 Regulation for Aquaculture Medicine 8 July 2015 Dr. Sasi Jaroenpioj Department of Livestock Medicine THAILAND
Regulation for Aquaculture Medicine 8 July 2015 Dr. Sasi Jaroenpioj Department of Livestock Medicine THAILAND
 Regulations under Drug Act Pre-marketing Control • Licensing • Registration • Adversetisment Control Post-marketing Control • Licensee’ , Pharmacists’ and Drug store surveillance as mentioned in the Act • GMP Audit • Drug Surveillance • ADR Monitoring • Drug Reevaluation
Regulations under Drug Act Pre-marketing Control • Licensing • Registration • Adversetisment Control Post-marketing Control • Licensee’ , Pharmacists’ and Drug store surveillance as mentioned in the Act • GMP Audit • Drug Surveillance • ADR Monitoring • Drug Reevaluation
 List of licensed antimicrobials for use in aquatic animal in Thailand • • • 3/19/2018 Amoxycillin Enrofloxcin Sarafloxacin Oxolinic acid Oxytetracycline Sulfadimethoxine sodium / Ormethoprim Sulfadimethoxine sodium / Trimethoprim Sulfadiazine and Trimethoprim Sulfadimidine and Trimetroprim Sulfamonomethoxine Sodium Toltrazuril
List of licensed antimicrobials for use in aquatic animal in Thailand • • • 3/19/2018 Amoxycillin Enrofloxcin Sarafloxacin Oxolinic acid Oxytetracycline Sulfadimethoxine sodium / Ormethoprim Sulfadimethoxine sodium / Trimethoprim Sulfadiazine and Trimethoprim Sulfadimidine and Trimetroprim Sulfamonomethoxine Sodium Toltrazuril
 List of banned antimicrobials in aquatic animal in Thailand • Chloramphenical • Nitrofuran and their Metabolites (Furazolidone, Furaltadone, Nitrofurazone and Nitrofurantoin) • Malachite green and Leuco-malachite green 3/19/2018
List of banned antimicrobials in aquatic animal in Thailand • Chloramphenical • Nitrofuran and their Metabolites (Furazolidone, Furaltadone, Nitrofurazone and Nitrofurantoin) • Malachite green and Leuco-malachite green 3/19/2018
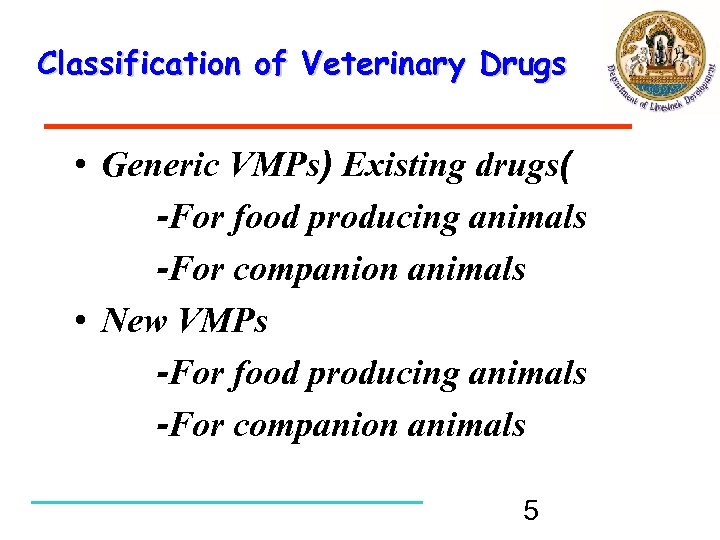 Classification of Veterinary Drugs • Generic VMPs) Existing drugs( -For food producing animals -For companion animals • New VMPs -For food producing animals -For companion animals 5
Classification of Veterinary Drugs • Generic VMPs) Existing drugs( -For food producing animals -For companion animals • New VMPs -For food producing animals -For companion animals 5
 VMPs Registration Procedure 6
VMPs Registration Procedure 6
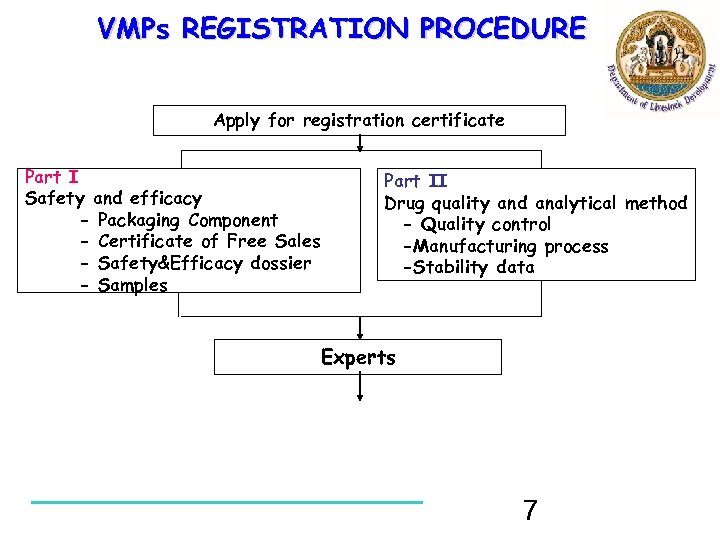 VMPs REGISTRATION PROCEDURE Apply for registration certificate Part I Safety and efficacy - Packaging Component - Certificate of Free Sales - Safety&Efficacy dossier - Samples Part II Drug quality and analytical method - Quality control -Manufacturing process -Stability data Experts 7
VMPs REGISTRATION PROCEDURE Apply for registration certificate Part I Safety and efficacy - Packaging Component - Certificate of Free Sales - Safety&Efficacy dossier - Samples Part II Drug quality and analytical method - Quality control -Manufacturing process -Stability data Experts 7
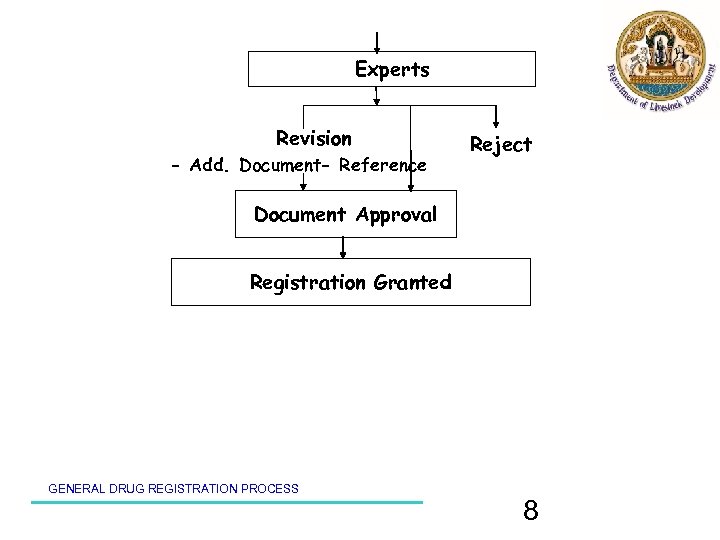 Experts Revision - Add. Document- Reference Reject Document Approval Registration Granted GENERAL DRUG REGISTRATION PROCESS 8
Experts Revision - Add. Document- Reference Reject Document Approval Registration Granted GENERAL DRUG REGISTRATION PROCESS 8
 Document required for New VMPs for food producing spp. submission 9
Document required for New VMPs for food producing spp. submission 9
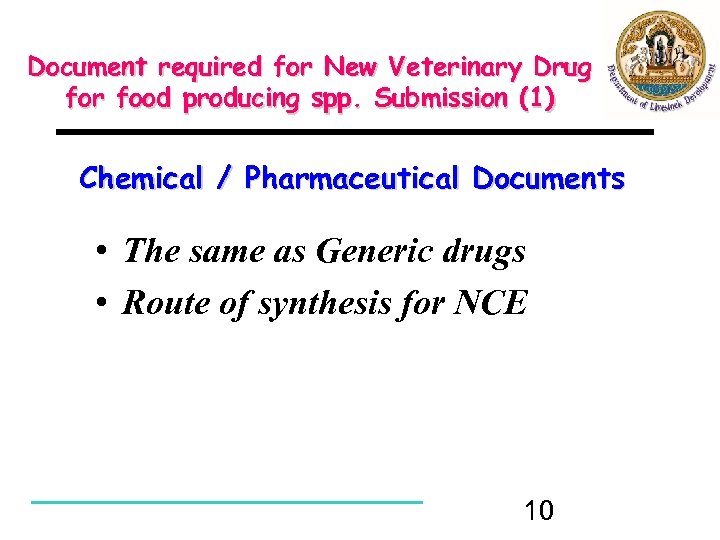 Document required for New Veterinary Drug for food producing spp. Submission (1) Chemical / Pharmaceutical Documents • The same as Generic drugs • Route of synthesis for NCE 10
Document required for New Veterinary Drug for food producing spp. Submission (1) Chemical / Pharmaceutical Documents • The same as Generic drugs • Route of synthesis for NCE 10
 Document required for New Veterinary Drug for food producing spp. Submission (2) Pharmacological Documents Pharmacokinetics Pharmacodynamics 11
Document required for New Veterinary Drug for food producing spp. Submission (2) Pharmacological Documents Pharmacokinetics Pharmacodynamics 11
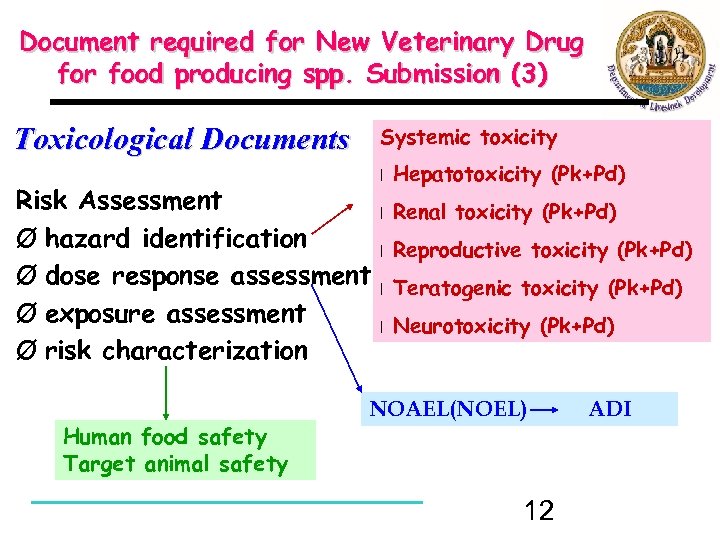 Document required for New Veterinary Drug for food producing spp. Submission (3) Toxicological Documents Systemic toxicity Risk Assessment Ø hazard identification Ø dose response assessment Ø exposure assessment Ø risk characterization Human food safety Target animal safety l Hepatotoxicity (Pk+Pd) l Renal toxicity (Pk+Pd) l Reproductive toxicity (Pk+Pd) l Teratogenic toxicity (Pk+Pd) l Neurotoxicity (Pk+Pd) NOAEL(NOEL) 12 ADI
Document required for New Veterinary Drug for food producing spp. Submission (3) Toxicological Documents Systemic toxicity Risk Assessment Ø hazard identification Ø dose response assessment Ø exposure assessment Ø risk characterization Human food safety Target animal safety l Hepatotoxicity (Pk+Pd) l Renal toxicity (Pk+Pd) l Reproductive toxicity (Pk+Pd) l Teratogenic toxicity (Pk+Pd) l Neurotoxicity (Pk+Pd) NOAEL(NOEL) 12 ADI
 Document required for New Veterinary Drug for food producing spp. Submission (4) Safety - Human Safety - Target Animal Safety - Environmental Safety - User Safety - The analytical method to determine the drug in edible tissue 13
Document required for New Veterinary Drug for food producing spp. Submission (4) Safety - Human Safety - Target Animal Safety - Environmental Safety - User Safety - The analytical method to determine the drug in edible tissue 13
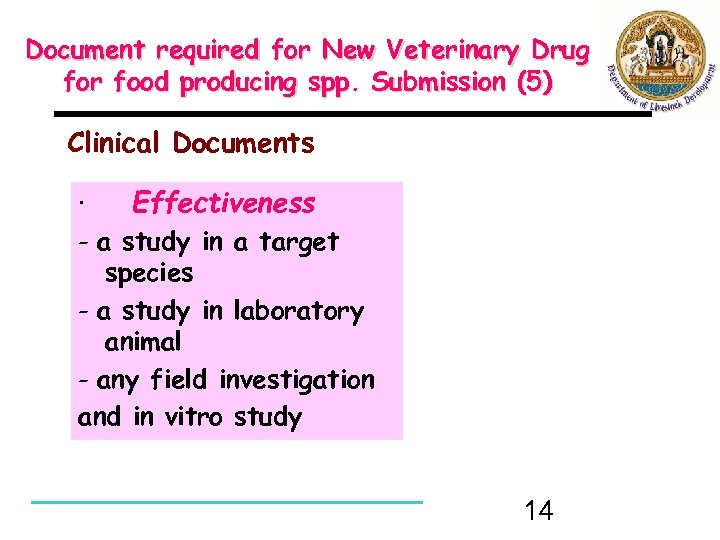 Document required for New Veterinary Drug for food producing spp. Submission (5) Clinical Documents · Effectiveness - a study in a target species - a study in laboratory animal - any field investigation and in vitro study 14
Document required for New Veterinary Drug for food producing spp. Submission (5) Clinical Documents · Effectiveness - a study in a target species - a study in laboratory animal - any field investigation and in vitro study 14
 Thank you
Thank you