROSTHETIC AND ORTHOTICS (RESOLUTION 1319 OF 2010) Manual of Good Manufacturing Practices for the development and adjustment of prescription medical devices for orthopedics prosthetic and orthotics, Ministry of Health and Social Protection - INVIMA Competencies – Health Secretaries Profile and requirements for the Technical Department The time of registration for manufacturers (Adding 3 months) PM 06 -CAT-G 17 V 4 01/07/2012 WHAT ARE WE IN?
 GOOD CLINICAL PRACTICE - GCP Legislative project is looking for regulating the use of medical devices in human research and medical device prototype. Creation of Proposed Resolution PM 06 -CAT-G 17 V 4 01/07/2012 WHAT ARE WE IN?
GOOD CLINICAL PRACTICE - GCP Legislative project is looking for regulating the use of medical devices in human research and medical device prototype. Creation of Proposed Resolution PM 06 -CAT-G 17 V 4 01/07/2012 WHAT ARE WE IN?
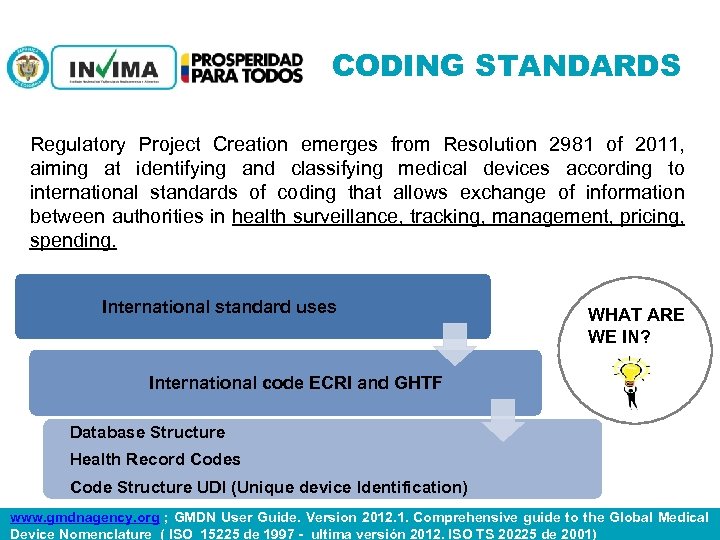 CODING STANDARDS Regulatory Project Creation emerges from Resolution 2981 of 2011, aiming at identifying and classifying medical devices according to international standards of coding that allows exchange of information between authorities in health surveillance, tracking, management, pricing, spending. International standard uses WHAT ARE WE IN? International code ECRI and GHTF Database Structure Health Record Codes Code Structure UDI (Unique device Identification) PM 06 -CAT-G 17 V 4 01/07/2012 www. gmdnagency. org ; GMDN User Guide. Version 2012. 1. Comprehensive guide to the Global Medical Device Nomenclature ( ISO 15225 de 1997 - ultima versión 2012. ISO TS 20225 de 2001)
CODING STANDARDS Regulatory Project Creation emerges from Resolution 2981 of 2011, aiming at identifying and classifying medical devices according to international standards of coding that allows exchange of information between authorities in health surveillance, tracking, management, pricing, spending. International standard uses WHAT ARE WE IN? International code ECRI and GHTF Database Structure Health Record Codes Code Structure UDI (Unique device Identification) PM 06 -CAT-G 17 V 4 01/07/2012 www. gmdnagency. org ; GMDN User Guide. Version 2012. 1. Comprehensive guide to the Global Medical Device Nomenclature ( ISO 15225 de 1997 - ultima versión 2012. ISO TS 20225 de 2001)
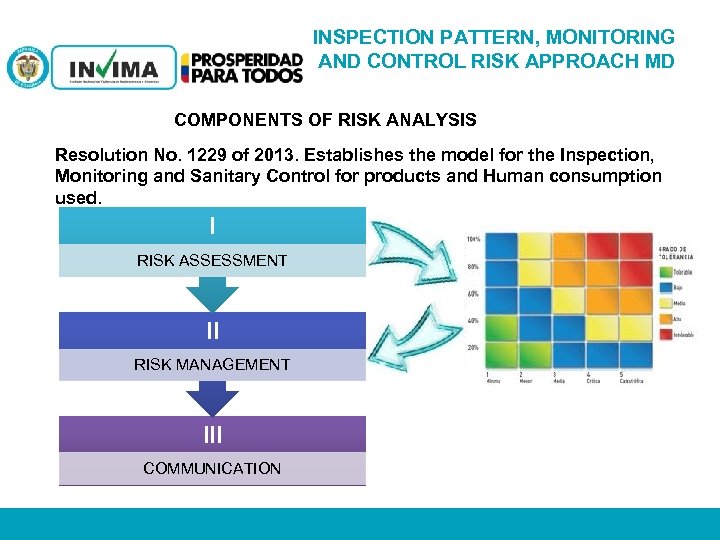 INSPECTION PATTERN, MONITORING AND CONTROL RISK APPROACH MD COMPONENTS OF RISK ANALYSIS Resolution No. 1229 of 2013. Establishes the model for the Inspection, Monitoring and Sanitary Control for products and Human consumption used. I RISK ASSESSMENT II RISK MANAGEMENT III COMMUNICATION PM 06 -CAT-G 17 V 4 01/07/2012
INSPECTION PATTERN, MONITORING AND CONTROL RISK APPROACH MD COMPONENTS OF RISK ANALYSIS Resolution No. 1229 of 2013. Establishes the model for the Inspection, Monitoring and Sanitary Control for products and Human consumption used. I RISK ASSESSMENT II RISK MANAGEMENT III COMMUNICATION PM 06 -CAT-G 17 V 4 01/07/2012
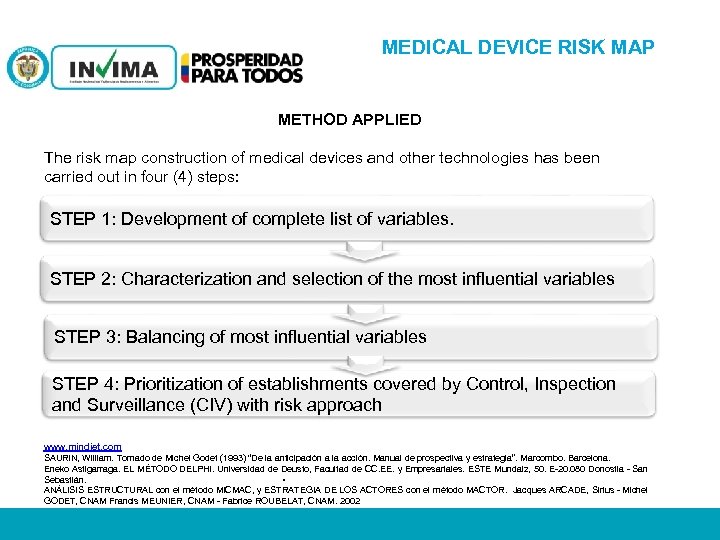 MEDICAL DEVICE RISK MAP METHOD APPLIED The risk map construction of medical devices and other technologies has been carried out in four (4) steps: STEP 1: Development of complete list of variables. STEP 2: Characterization and selection of the most influential variables STEP 3: Balancing of most influential variables STEP 4: Prioritization of establishments covered by Control, Inspection and Surveillance (CIV) with risk approach www. mindjet. com SAURIN, William. Tomado de Michel Godet (1993) “De la anticipación a la acción. Manual de prospectiva y estrategia”. Marcombo. Barcelona. Eneko Astigarraga. EL MÉTODO DELPHI. Universidad de Deusto, Facultad de CC. EE. y Empresariales. ESTE Mundaiz, 50. E-20. 080 Donostia - San Sebastián. ANÁLISIS ESTRUCTURAL con el método MICMAC, y ESTRATEGIA DE LOS ACTORES con el método MACTOR. Jacques ARCADE, Sirius - Michel GODET, CNAM Francis MEUNIER, CNAM - Fabrice ROUBELAT, CNAM. 2002 . PM 06 -CAT-G 17 V 4 01/07/2012
MEDICAL DEVICE RISK MAP METHOD APPLIED The risk map construction of medical devices and other technologies has been carried out in four (4) steps: STEP 1: Development of complete list of variables. STEP 2: Characterization and selection of the most influential variables STEP 3: Balancing of most influential variables STEP 4: Prioritization of establishments covered by Control, Inspection and Surveillance (CIV) with risk approach www. mindjet. com SAURIN, William. Tomado de Michel Godet (1993) “De la anticipación a la acción. Manual de prospectiva y estrategia”. Marcombo. Barcelona. Eneko Astigarraga. EL MÉTODO DELPHI. Universidad de Deusto, Facultad de CC. EE. y Empresariales. ESTE Mundaiz, 50. E-20. 080 Donostia - San Sebastián. ANÁLISIS ESTRUCTURAL con el método MICMAC, y ESTRATEGIA DE LOS ACTORES con el método MACTOR. Jacques ARCADE, Sirius - Michel GODET, CNAM Francis MEUNIER, CNAM - Fabrice ROUBELAT, CNAM. 2002 . PM 06 -CAT-G 17 V 4 01/07/2012
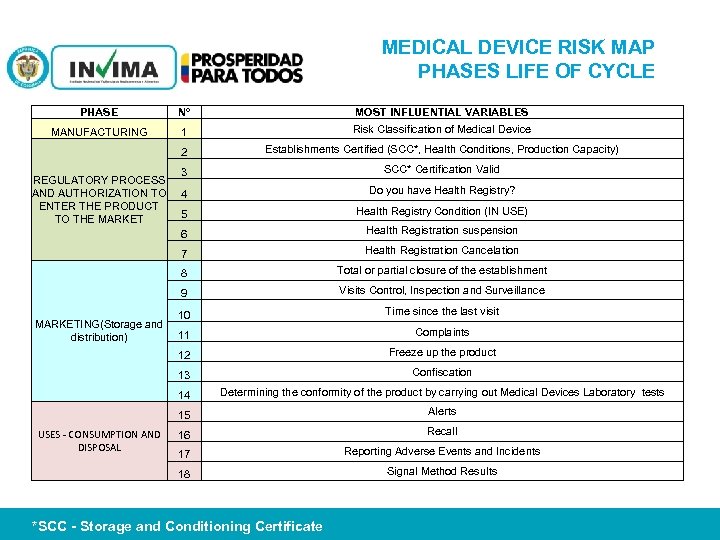 MEDICAL DEVICE RISK MAP PHASES LIFE OF CYCLE PHASE N° MOST INFLUENTIAL VARIABLES MANUFACTURING 1 Risk Classification of Medical Device 2 Establishments Certified (SCC*, Health Conditions, Production Capacity) 3 SCC* Certification Valid 4 Do you have Health Registry? 5 Health Registry Condition (IN USE) 6 Health Registration suspension 7 Health Registration Cancelation 8 Total or partial closure of the establishment 9 Visits Control, Inspection and Surveillance 10 Time since the last visit 11 Complaints 12 Freeze up the product 13 Confiscation 14 Determining the conformity of the product by carrying out Medical Devices Laboratory tests 15 Alerts 16 Recall 17 Reporting Adverse Events and Incidents 18 Signal Method Results REGULATORY PROCESS AND AUTHORIZATION TO ENTER THE PRODUCT TO THE MARKETING(Storage and distribution) USES - CONSUMPTION AND DISPOSAL PM 06 -CAT-G 17 V 4 01/07/2012 *SCC - Storage and Conditioning Certificate
MEDICAL DEVICE RISK MAP PHASES LIFE OF CYCLE PHASE N° MOST INFLUENTIAL VARIABLES MANUFACTURING 1 Risk Classification of Medical Device 2 Establishments Certified (SCC*, Health Conditions, Production Capacity) 3 SCC* Certification Valid 4 Do you have Health Registry? 5 Health Registry Condition (IN USE) 6 Health Registration suspension 7 Health Registration Cancelation 8 Total or partial closure of the establishment 9 Visits Control, Inspection and Surveillance 10 Time since the last visit 11 Complaints 12 Freeze up the product 13 Confiscation 14 Determining the conformity of the product by carrying out Medical Devices Laboratory tests 15 Alerts 16 Recall 17 Reporting Adverse Events and Incidents 18 Signal Method Results REGULATORY PROCESS AND AUTHORIZATION TO ENTER THE PRODUCT TO THE MARKETING(Storage and distribution) USES - CONSUMPTION AND DISPOSAL PM 06 -CAT-G 17 V 4 01/07/2012 *SCC - Storage and Conditioning Certificate
 II. CHALLENGES AND OPPORTUNITIES FOR STRENGTHENING THE REGULATION OF DEVICES IN THE REGIONAL AREA PM 06 -CAT-G 17 V 4 01/07/2012
II. CHALLENGES AND OPPORTUNITIES FOR STRENGTHENING THE REGULATION OF DEVICES IN THE REGIONAL AREA PM 06 -CAT-G 17 V 4 01/07/2012
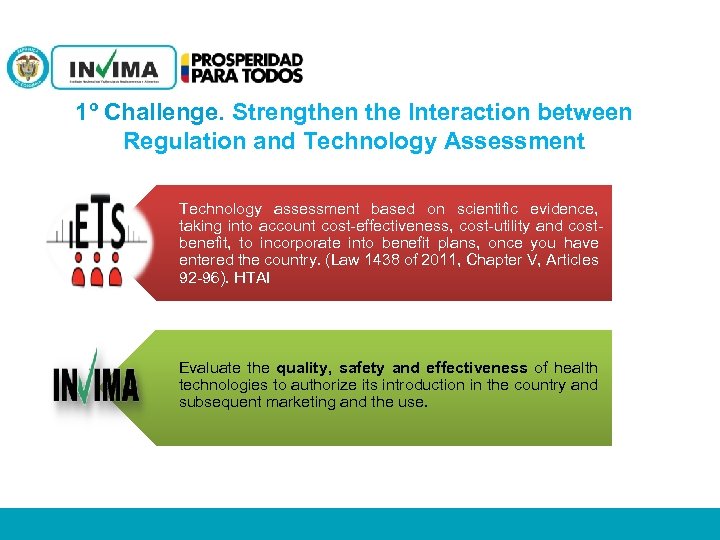 1º Challenge. Strengthen the Interaction between Regulation and Technology Assessment Technology assessment based on scientific evidence, taking into account cost-effectiveness, cost-utility and costbenefit, to incorporate into benefit plans, once you have entered the country. (Law 1438 of 2011, Chapter V, Articles 92 -96). HTAI Evaluate the quality, safety and effectiveness of health technologies to authorize its introduction in the country and subsequent marketing and the use. PM 06 -CAT-G 17 V 4 01/07/2012
1º Challenge. Strengthen the Interaction between Regulation and Technology Assessment Technology assessment based on scientific evidence, taking into account cost-effectiveness, cost-utility and costbenefit, to incorporate into benefit plans, once you have entered the country. (Law 1438 of 2011, Chapter V, Articles 92 -96). HTAI Evaluate the quality, safety and effectiveness of health technologies to authorize its introduction in the country and subsequent marketing and the use. PM 06 -CAT-G 17 V 4 01/07/2012
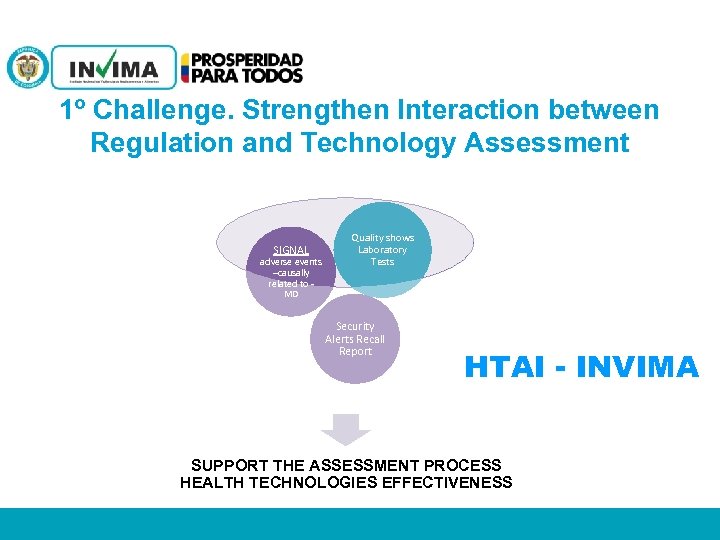 1º Challenge. Strengthen Interaction between Regulation and Technology Assessment SIGNAL adverse events –causally related to MD Quality shows Laboratory Tests Security Alerts Recall Report HTAI - INVIMA SUPPORT THE ASSESSMENT PROCESS HEALTH TECHNOLOGIES EFFECTIVENESS PM 06 -CAT-G 17 V 4 01/07/2012
1º Challenge. Strengthen Interaction between Regulation and Technology Assessment SIGNAL adverse events –causally related to MD Quality shows Laboratory Tests Security Alerts Recall Report HTAI - INVIMA SUPPORT THE ASSESSMENT PROCESS HEALTH TECHNOLOGIES EFFECTIVENESS PM 06 -CAT-G 17 V 4 01/07/2012
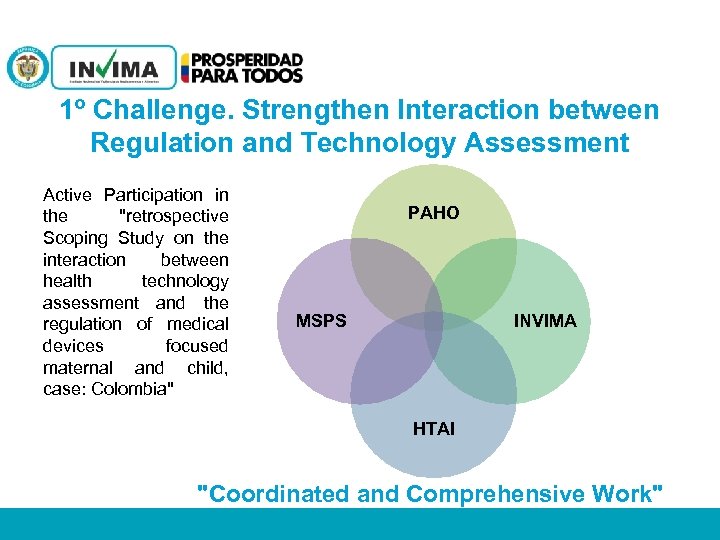 1º Challenge. Strengthen Interaction between Regulation and Technology Assessment Active Participation in the "retrospective Scoping Study on the interaction between health technology assessment and the regulation of medical devices focused maternal and child, case: Colombia" PAHO MSPS INVIMA HTAI "Coordinated and Comprehensive Work" PM 06 -CAT-G 17 V 4 01/07/2012
1º Challenge. Strengthen Interaction between Regulation and Technology Assessment Active Participation in the "retrospective Scoping Study on the interaction between health technology assessment and the regulation of medical devices focused maternal and child, case: Colombia" PAHO MSPS INVIMA HTAI "Coordinated and Comprehensive Work" PM 06 -CAT-G 17 V 4 01/07/2012
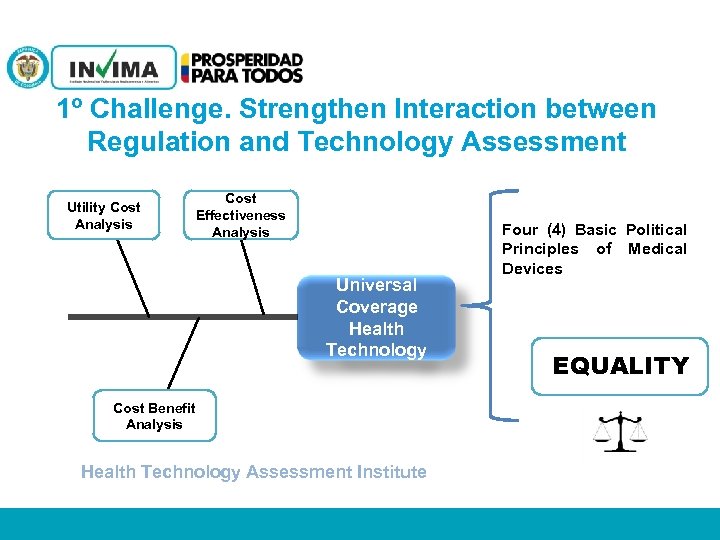 1º Challenge. Strengthen Interaction between Regulation and Technology Assessment Utility Cost Analysis Cost Effectiveness Analysis Universal Coverage Health Technology Cost Benefit Analysis Health Technology Assessment Institute PM 06 -CAT-G 17 V 4 01/07/2012 Four (4) Basic Political Principles of Medical Devices EQUALITY
1º Challenge. Strengthen Interaction between Regulation and Technology Assessment Utility Cost Analysis Cost Effectiveness Analysis Universal Coverage Health Technology Cost Benefit Analysis Health Technology Assessment Institute PM 06 -CAT-G 17 V 4 01/07/2012 Four (4) Basic Political Principles of Medical Devices EQUALITY
 2º Challenge. National Medical Device Policy The national health technology policy must consider four (4) basic principles: • Availability (ready to use) • Accessibility (easy access) • Appropriateness (Adequate and appropriate) EQUALITY • Affordability (can be achieved) PM 06 -CAT-G 17 V 4 01/07/2012 22
2º Challenge. National Medical Device Policy The national health technology policy must consider four (4) basic principles: • Availability (ready to use) • Accessibility (easy access) • Appropriateness (Adequate and appropriate) EQUALITY • Affordability (can be achieved) PM 06 -CAT-G 17 V 4 01/07/2012 22
 Tecnología Biomédica 2º Challenge. National Medical Device Policy Three (3) Components: I Strategic, conceptual and policy Components • • • view strategies action plans indicators monitoring and evaluation system II Technology Assessment Component • • Evaluation of medical technologies applied to medical devices Medical device needs assessment PM 06 -CAT-G 17 V 4 01/07/2012
Tecnología Biomédica 2º Challenge. National Medical Device Policy Three (3) Components: I Strategic, conceptual and policy Components • • • view strategies action plans indicators monitoring and evaluation system II Technology Assessment Component • • Evaluation of medical technologies applied to medical devices Medical device needs assessment PM 06 -CAT-G 17 V 4 01/07/2012
 Tecnología Biomédica 2º Challenge. National Medical Device Policy III Regulatory and Operational Management Components of the Life cycle of Medical Devices • • Good Manufacturing Practices Good Clinical Practice Coding Medical Devices Process. Incorporation Device Procurement Process Biomedical Equipment Maintenance Process Inventory Management Biomedical Equipment Computerized maintenance management Disposal of Medical Devices Process PM 06 -CAT-G 17 V 4 01/07/2012
Tecnología Biomédica 2º Challenge. National Medical Device Policy III Regulatory and Operational Management Components of the Life cycle of Medical Devices • • Good Manufacturing Practices Good Clinical Practice Coding Medical Devices Process. Incorporation Device Procurement Process Biomedical Equipment Maintenance Process Inventory Management Biomedical Equipment Computerized maintenance management Disposal of Medical Devices Process PM 06 -CAT-G 17 V 4 01/07/2012
 Tecnología Biomédica 3º Challenge. 2013 Regulatory Agenda 1. 2. 3. 4. 5. 6. 7. 8. Good Manufacturing Practices for Medical Devices. Good Clinical Practice for Medical Devices. Coding of Medical Devices. Good Manufacturing Practices for Medical Devices on Health Hearing Aids. Amendment of Resolution 4396 of 2008. (Reading Glasses) Modification Resolution 1319 of 2010 (Prosthetics and Orthotics). Model Implementation Inspection, Monitoring and Control. National Medical Device Policy PM 06 -CAT-G 17 V 4 01/07/2012
Tecnología Biomédica 3º Challenge. 2013 Regulatory Agenda 1. 2. 3. 4. 5. 6. 7. 8. Good Manufacturing Practices for Medical Devices. Good Clinical Practice for Medical Devices. Coding of Medical Devices. Good Manufacturing Practices for Medical Devices on Health Hearing Aids. Amendment of Resolution 4396 of 2008. (Reading Glasses) Modification Resolution 1319 of 2010 (Prosthetics and Orthotics). Model Implementation Inspection, Monitoring and Control. National Medical Device Policy PM 06 -CAT-G 17 V 4 01/07/2012
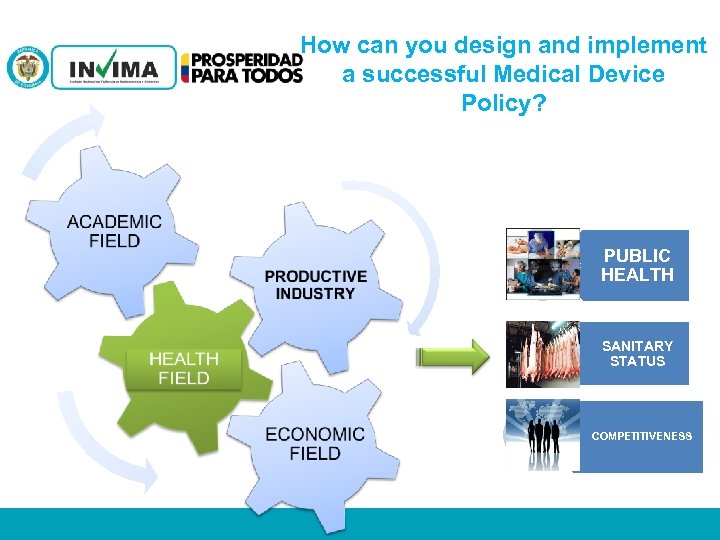 How can you design and implement a successful Medical Device Policy? PUBLIC HEALTH SANITARY STATUS COMPETITIVENESS PM 06 -CAT-G 17 V 4 01/07/2012
How can you design and implement a successful Medical Device Policy? PUBLIC HEALTH SANITARY STATUS COMPETITIVENESS PM 06 -CAT-G 17 V 4 01/07/2012
 THANK YOU FOR YOUR ATTENTION www. invima. gov. co Doctor Blanca Elvira Cajigas General Director INVIMA Email: bcajigasa@invima. gov. co Carrera 68 D No. 17 -11 - Bogotá, D. C. Colombia. Telephone: (1)2948700 PM 06 -CAT-G 17 V 4 01/07/2012
THANK YOU FOR YOUR ATTENTION www. invima. gov. co Doctor Blanca Elvira Cajigas General Director INVIMA Email: bcajigasa@invima. gov. co Carrera 68 D No. 17 -11 - Bogotá, D. C. Colombia. Telephone: (1)2948700 PM 06 -CAT-G 17 V 4 01/07/2012


